Abstract
Although males and females share much of the same genome, selection is often distinct in the two sexes. Sexually antagonistic loci will in theory cause a gender load in populations, because sex-specific selection on a given trait in one sex will compromise the adaptive evolution of the same trait in the other sex. However, it is currently not clear whether such intralocus sexual conflict (ISC) represents a transient evolutionary state, where conflict is rapidly resolved by the evolution of sexual dimorphism (SD), or whether it is a more chronic impediment to adaptation. All else being equal, ISC should manifest itself as correlated evolution between population fitness and SD in traits expressed in both sexes. However, comparative tests of this prediction are problematic and have been unfeasible. Here, we assess the effects of ISC by comparing fitness and SD across distinct laboratory populations of seed beetles that should be well adapted to a shared environment. We show that SD in juvenile development time, a key life-history trait with a history of sexually antagonistic selection in this model system, is positively related to fitness. This effect is due to a correlated evolution between population fitness and development time that is positive in females but negative in males. Loosening the genetic bind between the sexes has evidently allowed the sexes to approach their distinct adaptive peaks.
Keywords: sexual selection, sexual conflict, genetic constraints, adaptation
1. Introduction
Intralocus sexual conflict (ISC) occurs when the direction of selection on an allele depends upon which sex it is expressed in (Rice 1992), and is due to the fact that males and females may have different optimal trait values for phenotypic traits expressed in both sexes (Rice & Chippindale 2001). Although recent studies have demonstrated standing genetic variation in sexually antagonistic loci within populations in diverse taxa (Chippindale et al. 2001; Fedorka & Mousseau 2004; Pischedda & Chippindale 2006; Brommer et al. 2007; Foerster et al. 2007; Prasad et al. 2007; Bilde et al. 2009; Delcourt et al. 2009), the impact of ISC on population-level processes remains unexplored (Bonduriansky & Chenoweth 2009). This is unfortunate because ISC may affect fundamental evolutionary processes such as adaptation (Rice 1992) and speciation (Rice & Chippindale 2002). ISC is particularly likely to play an important role in the evolution of key life-history traits (Rice & Chippindale 2001)—such as growth/metabolic rate, age/size at maturation/emergence, reproductive rate and rate of senescence—where optimal male and female phenotypes typically differ (Wedell et al. 2006). Since males and females play different roles in reproduction and maximize fitness in ways that are partly distinct (Arnqvist & Rowe 2005), the optimal trade-offs between growth, survival and reproduction are often different in the two sexes (Svensson & Sheldon 1998; Crowley & Johansson 2002; Bonduriansky et al. 2008; Gotthard 2008). ISC can be resolved by the evolution of sexual dimorphism (figure 1). The fact that sexual dimorphism (SD) can evolve by several different genetic routes (Day & Bonduriansky 2004; Bonduriansky & Rowe 2005), allowing each sex to reach its distinct adaptive peak, suggests that mitigation of ISC might be rapid. On the other hand, there are reasons to believe that the evolution of SD may often be constrained (Lande 1987; Rhen 2000; Bedhomme & Chippindale 2008; Mank et al. 2008; Cox & Calsbeek 2009), and whether ISC represents a persistent impediment to sex-specific adaptation is an unsettled key issue in evolutionary biology (Bedhomme & Chippindale 2008).
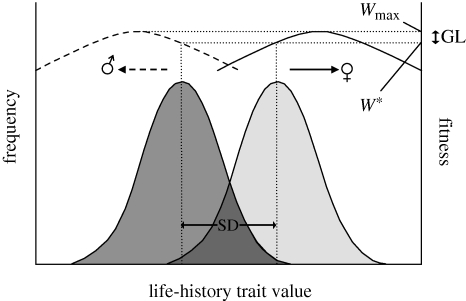
Figure 1.
Schematic illustration of the gender load. Because of differences in the optimal life-history trade-offs between the sexes, male (dashed line) and female (solid line) fitness functions typically differ. Whenever the evolution of SD in a trait expressed in both sexes is constrained, males (dark grey distribution) and females (light grey distribution) will be unable to reach their optimal sex-specific phenotypes. This results in a population fitness (W*) that is lower than that achieved when both sexes are free to evolve to reside on their adaptive peaks (Wmax). Although sexually antagonistic selection (arrows) will act to increase SD in an evolutionary tug-of-war known as intralocus sexual conflict, the genetic bind between the sexes causes a fitness depression constituting the gender load (GL), or SD load (Rice 1992). Thus, the evolution of increased SD should be associated with elevated population fitness. This key prediction is, however, contingent upon all else being equal, which complicates the interpretation of comparative tests of this prediction.
Several authors have suggested that important insights may be gained from comparative studies of the effects of ISC (Wedell et al. 2006; Bonduriansky & Chenoweth 2009). Comparative studies may, for example, help expose a history of ISC that is hidden within populations owing to the fixation of different sexually antagonistic alleles in different populations. Here, we adopt a comparative approach to study the evolutionary footprints of ISC across populations. It is based on the fundamental presumption that, given sexually antagonistic selection in a population, the evolution of a larger degree of SD in life-history traits allows both sexes to approach their distinct adaptive peaks (Rice & Chippindale 2001); all else being equal, current SD should therefore be positively associated with population fitness (figure 1). At first glance, this key prediction may seem straightforward. However, several factors will tend to obscure or confound any relationship between SD and population fitness brought about by ISC. First, because populations or higher-order taxa are invariably adapted to distinct environments, comparable measures of population fitness may be unattainable. Assays in different environments suffer from confounding environmental effects, and common garden assays will also be problematic. Second, the effect of ISC is generally obscured by a positive genetic correlation between fitness in the two sexes, built by varying degrees of sexually concordant adaptation (Bedhomme & Chippindale 2008; Morrow et al. 2008), such that a large proportion of variance in fitness across populations or higher-order taxa will be due to alleles with similar effects on fitness in the two sexes. This problem is aggravated if the selective regime varies across populations or taxa. Third, although the population consequences of sexual selection are poorly explored empirically, several forms of sexual selection are expected to generate covariance between SD and population fitness (Rankin & Arnqvist 2008). Any inference regarding the role of sexual conflict that is based on comparative data must thus, to some extent, rely on a sound understanding of the model system and of the function of the traits in question (Arnqvist & Rowe 2002). We return to this issue in §4.
