Abstract
Cuckoo–host interactions provide classical examples of coevolution. Cuckoos place hosts under selection to detect and reject foreign eggs, while host defences result in the evolution of host-egg mimicry in cuckoos. Despite a long history of research, egg pattern mimicry has never been objectively quantified, and so its coevolution with host defences has not been properly assessed. Here, we use digital image analysis and modelling of avian vision to quantify the level of pattern mimicry in eight host species of the common cuckoo Cuculus canorus and their respective cuckoo host-races. We measure a range of pattern attributes, including marking size, diversity in size, contrast, coverage and dispersion. This new technique reveals hitherto unnoticed sophistication in egg pattern mimicry. We show that various features of host egg pattern are mimicked by the eggs of their respective cuckoo host-races, and that cuckoos have evolved better pattern mimicry for host species that exhibit stronger egg rejection. Pattern differs relatively more between eggs of different host species than between their respective cuckoo host-races. We suggest that cuckoos may have more ‘average’ markings in order to be able to use subsidiary hosts. Our study sheds new light on cuckoo–host coevolution and illustrates a new technique for quantifying animal markings with respect to the relevant animal visual system.
Keywords: pattern mimicry, brood parasitism, bird vision, egg rejection, cuckoos, digital image analysis
1. Introduction
Brood parasitic birds lay their eggs in the nest of another species, so that the parasitized parents rear the foreign young to fledging (Davies 2000). Coevolution between brood parasites and their hosts provides many classical examples of evolutionary arms races, whereby selection pressure imposed by parasitism leads to host adaptations to detect and reject parasitic young, and to parasite counter-adaptations, such as egg mimicry (Rothstein 1990). Arguably, the most extensively studied brood parasite is the common cuckoo Cuculus canorus, which comprises several different host-races or gentes, with each female cuckoo specializing on a particular host species. To human eyes, females of a given gens often (but not always) lay an egg that mimics the appearance of the host egg in both colour and pattern, because many hosts have evolved the ability to discriminate accurately between their own and a foreign egg (Brooke & Davies 1988).
Although there have been a number of studies of egg mimicry in brood parasites, especially common cuckoos (e.g. Brooke & Davies 1988; Davies & Brooke 1989), the vast majority of work has been based on human assessments of colour and pattern, despite the well-known differences between human and avian vision (Bennett et al. 1994). Recently, several studies have analysed the level of match between foreign and host eggs using models of avian colour and luminance visual discrimination (Avilés 2008; Cassey et al. 2008; Langmore et al. 2009). Such models more accurately predict differences in egg appearance and egg rejection behaviour in hosts than do human assessments and can ‘revolutionize the investigation of host–brood parasite relationships’ (Safran & Vitousek 2008). However, previous work indicates that pattern also plays a crucial role in egg rejection in a range of brood parasitic systems (e.g. Lahti & Lahti 2002; López-de-Hierro & Moreno-Rueda in press). While many biologists have adopted methods of studying the colour and luminance of visual signals from the correct receiver's perspective (or at least objectively), it is still strikingly rare to find quantifications of animal patterns not based on human subjective assessment (but see for example: Godfrey et al. 1987; Stevens & Cuthill 2006; Barbosa et al. 2008). Analyses of egg pattern have almost always been based on human vision, with quantification of egg markings based on human-produced ordinal rankings of spottiness or dispersion, either from the eggs themselves (e.g. Davies & Brooke 1989; Moksnes & Røskaft 1995; Gosler et al. 2000) or based on apparently uncalibrated photographs (e.g. Nguyen et al. 2007; Sanz & García-Navas 2009). Just as human subjective assessments of visual signals are inappropriate with respect to colour and luminance (Bennett et al. 1994; Safran & Vitousek 2008), the same is likely to be true for pattern. The lack of research into the function of the two- or three-dimensional patterning of markings on an object represents a key shortcoming of current work on visual signals. Common methods used to obtain objective colour information (e.g. reflectance spectrometry) are unsuited to the task of capturing complex patterns (Stevens et al. 2007), yet advances in digital photography, computer vision and image-processing now provide a suite of ideal techniques to quantify objectively two-dimensional visual signals, and can be analysed in conjunction with specific models of visual processing (Godfrey et al. 1987; Stevens & Cuthill 2006; Stevens et al. 2007).
