Abstract
Sensory plasticity, whereby individuals compensate for sensory deprivation in one sense by an improvement in the performance of an alternative sense, is a well-documented phenomenon in nature. Despite this, the behavioural and ecological consequences of sensory plasticity have not been addressed. Here we show experimentally that some components (vision and chemoreception) of the sensory system of guppies are developmentally plastic, and that this plasticity has important consequences for foraging behaviour. Guppies reared under low light conditions had a significantly stronger response to chemical food cues encountered in isolation than fish reared at higher light levels. Conversely, they exhibited a weaker response to visual-only cues. When visual and olfactory/gustatory cues were presented together, no difference between the strength of response for fish reared at different light intensities was evident. Our data suggest that guppies can compensate for experience of a visually poor, low light environment via a sensory switch from vision to olfaction/gustation. This switch from sight to chemoreception may allow individuals to carry out the foraging behaviour that is essential to their survival in a visually poor environment. These considerations are especially important given the increasing frequency of anthropogenic changes to ecosystems. Compensatory phenotypic plasticity as demonstrated by our study may provide a hitherto unconsidered buffer that could allow animals to perform fundamental behaviours in the face of considerable change to the sensory environment.
Keywords: sensory plasticity, foraging behaviour, light environment, development
1. Introduction
Phenotypic development is the result of a complex interaction between genetic and environmental factors. These environmental effects can constrain development (through, for example, poor nutrition in early life: Monaghan 2008) but it is also possible that environmental conditions during early development may adaptively shape individuals’ phenotypes in such a way as to prepare them for conditions they are likely to encounter during their lives. For example, terrestrial snakes reared in an aquatic environment showed enhanced locomotor performance in water compared with snakes reared on land (Aubret et al. 2007; Aubret & Shine 2008). Compensatory phenotypic plasticity provides the potential for organisms to respond effectively to environmental change. Hence understanding the limits of trait plasticity is crucial to predict to what extent animals can mediate against environmental change via phenotypic plasticity.
Animals extract information from the environment using a diverse array of senses, and sensory information is of critical importance in locating food, finding mates and avoiding predators. A growing body of evidence in neurobiology highlights the brain's remarkable capacity to be shaped by the environment: for example, humans experiencing visual deficiencies early in life have been shown to compensate to some degree via sensory switches from vision to other senses such as hearing (Lessard et al. 1998) or touch (van Boven et al. 2000). This phenomenon (known as sensory plasticity) has also been documented in various other model species in neurobiology (e.g. cats, Rauschecker 1995). Given the importance of sensory performance in fitness-related behaviours such as foraging and predator avoidance it is perhaps surprising that the behavioural and ecological consequences of such sensory plasticity are not well understood. Here we provide evidence that a model species of poeciliid fish, the guppy, is capable of making a sensory switch from vision to chemoreception (smell/taste) following rearing in low light conditions, and show that this can have profound implications for fundamental behaviours such as locating food.
In wild animals, the ability to compensate for a loss or deficit in one sense by a sharpened acuity in another (sensory plasticity) is likely to be of importance in environments where vision is hampered. In aquatic ecosystems light intensity can vary dramatically over relatively small spatial scales as a function of depth, canopy cover and habitat structure. Furthermore, human disturbance and organic pollution are key factors that drive increases in turbidity which acts to reduce light intensity (Nurminen & Horppila 2006), reducing the visual performance of a variety of aquatic species (Engstrom-Ost & Candolin 2006). Reduction in visual cues can have powerful, detrimental effects upon a suite of behaviours and ultimately an individual's survival. Many animals primarily rely upon visual cues to forage, and a number of studies have found that capture efficiency of prey is reduced at lower light intensities in a variety of species (e.g. ruffe Gymnocephalus cernuus (Bergman 1988), perch Perca fluviatus (Diehl 1988), juvenile salmon Salmo salar (Fraser & Metcalfe 1997), largemouth bass Micropterus salmoides (McMahon & Holanov 2005)). Sensory plasticity has the potential to help animals overcome the reduction in visual cues associated with low light intensity and is hence likely to be important in visually mediated behaviours such as foraging.
