Abstract
We present a general strategy for the synthesis of stable 3-D metallosupramolecular tetragonal prisms in which multicomponent coordination-driven self-assembly allows for single supramolecular species to be formed. The appropriate stoichiometric combination of tetraphenylethylene based tetratopic donor (1), linear dipyridine donor (2) and 90° platinum metal complex(3)afford tetragonal prisms (4) as single products. The compounds have been characterized by multinuclear NMR spectroscopy and electrospray ionization mass spectrometry, the size of supramolecules was determined by PGSE and modeled with molecular force field simulation methods.
Nature has developed the construction of functional biological systems via the assembly of multiple distinct molecular subunits, for example, the proteasome of yeast Saccharomyces cerevisiae are assembled from pairs of seven different proteins.1 In the past two decades, coordination-driven self-assembly has emerged as a powerful new tool simulating nature’s own complexity to construct abiological supramolecular architectures with well-defined shape and size.2 Remarkable progress has been made in the self-assembly of two elaborately designed building blocks encoded with specific information such as coordination geometry and directionality in stoichiometric ratios to give preprogrammed supramolecular structures.3 However, for multicomponent coordination-driven self-assembly with more than one metal center or individual donors, an equilibration between numerous supramolecular structures generally exists,4 resulting in a self-organized mixture via self-recognition and self-selection.5 Thus, the preparation of a single and discrete supramolecular architecture capable of mimicking the natural systems via multicomponent coordination-driven self-assembly still remains challenging.6
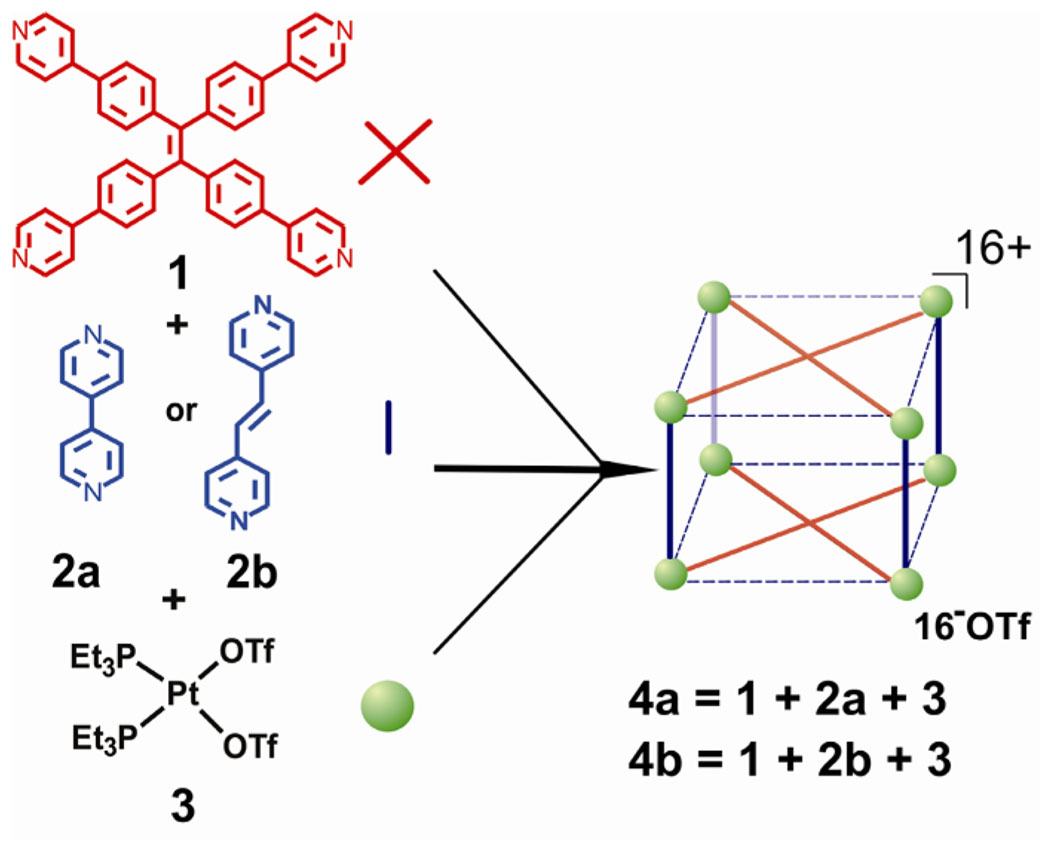
Till now, the efficient preparation of metallosupramolecules via multicomponent abiological self-assembly are largely limited to 2-D structures.6–9 We recently demonstrated the formation of 2-D fused metallacyclic polygons via the combination of properly designed multiple different tectons and in specific stoichiometric ratios,7 Schmittel et al also reported a five-component