Abstract
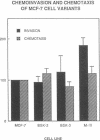
We have isolated a series of sublines of the hormone-dependent MCF-7 human breast cancer cell line after selection both in vivo and in vitro for growth in the presence of subphysiological concentrations of estrogens. These sublines represent a model system for study of the processes leading to hormonal autonomy. The cells form growing tumors in ovariectomized athymic nude mice in the absence of estrogen supplementation but retain some responsivity to estrogen as determined by stimulation of the rate of tumor growth in vivo and by induction of progesterone receptor. An ovarian-independent but hormone-responsive phenotype may occur early in the natural progression to hormone-independent and unresponsive growth in breast cancer. We observed no change in the affinity or decrease in the level of expression of estrogen receptors and progesterone receptors among the sublines and the parental cells. Epidermal growth factor receptors are not overexpressed in ovarian-independent cells. Thus, altered hormone receptor expression may be a late event in the acquisition of a hormone-independent and unresponsive phenotype. Sublines isolated by in vivo but not in vitro selection are more invasive than the parental cells both in vivo and across an artificial basement membrane in vitro. Thus, as yet unknown tumor-host interactions may be important in the development of an invasive phenotype. Furthermore, acquisition of the ovarian-independent and invasive phenotypes can occur independently.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Allegra J. C., Barlock A., Huff K. K., Lippman M. E. Changes in multiple or sequential estrogen receptor determinations in breast cancer. Cancer. 1980 Feb 15;45(4):792–794. doi: 10.1002/1097-0142(19800215)45:4<792::aid-cncr2820450430>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Bradlow H. L. A reassessment of the role of breast tumor aromatization. Cancer Res. 1982 Aug;42(8 Suppl):3382s–3386s. [PubMed] [Google Scholar]
- Briand P. Hormone-dependent mammary tumors in mice and rats as a model for human breast cancer (review). Anticancer Res. 1983 Jul-Aug;3(4):273–281. [PubMed] [Google Scholar]
- Brünner N., Svenstrup B., Spang-Thomsen M., Bennett P., Nielsen A., Nielsen J. Serum steroid levels in intact and endocrine ablated BALB/c nude mice and their intact littermates. J Steroid Biochem. 1986 Sep;25(3):429–432. doi: 10.1016/0022-4731(86)90257-8. [DOI] [PubMed] [Google Scholar]
- Clarke R., Brünner N., Katz D., Glanz P., Dickson R. B., Lippman M. E., Kern F. G. The effects of a constitutive expression of transforming growth factor-alpha on the growth of MCF-7 human breast cancer cells in vitro and in vivo. Mol Endocrinol. 1989 Feb;3(2):372–380. doi: 10.1210/mend-3-2-372. [DOI] [PubMed] [Google Scholar]
- Clarke R., Morwood J., van den Berg H. W., Nelson J., Murphy R. F. Effect of cytotoxic drugs on estrogen receptor expression and response to tamoxifen in MCF-7 cells. Cancer Res. 1986 Dec;46(12 Pt 1):6116–6119. [PubMed] [Google Scholar]
- Darbre P. D., King R. J. Progression to steroid insensitivity can occur irrespective of the presence of functional steroid receptors. Cell. 1987 Nov 20;51(4):521–528. doi: 10.1016/0092-8674(87)90121-8. [DOI] [PubMed] [Google Scholar]
- Darbre P., Yates J., Curtis S., King R. J. Effect of estradiol on human breast cancer cells in culture. Cancer Res. 1983 Jan;43(1):349–354. [PubMed] [Google Scholar]