Here, we test whether SD is related to population fitness by comparing SD in male and female juvenile development time and population fitness across a series of geographically distinct laboratory populations of the seed beetle Callosobruchus maculatus (Coleoptera, Bruchidae). We steer clear of most obstructing methodological complications by (i) using populations that originally occupied the same ecological niche in the wild, (ii) using artificial selection to allow all populations to adapt to the same adaptive peak and (iii) further minimizing variation in sexually congruent adaptation across populations by allowing all populations a long time to adapt to the shared selection regime. The focus is on development time, a key life-history trait with a history of sexually antagonistic selection in this species (see §4), as well as in many other insects (Blanckenhorn et al. 2007; Gotthard 2008; Jarosik & Honek 2008).
2. Material and methods
(a). Model system and rearing
We used 12 geographically distinct populations of C. maculatus: Benin, Brazil (London), California, IITA (Nigeria), Lossa, Mali, Oman, Oyo, Uganda, Upper Volta, Yemen and Zaire. These populations are genetically distinct (Dowling et al. 2007; see the electronic supplementary material) and differ somewhat in external morphology (Rankin & Arnqvist 2008) but show no signs of inbreeding or outbreeding depression and are fully reproductively compatible (hatching rate of eggs within populations as well as in crosses between populations is invariably 95% or more). They were all collected as pests in bean storage sites and were brought into the laboratory at various times (range: 1975–2000), but time since collection is not significantly related to current population fitness (r = 0.23, p = 0.47) or to morphology (Rankin & Arnqvist 2008) across these populations. These facts collectively suggest that differences in the laboratory history of these populations are not a major determinant of variation in population fitness. To minimize variance in sexually concordant adaptation across populations, all populations were exposed to a single common artificial selection regime in our laboratory for 90–125 generations prior to the experiments reported here: beetles were reared under a standardized population size (250–300 individuals) and density on black-eyed beans (Vigna unguiculata), at 30°C, 60 per cent RH and a 12 L : 12 D light cycle, under a discrete generation protocol. Prior to this, the populations had been reared an additional 50–450 generations under very similar conditions in other laboratories, such that populations had in total been adapting to the laboratory environment for 150–450 generations. The response to the selection pressures of domestication in Drosophila reaches a plateau in less than 100 generations (e.g. Simoes et al. 2007) and in C. maculatus in even less time (e.g. Messina et al. 2009). Further, the black-eyed bean is the main natural host for this species and the rearing conditions used (including non-overlapping generations and abiotic conditions) mimic natural conditions (Southgate 1979; Messina 1991). These facts collectively suggest that the populations used should be uniformly and well adapted to their shared laboratory environment. Yet, in a previous study, using a subset of these populations, Rankin & Arnqvist (2008) documented a positive relationship between SD in overall size and population fitness across populations. Because body size per se is not a target of sexually antagonistic selection in this system (Eady 1994; Savalli & Fox 1999), however, this study was interpreted as suggesting that fitness variation across populations is rooted in SD in causal life-history traits such as development time (Rankin & Arnqvist 2008).
(b). Assays of development time and body size
Approximately 100 virgin adult beetles from each population were placed with 100 g black-eyed beans (approx. 500 beans) for 6 h, for oviposition, in a 1 l glass stock jar. Following this brief period of oviposition, 96 beans, each carrying only a single egg (to avoid developmental effects of larval competition), were isolated individually in 24-compartment Petri dishes. These beans were subsequently scanned during spot checks, performed three to six times (evenly spaced) per 24 h, during the entire period of adult hatching. All newly hatched adults were collected and preserved by freezing. For each individual, the inverse of the time elapsed between the spot check at which an individual had hatched and the one immediately previous to this spot check (i.e. the inter-spot-check interval) was used as a measure of the precision of the recorded development time (see below). The average inter-spot-check interval across all individuals was 4.796 h (s.e. = 0.109). Adult beetles were later sexed and the mean length of the left and right elytra, measured using a digitizing tablet placed under a side-mounted camera lucida attached to a dissecting microscope, was used as a measure of body size of each individual. In total, data on development time and body size were collected for an average of 47.5 (range 36–54) females and 47.1 males (range 40–58) per population.
(c). Population fitness assays
In order to minimize variance in adult fitness owing to variation in their competitive environment as juveniles, only adults that had experienced no juvenile competition were used in this assay. As in the assay described above, approximately 100 virgin adult beetles from each population were first placed with 100 g black-eyed beans (approx. 500 beans) for oviposition. From these, beans carrying only a single egg were then isolated individually. As adults started to hatch from these beans, virgin males and females were introduced in pairs (n = 25–29 pairs per population) into Petri dishes containing 20 g black-eyed beans (approx. 100 beans). After 38 days, the number of adult progeny produced by each pair was recorded and used as a measure of lifetime offspring production. Adult lifespan ranges between 6 and 10 days (Maklakov et al. 2007a) and the development time between 19 and 25 days under these conditions. Because female C. maculatus produce approximately 100 eggs during their lifetime under our experimental conditions, which are evenly distributed among available beans, and because each bean can harbour several (four to five) larvae without reduced survival, this design minimizes the effects of differential larval survival owing to juvenile competition within beans (Toquenaga & Fujii 1990).