supramolecular trapezoid through integrative self-sorting.8,9 However, self-assembled 3-D structures are more prevalent and important in nature and biology. For example, polypeptides have to fold into a 3-D structure to ensure their function, and the viral coats of many viruses have structures with icosahedral symmetry.10 Furthermore, functional 3-D supramolecules play significant roles in a wide range of applications such as host-guest chemistry,11 supramolecular catalysis 12 and as micro-reaction vessels.13 Although two-component coordination-driven self-assembly is an efficient method to prepare 3-D metallosupramolecules, construction of 3-D supramolecular structures by multicomponent self-assembly still remains limited.14 For example, trigonal prisms could be prepared via three component self-assembly but accessible only in the presence of aromatic templates.15 Herein, we present the multicomponent coordination-driven self-assembly of 3-D tetragonal prisms by reaction of two donors and one metal acceptor in stoichiometric ratios without use of any templates.
The design rationale for multicomponent coordination-driven self-assembly of tetragonal prism is illustrated in Scheme 1.Tetra-(4-pyridylphenyl)-ethylene compound 1 is designed incorporating four pyridine groups that can act as the faces of a tetragonal prism during the self-assembly, while the linear donor 4, 4′-bipyridine (2a) or trans-1,2-Di(4-pyridyl)ethylene (2b) and 90° platinum triflate 3 are selected as pillars and corners of the tetragonal prism respectively under the combined interaction of edge-directed and face-directed self-assembly.2d When the three building blocks 1, 2 and 3 are reacted in a stoichiometric ratio of 1 : 2 : 4, the formation of tetragonal prism can be expected as shown in Scheme 1.
Scheme 1.
Multicomponent coordination-driven self-assembly of tetragonal prisms
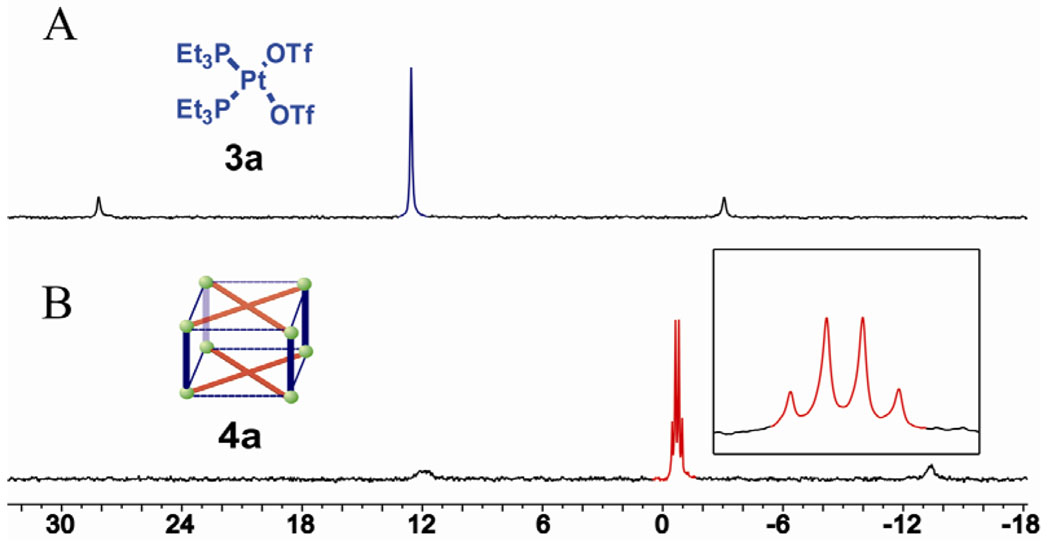
The self-assembly behavior of the three-component system composed of coplanar tetratopic donor 1, linear donor 2 and acceptor 3 was investigated by addition of the CD2Cl2 solution of donors 1 and 2 into the CD3NO2 solution of acceptor 3 in the ratio of 1: 2: 4, followed by a further 12 hours of reaction at room temperature. 