Abstract
Coevolution of male and female genitalia in waterfowl has been hypothesized to occur through sexual conflict. This hypothesis raises questions about the functional morphology of the waterfowl penis and the mechanics of copulation in waterfowl, which are poorly understood. We used high-speed video of phallus eversion and histology to describe for the first time the functional morphology of the avian penis. Eversion of the 20 cm muscovy duck penis is explosive, taking an average of 0.36 s, and achieving a maximum velocity of 1.6 m s−1. The collagen matrix of the penis is very thin and not arranged in an axial-orthogonal array, resulting in a penis that is flexible when erect. To test the hypothesis that female genital novelties make intromission difficult during forced copulations, we investigated penile eversion into glass tubes that presented different mechanical challenges to eversion. Eversion occurred successfully in a straight tube and a counterclockwise spiral tube that matched the chirality of the waterfowl penis, but eversion was significantly less successful into glass tubes with a clockwise spiral or a 135° bend, which mimicked female vaginal geometry. Our results support the hypothesis that duck vaginal complexity functions to exclude the penis during forced copulations, and coevolved with the waterfowl penis via antagonistic sexual conflict.
Keywords: sexual conflict, penis functional morphology, hydrostatic skeleton
1. Introduction
Sexual conflict is a common consequence of the differences in the reproductive interests of the sexes (Parker 1979), and it can result in antagonistic coevolution in genital morphology (Arnqvist & Rowe 2002; Morrow & Arnqvist 2003; Hosken & Stockley 2004). Recently, we described the first comparative evidence of coevolution in vertebrate genital morphology from male and female waterfowl, and proposed that waterfowl genital morphologies have coevolved through antagonistic sexual conflict (Brennan et al. 2007).
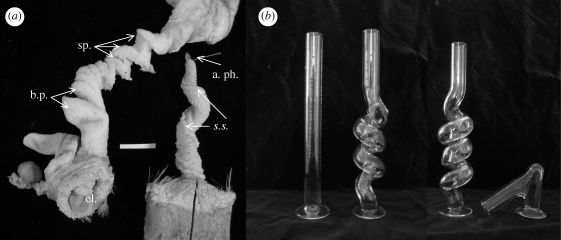
Waterfowl are among the few birds that have a penis (King 1981; Briskie & Montgomerie 1997). Penis length and surface elaboration are correlated with frequency of forced copulations among waterfowl species (Coker et al. 2002). Forced copulations can be very common in some waterfowl (McKinney et al. 1983), and may be facilitated by having an intromittent phallus (Briskie & Montgomerie 1997; Coker et al. 2002). Forced copulations result in overt sexual conflict between males and females to control fertilization. This conflict appears to have generated sexually antagonistic genital coevolution (Brennan et al. 2007), where the sexes evolve traits that allow them to control the outcome of fertilization (e.g. Parker 2006). The vaginas of females of some species of waterfowl have blind-ending pouches and a series of spirals that are opposite to the chirality of the corkscrew-shaped penis (figure 1a) (Brennan et al. 2007). We hypothesized that these anatomical structures can block and/or delay the progress of the phallus as it everts inside the vagina (Brennan et al. 2007).
Figure 1.
Duck genitalia and mechanical barriers. (a) Male and female genitalia in a Pekin duck (Anas sp.). The male phallus (right) spirals in a counterclockwise direction and the female oviduct (left) spirals in a clockwise direction. The female vagina has blind pouches (b.p.) proximal to the cloacal entrance, followed by a series of spirals (sp.). s.s., sulcus spermaticus; a. ph., tip of the penis; cl, cloaca. Scale bar, 2 cm. (b) Diameter glass tubes (10 mm) of different shapes used to test male penis eversion; from left to right, straight, anticlockwise (male-like), clockwise and 135° bend (female-like).