The ‘compensatory plasticity hypothesis’ (Rauschecker & Kniepert 1994) proposes that a loss or deficit in one sense leads to a heightened capacity in another. Hence we might predict that animals experiencing low light environments during ontogeny can compensate for a lack of visual acuity by switching to reliance upon an alternative sensory modality such as olfaction (smell). Alternatively, given the importance of vision in the development of spatial concepts, we might predict that early experience of a low light environment would severely impair an individual's behaviour in spatial tasks (Axelrod 1959) such as locating food (the ‘sensory constraint hypothesis’). Here we experimentally test these competing hypotheses in a foraging context: firstly, we rear newly born guppies Poecilia reticulata, at low and relatively high light intensities over a period of 72 days (figure 1). Secondly, we provide the first ecologically relevant test of the importance of sensory plasticity upon a fundamental behaviour—the ability to locate food in a novel environment.
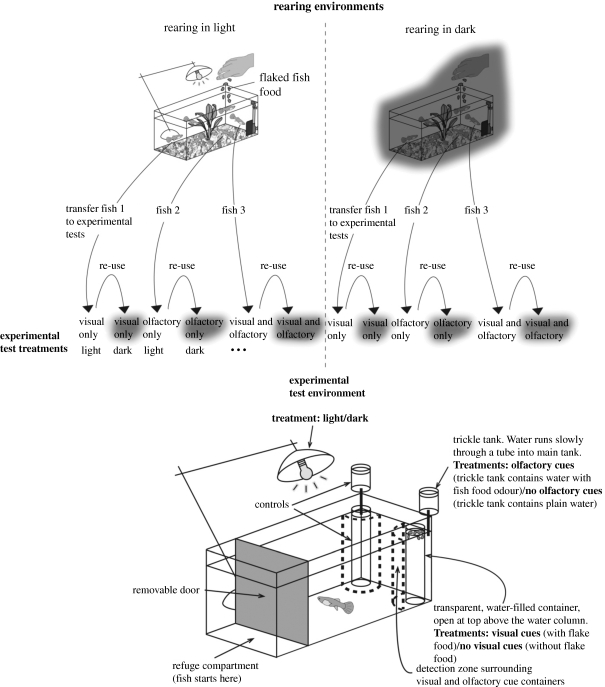
Figure 1.
Experimental design and foraging tank setup. Fish reared in the two light environments were assigned to different cue treatments (top half), before foraging behaviour was assayed in a test tank (bottom half). Note that the order of experimental lighting conditions was systematically alternated so half the fish experienced a light environment first, and half a dark environment first.
2. Material and methods
(a). Rearing environment
All of the fish used in this experiment were descended from wild-type guppies from a high predation population (the Tacarigua river in Trinidad: Trinidad national grid reference: PS 787 804; coordinates: 10°40.736′ N, 61°19.168′ W).
We randomly assigned neonate fry of less than or equal to 10 mm (mean ± s.d. = 7.6 ± 1.1 mm) to one of two light intensity environments: relatively high light intensity (307 ± 136 lx: from now on the ‘light’ treatment) and relatively low light intensity (1.545 ± 0.11 lx: from now on the ‘dark’ treatment) for a period of 72 days. Fish were reared at an initial density of six individuals per tank and fed daily with a 5 mm2 spatula on ZM200 fry food for 40 days before switching to Aquarian brand flake food for the remaining 32 days. Rearing tanks were 20 × 20 × 60 cm and contained a foam filter, plastic aquarium plant and gravel substrate. Water depth was maintained at 11 cm. We added 1 ml of a copper sulphate-based algal control (King British) to all tanks at 14 day intervals following 38 days to control for differences in food availability due to potential differential algal growth between treatments. It should be noted that at high concentrations (10 mg l−1) copper sulphate can cause anosmia in fish (Belanger et al. 2006); here we used very low concentrations (0.076 mg l−1) that did not cause anosmia (see §3). We reared 16 replicates of high light and 17 replicates of low light treatments. After 72 days, we randomly selected three fry from each tank to test in the foraging trials. One fry from each tank was assigned to each of the three cue treatments: exposure to visual food cues only, olfactory food cues only and visual and olfactory cues together.