We derived three alternative measures of population fitness, representing both rate-insensitive and rate-sensitive metrics (Heesterbeek 2002; Brommer et al. 2004; Roff 2008). As a rate-insensitive measure of net reproductive output (R0), we used (i) the mean lifetime offspring production per pair. The mean lifetime offspring production per pair was also divided by either (ii) mean development time in the two sexes or (iii) the mean development time in females, to form two alternative rate-sensitive measures of population fitness (rmax). The three measures yielded quantitatively very similar and qualitatively identical results (in terms of our ability or inability to reject null hypotheses at α) and, thus, we only present analyses of the third measure throughout this contribution. We note that the fitness assay was conducted under conditions different from those of our artificial selection regime, where juvenile competition within beans was more common. Yet the maximum population growth rate forms a biologically relevant measure of population fitness in seed beetles, where intermittent pulses of large amounts of resources occur when legume seeds mature and/or are brought into storage (Southgate 1979; Fujii et al. 1990).
(d). Statistical methods
For all inferential general linear models, we assessed whether data fulfilled the homogeneity of variance assumption (Levene's tests) and whether residuals showed a normal distribution (Shapiro–Wilk's tests). In cases where any of these assumptions were not fulfilled, models were also evaluated by resampling tests (denoted prand) involving bootstrapping (10 000 replicates) the residuals of the original models (ter Braak 1992; Manly 1997). Differences in development time across populations and between the sexes were assessed in an analysis of variance, and differences in growth rate in an analysis of covariance, using adult body size as a covariate. Both models used log-transformed development times, all cases were weighted by their precision and observations with an unsigned studentized residual greater than 2.5 (less than 3% of all observations) were not included in the final inferential models. To characterize variation in life-history traits across populations, we first performed principal component (PC) analyses of mean values for males and females across populations, and retained both PCs from these analyses. Note that no variance in the data is lost in this analytical procedure. It merely involves converting variation in a given life-history trait across sexes and populations into two uncorrelated variables, one of which measures magnitude (PC1) and the other SD (PC2) of the trait in question. This is because PC1 will describe the major axis of variation (i.e. the positive covariation between the sexes across populations) and PC2 will be orthogonal to this: the correlation between PC1 on one hand and male and female trait values on the other was high and positive in all cases (i.e. all r > 0.85). We note that the most well-behaved ratio-based measure of SD (Smith 1999)—the SD index of Lovich & Gibbons (1992)—correlated very closely with PC2 (r = 0.96 for development time, r = 0.99 for size and r = 0.95 for growth rate).
The prediction that mean population fitness should be positively related to variation in life-history traits was then tested by means of conventional multiple linear regression models including both the magnitude of a trait (PC1) and its SD (PC2) as independent variables. This procedure (i.e. a PC regression; Jolliffe 1982) allows accurate tests of the effects of SD while circumventing inherent problems with multi-collinearity between male and female life-history traits. When testing hypotheses in which the sign of the effect was predicted a priori, we used directed tests (Rice & Gaines 1994). Directed tests enable detection of patterns that are opposite to predictions while retaining much of the statistical power of one-tailed tests. In all directed tests (denoted pdir), we followed the convention of setting γ/α = 0.8 (Rice & Gaines 1994).
3. Results
Populations differed both in development time (table 1A) and growth rate (table 1B). The sexes also differed both in development time and growth rate, although SD in development time was larger than in growth rate (F-ratio: 160 versus 33). Across all populations, the mean development time and body size was 21.26 days (s.e. = 0.04) and 2.10 mm (s.e. = 0.003) in females, and 20.82 days (s.e. = 0.04) and 1.93 mm (s.e. = 0.004) in males, respectively. Further, the significant interactions between population and sex showed that SD in these key life-history traits differed across populations (table 1). For subsequent analyses of variation across populations, we estimated mean development time and body size for each sex and population. To secure a measure of growth rate, body size was first regressed on development time (b′ = 0.17, t = 5.8, n = 1173, p < 0.001) and residual body size from this global regression model was retained. Mean residual body size for each sex and population was then used as a measure of growth rate. Mean growth rate was 99 × 10−3 mm d−1 (s.e. = 0.26 × 10−3) for females and 92.8 × 10−3 mm d−1 (s.e. = 0.24 × 10−3) for males. Analyses of the pattern of allometry across populations showed that, as in other beetles (Blanckenhorn et al. 2007), variation in SD in body size across populations was to a large extent caused by variation in SD in development time (see the electronic supplementary material).
Table 1.
Analysis of variance (A) and analysis of covariance (B) of development time.
| SS | d.f. | F | p | prand | |
|---|---|---|---|---|---|
| A | |||||
| population | 31.9 | 11 | 55.4 | <0.001 | <0.001 |
| sex | 8.38 | 1 | 160.1 | <0.001 | <0.001 |
| population × sex | 1.13 | 11 | 2.0 | 0.029 | 0.029 |
| residual | 55.5 | 1060 | |||
| Ba | |||||
| population | 32.0 | 11 | 55.9 | <0.001 | <0.001 |
| sex | 1.71 | 1 | 32.9 | <0.001 | <0.001 |
| population × sex | 1.10 | 11 | 1.9 | 0.033 | 0.034 |
| body size | 0.41 | 1 | 7.8 | 0.005 | 0.007 |
| residual | 55.1 | 1059 | |||
aInteractions involving body size (two- and three-way) did not improve model fit (F23,1036 = 1.41, p = 0.095) and were thus not included in the inferential ANCOVA.