Here, for the first time, to our knowledge, we use methods based on avian visual perception and digital image analysis to quantify egg pattern mimicry between a brood parasite and its main hosts. We do this in the common cuckoo and eight of its principal hosts, and investigate the extent to which the level of pattern match between cuckoo and host eggs can be explained by host rejection rates reported in the literature (Avilés & Garamszegi 2007).
2. Material and methods
(a). Data collection
We photographed 205 parasitized clutches of host eggs held in the Natural History Museum (NHM; Tring, Hertfordshire, UK), with clutches belonging to eight principal cuckoo hosts in Europe: great reed warbler (Acrocephalus arundinaceus, n = 27: all from Hungary), reed warbler (Acrocephalus scirpaceus, n = 29: all from England), meadow pipit (Anthus pratensis, n = 30: all from England), brambling (Fringilla montifringilla, n = 13: 12 from Finland, 1 Russia), red-backed shrike (Lanius collurio, n = 26: 16 from Germany, 6 England, 2 Czech Republic, 1 Hungary, 1 Pomerania), pied wagtail (Motacilla alba, n = 28: all from England), dunnock (Prunella modularis, n = 30: all from England) and garden warbler (Sylvia borin, n=22: 10 from Germany, 6 England, 2 Czech Republic, 2 Pomerania, 1 France, 1 Poland). Almost all eggs were collected between 1880 and 1940, with more than half collected between 1880 and 1910. Egg pigmentation may be affected by fading or variable environmental conditions (Avilés et al. 2007). However, the effects of these potential sources of bias were probably limited because eggs (i) were stored in the dark under controlled conditions to minimize fading, and (ii) were sampled from many different localities and in different years. To avoid measuring more than one cuckoo egg laid by the same female, we selected clutches from different localities. When overlap by locality was unavoidable, we only used clutches obtained several years apart or by different collectors. We photographed the entire clutch, but randomly chose one host egg per clutch for subsequent analyses.
(b). Image acquisition and calibration
Images were taken with a Fujifilm IS Pro ultraviolet (UV)-sensitive digital camera with a quartz CoastalOpt UV lens (Coastal Optical Systems), fitted with a UV and infrared (IR) blocking filter for photographs in the human visible spectrum (Baader UV/IR Cut filter; transmitting between 400 and 700 nm), and with a UV pass filter (Baader U filter; transmitting between 300 and 400 nm) for the UV images. Each image included a Spectralon grey reflectance standard (Labsphere, Congleton, UK), reflecting light equally at 40 per cent between 300 and 750 nm. All images were taken at the same distance and angle from the eggs, and therefore all markings were at the same scale. Two UV-emitting lamps (Kaiser RB260 Digital Lighting Unit) kept at a fixed distance from the eggs provided standard, constant illumination. Each image was linearized with respect to light intensity, because most cameras show a nonlinear response in image value with changes in radiance (see Stevens et al. (2007) for details). Generally, perception of pattern and texture is primarily a function of achromatic (luminance) vision; in birds, evidence indicates that luminance is encoded by the double cones (Jones & Osorio 2004; Osorio & Vorobyev 2005). Therefore, we analysed pattern just in terms of this luminance channel, but also undertook analysis to confirm that we were not missing pattern information in other parts of the avian visible spectrum (see electronic supplementary material). Since we know the spectral sensitivity of our camera set-up (M. Stevens 2007, unpublished data), the images were transformed from camera colour space to correspond to the relative photon catches of a bird's double cones (Stevens & Cuthill 2006; Stevens et al. 2007), using the spectral sensitivity of a blue tit Cyanistes caeruleus (Hart et al. 2000). The blue tit is a relatively well studied bird in terms of its visual system, and seems representative of most higher passerines (Hart & Hunt 2007). All calibrations and pattern analyses were undertaken with self-written programmes in MATLAB1 (The MathWorks, Inc., MA, USA) and its associated Image Processing toolbox.
For each image of an egg, we extracted three sub-images of equal size (sizes identical across all eggs analysed), corresponding to the upper, middle and base sections (thirds) of the eggs. We initially analysed these regions separately because characteristics of the markings can vary across these regions, with markings usually densest at the base.