Surprisingly, little is known about the functional morphology of the avian penis under natural conditions (King 1981). However, the basic anatomy of the avian penis is well described, and is characterized by several distinct and evolutionary derived features. At rest, the penis is kept inverted (i.e. outside-in) within the phallic sac (saccus phalli) in the ventral wall of the cloaca. Unlike other amniotes, the erectile mechanism of the avian penis is lymphatic rather than vascular (Gerhardt 1933). During eversion, lymph accumulates in two lymphatic cavities at the base of the cloaca and enters a lymphatic lumen inside the penis forcing it out of the phallic sac (Gerhardt 1933). In waterfowl, males do not have an erection prior to copulation. Rather, intromission is accomplished by eversion of the penis into female reproductive tract. In birds and reptiles, semen is transported along the penis in an external groove (sulcus spermaticus), instead of an enclosed urethra as in mammals (figure 1a). However, during manual eversion, semen flows from the base of the penis, rather than from its tip at the end of the sulcus (apex phalli), and therefore there have been questions over whether the sulcus is functional (King 1981).
The erect penises of mammals and turtles are stiff hydrostats supported by axial-orthogonal layers of inextensible collagen fibres (Kelly 2002, 2004; Babinski et al. 2005). Unlike mammals and turtles, the waterfowl penis is flexible when erect, suggesting that the collagen fibres are not arranged in axial-orthogonal arrays. Flexible hydrostats such as sea anemones or earthworms are generally reinforced with cross-helical arrays of fibres (Koehl et al. 2000).
Here, we used high-speed video to describe the natural eversion in male muscovy duck (Cairina moschata) and histology to examine the collagen fibres of the flexible hydrostatic tissue of a duck penis. Further, we observed penis eversion into glass tubes of different shapes to test the hypothesis that derived vaginal morphologies of female waterfowl present physical challenges to intromission of the penis.
2. Material and methods
(a). Observations
We studied the penile eversion of muscovy drakes at a commercial duck farm in California. Drakes were trained to provide semen for artificial insemination for commercial production (Sellier et al. 2005). During the spring, drakes were kept in individual cages, and four days a week sperm was collected. A female muscovy was introduced into a cage and the drake was allowed to mount the female. As the male treaded on the back of the female, the swelling of the paired lymphatic bodies on either side of the male cloaca and the lymph flow into the base of the left fibrolymphatic body were observed. Once the cloaca swelling indicated that the male was ready to copulate, the duck handler held a glass container up to the male cloaca and touched the paired lymphatic bodies on the sides of the cloaca, triggering penile eversion and ejaculation into the container. We measured the straight length of the penis of 15 males immediately after ejaculation using a handheld ruler.
Penile eversions of 56 muscovy ducks were filmed with a high-speed video camera (AOS X-PRI2; 1280 × 1024 pixel resolution) at 250 frames per second using a 50 mm Navitar lens and illuminated with a 500 W halogen lamp (Lowel V). Each male was filmed only once. Eversion into the air was recorded 11 times, while eversion into mechanical challenges was recorded 45 times. The velocity of eversion could only be estimated for those observations in which the penis was approximately parallel to the ruler (in air) and continuously in view (in air and all mechanical barriers). Therefore, our sample sizes from the video analyses vary, but actual sample sizes accompany each statistical test. After eversion/ejaculation, we video recorded the reversion of the penis back into the cloaca using a Canon Powershot SD1100 IS digital video camera (n = 13).
(b). Mechanical challenges
We video recorded duck penile eversion into glass tubes of four different shapes to examine functional response to different mechanical challenges (figure 1b). Each glass tube had a 15 mm outer diameter, 2.5 mm wall thickness and 10 mm inner diameter. The first tube was straight. The second was an anticlockwise spiral that matched the direction of the spiral of the waterfowl penis. The third and fourth tubes were designed to present physical challenges to eversion that are similar to those produced by coevolved waterfowl vaginal morphologies (Brennan et al. 2007). The third tube was a clockwise spiral in the opposite direction of the duck penis. This tube was the same diameter as the third tube, but spiraled in the opposite direction. The fourth tube had a 135° bend, 2 cm from the tube entrance, similar to the distance to the female's first blind pouch. Prior to testing, each glass tube was coated with mineral oil. The time to completion of eversion was quantified from high-speed video. Eversion was considered successful if the tip of the penis (apex phalli) was completely exposed and the penis had not folded back onto itself. We compared the rates of successful eversion between the control and each mechanical barrier using two-tailed Fisher exact tests.