(b). Foraging trial
The foraging trial consisted of a plastic tank of dimensions 46 × 37 × 30 cm that was divided into two compartments by an opaque plastic divider which could be lifted remotely by an observer (figure 1). One compartment (the refuge) was shaded from above. The second compartment (food compartment) contained two transparent 250 ml cylindrical containers filled with water (diameter 7.5 cm, height 9.5 cm) which contained visual food cues or a control of an empty container with no cues during the trial. The cue containers were placed 5 cm from the corner farthest from the refuge compartment. Visual food cues consisted of finely crushed flake food (0.6 g) sprinkled on the surface of the water in the cue container. We manufactured olfactory cues by filtering a solution of finely crushed flake food (10 g l−1). Four hundred millilitres of olfactory cue were used in each trial. These olfactory cues were delivered via a simple delivery system which consisted of a plastic container with a small hole at the base through which cues dripped through plastic tubing at a known rate (1 ml s−1) beneath the water level of the test tank. A container was fixed above each of the two corners of the tank containing the visual cue containers, one of which contained an olfactory cue solution and a control which contained aerated water. An area of 5 cm (approx. three body lengths) around the visual cue containers was designated the ‘detection zone’. An overflow pipe at the back of the refuge compartment meant that water levels remained constant throughout the trials. Trials were observed via a mirror angled at 45° above the test tank. The test tank had opaque sides to minimize disturbance to the focal fish. Each test fish was trialled under both light and dark conditions which matched light and dark rearing conditions on consecutive days in a repeated measures design to assess the importance of rearing environment, cue use and current light environment. The lighting conditions test fish experienced on the first day was alternated between replicates.
(c). Experimental protocol
We isolated test fish 24 h prior to the trial in a transparent, perforated bottle (250 ml) within their home tank. Prior to the beginning of the trial, fish were acclimatized for 1 h under trial lighting conditions before being placed into the experimental tank. Individuals were given a further 2 min to acclimatize in the refuge compartment before the door was lifted remotely by the observer. Once the fish had left the refuge zone, a 5 min trial began. We measured the proportion of time spent in the cue zone out of the total time spent in either zone as an index of cue detection. At the end of the trial, fish were returned to their holding bottle and placed in their rearing tank and fed. After 24 h, the focal fish was tested again in the alternate light intensity conditions.
(d). Statistical analysis
All data were analysed using R 2.7.0. Linear mixed effect models (LME) were used to statistically assess the importance of rearing environment (high and low light), experimental lighting condition (high and low light), sex and rearing tank final density upon our index of cue detection (which was arcsin square root transformed to satisfy parametric assumptions). We included final tank density into our analysis as there were mortalities during the rearing period (well within the expected range for this species (Chapman et al. 2008a,b). All of these analyses included rearing treatment, final tank density and experimental lighting condition as main effects, all interaction terms and individual ID as a random effect in this repeated measures analysis. We included all main effects and interaction terms in the initial model, which was then simplified by sequentially removing all non-significant terms to achieve the minimal adequate model. We found no significant interaction effects, and so only report main effects. Fish that failed to enter the detection zone during the foraging trial were excluded from data analysis (n = 12; this occurred in 5.9% of trials), as were fish that froze for more than 30 s during the trial (n = 3; 1.5% of trials).