Mean lifetime offspring production per pair differed across populations and ranged from 92.7 to 117 (F11 280 = 5.3, p < 0.001). Population fitness was, however, not significantly related to either body size (F2,9 = 0.62, pdir = 0.35) or growth rate (F2,9 = 0.56, pdir = 0.37) across populations. In contrast, variation in development time was significantly related to population fitness (F2,9 = 5.12, pdir = 0.020). The standardized regression coefficients of this model showed that this relationship was caused by SD in development time (PC2; b′ = 0.50, t = 2.16, pdir = 0.036) rather than by overall development time (PC1; b′ = −0.22, t = 1.46, pdir = 0.11). Importantly, the phylogenetic signal across populations was low for these life-history trait variables and phylogenetic comparative analyses yielded qualitatively identical results to those above (see the electronic supplementary material), demonstrating correlated evolution between population fitness and SD in development time. In theory, this pattern (figure 2) could be due to variation in male development time, female development time or to independent effects of both. A multiple regression model of population fitness, including male and female development time as independent variables, suggested that the independent effects of variation in development time was of comparable magnitude in the two sexes (females: b′ = 1.38, t = 1.79; males: b′ = −1.91, t = 2.49; figure 3). Because of potential inferential problems owing to multi-collinearity (tolerance = 0.09), this model was replicated using ridge regression employing the Hoerl–Kennard–Baldwin (HKB) estimator of the ridge complexity parameter (λ). Ridge regression is a common remedy for potential multi-collinearity problems (Draper & Smith 1998). The ridge regression coefficients were, again, of comparable magnitude in the two sexes (females: b′ = 0.86; males: b′ = −1.38). Thus, a long development time in females and a short development time in males were both associated with high population fitness (figure 3).
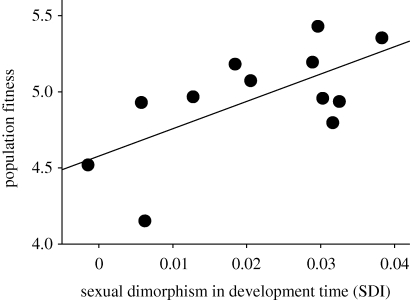
Figure 2.
The relationship between SD and population fitness in seed beetles. Here, population fitness is plotted against SD in juvenile development time across populations, measured as the SD index (SDI = F/M − 1; rp = 0.65, pdir = 0.011; randomization test based on 10 000 random permutations). Line represents conventional linear regression.
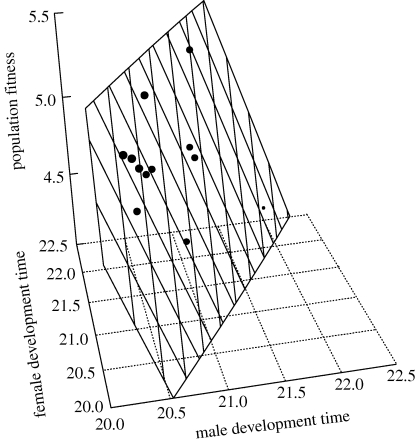
Figure 3.
The relationship between male and female development time (in days) and population fitness in seed beetles. Note that elevated population fitness is associated with a relatively long development time in females and a relatively short development time in males.
4. Discussion
Development time is a key life-history trait with important and direct associations with fitness in both sexes (Wedell et al. 2006). Selection on development time in insects is often sexually antagonistic (Blanckenhorn et al. 2007; Gotthard 2008; Jarosik & Honek 2008) and males emerge before females (protandry) in many groups of insects (Thornhill & Alcock 1983; Morbey & Ydenberg 2001). In general, a long juvenile development time rewards female insects with a high fecundity (Blanckenhorn et al. 2007; Gotthard 2008). Seed beetles are capital breeders, and a longer juvenile development time, when reproductive resources are accumulated, has been shown to be genetically correlated with a higher lifetime fecundity in females in both C. maculatus (Møller et al. 1989) and other seed beetles (Seslija & Tucic 2003). In females, thus, genes that are associated with a long development time are also associated with high fecundity. In contrast, males that emerge early are often rewarded with greater fertilization opportunities in insects (Dickinson 1992; Del Castillo & Nunez-Farfan 1999; Crowley & Johansson 2002; Gotthard 2008). Five factors are known to promote selection for protandry in males (Morbey & Ydenberg 2001; Blanckenhorn et al. 2007). First, early emergence is favoured in males when females have higher residual fecundity early during the reproductive period. This effect is very strong in C. maculatus, where females lay more than 60 per cent of their entire lifetime egg production during the first 2 days of their adult life (Fricke et al. 2006). Second, early emergence is favoured when encountering virgin females is advantageous. In C. maculatus, female mating frequency is highest during their first day of adult life (Pushpinder 1986) because virgin females mate readily and immediately upon their emergence from their pupae within the seeds (Rönn et al. 2008) but then become very reluctant to remate with other males for several days (Edvardsson & Tregenza 2005). Further, the sperm of the first male is not fully displaced should the female remate with a second male (Eady 1994). Third, polygyny generally favours protandry. Male C. maculatus are highly polygynous, and can mate and successfully fertilize the eggs of a large number of virgin females in rapid succession (more than 10 females per day; Ofuya 1995; Rönn et al. 2008). In fact, early-emerging males patrol infested beans where they mate with all emerging virgin females encountered (Pushpinder 1986). Fourth, because of life-history trade-offs between body size and development time, early emergence will be more favourable when sexual selection for large body size in males is weak or absent (Zonneveld 1996). This is the case in C. maculatus, where there is no detectable large male advantage either in pre-mating (Savalli & Fox 1999) or post-mating (Eady 1994) sexual selection. This is also supported by the fact that body size is much less canalized (i.e. more phenotypically plastic) in males than in females (Stillwell & Fox 2007). Fifth, selection for protandry will be stronger when generations are discrete rather than overlapping. The populations of C. maculatus studied here have been maintained under a discrete generation protocol for more than 150 generations (§2) and conditions in the field, where populations live on ephemeral resources (leguminous seeds), are also characterized by discrete generation population dynamics (Fujii et al. 1990). In conclusion, the evolutionary history of C. maculatus has, at least to some extent, been characterized by sexually antagonistic selection on development time.