(c). Video analysis
Time estimates for all observations were made by counting frames of the high-speed video. We calculated differences in speed of eversion along the length of the penis using a marked straight glass tube (0.5 cm scale). We estimated the instantaneous erection velocity by digitizing the position of the tip of the penis over time as it everted through the straight glass tube (n = 8 videos), relative to the tick marks. Speed of ejaculate was estimated by digitizing the position of individual semen droplets over time in air. Only videos (n = 3) in which the penis tip was relatively immobile, and both the ruler and apparent ejaculate trajectory were approximately coincident with the image plane were used. All data are reported as average ±95% confidence interval.
(d). Histology
Following Kelly (1997), we injected the penis of two fresh specimens of male mallard (Anas platyrhyncos) with saline solution and preserved them in 10 per cent buffered formaline for histology sectioning. Specimens were embedded in paraffin; 5 µm thick histological sections were cut and stained with Masson trichrome to visualize the collagen fibres (blue). We examined 40 histological transverse cross sections and 15 longitudinal sections of the penis at different regions including the base close to the cloaca, the midpoint and close to the tip.
3. Results
(a). Eversion, ejaculation and repositioning
Full eversion of the waterfowl penis was explosive (see video S1V in the electronic supplementary material). Domestic muscovy drakes have an average penis length of 19.23 ± 0.70 cm (n = 15). Measured in air, eversion of the penis occurred in 0.346 ± 0.07 s, with a mean speed of 1.3 m s−1 (n = 8), about 60 times faster than previously reported (20 s; King 1981). The average time of penis eversion was no different between the air and a straight glass tube (0.36 ± 0.033 s, n = 10, t-test equal variance: T-ratio = 1.77, p = 0.10).
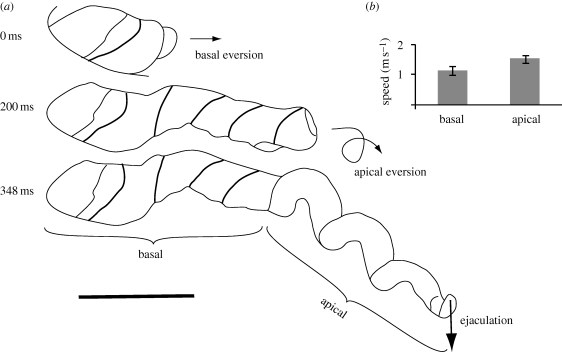
There are two distinct regions in the duck penis that differ in the curvature of the anticlockwise coils (figure 2a), and they evert at different speeds as measured in the straight glass tubes. The basal region of tight coils (approx. 7–8 cm long) everts on a linear path with an average linear velocity of 0.92 m s−1 (time to evert: 0.16 ± 0.026 s, n = 9). The apical region of more open coils (approx. 11–12 cm long) everts following a more circuitous path with an average linear velocity of 1.6 m s−1 (time to evert: 0.186 ± 0.048 s, n = 6) (figure 2b, see video S1V in the electronic supplementary material).
Figure 2.
The explosive eversion of the muscovy duck penis in air. (a) Tracings of the penis at the start of the eversion (prior to the eversion of the basal portion of the penis), midway through the eversion (prior to the eversion of the apical portion of the penis) and at the end of the eversion just before ejaculation. Tracings are from high-speed video. Arrows indicate direction of movement. Scale bar, 5 cm. (b) Average speed of the penis during the basal and apical stages (eversions through the straight tube). Error bars = 95% CI.
