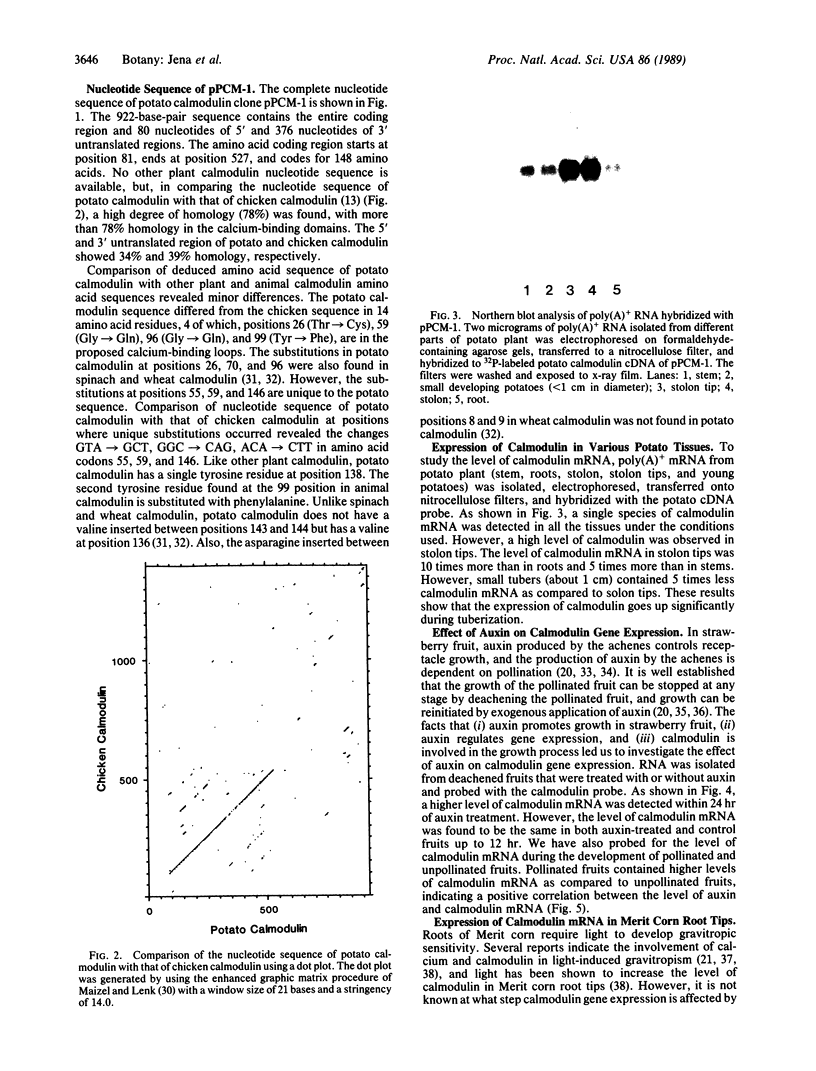
Abstract
A cDNA clone (pPCM-1) for plant calmodulin was isolated by screening a potato stolon tip cDNA library with a chicken calmodulin cDNA. Nucleotide sequence analysis of pPCM-1 revealed that it contained 80 base pairs of 5' untranslated region, the entire coding region, and 376 base pairs of 3' untranslated region. Comparison of the nucleotide sequence of coding regions of potato and chicken calmodulin mRNA showed 78% homology. Comparison of the predicted amino acid sequence of potato calmodulin with other known calmodulin sequences indicated a high degree of homology with a few exceptions. Three changes in the amino acid sequence were found to be unique to the potato calmodulin sequence. In our earlier studies we showed the involvement of calcium and calmodulin in potato tuberization. The pPCM-1 clone was used as a probe to study the expression of calmodulin mRNA during tuberization and to monitor calmodulin mRNA level in various parts of the potato plant. Stolon tips showed the highest levels of calmodulin mRNA, suggesting a role for calmodulin in the tuberization process. In addition, pPCM-1 was used to investigate the effect of auxin and light on calmodulin gene expression in auxin-responsive strawberry fruit and light-responsive Merit corn roots, respectively. Both auxin and light signals were found to increase the level of mRNA for calmodulin. These results suggest that the altered calmodulin gene expression could be one of the molecular events involved in the signal transduction process in plants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arteca R. N., Poovaiah B. W., Smith O. E. Changes in Carbon Fixation, Tuberization, and Growth Induced by CO2 Applications to the Root Zone of Potato Plants. Science. 1979 Sep 21;205(4412):1279–1280. doi: 10.1126/science.205.4412.1279. [DOI] [PubMed] [Google Scholar]
- Balamani V., Veluthambi K., Poovaiah B. W. Effect of Calcium on Tuberization in Potato (Solanum tuberosum L.). Plant Physiol. 1986 Apr;80(4):856–858. doi: 10.1104/pp.80.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Hidaka H., Boyd A. E., 3rd, Means A. R. Calmodulin and the cell cycle: involvement in regulation of cell-cycle progression. Cell. 1982 Jan;28(1):41–50. doi: 10.1016/0092-8674(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Pardue R. L., Brinkley B. R., Dedman J. R., Means A. R. Regulation of intracellular levels of calmodulin and tubulin in normal and transformed cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):996–1000. doi: 10.1073/pnas.78.2.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwalder M., Roux S. J., Hardison L. Distribution of calmodulin in pea seedlings: immunocytochemical localization in plumules and root apices. Planta. 1986;168:461–470. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Lagacé L., Chandra T., Woo S. L., Means A. R. Identification of multiple species of calmodulin messenger RNA using a full length complementary DNA. J Biol Chem. 1983 Feb 10;258(3):1684–1688. [PubMed] [Google Scholar]
- Lukas T. J., Iverson D. B., Schleicher M., Watterson D. M. Structural characterization of a higher plant calmodulin : spinacia oleracea. Plant Physiol. 1984 Jul;75(3):788–795. doi: 10.1104/pp.75.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Munjaal R. P., Chandra T., Woo S. L., Dedman J. R., Means A. R. A cloned calmodulin structural gene probe is complementary to DNA sequences from diverse species. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2330–2334. doi: 10.1073/pnas.78.4.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch J. P. Free Auxins and Free Tryptophane in the Strawberry. Plant Physiol. 1955 Jan;30(1):33–39. doi: 10.1104/pp.30.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliyath G., Poovaiah B. W. Calmodulin inhibitor in senescing apples and its physiological and pharmacological significance. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2065–2069. doi: 10.1073/pnas.81.7.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliyath G., Poovaiah B. W. Identification of naturally occurring calmodulin inhibitors in plants and their effects on calcium- and calmodulin-promoted protein phosphorylation. Plant Cell Physiol. 1985;26(1):201–209. [PubMed] [Google Scholar]