The results of this study are consistent with a scenario where ISC over development time has led to somewhat different evolutionary trajectories in different populations. Apparently, populations differ in the degree to which the evolutionary tug-of-war between the sexes has been ‘resolved’ by the evolution of SD, such that adaptive evolution by one sex is more constrained by selection in the other sex in some populations and less in others (Bedhomme & Chippindale 2008). We suggest that this is due to differences in the genetic architecture, defined broadly, across populations (Bieri & Kawecki 2003; Long et al. 2006; Simoes et al. 2008) that have allowed more adaptive sex-specific evolution in some populations compared with others. We note that the phylogenetic signal was low and non-significant for both population fitness and SD in development time (see the electronic supplementary material). This implies that shared ancestry is not a major determinant of variation in genetic architecture across populations. Theory suggests that minor differences in genetic architecture can have large and unanticipated effects on the degree of trait divergence between the sexes (Rhen 2000). Such differences may involve the amount of additive genetic variation (Reeve & Fairbairn 2001), although marked differences in genetic variance across the populations studied here are not expected considering the fact that they have shared selection regime and have had the same effective population size for more than 100 generations (Zhang & Hill 2005; Simoes et al. 2007). Instead, we suggest that the evolvability of SD may differ because populations differ in the degree to which loci affected by ISC are involved in epistatic interactions (Rhen 2000) or the degree to which they are pleiotropic (Mank et al. 2008). Minor but consequential differences in the genetic architecture could to a large extent reflect contingencies owing to random variation in the input of spontaneous mutations in different populations. Variance in mutational input is known to be capable of rapidly building variation in the pattern of genetic covariance across life-history traits in other model systems (Houle et al. 1994; Estes et al. 2005). Although there is no detailed data on the genetic architecture of fitness from the populations used here, a previous study has revealed genetic variation across these populations in development time (Dowling et al. 2007). Further, studies of other laboratory populations of C. maculatus have shown that epistasis is a major contributor to genetic variation in fitness (Bilde et al. 2008), and that intersexual genetic correlations for life-history traits do differ between populations (Fox et al. 2004).
Variation in the strength of sexual selection across populations can also generate covariance between SD and population fitness (Rankin & Arnqvist 2008), and this can complicate interpretations of comparative data of the type reported here. In particular, evolution of the economics of reproduction may be affected by sexual selection in the form of interlocus sexual conflict, where sex-limited and antagonistically interacting traits in males and females coevolve (Rice 1996; Arnqvist & Rowe 2005). Although interlocus sexual conflict involves different sex-limited traits in the two sexes, coevolution could potentially affect also the expression of traits expressed by both sexes. Because the evolution of male persistence adaptations should depress female fitness (Parker 1979; Arnqvist & Rowe 2005), one may be led to predict that interlocus sexual conflict should result in a negative relationship between SD and population fitness (Rankin & Arnqvist 2008). However, theory predicts that this effect should be effectively nullified by the evolution of female resistance adaptations (Rice 1996), and this has been confirmed in two comparative studies of insects (Arnqvist & Rowe 2002; Rönn et al. 2007). In seed beetles, this form of intersexual coevolutionary ‘arms race’ has generated correlated evolution between genital spines in males that injure females internally during mating and female counteradaptation to such harm (Rönn et al. 2007; Hotzy & Arnqvist 2009). Such interlocus sexual conflict could contribute to the patterns detected here if, and only if, SD in development time was correlated with the expression of the sex-limited traits that mediate interlocus sexual conflict. However, SD in development time was not significantly correlated with the length of ventral (r = −0.08, p = 0.79) or dorsal (r = −0.21, p = 0.51) genital spines in males across these populations (data from Hotzy & Arnqvist 2009). Similarly, population fitness was not related to either of these two measures of the harmfulness of male genitalia (r = −0.09, p = 0.78; and r = −0.21, p = 0.51 respectively), suggesting that females have evolved counteradaptations to avoid harm imposed by males (Rönn et al. 2007). Thus, available data do not directly support a confounding role of interlocus sexual conflict. This does not, of course, preclude the possibility that other types of sex-limited and sexually antagonistic traits in males and females could contribute to our results.
The independent and negative relationship between development time in males and our measure of population fitness could in theory be due to two non-mutually exclusive effects. First, short development time in males could be associated with ‘genetic release’ from intralocus conflict, permitting the evolution of more optimal female phenotypes (see above). Second, short development time in males could have direct positive effects on female productivity; for example, if it was beneficial to females to mate immediately upon eclosion, or if the cost of mating to females was lower with early-maturing males. However, four observations strongly suggest that direct positive effects are unlikely: (i) mating very early in adult life actually causes a reduction of lifetime fecundity in female seed beetles (Maklakov et al. 2007b); (ii) the morphology of harmful male genitalia is not related to SD in development time across populations (see above); (iii) the length of genital spines in males is not closely correlated with male body size either within or across populations (Hotzy & Arnqvist 2009); and (iv) the effects of male size (Moya-Larano & Fox 2006) and ejaculate weight (Rönn et al. 2008) on female fitness are both positive in this model system. Because male size covaries with male development time (see the electronic supplementary material), the latter observation is inconsistent with direct positive effects of short development time in males on female productivity.
The demonstration of the correlated evolution between population fitness and SD in development time reported here represents the first comparative corroboration of a fundamental prediction of the ISC theory (Rice & Chippindale 2001; Bedhomme & Chippindale 2008): that adaptation by each sex is persistently compromised by antagonistic sex-specific selection in the other sex. The fact that this became evident when evading the inherent problems with testing this prediction, which normally obscures the importance of ISC, suggests that the importance of ISC is unappreciated (Bonduriansky & Chenoweth 2009). A sizeable gender load may be a much more widespread phenomenon than previously believed.
Acknowledgements
We thank Peter Credland, Robert Smith and George Keeney for providing the beetle populations used and Mirjam Amcoff for invaluable laboratory assistance. Theodore Garland kindly provided the MATLAB code used for the phylogenetic comparative analyses. This work was funded by the Swedish Research Council and the Japan Society for the Promotion of Science.