Ejaculation is synchronized with maximum eversion and ejaculate velocity in air was 0.75–1.56 m s−1. Unlike previous descriptions of manual ejaculation, during natural ejaculation, the sulcus spermaticus formed a functionally closed channel inside of which the semen travelled at high speed.
Reversion began immediately (less than 1 s) after ejaculation. The tip of the penis inverted, and the male began pulsating the cloacal muscles and withdrawing the base of the penis into the cloaca. Reversion of the penis back inside their cloaca took an average of 124 ± 53.27 s, n = 13), or more than 190 times longer than eversion.
(b). Mechanical challenges
There were no significant differences between successful eversion in the control straight tube and eversion inside of an anticlockwise spiral. Males were able to evert in both of these tubes in the majority of the trials (n = 9/1 and 7/2; two-tailed Fisher p = 0.58). The three instances of unsuccessful eversion during these ‘easy’ challenges (n = 3) were caused by the penis tip turning back in the wrong direction in the apical region just before it was fully everted. As predicted, there were significant differences between eversion in the control and eversion in the female-like challenges, where the penis did not fully evert in 12 of 15 trials (clockwise spiral: 6/7, and 135° angle: 6/8; two-tailed Fisher, p = 0.003 and 0.01, respectively) (see video S2V in the electronic supplementary material). The penis was completely blocked in the basal region (n = 7), or continued everting back upon itself in the wrong direction after a brief pause (n = 5). When the penis successfully everted in these female-like challenges, eversion took longer than the average in the straight tube (0.516 and 0.812 s versus 0.36 s, respectively).
Mechanical barriers did not prevent ejaculation. Even if the tip of the penis was not fully everted, semen was always ejaculated from the exposed end of the sulcus spermaticus at any point along the penis length. Only 2 of 56 observed eversions did not end in ejaculation.
(c). Female behaviour
While males were treading on the backs of the females prior to eversion, the female muscovy ducks showed multiple indications of sexual receptivity. Females assumed the well-described pre-copulatory posture with the body prone, and the tail lifted high. Females also contracted and relaxed their cloacal muscles at a rate of 41 times min−1 (±7.49, n = 5) (see video S3V in the electronic supplementary material).
(d). Histology
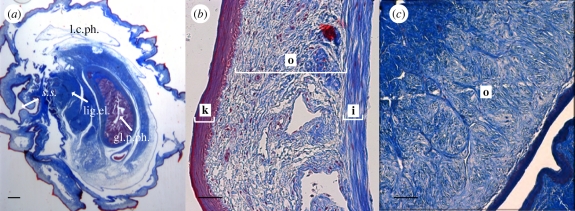
The fluid-filled lumen of the duck penis occupies a proportionally larger cross-sectional area than the collagen fibres (figure 3a), the opposite to the pattern present in mammals. The large lymphatic lumen of the waterfowl penis is surrounded by a thin layer of collagen fibres only 200–300 µm thick (figure 3a). This collagen layer is composed of two distinct layers: an inner layer (40–50 µm thick) next to the lumen which is made of circumferential fibres that encircle the lumen, and an outer layer (150–250 µm thick) that lacks any recognizable organization in either cross or longitudinal sections (figure 3b,c). This collagen organization is distinctly different from the mammal and turtle penis, where the collagen fibres surrounding the lumen are arranged in axial-orthogonal layers. The surface of the penis is covered with keratinized ridges and spines. The ridges run around the circumference of the penis, but vary in precise position and orientation on the outer surface of the spiral. The spines are oriented with their tips facing backward towards the base of the penis.
Figure 3.