3. Results
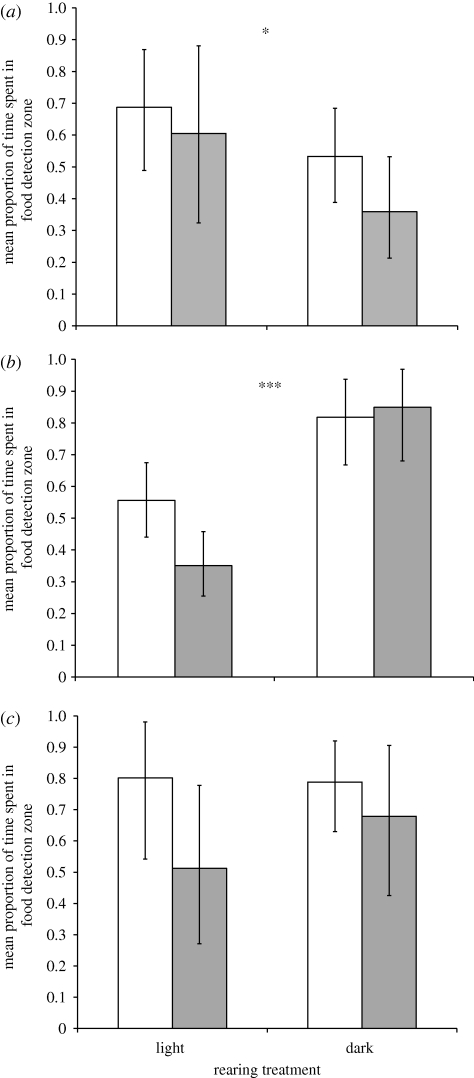
Guppies reared at higher light intensities responded significantly stronger to visual cues, irrespective of trial lighting condition (p < 0.05; table 1 and figure 2a). Conversely, fish reared at low light intensities responded significantly stronger to olfactory cues than fish reared at higher light intensities, also irrespective of trial lighting condition (p < 0.0001; table 1 and figure 2b). Finally we found no effect of rearing treatment on the strength of response to a combination of visual and olfactory cues (p > 0.05; table 1 and figure 2c), although irrespective of rearing treatment individuals responded more strongly at higher light intensities (p < 0.05; table 1). These results are consistent with the compensatory sensory plasticity hypothesis and show that animals reared at low light intensities make a sensory switch to olfaction, while maintaining a comparable response when both cues are present. We also found a positive relationship between final rearing density and strength of response to cues when olfactory cues were isolated (p < 0.05; table 1).
Table 1.
A summary of the results from the three foraging trials. All main effects are shown following model simplification. Emboldened p-values denote significance.
| visual cues only |
olfactory cues only |
visual and olfactory |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| F | d.f. | p | F | d.f. | p | F | d.f. | p | |
| rearing treatment | 6.68 | 1,28 | 0.02 | 27.52 | 1,28 | <0.001 | 0.41 | 1,22 | 0.53 |
| trial conditions | 1.12 | 1,25 | 0.30 | 1.21 | 1,28 | 0.28 | 5.10 | 1,23 | 0.03 |
| sex | 0.29 | 1,28 | 0.60 | 0.47 | 1,28 | 0.50 | 0.28 | 1,22 | 0.60 |
| density | 1.46 | 1,28 | 0.24 | 6.27 | 1,28 | 0.02 | 4.20 | 1,23 | 0.05 |
Figure 2.
Mean±2 s.e. proportion of time spent in the food detection zone back-transformed following an arcsin squareroot transformation) with (a) only visual cues available, (b) only olfactory cues available and (c) both visual and olfactory cues. Shaded bars indicate dark trial conditions; unshaded bars indicate high light trial conditions. Note that *p < 0.05; ***p < 0.001.
4. Discussion
Here we provide one of the first ecologically relevant tests of the consequences of sensory plasticity and show that fish reared at low light levels can compensate for a reduction of visual cues in foraging via a sensory switch from vision to chemoreception (olfaction/gestation). Our results suggest that following degradation of the visual environment, guppies can potentially maintain feeding rates via developmental sensory plasticity. This plasticity enables fish to maintain a strong response to food cues using an alternative sensory mode, and hence is likely to be of critical importance to individual survival and growth potential/fecundity. In turn this will potentially also impact upon population dynamics and persistence and ultimately the structuring of ecological communities. Guppies primarily rely upon vision in various other important behaviours such as mate choice, social behaviour and predator detection, but also use olfactory cues to mediate these behaviours (Brown & Godin 1999; Shohet & Watt 2004; Guevara-Fiore et al. 2009). Hence the sensory plasticity we document here could have implications beyond simply locating food to being beneficial to other fitness-related behaviours in this species. Many animals use a combination of sensory cue modalities in behaviours as diverse as assessing mate quality to navigation (e.g. Candolin 2003 and references within). Evidence suggests that early sensory experience is important in the development of neuronal circuits and sensory physiology in a number of species (e.g. mice, ferrets and turtles: Grubb & Thompson 2004). Hence the compensatory sensory plasticity we document here is potentially a broad phenomenon (at least among higher vertebrates) which means that the behavioural consequences of such plasticity could have far-reaching implications for a wide range of taxa. As yet data upon the role of plasticity in mediating fitness-related behaviour is lacking, but indirect evidence suggests that this phenomenon has powerful consequences for species other than the guppy. In a foraging context, sensory compensation may occur in wild populations of sticklebacks Gasterosteus aculeatus. Individuals from a number of UK populations were able to maintain prey capture rate despite increasing turbidity (Webster et al. 2007). Furthermore, when the larvae of a marine fish, the striped trumpeter Latris lineata, were reared in clear water individuals suffered a reduced foraging efficiency in algal cell-induced turbid water (greenwater). However, larvae reared in greenwater showed no negative effects when foraging in turbid conditions, a pattern that could be explained by individuals reared in turbid conditions making a switch to an alternative sensory modality (Cobcroft et al. 2001).