(c). Pattern analysis: granularity
To analyse the pattern sizes and contrasts of the egg markings, we adopted a ‘granularity’ analysis similar to that recently used to analyse cuttlefish camouflage markings (Barbosa et al. 2008; Chiao et al. 2009), which is ideal for analysing the contribution that different marking sizes make to a given pattern. For each calibrated image of an egg region, we produced seven new images, each containing information at different spatial scales, by fast Fourier transforming the original image (Godfrey et al. 1987) and applying seven octave-wide, isotropic band-pass filters (Barbosa et al. 2008). These filters function like a sieve, capturing information at different spatial scales (different sized markings), with smaller filter sizes corresponding to larger (low spatial frequency) markings and larger filter sizes corresponding to smaller (high spatial frequency) markings. Although real visual systems do not directly filter spatial information in the same way as a Fourier transform, early-stage visual processing does break down information in a scene into different spatial frequencies by virtue of receptive fields (Campbell & Robson 1968; Godfrey et al. 1987). Adding together the seven different filtered images produces a new image that is a close approximation to the original unfiltered image, with only a small loss of information. Analysing these seven different images (‘granularity bands’; Barbosa et al. 2008) allows us to determine the relative contribution of different marking sizes to the overall egg pattern, and to quantify the level of match of each cuckoo gens and host.
After filtering an image, we calculated a range of pattern information. First, for each granularity band (1–7), we calculated the overall pattern ‘energy’ (e), as the sum of the squared pixel values in each image divided by the number of pixels in the image, with the actual scale being arbitrary (Chiao et al. 2009). The values of e across all seven band-pass filtered images produce a ‘granularity spectrum’ (Chiao et al. 2009). From each granularity spectrum, we can calculate a range of information about an egg's markings. First, we calculated the maximum value of e in the spectrum (maximum energy; emax), as the filter size containing the highest energy, which thus corresponds to the predominant marking size. We also calculated the proportion of the total energy across all scales corresponding to emax (proportion energy; eprop). This value provides a measure of how important the main marking size is to the overall egg pattern; a high value indicates that the egg pattern is dominated by this marking size. The total energy (etot) across all filter sizes corresponds to the overall amplitude of the spectrum, and provides a measure of overall pattern contrast (Chiao et al. 2009), with higher values indicating more contrasting markings.
(d). Pattern analysis: pattern coverage and dispersion
In addition to the granularity analysis, we calculated the relative proportion of each egg region covered by markings. To do this, we thresholded the calibrated images into a binary format, with a pixel value of one corresponding to a marking and zero to the background egg colour. Although it would have been ideal to threshold each image automatically/adaptively, deviations in ambient lighting and the curvature of the egg (even on the relatively ‘flatter’ regions selected) prevented this, with this approach producing highly inaccurate representations of pattern. We interactively chose a thresholding value that, to the human eye, reproduced the egg pattern coverage. Although this introduces some subjectivity, any error associated should be minor because: (i) there should be no bias towards a particular direction of marking coverage; (ii) patterns in the UV not represented in the analysed images coincided with the human visible pattern; and (iii) the actual values for pattern coverage were calculated from the thresholded images, and not by human eye. Pattern coverage was calculated as the proportion of the pixels corresponding to a marking (values of one) compared with the overall image size (total number of pixels). In addition, we calculated pattern dispersion, described by the standard deviation of pattern coverage for each of the three egg regions. A low standard deviation indicates uniform pattern coverage across the egg, while a high value indicates that one or two regions are disproportionately covered by markings.
(e). Statistical methods
For each cuckoo and host egg, we calculated marking filter size (emax), proportion energy (eprop), total energy (etot) and pattern coverage for each egg region (upper, middle, base), in addition to overall pattern dispersion among regions. Initial analyses indicated that differences between egg regions were minor for marking filter size, proportion energy and total energy (electronic supplementary material, figure S1). Consequently, we averaged these upper, middle and base measurements for each egg to yield overall measures of egg pattern and contrast. These different pattern attributes are not highly correlated with each other (electronic supplementary material, table S1). One-way analysis of variances tested whether eggs of cuckoo gentes differ significantly for each measure of pattern.