- Poovaiah B. W., McFadden J. J., Reddy A. S. The role of calcium ions in gravity signal perception and transduction. Physiol Plant. 1987;71:401–407. doi: 10.1111/j.1399-3054.1987.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S. Calcium messenger system in plants. CRC Crit Rev Plant Sci. 1987;6(1):47–103. doi: 10.1080/07352688709382247. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S., McFadden J. J. Calcium messenger system: role of protein phosphorylation and inositol bisphospholipids. Physiol Plant. 1987;69:569–573. doi: 10.1111/j.1399-3054.1987.tb09241.x. [DOI] [PubMed] [Google Scholar]
- Putkey J. A., Ts'ui K. F., Tanaka T., Lagacé L., Stein J. P., Lai E. C., Means A. R. Chicken calmodulin genes. A species comparison of cDNA sequences and isolation of a genomic clone. J Biol Chem. 1983 Oct 10;258(19):11864–11870. [PubMed] [Google Scholar]
- Reddy A. S., McFadden J. J., Friedmann M., Poovaiah B. W. Signal transduction in plants: evidence for the involvement of calcium and turnover of inositol phospholipids. Biochem Biophys Res Commun. 1987 Dec 16;149(2):334–339. doi: 10.1016/0006-291x(87)90371-8. [DOI] [PubMed] [Google Scholar]
- Reddy A. S., Poovaiah B. W. Accumulation of a glycine rich protein in auxin-deprived strawberry fruits. Biochem Biophys Res Commun. 1987 Sep 30;147(3):885–891. doi: 10.1016/s0006-291x(87)80153-5. [DOI] [PubMed] [Google Scholar]
- Roux S. J., Serlin B. S. Cellular mechanisms controlling light-stimulated gravitropism: role of calcium. CRC Crit Rev Plant Sci. 1987;5(3):205–236. doi: 10.1080/07352688709382240. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinemetz C. L., Kuzmanoff K. M., Evans M. L., Jarrett H. W. Correlation between calmodulin activity and gravitropic sensitivity in primary roots of maize. Plant Physiol. 1987;84:1337–1342. doi: 10.1104/pp.84.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan D. G., Hale R. S., Dhillon N., Leadlay P. F. A bacterial calcium-binding protein homologous to calmodulin. Nature. 1987 Sep 3;329(6134):84–85. doi: 10.1038/329084a0. [DOI] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Toda H., Yazawa M., Sakiyama F., Yagi K. Amino acid sequence of calmodulin from wheat germ. J Biochem. 1985 Sep;98(3):833–842. doi: 10.1093/oxfordjournals.jbchem.a135342. [DOI] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Auxin-regulated polypeptide changes at different stages of strawberry fruit development. Plant Physiol. 1984 Jun;75(2):349–353. doi: 10.1104/pp.75.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium-promoted protein phosphorylation in plants. Science. 1984 Jan 13;223(4632):167–169. doi: 10.1126/science.223.4632.167. [DOI] [PubMed] [Google Scholar]
- Zendegui J. G., Zielinski R. E., Watterson D. M., Van Eldik L. J. Biosynthesis of calmodulin in normal and virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1984 May;4(5):883–889. doi: 10.1128/mcb.4.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski R. E. Calmodulin mRNA in Barley (Hordeum vulgare L.) : Apparent Regulation by Cell Proliferation and Light. Plant Physiol. 1987 Jul;84(3):937–943. doi: 10.1104/pp.84.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]