- Davidson N. E., Gelmann E. P., Lippman M. E., Dickson R. B. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol Endocrinol. 1987 Mar;1(3):216–223. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- Devleeschouwer N., Legros N., Olea-Serrano N., Paridaens R., Leclercq G. Estrogen conjugates and serum factors mediating the estrogenic trophic effect on MCF-7 cell growth. Cancer Res. 1987 Nov 15;47(22):5883–5887. [PubMed] [Google Scholar]
- Fulton A. M., Loveless S. E., Heppner G. H. Mutagenic activity of tumor-associated macrophages in Salmonella typhimurium strains TA98 and TA 100. Cancer Res. 1984 Oct;44(10):4308–4311. [PubMed] [Google Scholar]
- Humphries J. E., Isaacs J. T. Unusual androgen sensitivity of the androgen-independent Dunning R-3327-G rat prostatic adenocarcinoma: androgen effect on tumor cell loss. Cancer Res. 1982 Aug;42(8):3148–3156. [PubMed] [Google Scholar]
- Jordan V. C. The control of hormone-dependent breast cancer growth--are we talking about estrogen alone? Eur J Cancer Clin Oncol. 1988 Aug;24(8):1245–1248. doi: 10.1016/0277-5379(88)90210-6. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Kendra K. L., Norman M. J., Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987 Aug 15;47(16):4355–4360. [PubMed] [Google Scholar]
- Kim U., Depowski M. J. Progression from hormone dependence to autonomy in mammary tumors as an in vivo manifestation of sequential clonal selection. Cancer Res. 1975 Aug;35(8):2068–2077. [PubMed] [Google Scholar]
- Manni A., Rainieri J., Arafah B. M., Finegan H. M., Pearson O. H. Role of estrogen and prolactin in the growth and receptor levels of N-nitrosomethylurea-induced rat mammary tumors. Cancer Res. 1982 Sep;42(9):3492–3495. [PubMed] [Google Scholar]
- Matsuzawa A., Kaneko T., Ikeda Y. Accelerated progression to autonomy of a pregnancy-dependent mouse mammary tumor (TPDMT-4) by hormones. Cancer Res. 1983 May;43(5):2283–2289. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Sherbet G. V., Harris A. L. Epidermal-growth-factor receptors and oestrogen receptors in human breast cancer. Lancet. 1985 Feb 16;1(8425):364–366. doi: 10.1016/s0140-6736(85)91385-6. [DOI] [PubMed] [Google Scholar]
- Seibert K., Shafie S. M., Triche T. J., Whang-Peng J. J., O'Brien S. J., Toney J. H., Huff K. K., Lippman M. E. Clonal variation of MCF-7 breast cancer cells in vitro and in athymic nude mice. Cancer Res. 1983 May;43(5):2223–2239. [PubMed] [Google Scholar]
- Shafie S. M., Grantham F. H. Role of hormones in the growth and regression of human breast cancer cells (MCF-7) transplanted into athymic nude mice. J Natl Cancer Inst. 1981 Jul;67(1):51–56. [PubMed] [Google Scholar]
- Sluyser M., Van Nie R. Estrogen receptor content and hormone-responsive growth of mouse mammary tumors. Cancer Res. 1974 Dec;34(12):3253–3257. [PubMed] [Google Scholar]
- Soule H. D., McGrath C. M. Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett. 1980 Aug;10(2):177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- Vickers P. J., Dickson R. B., Shoemaker R., Cowan K. H. A multidrug-resistant MCF-7 human breast cancer cell line which exhibits cross-resistance to antiestrogens and hormone-independent tumor growth in vivo. Mol Endocrinol. 1988 Oct;2(10):886–892. doi: 10.1210/mend-2-10-886. [DOI] [PubMed] [Google Scholar]
- van den Berg H. W., Leahey W. J., Lynch M., Clarke R., Nelson J. Recombinant human interferon alpha increases oestrogen receptor expression in human breast cancer cells (ZR-75-1) and sensitizes them to the anti-proliferative effects of tamoxifen. Br J Cancer. 1987 Mar;55(3):255–257. doi: 10.1038/bjc.1987.49. [DOI] [PMC free article] [PubMed] [Google Scholar]