We then compared the values of cuckoos and hosts to assess egg pattern mimicry for each attribute. Following Nakagawa & Cuthill (2007), we determined the magnitude of difference between patterns for each cuckoo–host pair, and calculated Cohen's d (standardized mean difference) with regard to each pattern measure (Cohen 1988). Cohen's d is an effect size measure used to describe the overlap of distributions, with larger effect sizes indicating a smaller overlap. Following Cohen's (1988) description of ‘large’ effects (d = 0.8), we determined whether cuckoo–host pairs ‘matched’ (d < 0.8) for each pattern attribute.
Interval plots illustrate the degree of overlap for cuckoos and hosts for each measure (see electronic supplementary material, figures S2 and S3). To determine if variation between cuckoo and host eggs differed for each pattern attribute, we used Levene's tests for equal variances. Finally, we compared overall pattern mimicry to previously established rejection rates of non-mimetic eggs by hosts. Since the likelihood of rejection can vary depending on the context (such as being affected by the rate of parasitism in the population or the experience of the host; Davies 2000), we refer both to rates measured directly by Davies & Brooke (1989) and to rates calculated from several published and unpublished sources compiled in Avilés & Garamszegi (2007). When plotting rejection rate versus pattern-matching, we use rates calculated by Avilés & Garamszegi (2007) only, since this study alone provides rejection rates for all eight hosts.
3. Results
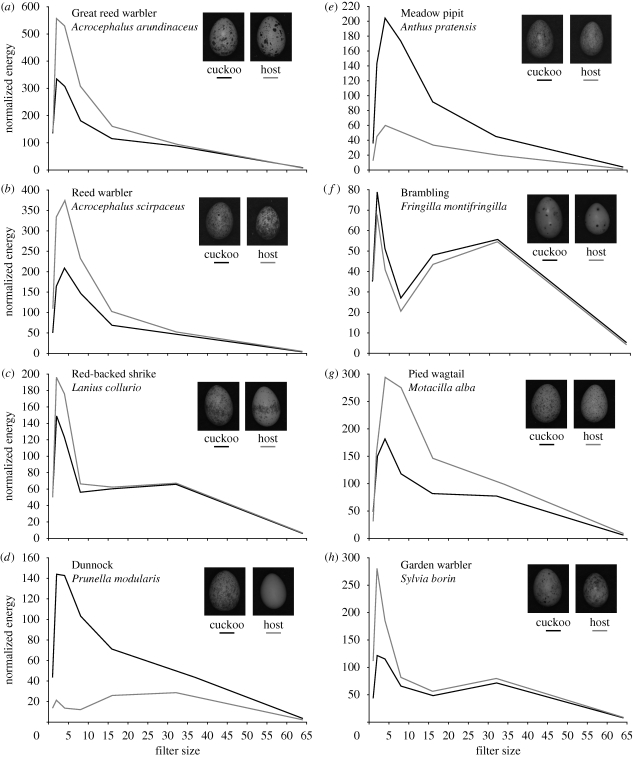
There were significant differences between cuckoo egg-morphs for all five variables measured (electronic supplementary material, table S2): marking filter size (F7,197 = 2.18, p = 0.038), proportion energy (F7,197 = 2.64, p = 0.012), total energy (F7,197 = 11.92, p < 0.001), pattern coverage (F7,197 = 23, p < 0.001), and pattern dispersion (F7,197 = 2.74, p = 0.01). Variation among host eggs was higher than the variation among the cuckoo gentes for all five pattern attributes (n = 205 for both hosts and cuckoo; electronic supplementary material, table S3): marking filter size (W = 13.77, p < 0.001), proportion energy (W = 10.14, p = 0.002), total energy (W = 34.75, p < 0.001), pattern coverage (W = 41.47, p < 0.001), and pattern dispersion (W = 8.14, p = 0.005). Granularity profiles revealed varying degrees of matching between cuckoo and host eggs, with the shape and amplitude of the cuckoo granularity spectrum yielding a nearly identical match to its host in some instances (i.e. brambling) but deviating considerably in others (see figure 1 and §4). Magnitudes of difference (Cohen's d values) showed the extent to which cuckoo and host distributions overlap for each pattern variable, with cuckoo patterns matching certain features of host pattern better than others (table 1). A comparison of egg pattern mimicry to host rejection data (Avilés & Garamszegi 2007) indicated that mimicry improves with increasingly strong host rejection (figure 2).