31P and 1H NMR multinuclear analysis of the reaction mixture indicated the formation of single and discrete assemblies with high symmetry. The 31P{1H} NMR spectra of self-assembly product 4a and 4b show two different doublets at −0.7 ppm and −0.6 ppm with concomitant 195Pt satellites respectively, upfield shifted about 13.5 ppm compared to that of the staring acceptor 3 due to coordination with the pyridine rings(Figure 1 and Figure S2); the two doublets were confirmed by different field strength and variable temperature 31P{1H} NMR spectra (Figure S3 and S4). The two different doublets in the 31P{1H} NMR indicates that the phosphorus nuclei connected to the platinum atom in 4 are magnetically nonequivalent and these results could only be explained by considering that each platinum center is coordinating with two different pyridines belonging to donors 1 and 2 respectively in the tetragonal prism structure 4(Scheme 1). In the corresponding 1H NMR spectra of product 4, signals for the protons of the pyridine rings in donor ligand 1 and 2 exhibit downfield shifts (Hα-Py, 0.20 – 0.30 ppm for 4a and 4b; Hβ-Py, 0.40 – 0.45 ppm for 4a and 0.20 – 0.25 ppm for 4b) resulting from the loss of electron density upon coordination of the pyridine nitrogen atoms with the platinum metal center(Figure S5, S6). The sharp NMR signals in both of the 31P{1H} and 1H NMR spectra along with the solubility of these species ruled out the formation of oligomers. Moreover, the observed NMR data exclude the formation of either simple prisms or squares between donor 1 and 3, as well as donor 2 and 3 respectively.
Figure 1.
The 31P{1H} NMR spectra (121.4 MHz, 298K, CD2Cl2/CD3NO2 v : v = 2 : 1) of acceptor 3 (A) and tetragonal prism 4a (B)
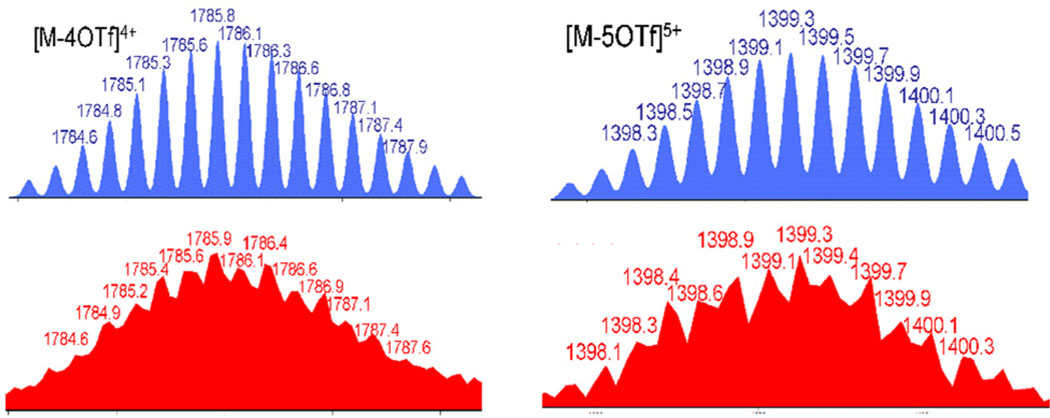
Electrospray ionization (ESI) mass spectra of 4 were measured to provide evidence for the formation of tetragonal prism structures. In the mass spectrum of assembly 4a, peaks at m/z = 1786.1 and 1399.3 that corresponding to the [M-4OTf]4+ and [M-5OTf]5+ were observed and their isotopic distributions are in good agreement with the theoretical expectation (Figure 2). The mass spectrum of tetragonal prism 4b shows [M-5OTf]5+ peak at m/z = 1420.1 with good agreement with its theoretical isotopic distribution(Figure S7).