Interestingly, guppies were unable to plastically respond to short-term changes in the sensory environment. Fish reared under low light conditions assayed under high light conditions did not show a significant increase in the strength of response to visual only food cues. This could be due to the costs involved in switching between reliance upon different sensory modes, irreversible neurophysiological changes or the inflexibility imposed by the learning of associations and behavioural rules by fish. The precise mechanisms of the compensatory sensory plasticity we report here warrant further investigation. The stronger response to olfactory cues exhibited by dark-reared fish may be due to increased attention to olfactory signals, a complex learning process whereby available cues are more effectively used, neurophysiological changes in the hard-wiring of sensory circuits or potentially structural changes in the olfactory epithelium such as increased lamellar folding. Some evidence from humans suggests that structural differences occur in the brain in response to early visual deprivation leading to a reduced grey matter volume in the visual cortex (Noppeney et al. 2005). Such morphological differences may be indicative of changes in synaptic density, dendritic spine numbers or axonal arborizations (Globus & Scheibel 1966). Rats experiencing visual deprivation during ontogeny were reported to have a higher neuron density and spine density in the auditory cortex (Ryogu et al. 1975). Recent work also supports the idea that the developmental light environment is important in mediating plasticity in the visual system (Fuller et al. 2005).
Analysis of the consequences of environmental change upon individual fitness has mostly relied upon research carried out at a single stage in an organism's development (e.g. the effects of pollutants upon fish: Ward et al. 2008). If animals are able to flexibly respond to changing environments via sensory plasticity (as we show here in response to a reduction in light intensity), it is important that future studies of this kind must incorporate the developmental environment into experimental designs. In our study we focus on an aquatic environment, and indeed freshwater and marine ecosystems are particularly prone to shifts in the sensory environment. In the wild, aquatic environments are among the most heavily impacted ecosystems in the world (Vitousek et al. 1997), and changes to the visual environment can be driven by a number of factors including increased turbidity (due to algal growths or sediment load), brownification of waterbodies and changes in the canopy structure due to logging and deforestation. Terrestrial environments can also be subject to changes in the sensory environment: for example wind-farms, leisure activities, increased urbanization and construction can drive changes in the acoustic environment in both aquatic systems and on land. Plasticity in communication behaviour has been documented in birds in response to acoustic disruption: for example, great tits Parus major that live in noisy urban environments flexibly adjust their song patterns to attract mates by singing faster and at higher minimum frequencies (Slabbekoorn & Smith 2002).