Histological sections of the mallard penis. (a) Cross section of the base of a mallard penis. l.c.ph, lymphatic cavity of the phallus; s.s., sulcus spermaticus; gl.p.ph, glandular part of the phallus; lig.el, elastic ligament. Scale bar, 1 mm. (b) Close view of the wall of the penis at the base (20×). i, inner layer of collagen; o, outer layer of collagen; k, keratinized epithilium. (c) Longitudinal section of the outer layer (o) of the penis at the base (20×) showing collagen fibres not arranged in any particular pattern. Scale bar, (b,c) 100 µm.
4. Discussion
(a). Sexual conflict
Our experiments support the hypothesis that the convoluted vaginal morphologies that have coevolved with penis size in various duck lineages present physical, mechanical barriers to the full eversion of the waterfowl penis. The straight and anticlockwise spiral tubes provided minimal barriers to the progress of eversion. However, the clockwise spiral tube and the 135° angle tube—which mimic geometric aspects of the mechanical challenges present in female duck vaginas—created significant physical barriers to eversion. The two spiral tubes we used differ only in the chirality of the spiral, supporting an antagonistic function for vaginal morphologies that conflict with, rather than match, the natural spiral of the duck penis.
Although female-like mechanical challenges prevented full eversion, they did not prevent ejaculation. Semen was always ejaculated from the exposed end of the external sperm channel (sulcus spermaticus) regardless of the extent of eversion. Males that are prevented from fully everting inside the oviduct will deposit sperm at lower positions in the reproductive tract. Our observations support the hypothesis that novelties in waterfowl vaginal morphology can restrict forced intromission, and prevent the deposition of sperm deep within the reproductive tract where it would be more likely to achieve fertilization.
We hypothesize that the complex vaginal morphologies function during forced copulations and not during receptive copulations. Observations confirm that behaviour of sexually receptive female muscovy ducks includes several features that may make full intromission easier. Females assumed a characteristic receptive posture with the body prone and the tail lifted high to expose the cloaca (McKinney 1992). Females repeatedly contracted and relaxed their cloacal muscles, similar to contractions that allow passage of an egg down the oviduct (Shimada & Asai 1978). Contractions during receptive copulations may relax the oviduct wall enough for preferred males to achieve full penetration and increase the likelihood of fertilization. In contrast, females struggle vigorously during forced copulations and do not adopt a receptive posture (McKinney et al. 1983; McKinney & Evarts 1998).
Our data are consistent with the hypothesis that complex coevolved vaginal morphologies of waterfowl function in physically restricting forced intromission and reducing consequent likelihood of fertilization. Forced copulations result in high direct (viability) costs to the female from mate abandonment, injury and death (McKinney et al. 1983; McKinney & Evarts 1998). Females can also incur direct costs of mating after copulation if males manipulate female reproduction with seminal products (e.g. Drosophila, Chapman et al. 2003). It is possible that such manipulation occurs in waterfowl but this remains to be tested. However, vaginal morphology is unlikely to have evolved primarily by selection to limit the direct costs of mating (i.e. sexual conflict in the narrow sense; Chapman et al. 2003), because complex genital morphology does not directly help females to avoid forced copulations and does not prevent insemination. Rather, convoluted vaginal morphology could have evolved through the indirect (genetic via offspring) benefits to females of reasserting their own mating preferences (sexual conflict in the broad sense; Parker 2006) by preventing unwanted sperm from reaching fertilization sites. Our hypothesis that genital anatomy has coevolved through broad sexual conflict between female mating preference and male coercion is consistent with the details of waterfowl breeding systems such as elaborate and diversified courtship display behaviour in waterfowl (e.g. Lorenz 1971) and direct evidence of female mate preferences (e.g. Sorenson & Derrickson 1994).
An alternative hypothesis is that complex vaginal morphologies of waterfowl are a form of cryptic female choice, which has evolved by the indirect benefit to females of forced copulations through siring male offspring that would also be more successful at sexual coercion (e.g. Cordero & Eberhard 2003). However, this hypothesis is inconsistent with the evidence that females of many waterfowl species select and pair bond with mates weeks or months before the breeding season (e.g. Rohwer & Anderson 1988), and that direct costs of forced copulations are expected to be high for females as described above.