Figure 2.
Theoretical(blue) and experimental (red) ESI-MS spectra of tetragonal prism 4a
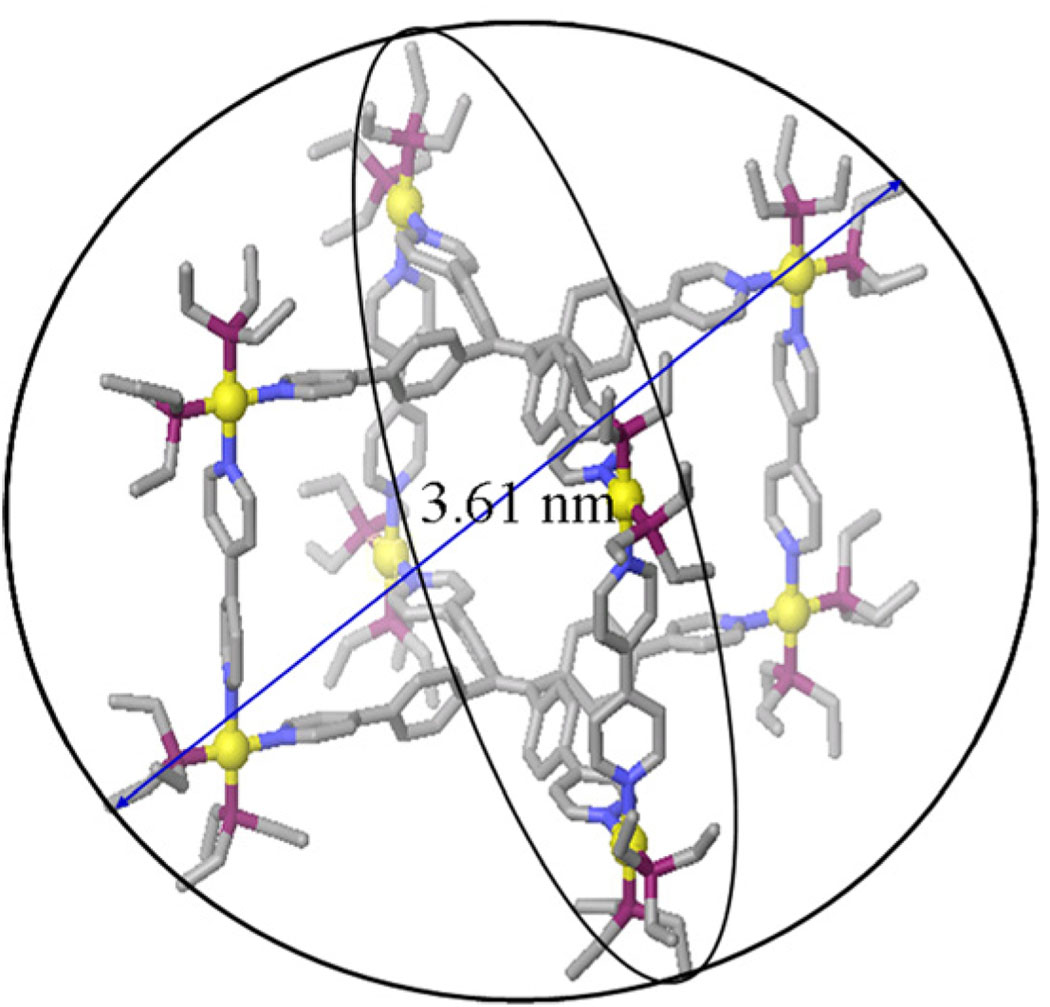
All attempts to get crystal structures of tetragonal prisms 4 were unsuccessful, and therefore molecular force field simulations were used to gain further insight into the structural characteristics of supramolecules 4. A 1.0 ns molecular dynamics simulation (MMFF force field) was used to equilibrate supramolecules 4a and 4b, followed by energy minimization of the resulting structures to full convergence. The model structure of 4a features a well-defined tetragonal prism with length, width and height of 1.66 nm, 1.55 nm, and 1.14 nm respectively, the modeling of prism 4a also indicated an diameter of 3.61 nm(Figure 3). The simulation of 4b shows a similar tetragonal structure and reveals an diameter of 3.65 nm (Figure S8). We also carried out the Pulsed-gradient spin-echo (PGSE) NMR measurements and in conjunction with the Stokes-Einstein equation (See Table S1 in SI) determined the approximate sizes of 4. The results from the PGSE NMR measurements (4a: 4.2 ± 0.1 nm; 4b: 4.3 ± 0.1 nm) are comparable to that obtained by molecular force field modeling for the diameter of 4 (Figure 3).
Figure 3.
Molecular Modeling of tetragonal prism 4a
In conclusion, we report herein the construction of discrete 3-D tetragonal prisms via multicomponent coordination-driven self-assembly from a combination of tetraphenylethylene based tetratopic donor (1), linear dipyridine donor (2a or 2b) and 90° platinum metal complex in appropriate stoichiometric ratios without using any template. Furthermore, the use of linear pillars of different length in the assembly allow for tetragonal prisms with different cavity size, which may result in applications in host-guest chemistry or as new micro-reactors. We are currently exploring the functionalization of these tetragonal prisms via multicomponent coordination-driven self-assembly.
Supplementary Material
Acknowledgment
P.J.S. thanks the NIH (Grant GM-057052) for financial support.
Footnotes
Supporting Information Available: Synthesis and analytical data for 1 and 4. PGSE measurement, strength and temperature-dependant 31P{1H} NMR for 4, ESI mass spectra and molecular modeling for 4b. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference
- 1.Groll M, Dizel L, Lowe J, Stock D, Bochter M, Bartunik HD, Huber R. Nature. 1997;386:463. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 2.(a) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502. [Google Scholar]; (b) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (c) Holliday BJ, Mirkin CA. Angew. Chem., Int. Ed. 2001;40:2022. [PubMed] [Google Scholar]; (d) Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (e) Fujita M, Tominaga M, Hori A, Terrien B. Acc. Chem. Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (f) Oliver CG, Ulman PA, Wiester MJ, Mirkin CA. Acc. Chem. Res. 2008;41:1618. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Parkash MJ, Lah MS. Chem. Commun. 2009:3326. doi: 10.1039/b902988e. [DOI] [PubMed] [Google Scholar]
- 3.(a) Ghosh K, Yang H-B, Northrop BH, Lyndon MM, Zheng Y-R, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2008;130:5320. doi: 10.1021/ja711502t. [DOI] [PubMed] [Google Scholar]; (b) Ghosh K, Hu J-M, White HS, Stang PJ. J. Am. Chem. Soc. 2009;131:6695. doi: 10.1021/ja902045q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vacek J, Caskey DC, Horinek D, Shoemaker RK, Stang PJ, Michl J. J. Am. Chem. Soc. 2008;130:7629. doi: 10.1021/ja801341m. [DOI] [PubMed] [Google Scholar]; (d) Pluth MD, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2008;130:11423. doi: 10.1021/ja802839v. [DOI] [PubMed] [Google Scholar]; (e) Mugridge JS, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2010;132:1182. doi: 10.1021/ja905170x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Lehn JM, Eliseev AV. Science. 2001;291:2331. doi: 10.1126/science.1060066. [DOI] [PubMed] [Google Scholar]; (b) Lehn JM. Chem. Eur. J. 1999;5:2455–2463. [Google Scholar]
- 5.(a) Northrop BH, Zheng Y-R, Chi K-W, Stang PJ. Acc. Chem. Res. 2009;42:1554. doi: 10.1021/ar900077c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zheng Y-R, Yang H-B, Ghosh K, Zhao L, Stang PJ. Chem. Eur. J. 2009;15:7203. doi: 10.1002/chem.200900230. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Northrop BH, Yang H-B, Stang PJ. Inorg. Chem. 2008;47:11257. doi: 10.1021/ic801711q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zheng Y-R, Yang H-B, Northrop BH, Ghosh K, Stang PJ. Inorg. Chem. 2008;47:4706. doi: 10.1021/ic800038j. [DOI] [PubMed] [Google Scholar]; (e) Zheng Y-R, Northrop BH, Yang H-B, Zhao L, Stang PJ. J. Org. Chem. 2009;74:3554. doi: 10.1021/jo9002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Christinat N, Scopelliti R, Severin K. Angew. Chem. Int. Ed. 2008;47:1848. doi: 10.1002/anie.200705272. [DOI] [PubMed] [Google Scholar]; (b) De S, Mahata K, Schmittel M. Chem. Soc. Rev. 2010 doi: 10.1039/b922293f. DOI: 10.1039/b922293f. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Ghosh K, Stang PJ. J. Am. Chem. Soc. 2009;131:12028. doi: 10.1021/ja903330j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahata K, Schmittel M. J. Am. Chem. Soc. 2009;131:16544. doi: 10.1021/ja907185k. [DOI] [PubMed] [Google Scholar]
- 9.(a) Schmittel M, Mahata K. Inorg. Chem. 2009;48:822. doi: 10.1021/ic8021084. [DOI] [PubMed] [Google Scholar]; (b) Kishore RSK, Paululat T, Schmittel M. Chem. Eur. J. 2006;12:8136. doi: 10.1002/chem.200600463. [DOI] [PubMed] [Google Scholar]
- 10.Principles of Molecular Virology. 2nd Ed. New York: Cann. A. J. Academic Press; 1997. [Google Scholar]
- 11.(a) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:349. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (b) Northrop BH, Yang H-B, Stang PJ. Chem. Commun. 2008:5896. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Pluth MD, Bergman RG, Raymond KN. Acc. Chem. Res. 2009;42:1650. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]; (b) Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Chem. Soc. Rev. 2009;38:1450. doi: 10.1039/b807080f. [DOI] [PubMed] [Google Scholar]
- 13.Pluth MD, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2008;130:6362. doi: 10.1021/ja076691h. [DOI] [PubMed] [Google Scholar]
- 14.Al-Rasbi NK, Tidmarsh IS, Argent SP, Adams H, Harding LP, Ward MD. J. Am. Chem. Soc. 2008;130:11641. doi: 10.1021/ja803847w. [DOI] [PubMed] [Google Scholar]
- 15.(a) Yoshizawa M, Nagao M, Kumazawa K, Fujita M. J. Organomet. Chem. 2005;690:5383. [Google Scholar]; (b) Yoshizawa M, Nakagawa J, Kurnazawa K, Nagao M, Kawano M, Ozeki T, Fujita M. Angew. Chem. Int. Ed. 2005;44:1810. doi: 10.1002/anie.200462171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.