References
- Arnqvist G., Rowe L.2002Antagonistic coevolution between the sexes in a group of insects. Nature 415, 787–789 (doi:10.1038/415787a) [DOI] [PubMed] [Google Scholar]
- Arnqvist G., Rowe L.2005Sexual conflict Princeton, NJ: Princeton University Press [Google Scholar]
- Bedhomme S., Chippindale A. K.2008Irreconcilable differences: when sexual dimorphism fails to resolve sexual conflict. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn D. J., Blanckenhorn W. U., Szekely T.), pp. 185–194 Oxford, UK: Oxford University Press [Google Scholar]
- Bieri J., Kawecki T. J.2003Genetic architecture of differences between populations of cowpea weevil (Callosobruchus maculatus) evolved in the same environment. Evolution 57, 274–287 (doi:10.1111/j.0014-3820.2003.tb00262.x) [DOI] [PubMed] [Google Scholar]
- Bilde T., Friberg U., Maklakov A. A., Fry J. D., Arnqvist G.2008The genetic architecture of fitness in a seed beetle: assessing the potential for indirect genetic benefits of female choice. BMC Evol. Biol. 8, 295 (doi:10.1186/1471-2148-8-295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilde T., Foged A., Schilling N., Arnqvist G.2009Postmating sexual selection favors males that sire offspring with low fitness. Science 324, 1705–1706 (doi:10.1126/science.1171675) [DOI] [PubMed] [Google Scholar]
- Blanckenhorn W. U., et al. 2007Proximate causes of Rensch's rule: does sexual size dimorphism in arthropods result from sex differences in development time? Am. Nat. 169, 245–257 (doi:10.1086/510597) [DOI] [PubMed] [Google Scholar]
- Bonduriansky R., Chenoweth S. F.2009Intralocus sexual conflict. Trends Ecol. Evol. 24, 280–288 (doi:10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- Bonduriansky R., Rowe L.2005Intralocus sexual conflict and the genetic architecture of sexually dimorphic traits in Prochyliza xanthostoma (Diptera: Piophilidae). Evolution 59, 1965–1975 (doi:10.1111/j.0014-3820.2005.tb01066.x) [PubMed] [Google Scholar]
- Bonduriansky R., Maklakov A., Zajitschek F., Brooks R.2008Sexual selection, sexual conflict and the evolution of ageing and life span. Funct. Ecol. 22, 443–453 (doi:10.1111/j.1365-2435.2008.01417.x) [Google Scholar]
- Brommer J. E., Gustafsson L., Pietiainen H., Merila J.2004Single-generation estimates of individual fitness as proxies for long-term genetic contribution. Am. Nat. 163, 505–517 (doi:10.1086/382547) [DOI] [PubMed] [Google Scholar]
- Brommer J. E., Kirkpatrick M., Qvarnström A., Gustafsson L.2007The intersexual genetic correlation for lifetime fitness in the wild and its implications for sexual selection. PLoS ONE 2, e744 (doi:10.1371/journal.pone.0000744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippindale A. K., Gibson J. R., Rice W. R.2001Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA 98, 1671–1675 (doi:10.1073/pnas.041378098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. M., Calsbeek R.2009Sexually antagonistic selection, sexual dimorphism, and the resolution of intralocus sexual conflict. Am. Nat. 173, 176–187 (doi:10.1086/595841) [DOI] [PubMed] [Google Scholar]
- Crowley P. H., Johansson F.2002Sexual dimorphism in Odonata: age, size, and sex ratio at emergence. Oikos 96, 364–378 (doi:10.1034/j.1600-0706.2002.960218.x) [Google Scholar]
- Day T., Bonduriansky R.2004Intralocus sexual conflict can drive the evolution of genomic imprinting. Genetics 167, 1537–1546 (doi:10.1534/genetics.103.026211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo R. C., Nunez-Farfan J.1999Sexual selection on maturation time and body size in Sphenarium purpurascens (Orthoptera: Pyrgomorphidae): correlated response to selection. Evolution 53, 209–215 (doi:10.2307/2640933) [DOI] [PubMed] [Google Scholar]
- Delcourt M., Blows M. W., Rundle H. D.2009Sexually antagonistic genetic variance for fitness in an ancestral and a novel environment. Proc. R. Soc. B 276, 2009–2014 (doi:10.1098/rspb.2008.1459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson J. L.1992Scramble competition polygyny in the milkweed leaf beetle—combat, mobility and the importance of being there. Behav. Ecol. 3, 32–41 (doi:10.1093/beheco/3.1.32) [Google Scholar]
- Dowling D. K., Abiega K. C., Arnqvist G.2007Temperature-specific outcomes of cytoplasmic–nuclear interactions on egg-to-adult development time in seed beetles. Evolution 61, 194–201 (doi:10.1111/j.1558-5646.2007.00016.x) [DOI] [PubMed] [Google Scholar]
- Draper N. R., Smith H.1998Applied regression analysis. New York, NY: John Wiley & Sons [Google Scholar]
- Eady P.1994Intraspecific variation in sperm precedence in the Bruchid beetle Callosobruchus maculatus. Ecol. Entomol. 19, 11–16 (doi:10.1111/j.1365-2311.1994.tb00384.x) [Google Scholar]
- Edvardsson M., Tregenza T.2005Why do male Callosobruchus maculatus harm their mates? Behav. Ecol. 16, 788–793 (doi:10.1093/beheco/ari055) [Google Scholar]
- Estes S., Ajie B. C., Lynch M., Phillips P. C.2005Spontaneous mutational correlations for life-history, morphological and behavioral characters in Caenorhabditis elegans. Genetics 170, 645–653 (doi:10.1534/genetics.104.040022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorka K. M., Mousseau T. A.2004Female mating bias results in conflicting sex-specific offspring fitness. Nature 429, 65–67 (doi:10.1038/nature02492) [DOI] [PubMed] [Google Scholar]