Human activity can also have major effects upon the olfactory environment animals experience through the release of pollutants from industry, manufacturing or agriculture. Recent research into the sub-lethal effects of common pollutants such as the surfactant 4-nonylphenol (Ward et al. 2008), and increased levels of humic acid (a by-product of eutrophication) in waterbodies (Fisher et al. 2006) have shown that these negatively impact upon social recognition in fish. Pesticides such as malathion and deltamethrin have been shown to reduce the strength of a male's response to female pheromones in Asian corn borer moths, Ostrinia furnacalis (malathion: Zhou et al. 2005; deltamethrin: Wei & Du 2004) and exposure to zinc and lead impaired fright response to predator cues in tadpoles of spotted frogs Rana luteiventris (Lefcort et al. 1998). Similarly, olfactory epithelium damage in fishes has been linked to pesticide use (Tierney et al. 2007), and exposure to metals such as cadmium (Scott et al. 2003; Sloman 2007). Whether phenotypic plasticity can counteract this loss of sensory function by sharpening other senses such as vision (as we report here in olfaction in response to low light conditions) is an intriguing prospect, and remains to be investigated. Given the ubiquity of chemical communication in the animal kingdom and its importance in sexual selection (Plenderleith et al. 2005), social behaviour (Ward et al. 2008) and predator detection (Coleman & Rosenthal 2006) in many species, a research focus in this area could have wide-reaching implications. So while here we have focused upon changes in the visual environment, it remains a challenge for sensory ecologists to understand how diverse changes to the sensory environment impact upon the development and behaviour of organisms. Understanding how individuals respond to such changes in environment is vital to our understanding of the consequences of anthropogenic environmental change in ecosystems.
We also found that fish spent significantly more time in the detection zone when both cues were present in higher light conditions, irrespective of early experience. This perhaps suggests that while sensory plasticity allows individuals to compensate for a poor visual rearing environment, all fish do better under the light conditions they have evolved to forage in. Furthermore, fish experiencing higher density tanks responded more strongly to olfactory cues. At these higher densities fish most probably experienced increased levels of competition within the rearing tank, which may drive individuals to respond more strongly to food cues. Early social environment is certainly crucial in the development of many behaviours (Chapman et al. 2008a,b) and further work investigating density, competitive level and foraging behaviour would certainly be useful.
In conclusion we have shown that sensory plasticity has positive consequences for foraging behaviour under visually poor conditions, which is likely to be extremely important in individual survival and reproduction and hence individual fitness. Our results indicate that sensory plasticity may have profound ecological consequences, potentially buffering populations, and ultimately species, against environmental change. We predict that further investigation into the behavioural and ecological importance of sensory plasticity will provide fertile ground for future research.
Acknowledgements
Many thanks to Neil Metcalfe, Keith Hamer, John Endler, Jon Ward, Jolyon Faria and Gwen Rodgers for useful discussions, Martin Stålhammer for graphics assistance and to two anonymous reviewers for comments that greatly improved the clarity of our manuscript! B.B.C. was funded by a University of Leeds scholarship, L.J.M. by a NERC fellowship NE/D008 921/1 and J.K. and C.R.T. by a NERC grant NE/DO11 035/1.
References
- Aubret F., Shine R.2008Early experience influences both habitat choice locomotor performance in tiger snakes. Am. Nat. 171, 524–531 (doi:10.1086/528969) [DOI] [PubMed] [Google Scholar]