Figure 1.
Average granularity spectra for each host (grey lines) and its respective cuckoo gens (black lines), illustrating the contribution of different marking sizes to the given pattern. Measurements were made at the following filter sizes: 1, 2, 4, 8, 16, 32, 64. Granularity profiles show varying degrees of match between cuckoo and host eggs, with the shape and amplitude of the cuckoo granularity spectrum yielding a nearly identical match to its host in some instances (e.g. brambling) but no match in others (e.g. dunnock). We averaged the top, middle and base spectra for each egg to yield overall measures of egg pattern and contrast (electronic supplementary material, figure S1). Photographs of eggs within the figure are copyright of the NHM and were taken by Mary Caswell Stoddard.
Table 1.
Summary of the magnitudes of difference (Cohen's d values, standardized mean difference) between egg pattern attributes for each cuckoo gens and its host. (Small d values indicate a large overlap of distributions, while large d values indicate a small overlap. Following Cohen (1988), we considered smaller effect sizes (d < 0.8) to indicate ‘matches’ between cuckoo and host patterns, shown here in bold and italics.)
| host | n | marking filter size; log-transformed | proportion energy | total energy | pattern coverage | pattern dispersion |
|---|---|---|---|---|---|---|
| great reed warbler | 27 | 0.0080 | 0.6631 | 0.8960 | 0.8963 | 0.0192 |
| reed warbler | 29 | 0.0898 | 0.6338 | 1.0538 | 1.5319 | 0.9430 |
| meadow pipit | 30 | 0.0814 | 1.0509 | 1.5535 | 1.7613 | 0.0641 |
| brambling | 13 | 0.5819 | 0.1483 | 0.2135 | 0.1534 | 0.2805 |
| red-backed shrike | 26 | 0.1675 | 0.7851 | 0.4488 | 0.9743 | 1.1477 |
| pied wagtail | 28 | 0.4207 | 0.3669 | 1.3111 | 1.8642 | 0.0952 |
| dunnock | 30 | 2.9727 | 0.2005 | 1.7698 | 6.6545 | 2.0360 |
| garden warbler | 22 | 0.1363 | 1.4256 | 1.1021 | 0.0999 | 0.0076 |
Figure 2.
The relationship between the likelihood that hosts will reject non-mimetic eggs and the number of matching pattern attributes. Pattern attributes are marking (filter) size, proportion energy, total pattern contrast (energy), pattern coverage and pattern dispersion. Rejection rates are taken from Avilés & Garamszegi (2007).
The absence of egg pattern mimicry is most striking in the dunnock, which rejects non-mimetic eggs at low rates ranging from 2 per cent (Avilés & Garamszegi 2007) to 6 per cent (Davies & Brooke 1989). The lack of pattern mimicry is evident in the divergent granularity profiles of the cuckoo and host (figure 1d), and because the dunnock-cuckoo egg matches its host in only one of the five pattern characteristics (table 1). Cuckoos parasitizing the meadow pipit, with moderate rejection rates between 18 per cent (Avilés & Garamszegi 2007) and 48 per cent (Davies & Brooke 1989), lay eggs that match host pattern in two categories: marking size and pattern dispersion (table 1), and have granularity profiles only roughly following the host spectrum shape (figure 1e). The reed warbler also has moderate egg rejection rates, from 31 per cent (Avilés & Garamszegi 2007) to 62 per cent (Davies & Brooke 1989), and its corresponding cuckoo egg matches the host pattern in marking size and proportion energy (table 1). Granularity profiles match in general shape, particularly at higher filter sizes (i.e. smaller markings; figure 1b). Cuckoos parasitizing the garden warbler, with a high rejection rate of 67 per cent (Avilés & Garamszegi 2007), match the host pattern in marking size, pattern coverage and pattern dispersion (table 1), but have a poor match in contrast (figure 1h). The great reed warbler rejects non-mimetic eggs at a high rate of 88 per cent (Avilés & Garamszegi 2007), and its cuckoo gens matches host egg pattern in marking size, proportion energy and pattern dispersion (table 1), and matches the host granularity spectra well in shape, particularly at higher filter sizes (figure 1a). The pied wagtail-cuckoo matches the marking size, proportion energy and pattern dispersion of eggs laid by the host, which has rejection rates ranging from 71 per cent (Davies & Brooke 1989) to 91 per cent (Avilés & Garamszegi 2007). The granularity profile of the cuckoo matches the shape of the host spectrum but not the amplitude (figure 1g). The red-backed shrike shows strong rejection (100%, Avilés & Garamszegi 2007), and its cuckoo matches the host in marking size, proportion energy and total contrast, but not pattern coverage or dispersion (table 1). This is because the cuckoo egg rarely mimics the ‘corona’ ring of markings that is prominent at the middle of red-backed shrike eggs. The granularity profiles match very closely for all but two of the sizes (figure 1c). In accordance with strong rejection shown by the brambling (90%, Avilés & Garamszegi 2007), its cuckoo matches the host eggs across all five pattern characteristics (table 1), with the granularity spectra essentially identical (figure 1f).