- Foerster K., Coulson T., Sheldon B. C., Pemberton J. M., Clutton-Brock T. H., Kruuk L. E. B.2007Sexually antagonistic genetic variation for fitness in red deer. Nature 447, 1107–1110 (doi:10.1038/nature05912) [DOI] [PubMed] [Google Scholar]
- Fox C. W., Bush M. L., Roff D. A., Wallin W. G.2004Evolutionary genetics of lifespan and mortality rates in two populations of the seed beetle Callosobruchus maculatus. Heredity 92, 170–181 (doi:10.1038/sj.hdy.6800383) [DOI] [PubMed] [Google Scholar]
- Fricke C., Arnqvist G., Amaro N.2006Female modulation of reproductive rate and its role in postmating prezygotic isolation in Callosobruchus maculatus. Func. Ecol. 20, 360–368 (doi:10.1111/j.1365-2435.2006.01102.x) [Google Scholar]
- Fujii K., Gatehouse A. M. R., Johnson C. D., Mitchel R., Yoshida T.1990Bruchids and legumes: economics, ecology and coevolution Dordrecht, The Netherlands: Kluwer [Google Scholar]
- Gotthard K.2008Adaptive growth decisions in butterflies. Bioscience 58, 222–230 (doi:10.1641/B580308) [Google Scholar]
- Heesterbeek J. A. P.2002A brief history of R0 and a recipe for its calculation. Acta Biotheor. 50, 189–204 (doi:10.1023/A:1016599411804) [DOI] [PubMed] [Google Scholar]
- Hotzy C., Arnqvist G.2009Sperm competition favors harmful males in seed beetles. Curr. Biol. 19, 404–407 (doi:10.1016/j.cub.2009.01.045) [DOI] [PubMed] [Google Scholar]
- Houle D., Hughes K. A., Hoffmaster D. K., Ihara J., Assimacopoulos S., Canada D., Charlesworth B.1994The effects of spontaneous mutation on quantitative traits. 1. Variances and covariances of life-history traits. Genetics 138, 773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosik V., Honek A.2008Sexual differences in insect development time in relation to sexual size dimorphism. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn D. J., Blanckenhorn W. U., Szekely T.), pp. 205–211 Oxford, UK: Oxford University Press [Google Scholar]
- Jolliffe I. T.1982A note on the use of principal components in regression. J. R. Stat. Soc. C 31, 300–303 [Google Scholar]
- Lande R.1987Genetic correlations between the sexes in the evolution of sexual dimorphism and mating preferences. In Sexual selection: testing the alternatives (eds Bradbury J. W., Andersson M. B.), pp. 83–94 Chichester, UK: John Wiley [Google Scholar]
- Long T. A. F., Montgomerie R., Chippindale A. K.2006Quantifying the gender load: can population crosses reveal interlocus sexual conflict? Phil. Trans. R. Soc. B 361, 363–374 (doi:10.1098/rstb.2005.1786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovich J. E., Gibbons J. W.1992A review of techniques for quantifying sexual size dimorphism. Growth Dev. Aging 56, 269–281 [PubMed] [Google Scholar]
- Maklakov A. A., Fricke C., Arnqvist G.2007aSexual selection affects lifespan and aging in the seed beetle. Aging Cell 6, 739–744 (doi:10.1111/j.1474-9726.2007.00333.x) [DOI] [PubMed] [Google Scholar]
- Maklakov A. A., Kremer N., Arnqvist G.2007bThe effects of age at mating on female life-history traits in a seed beetle. Behav. Ecol. 18, 551–555 (doi:10.1093/beheco/arm016) [Google Scholar]
- Mank J. E., Hultin-Rosenberg L., Zwahlen M., Ellegren H.2008Pleiotropic constraint hampers the resolution of sexual antagonism in vertebrate gene expression. Am. Nat. 171, 35–43 (doi:10.1086/523954) [DOI] [PubMed] [Google Scholar]
- Manly B.1997Randomization, bootstrap and Monte Carlo methods in biology London, UK: Chapman and Hall [Google Scholar]
- Messina F. J.1991Life-history variation in a seed beetle: adult egg-laying vs. larval competition ability. Oecologia 85, 447–455 (doi:10.1007/BF00320624) [DOI] [PubMed] [Google Scholar]
- Messina F. J., Mendenhall M., Jones J. C.2009An experimentally induced host shift in a seed beetle. Entomol. Exp. Appl. 132, 39–49 (doi:10.1111/j.1570-7458.2009.00864.x) [Google Scholar]
- Møller H., Smith R. H., Sibly R. M.1989Evolutionary demography of a bruchid beetle. I. Quantitative genetical analysis of the female life history. Funct. Ecol. 3, 673–681 (doi:10.2307/2389499) [Google Scholar]
- Morbey Y. E., Ydenberg R. C.2001Protandrous arrival timing to breeding areas: a review. Ecol. Lett. 4, 663–673 (doi:10.1046/j.1461-0248.2001.00265.x) [Google Scholar]
- Morrow E. H., Stewart A. D., Rice W. R.2008Assessing the extent of genome-wide intralocus sexual conflict via experimentally enforced gender-limited selection. J. Evol. Biol. 21, 1046–1054 (doi:10.1111/j.1420-9101.2008.01542.x) [DOI] [PubMed] [Google Scholar]
- Moya-Larano J., Fox C. W.2006Ejaculate size, second male size, and moderate polyandry increase female fecundity in a seed beetle. Behav. Ecol. 17, 940–946 (doi:10.1093/beheco/arl029) [Google Scholar]
- Ofuya T. I.1995Multiple mating and its consequences in males of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stored Prod. Res. 31, 71–75 (doi:10.1016/0022-474X(94)00031-N) [Google Scholar]
- Parker G. A.1979Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum M. S., Blum N. A.), pp. 123–166 New York, NY: Academic Press [Google Scholar]