- Aubret F., Bonnet X., Shine R.2007The role of adaptive plasticity in major evolutionary transition: early aquatic experience affects locomotor performance of terrestrial snakes. Funct. Ecol. 21, 1154–1161 (doi:10.1111/j.1365-2435.2007.01310.x) [Google Scholar]
- Axelrod S.1959Effects of early blindness. New York, NY: American Foundation for the Blind [Google Scholar]
- Belanger R. M., Corkum L. D., Li W., Zielinski B. S.2006Olfactory sensory input increases gill ventilation in male round gobies (Neogobius melanostomus) during exposure to steroids. Comp. Biochem. Physiol. Part A 144, 196–202 (doi:10.1016/j.cbpa.2006.02.027) [DOI] [PubMed] [Google Scholar]
- Bergman E.1988Foraging abilities and niche breadths of two percids, Perca fluviatilis and Gymocephalus cernua under different environmental conditions. J. Anim. Ecol. 57, 443–453 [Google Scholar]
- Brown G. E., Godin G. J.1999Chemical alarm signals in wild Trinidadian guppies (Poecilia reticulata). Can. J. Zool. 77, 562–570 (doi:10.1139/cjz-77-4-562) [Google Scholar]
- Candolin U.2003The use of multiple cues in mate choice. Biol. Rev. 78, 575–595 (doi:10.1017/S1464793103006158) [DOI] [PubMed] [Google Scholar]
- Chapman B. B., Morrell L. J., Benton T. B., Krause J.2008aEarly interactions with adults mediate the development of predator defenses in guppies. Behav. Ecol. 19, 87–93 (doi:10.1093/beheco/arm111) [Google Scholar]
- Chapman B. B., Ward A. J. W., Krause J.2008bSchooling and learning: early social environment predicts social learning ability in the guppy Poecilia reticulata. Anim. Behav. 76, 923–929 (doi:10.1016/j.anbehav.2008.03.022) [Google Scholar]
- Cobcroft J. M., Pankhurst P. M., Hart P. R., Battaglene S. C.2001The effects of light intensity and algae induced turbidity on feeding behaviour of larval striped trumpeter. J. Fish Biol. 59, 1181–1197 (doi:10.1111/j.1095-8649.2001.tb00185.x) [Google Scholar]
- Coleman S. W., Rosenthal G. G.2006Swordtail fry attend to chemical and visual cues in detecting predators and conspecifics. PLoS ONE 1, e118 (doi:10.1371/journal.pone.0000118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl S.1988Foraging efficiency of three freshwater fishes: effects of structural complexity and light. Oikos 53, 207–214 (doi:10.2307/3566064) [Google Scholar]
- Engstrom-Ost J., Candolin U.2006Human-induced water turbidity alters selection on sexual displays in sticklebacks. Behav. Ecol. 18, 393–398 (doi:10.1093/beheco/arl097) [Google Scholar]
- Fisher H. S., Wong B. M., Rosenthal G. G.2006Alteration of the chemical environment disrupts communication in a freshwater fish. Proc. R. Soc. B 273, 1187–1193 (doi:10.1098/rspb.2005.3406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. H. C., Metcalfe N. B.1997The costs of becoming nocturnal: feeding efficiency in relation to light intensity in juvenile Atlantic Salmon. Funct. Ecol. 11, 385–391 (doi:10.1046/j.1365-2435.1997.00098.x) [Google Scholar]
- Fuller R. C., Carleton K. L., Fadool J. M., Spady T. C., Travis J.2005Genetic and environmental variation in the visual properties of the bluefin killifish Luciana goodei. J. Evol. Biol. 18, 516–523 (doi:10.1111/j.1420-9101.2005.00886.x) [DOI] [PubMed] [Google Scholar]
- Globus A., Scheibel A. B.1966Loss of dendrite spines as an index of pre-synaptic terminal patterns. Nature 212, 463–465 (doi:10.1038/212463a0) [DOI] [PubMed] [Google Scholar]
- Grubb M. S., Thompson I. D.2004The influence of early experience on the development of sensory systems. Curr. Opin. Neurobiol. 14, 503–512 (doi:10.1016/j.conb.2004.06.006) [DOI] [PubMed] [Google Scholar]
- Guevara-Fiore P., Skinner A., Watt P. J.2009Do male guppies distinguish virgin females from recently mated ones? Anim. Behav. 77, 425–431 [Google Scholar]
- Lefcort H., Meguire R. A., Wilson L. H., Ettinger W. F.1998Heavy metals alter the survival, growth, metamorphosis, and antipredator behavior of Columbia spotted frog (Rana luteiventris) tadpoles. Arch. Environ. Contam. Toxicol. 35, 447–456 (doi:10.1007/s002449900401) [DOI] [PubMed] [Google Scholar]
- Lessard N., Pare M., Lepore F., Lassonde M.1998Early-blind human subjects localize sound sources better than sighted subjects. Nature 395, 278–280 (doi:10.1038/26228) [DOI] [PubMed] [Google Scholar]