4. Discussion
Here, we quantified five different characteristics of egg pattern mimicry between the common cuckoo and eight of its principal hosts, and revealed varying degrees of matching between each cuckoo gens and host (figure 1). Previous studies using subjective human criteria have usually focused on a basic ‘matching’ score, which ignores the relative contribution of different pattern attributes to overall pattern mimicry. Our technique demonstrates that pattern is composed of multiple different attributes (e.g. marking size, dispersion, etc.) that should be considered independently. Furthermore, different components of a pattern may be relatively more important in general (across most species), such as marking size, whereas other characteristics appear to be more important to different host species in achieving mimicry (table 1). Our analyses support the conclusion that cuckoos have evolved better mimetic egg patterns where host species show strong egg rejection (figure 2). In addition, there are significant differences in pattern among the cuckoo gentes (electronic supplementary material, table S2), but cuckoos have significantly less diverse patterns than hosts.
Gentes of the common cuckoo and their hosts appear to be at various stages of an evolutionary arms race, in which the cuckoo's match to host eggs improves as the hosts evolve better rejection defences (Davies 2000). Early studies indicated that cuckoos lay a better mimetic egg where the host species is more discriminating (Brooke & Davies 1988; Davies & Brooke 1989). Our pattern analyses support this. The cuckoo has no pattern mimicry where the hosts show no rejection, moderate pattern mimicry where the hosts show modest rejection, and excellent pattern mimicry with strong host rejection (figure 2). The cuckoo gentes match the predominant marking size for all hosts except the dunnock, suggesting that a good match in marking size is crucial for evolving mimetic eggs across species. However, differences in the nature and extent of pattern-matching suggest that different hosts may rely on different pattern attributes in rejecting eggs, with more sophisticated mimicry achieved when the cuckoo egg matches not only marking size but also more nuanced aspects of pattern. Whereas previous studies have often treated pattern as a single entity (e.g. Brooke & Davies 1988; Nguyen et al. 2007), our results demonstrate that some cues (e.g. marking size) may be relatively more important in general, with others used differentially across host species. Some species, such as the dunnock, fail to reject cuckoo eggs in spite of low pattern-matching and high fitness consequences of rejection, possibly because dunnocks are in an earlier stage of the arms race with the cuckoo and the lack of egg rejection may be owing to a time lag in developing host defences (Davies & Brooke 1989). In other host species, lack of rejection may stem from costs associated with recognition errors, or if hosts cannot see the eggs; for example, some hosts of Australian bronze-cuckoos, Chalcites, do not reject dark-coloured cuckoo eggs that look very different from the host eggs, possibly because the cuckoo eggs are well camouflaged in the nest (Langmore et al. 2009).
We found statistically significant differences between the cuckoo gentes for all pattern characteristics measured (electronic supplementary material, table S2), corroborating the existence of distinct cuckoo egg-morphs for the eight gentes studied (Moksnes & Røskaft 1995; Davies 2000). Interestingly, our analyses showed that the eggs of the different cuckoo gentes were significantly less variable than were the host eggs. A more general pattern could permit cuckoos to parasitize subsidiary hosts successfully when the primary host is unavailable (Brooke & Davies 1991; Moksnes & Røskaft 1995). Alternatively, the relative similarity of cuckoo eggs could result from genetic constraints on egg-patterning in cuckoos or from evolutionary lag (Davies 2000).