(b). Functional morphology
Our observations constitute the first description of the functional morphology of penile eversion and ejaculation in birds. Eversion of the penis is explosive, occurring in approximately a third of a second in air, and less than half a second inside glass barriers. It is difficult to predict whether eversion inside of the female is faster or slower, because we do not know enough about how the female oviduct functions during intromission and the interactions between the male and female genitalia (see below). The explosive nature of penis eversion and ejaculation in waterfowl provides males with a mechanism to forcefully achieve insemination in a short period of time. Previous description of eversion in 15–20 s described by Liebe (1914) (cited in King 1981) is inaccurate and artifactual.
Our observations document that the s. spermaticus forms an efficient channel for sperm transport. Semen is ejected from the tip of the penis at a speed of up to 1.6 m s−1. Ejaculation is exactly timed relative to the contraction of the muscles of the lymphatic bodies to insure that it takes place at the brief moment of maximum eversion. This mechanism differs from that found in mammals and turtles, where either thrusting or the establishment of a genital lock is required for ejaculation to take place (e.g. Davis & Jackson 1970; Dewsbury 1975).
The distinct basal and apical regions of the duck penis that we describe here correlate with the structure of the female vagina. The length of the female region of blind pockets (7 cm) is similar to that of the basal region of the penis (7–8 cm), whereas the length of the region with spirals (11.5 cm) is similar to the apical region (11–12 cm) (P. Brennan 2009, unpublished data). The different shape between these two distinct regions may have functional significance within the context of copulation. In addition, the shape and position of the keratinized ridges, bumps and spines on the surface of waterfowl penis suggests that they engage the surface of the lumen of the vagina and function mechanically in preventing the penis from slipping during eversion. The unrealistically rigid surfaces of the glass tubes prevent these mechanical interactions during our observations. However, the glass tubes allow us to test the hypothesis that the shapes found in the female vagina make eversion of the penis more difficult.
Unlike the stiff hydrostat penis of mammals and turtles, the duck penis functions as a flexible hydrostat, and remains flexible when fully everted. The curved, flexible penis of male waterfowl apparently functions in the navigation of the inside of the folded female vagina during rapid eversion. The collagen matrix that surrounds the central lumen of the penis lacks the axial-orthogonal layers that provide rigidity in mammal and turtle erections (Kelly 2004). However, we did not find the typical arrangement of cross-helical fibres of flexible hydrostats in the duck penis. Instead, we found a circumferential inner collagen layer (at a 90° angle from the longitudinal axis of the penis), and a second outer layer that was not organized in a discernable pattern. This unusual organization could be related to spiral shape of the waterfowl penis. Hydrostatic cross-helical skeletons usually do not twist or spiral when they extend because when the fibres perfectly oppose one another, they cause the clockwise and anticlockwise torques to balance (Koehl et al. 2000; Wolgemuth et al. 2005). In the waterfowl penis, when pressurized lymph is forced into the penis, the inner circumferential collagen layer probably restricts the expansion of the lymphatic lumen to a fixed maximum diameter, thereby forcing additional lymph towards the apical portions of the penis. The outer collagen layer in the duck penis may also contribute to the apical portion's spiral shape. A biomechanical model for the development of spiral bacteria suggests that unbalanced torque between ‘sub-orthogonal’ fibres results in a spiral shape; for example, if stretch-resistant fibres all wrap helically in the same direction, resulting in a spiral shape when the interior is pressurized (Wolgemuth et al. 2005). More data are required to test this mechanism in the waterfowl penis. In addition to collagen, β-keratin in the epithelium of the penis probably offers additional structural support for the hydrostatic skeleton of the waterfowl penis, as keratin is an inextensible polymer.