- Pischedda A., Chippindale A. K.2006Intralocus sexual conflict diminishes the benefits of sexual selection. PLoS Biol. 4, 2099–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad N. G., Bedhomme S., Day T., Chippindale A. K.2007An evolutionary cost of separate genders revealed by male-limited evolution. Am. Nat. 169, 29–37 (doi:10.1086/509941) [DOI] [PubMed] [Google Scholar]
- Pushpinder J. R.1986Mating and its attendant behaviour in Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stored Prod. Res. 22, 77–79 [Google Scholar]
- Rankin D. J., Arnqvist G.2008Sexual dimorphism is associated with population fitness in the seed beetle Callosobruchus maculatus. Evolution 62, 622–630 (doi:10.1111/j.1558-5646.2007.00315.x) [DOI] [PubMed] [Google Scholar]
- Reeve J. P., Fairbairn D. J.2001Predicting the evolution of sexual size dimorphism. J. Evol. Biol. 14, 244–254 (doi:10.1046/j.1420-9101.2001.00276.x) [Google Scholar]
- Rhen T.2000Sex-limited mutations and the evolution of sexual dimorphism. Evolution 54, 37–43 (doi:10.1111/j.0014-3820.2000.tb00005.x) [DOI] [PubMed] [Google Scholar]
- Rice W. R.1992Sexually antagonistic genes: experimental evidence. Science 256, 1436–1439 (doi:10.1126/science.1604317) [DOI] [PubMed] [Google Scholar]
- Rice W. R.1996Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232–234 (doi:10.1038/381232a0) [DOI] [PubMed] [Google Scholar]
- Rice W. R., Chippindale A. K.2001Intersexual ontogenetic conflict. J. Evol. Biol. 14, 685–693 (doi:10.1046/j.1420-9101.2001.00319.x) [Google Scholar]
- Rice W. R., Chippindale A. K.2002The evolution of hybrid infertility: perpetual coevolution between gender-specific and sexually antagonistic genes. Genetica 116, 179–188 (doi:10.1023/A:1021205130926) [PubMed] [Google Scholar]
- Rice W. R., Gaines S. D.1994Heads I win, tails you lose—testing directional alternative hypotheses in ecological and evolutionary research. Trends Ecol. Evol. 9, 235–237 (doi:10.1016/0169-5347(94)90258-5) [DOI] [PubMed] [Google Scholar]
- Roff D. A.2008Defining fitness in evolutionary models. J. Genet. 87, 339–348 (doi:10.1007/s12041-008-0056-9) [DOI] [PubMed] [Google Scholar]
- Rönn J., Katvala M., Arnqvist G.2007Coevolution between harmful male genitalia and female resistance in seed beetles. Proc. Natl Acad. Sci. USA 104, 10 921–10 925 (doi:10.1073/pnas.0701170104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönn J. L., Katvala M., Arnqvist G.2008Interspecific variation in ejaculate allocation and associated effects on female fitness in seed beetles. J. Evol. Biol. 21, 461–470 (doi:10.1111/j.1420-9101.2007.01493.x) [DOI] [PubMed] [Google Scholar]
- Savalli U. M., Fox C. W.1999The effect of male size, age, and mating behavior on sexual selection in the seed beetle Callosobruchus maculatus. Ethol. Ecol. Evol. 11, 49–60 [Google Scholar]
- Seslija D., Tucic N.2003Selection for developmental time in bean weevil (Acanthoscelides obtectus): correlated responses for other life history traits and genetic architecture of line differentiation. Ent. Exp. Appl. 106, 19–35 (doi:10.1046/j.1570-7458.2003.00007.x) [Google Scholar]
- Simoes P., Rose M. R., Duarte A., Goncalves R., Matos M.2007Evolutionary domestication in Drosophila subobscura. J. Evol. Biol. 20, 758–766 (doi:10.1111/j.1420-9101.2006.01244.x) [DOI] [PubMed] [Google Scholar]
- Simoes P., Santos J., Fragata I., Mueller L. D., Rose M. R., Matos M.2008How repeatable is adaptive evolution? The role of geographical origin and founder effects in laboratory adaptation. Evolution 62, 1817–1829 (doi:10.1111/j.1558-5646.2008.00423.x) [DOI] [PubMed] [Google Scholar]
- Smith R. J.1999Statistics of sexual size dimorphism. J. Hum. Evol. 36, 423–459 (doi:10.1006/jhev.1998.0281) [DOI] [PubMed] [Google Scholar]
- Southgate B. J.1979Biology of the Bruchidae. Ann. Rev. Entomol. 24, 449–473 (doi:10.1146/annurev.en.24.010179.002313) [Google Scholar]
- Stillwell R. C., Fox C. W.2007Environmental effects on sexual size dimorphism of a seed-feeding beetle. Oecologia 153, 273–280 (doi:10.1007/s00442-007-0724-0) [DOI] [PubMed] [Google Scholar]
- Svensson E., Sheldon B. C.1998The social context of life history evolution. Oikos 83, 466–477 (doi:10.2307/3546674) [Google Scholar]
- ter Braak C. J. F.1992Permutation versus bootstrap significance tests in multiple regression and ANOVA. In Bootstrapping and related techniques (eds Jockel K. H., Rothe G., Sendler W.), pp. 79–86 Berlin, Germany: Springer [Google Scholar]
- Thornhill R., Alcock J.1983The evolution of insect mating systems Cambridge, MA: Harvard University Press [Google Scholar]
- Toquenaga Y., Fujii K.1990Contest and scramble competition in two bruchid species, Callosobruchus analis and C. phaseoli (Coleoptera: Bruchidae). I. Larval competition curves and interference mechanisms. Res. Pop. Ecol. 32, 349–363 (doi:10.1007/BF02512569) [Google Scholar]
- Wedell N., Kvarnemo C., Lessells C. K. M., Tregenza T.2006Sexual conflict and life histories. Anim. Behav. 71, 999–1011 (doi:10.1016/j.anbehav.2005.06.023) [Google Scholar]
- Zhang X. S., Hill W. G.2005Genetic variability under mutation selection balance. Trends Ecol. Evol. 20, 468–470 (doi:10.1016/j.tree.2005.06.010) [DOI] [PubMed] [Google Scholar]
- Zonneveld C.1996Being big or emerging early? Polyandry and the trade-off between size and emergence in male butterflies. Am. Nat. 147, 946–965 (doi:10.1086/285887) [Google Scholar]