To date, few experiments have established which visual cues are important in determining egg rejection behaviour by hosts (but see Polačiková et al. 2007; Moskát et al. 2008), partly stemming from the lack of consistent methods for quantifying egg appearance. Future tests must incorporate pattern, measured either objectively or through a bird's own eyes. Of course, pattern should not be considered in isolation: selection has acted on egg size, shape, colour, luminance and pattern simultaneously, and these cues together contribute to egg detection and rejection behaviour. Overall, to understand the form and evolution of visual signals and how they relate to behaviour, we should further incorporate the spatial component of animal markings in future work.
Acknowledgements
We thank the editor T. Price and two anonymous referees for a range of helpful comments and suggestions. We also thank Douglas Russell, Robert Prys-Jones and the staff of the NHM, Tring, for kindly allowing access to collections, and Nick Davies and Rebecca Kilner for a range of advice. M.C.S. was supported by a Marshall Scholarship, the Cambridge Overseas Trust, and the Hanne and Torkel Weis-Fogh Fund. M.S. was supported by a Biotechnology and Biological Sciences Research Council David Phillips Fellowship (BB/G022887/1) and Girton College, Cambridge.
Endnote
MATLAB functions for undertaking the pattern analysis outlined in this paper are available from M.S. (ms726@cam.ac.uk).
References
- Avilés J. M.2008Egg colour mimicry in the common cuckoo Cuculus canorus as revealed by modelling host retinal function. Proc. R. Soc. B 275, 2345–2352 (doi:10.1098/rspb.2008.0720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilés J. M., Garamszegi L. Z.2007Egg rejection and brain size among potential hosts of the common cuckoo. Ethology 113, 562–572 (doi:10.1111/j.1439-0310.2007.01359.x) [Google Scholar]
- Avilés J., Stokke B., Moksnes A., Røskaft E., Møller A.2007Environmental conditions influence egg color of reed warblers Acrocephalus scirpaceus and their parasite, the common cuckoo Cuculus canorus. Behav. Ecol. Sociobiol. 61, 475–485 (doi:10.1007/s00265-006-0275-0) [Google Scholar]
- Barbosa A., Mäthger L. M., Buresch K. C., Kelly J., Chubb C., Chiao C.-C., Hanlon R. T.2008Cuttlefish camouflage: the effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vis. Res. 48, 1242–1253 (doi:10.1016/j.visres.2008.02.011) [DOI] [PubMed] [Google Scholar]
- Bennett A. T. D., Cuthill I. C., Norris K. J.1994Sexual selection and the mismeasure of color. Am. Nat. 144, 848–860 (doi:10.1086/285711) [Google Scholar]
- Brooke M. de L., Davies N. B.1988Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630–632 [Google Scholar]
- Brooke M. de L., Davies N. B.1991A failure to demonstrate host imprinting in the cuckoo (Cuculus canorus) and alternative hypotheses for the maintenance of egg mimicry. Ethology 89, 154–166 [Google Scholar]
- Campbell F. W., Robson J. G.1968Applications of Fourier analysis to the visibility of gratings. J. Physiol. 197, 551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassey P., Honza M., Grim T., Hauber M. E.2008The modelling of avian visual perception predicts behavioural rejection responses to foreign egg colours. Biol. Lett. 4, 515–517 (doi:10.1098/rsbl.2008.0279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao C.-C., Chubb C., Buresch K. C., Siemann L., Hanlon R. T.2009The scaling effects of substrate texture on camouflage patterning in cuttlefish. Vis. Res. 49, 1647–1656 (doi:10.1016/j.visres.2009.04.002) [DOI] [PubMed] [Google Scholar]
- Cohen J.1988Statistical power analysis for the behavioral sciences Hillsdale, NJ: Lawrence Earlbaum Associates [Google Scholar]
- Davies N. B.2000Cuckoos, cowbirds and other cheats London, UK: T & A D Poyser [Google Scholar]