The functional novelties of copulation in waterfowl include a combination of features that have evolved in earlier avian ancestors and those that have probably evolved within waterfowl. The early evolutionary origin of the flexible hydrostat avian penis was probably coincident with the evolution of lymphatic erection and the associated loss of the mechanical capacity to maintain higher pressure in the hydraulic lumen of the penis for extended periods of time.
Acknowledgements
This research followed all the guidelines for ethical treatment of animals in research under IACUC approval # 2008-10906.
The commercial farm where we conducted our research wishes to remain anonymous, but we are grateful for their full cooperation during our research. We thank Tom Libby and the Center for Integrative Biomechanics Education and Research of the University of California at Berkeley for the use of their high-speed camera. Histological sections were made at the Yale Histopathology Laboratory. Research support was provided by the William Robertson Coe Fund from Yale University. We are grateful to Michael Anderson and Daryl Smith of Yale University for their assistance in making silicone and glass tubes.
References
- Arnqvist G., Rowe L.2002Antagonistic coevolution between the sexes in a group of insects. Nature 415, 787–789 (doi:10.1038/415787a) [DOI] [PubMed] [Google Scholar]
- Babinski M. A., deBrito-Gitirana L., Chagas M. A., Abidú-Figueiredo M., Costa W. S., Sampaio F. J. B.2005Immunohistochemical analysis of smooth muscle cells and volumetric density of the elastic system fibers of wild boar (Sus scrofa) penis. An. Reprod. Sci. 86, 317–328 (doi:10.1016/j.anireprosci.2004.08.002) [DOI] [PubMed] [Google Scholar]
- Brennan P. L. R., Prum R. O., McCracken K. G., Sorenson M. D., Wilson R. E., Birkhead T. R.2007Coevolution of male and female genital morphology in waterfowl. PLoS ONE 2, e418 (doi:10.1371/journal.pone.0000418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskie J. V., Montgomerie R.1997Sexual selection and the intromittent organ of birds. J. Av. Biol. 28, 73–86 (doi:10.2307/3677097) [Google Scholar]
- Chapman T., Arnqvist G., Bangham J., Rowe L.2003Sexual conflict. Trends Ecol. Evol. 18, 41–48 (doi:10.1016/S0169-5347(02)00004-6) [Google Scholar]
- Coker C. R., McKinney F., Hays H., Briggs S., Cheng K.2002Intromittent organ morphology and testis size in relation to mating system in waterfowl. Auk 119, 403–413 (doi:10.1642/0004-8038(2002)119[0403:IOMATS]2.0.CO;2) [Google Scholar]
- Cordero C., Eberhard W. G.2003Female choice of sexually antagonistic male adaptations: a critical review of some current research. J. Evol. Biol. 16, 1–6 (doi:10.1046/j.1420-9101.2003.00506.x) [DOI] [PubMed] [Google Scholar]
- Davis J. D., Jackson C. G.1970Copulatory behavior in the red-eared turtle Pseudemys scripta elegans. Herpetologica 26, 238–240 [Google Scholar]
- Dewsbury D. A.1975Diversity and adaptation in rodent copulatory behavior. Science 190, 947–954 (doi:10.1126/science.1188377) [DOI] [PubMed] [Google Scholar]
- Gerhardt U.1933Kloake und Begattungsorgane. In Hanbuch der vergleichenden Anatomie der Wirbeltiere (eds Bolk L., Goppert E., Kallius E., Lubosch W.), pp. 37–54 Berlin, Germany: Urban and Schwarzenberg [Google Scholar]
- Hosken D., Stockley P.2004Sexual selection and genital evolution. Trends Ecol. Evol. 19, 87–93 (doi:10.1016/j.tree.2003.11.012) [DOI] [PubMed] [Google Scholar]