- McMahon T. E., Holanov S. H.2005Foraging success of largemouth bass at different light intensities: implications for time and depth of feeding. J. Fish Biol. 46, 759–767 (doi:10.1111/j.1095-8649.1995.tb01599.x) [Google Scholar]
- Monaghan P.2008Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645 (doi:10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U., Friston K., Ashburner K., Frackowiak R., Price C.2005Early visual deprivation induces structural plasticity in gray and white matter. Curr. Biol. 15, 488–490 [DOI] [PubMed] [Google Scholar]
- Nurminen L., Horppila J.2006Efficiency of fish feeding on plant-attached prey: effects of inorganic turbidity and plant-mediated changes in the light environment. Limnol. Oceanogr. 51, 1550–1555 [Google Scholar]
- Plenderleith M., van Oosterhout C., Robinson R. L., Turner G. F.2005Female preference for conspecific males based on olfactory cues in a Lake Malawi cichlid fish. Biol. Lett. 1, 411–414 (doi:10.1098/rsbl.2005.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J. P.1995Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 18, 36–43 (doi:10.1016/0166-2236(95)93948-W) [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Kniepert U.1994Auditory localization behaviour in visually deprived cats. Euro. J. Neurosci. 6, 149–160 (doi:10.1111/j.1460-9568.1994.tb00256.x) [DOI] [PubMed] [Google Scholar]
- Ryogu D. K., Ryugo R., Globus A., Killackey H. P.1975Increased spine density in auditory cortex following visual or somatic deafferentation. Brain Res. 90, 143–146 [DOI] [PubMed] [Google Scholar]
- Scott G. R., Sloman K. A., Rouleau C., Wood C. M.2003Cadmium disrupts behavioural and physiological responses to alarm substance in juvenile rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 206, 1779–1790 (doi:10.1242/jeb.00353) [DOI] [PubMed] [Google Scholar]
- Shohet A. J., Watt P. J.2004Female association preferences based on olfactory cues in the guppy Poecilia reticulata. Behav. Ecol. Sociobiol. 55, 363–369 (doi:10.1007/s00265-003-0722-0) [Google Scholar]
- Slabbekoorn H., Smith T. B.2002Habitat-dependent song divergence in the little greenbul: an analysis of environmental selection pressures on acoustic signals. Evolution 56, 1849–1853 [DOI] [PubMed] [Google Scholar]
- Sloman K. A.2007Effects of trace metals on salmonid fish: the role of social hierarchies. Appl. Anim. Behav. Sci. 104, 326–345 (doi:10.1016/j.applanim.2006.09.003) [Google Scholar]
- Tierney K. B., Singh C. R., Ross P. S., Kennedy C. J.2007Relating olfactory neurotoxicity to altered olfactory-mediated behaviors in rainbow trout exposed to three currently-used pesticides. Aquat. Toxicol. 81, 55–64 (doi:10.1016/j.aquatox.2006.11.006) [DOI] [PubMed] [Google Scholar]
- van Boven R. W., Hamilton R. H., Kauffman T., Keenan J. P., Pascual–Leone A.2000Tactile spatial resolution in blind Braille readers. Neurology 54, 2230–2236 [DOI] [PubMed] [Google Scholar]
- Vitousek P. M., Mooney H. A., Lubchenco J., Melillo J. M.1997Human domination of Earth's ecosystems. Science 277, 494–499 (doi:10.1126/science.277.5325.494) [Google Scholar]
- Ward A. J. W., Duff A. J., Horsfall J. S., Currie S.2008Scents and scents-ability—pollution disrupts chemical social recognition and shoaling in fish. Proc. R. Soc. B 275, 101–105 (doi:10.1098/rspb.2007.1283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M. M., Atton N., Ward A. J. W., Hart P. J. B.2007Turbidity and foraging rate in three-spined sticklebacks: the importance of visual and chemical cues. Behaviour 144, 1347–1360 (doi:10.1163/156853907782418222) [Google Scholar]
- Wei H., Du J.2004Sublethal effects of larval treatment with deltamethrin on moth sex pheromone communication system of the Asian corn borer, Ostrinia furnacalis. Pest. Biochem. Physiol. 80, 12–20 (doi:10.1016/j.pestbp.2004.05.001) [Google Scholar]
- Zhou H.2005Effects of sublethal doses of malathion on responses to sex pheromones by male Asian corn borer moths, Ostrinia furnacalis (Guenee). J. Chem. Ecol. 31, 1645–1656 (doi:10.1007/s10886-005-5804-1) [DOI] [PubMed] [Google Scholar]