- Davies N. B., Brooke M. de L.1989An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J. Anim. Ecol. 58, 207–224 [Google Scholar]
- Godfrey D., Lythgoe J. N., Rumball D. A.1987Zebra stripes and tiger stripes: the spatial frequency distribution of the pattern compared to that of the background is significant in display and crypsis. Biol. J. Linn. Soc. 32, 427–433 (doi:10.1111/j.1095-8312.1987.tb00442.x) [Google Scholar]
- Gosler A. G., Barnett P. R., Reynolds S. J.2000Inheritance and variation in eggshell patterning in the great tit, Parus major. Proc. R. Soc. Lond. B 267, 2469–2473 (doi:10.1098/rspb.2000.1307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart N., Hunt D.2007Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 169, 7–26 [DOI] [PubMed] [Google Scholar]
- Hart N. S., Partridge J. C., Cuthill I. C., Bennett A. T. D.2000Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J. Comp. Physiol. A 186, 375–387 (doi:10.1007/s003590050437) [DOI] [PubMed] [Google Scholar]
- Jones C. D., Osorio D.2004Discrimination of orientated visual textures by poultry chicks. Vis. Res. 44, 83–89 (doi:10.1016/j.visres.2003.08.014) [DOI] [PubMed] [Google Scholar]
- Lahti D., Lahti A.2002How precise is egg discrimination in weaverbirds? Anim. Behav. 63, 1135–1142 (doi:10.1006/anbe.2002.3009) [Google Scholar]
- Langmore N. E., Stevens M., Maurer G., Kilner R. M.2009Are dark cuckoo eggs cryptic in host nests? Anim. Behav. 78, 461–468 (doi:10.1016/j.anbehav.2009.06.003) [Google Scholar]
- López-de-Hierro M., Moreno-Rueda G.In press Egg-spot pattern rather than egg colour affects conspecific egg rejection in the house sparrow (Passer domesticus). Behav. Ecol. Sociobiol. (doi:10.1007/s00265-009-0811-9) [Google Scholar]
- Moksnes A., Røskaft E.1995Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J. Zool. 236, 625–648 (doi:10.1111/j.1469-7998.1995.tb02736.x) [Google Scholar]
- Moskát C., Székely T., Cuthill I., Kisbenedek T.2008Hosts' responses to parasitic eggs: which cues elicit hosts' egg discrimination? Ethology 114, 186–194 [Google Scholar]
- Nakagawa S., Cuthill I.2007Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605 (doi:10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- Nguyen L. P., Nol E., Abraham K. F.2007Using digital photographs to evaluate the effectiveness of plover egg crypsis. J. Wild. Manage. 71, 2084–2089 (doi:10.2193/2006-471) [Google Scholar]
- Osorio D., Vorobyev M.2005Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B 272, 1745–1752 (doi:10.1098/rspb.2005.3156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polačiková L., Honza M., Procházka P., Topercer J., Stokke B. G.2007Colour characteristics of the blunt egg pole: cues for recognition of parasitic eggs as revealed by reflectance spectrophotometry. Anim. Behav. 74, 419–427 (doi:10.1016/j.anbehav.2006.10.023) [Google Scholar]
- Rothstein S. I.1990A model system for coevolution: avian brood parasitism. Ann. Rev. Ecol. 21, 481–508 (doi:10.1146/annurev.es.21.110190.002405) [Google Scholar]
- Safran R. J., Vitousek M. N.2008Evolutionary biology: arms races in the eye of the beholder. Curr. Biol. 18, R734–R736 (doi:10.1016/j.cub.2008.07.045) [DOI] [PubMed] [Google Scholar]
- Sanz J. J., García-Navas V.2009Eggshell pigmentation pattern in relation to breeding performance of blue tits Cyanistes caeruleus. J. Anim. Ecol. 78, 31–41 (doi:10.1111/j.1365-2656.2008.01465.x) [DOI] [PubMed] [Google Scholar]
- Stevens M., Cuthill I. C.2006Disruptive coloration, crypsis and edge detection in early visual processing. Proc. R. Soc. B 273, 2141–2147 (doi:10.1098/rspb.2006.3556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Párraga C. A., Cuthill I. C., Partridge J. C., Troscianko T. S.2007Using digital photography to study animal coloration. Biol. J. Linn. Soc. 90, 211–237 (doi:10.1111/j.1095-8312.2007.00725.x) [Google Scholar]