- Kelly D. A.1997Axial orthogonal fiber reinforcement in the penis of the nine-banded armadillo (Dasypus novemcinctus). J. Morph. 233, 249–255 (doi:10.1002/(SICI)1097-4687(199709)233:3<249::AID-JMOR4>3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- Kelly D. A.2002The functional morphology of penile erection: tissue designs for increasing and maintaining stiffness. Int. Comp. Biol. 42, 216–221 (doi:10.1093/icb/42.2.216) [DOI] [PubMed] [Google Scholar]
- Kelly D. A.2004Turtle and mammal penis designs are anatomically convergent. Proc. R. Soc. Lond. B 271, S293–S295 (doi:10.1098/rsbl.2004.0161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. S.1981Phallus. In Form and function in birds (eds King A. S., McLelland J.), pp. 107–148 New York, NY: Academic Press [Google Scholar]
- Koehl M. A. R., Quillin K. J., Pell C. A.2000Mechanical design of fiber-wound hydraulic skeletons: the stiffening and straightening of embryonic nothocords. Am. Zool. 40, 28–41 (doi:10.1668/0003-1569(2000)040[0028:MDOFWH]2.0.CO;2) [Google Scholar]
- Lorenz K.1971Comparative studies of the motor patterns of the Anatinae 1941. Studies in animal and human behavior, pp. 14–114 London, UK: Harvard University Press; [Translation by Martin R. Methuen.] [Google Scholar]
- McKinney F.1992Courtship, pair fomation and signal systems. In Ecology and management of breeding waterfowl (ed. Batt B. D. J.), pp. 214–250 Minneapolis, MN: University of Minnesota Press [Google Scholar]
- McKinney F., Evarts S.1998Sexual coercion in waterfowl and other birds. In Avian reproductive tactics: female and male perspectives (eds Parker P. G., Tyler Burley N.), pp. 163–195 Washington, DC: American Ornithologist Union [Google Scholar]
- McKinney F., Derrickson S. R., Mineau P.1983Forced copulation in waterfowl. Behavior 86, 250–294 (doi:10.1163/156853983X00390) [Google Scholar]
- Morrow E. H., Arnqvist G.2003Costly traumatic insemination and a female counter-adaptation in bed bugs. Proc. R. Soc. Lond. B 270, 2377–2381 (doi:10.1098/rspb.2003.2514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. A.1979Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum M. S., Blum N. B.), pp. 123–166 New York, NY: Academic Press [Google Scholar]
- Parker G. A.2006Sexual conflict over mating and fertilization: an overview. Phil. Trans. R. Soc. B 361, 235 (doi:10.1098/rstb.2005.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer F. C., Anderson M. G.1988Female-biased philopatry, monogamy, and the timing of pair formation in migratory waterfowl. Curr. Ornith. 5, 187–221 [Google Scholar]
- Sellier N., Brun J., Richard M., Batellier F., Dupuy V., Brillard J.2005Comparison of fertility and embryo mortality following artificial insemination of common duck females (Anas platyrhynchos) with semen form muscovy (Cairina moschata) drakes. Theriogenology 64, 429–439 (doi:10.1016/j.theriogenology.2004.12.010) [DOI] [PubMed] [Google Scholar]
- Shimada K., Asai I.1978Uterine contraction during the ovulatory cycle of the hen. Biol. Reprod. 19, 1057–1062 (doi:10.1095/biolreprod19.5.1057) [DOI] [PubMed] [Google Scholar]
- Sorenson L., Derrickson D. E.1994Sexual selection in the northern pintail Anas acuta: the importance of female choice versus male–male competition in the evolution of sexually selected traits. Behav. Ecol. Sociobiol. 35, 389–400 (doi:10.1007/BF00165841) [Google Scholar]
- Wolgemuth C. W., Inclan Y. F., Quan J., Mukherjee S., Oster G., Koehl M. A. R.2005How to make a spiral bacterium. Phys. Biol. 2, 189–199 (doi:10.1088/1478-3975/2/3/006) [DOI] [PubMed] [Google Scholar]