Abstract
The transcription factor TBX1 is a key mediator of developmental abnormalities associated with DiGeorge/Velocardiofacial Syndrome. Studies in mice have demonstrated that decreased dosage of Tbx1 results in defects in pharyngeal arch, cardiovascular, and craniofacial development. The role of Tbx1 in cardiac development has been intensely studied; however, its role in palatal development is poorly understood. By studying the Tbx1-/- mice we found defects during the critical points of palate elongation and elevation. The intrinsic palate defects in the Tbx1-/- mice were determined by measuring changes in palate shelf length, proliferation, apoptosis, expression of relevant growth factors, and in palate fusion assays. Tbx1-/- embryos exhibit cleft palate with failed palate elevation in 100% and abnormal palatal-oral fusions in 50%. In the Tbx1-/- mice the palate shelf length was reduced and tongue height was greater, demonstrating a physical impediment to palate elevation and apposition. In vitro palate fusion assays demonstrate that Tbx1-/- palate shelves are capable of fusion but a roller culture assay showed that the null palatal shelves were unable to elongate. Diminished hyaluronic acid production in the Tbx1-/- palate shelves may explain failed palate shelf elevation. In addition, cell proliferation and apoptosis were perturbed in Tbx1-/- palates. A sharp decrease of Fgf8 expression was detected in the Tbx1-/- palate shelves, suggesting that Fgf8 is dependent on Tbx1 in the palate. Fgf10 is also up-regulated in the Tbx1-/- palate shelves and tongue. These data demonstrate that Tbx1 is a critical transcription factor that guides palatal elongation and elevation and that Fgf8 expression in the palate is Tbx1-dependent.
Keywords: palate, Tbx1, Fgf8, Tgfb3, Fgf10, Shh, Hyaluronic Acid
Introduction
Cleft palate is one of the most commonly observed congenital malformations in humans, occurring in up to 1 in 500 live births(Schutte and Murray, 1999). The function of the palate is to separate the oral cavity and nasal cavity to allow respiration, deglutition and phonation to occur. Craniofacial development is a highly regulated process involving the growth and fusion of the maxillary, mandibular and nasal processes(Chai and Maxson, 2006). Specifically, the migration of the cranial neural crest mesenchyme through the facial primordium guides proper facial growth(Trainor, 2005). During facial morphogenesis, paired first brachial arches elongate and fuse in the midline to create the palate and mandible.
During palate development, paired palate shelves under go a carefully orchestrated expansion, elevation, and midline fusion between E13.5 and E15.5 in mice. This process involves proliferation of mesenchyme within the palate shelves, and dissolution of the epithelium during palate fusion(Nawshad, 2008). Interruption of the normal palate proliferation, elevation or fusion process leads to cleft palate formation. Many genes have been identified as critical to palate development, including the transcription factor Tbx1(Gritli-Linde, 2007).
The T-box transcription factor TBX1 has been linked to DiGeorge and Velocardiofacial Syndrome in humans, both of which have cardiac and cleft palate phenotypes, similar to the mouse phenotype (Jerome and Papaioannou, 2001; Merscher et al., 2001). The cardiac anomalies in Tbx1-/- mice occur, in part, due to changes in Fgf8 and Fgf10 expression, suggesting that Fgf8 and Fgf10 are involved in Tbx1 signaling (Aggarwal et al., 2006; Brown et al., 2004). Tbx1 expression is observed throughout the developing face, including the epithelium and mesenchyme of the maxillary and mandibular prominences, as well as the endoderm of the pharyngeal pouches (Chapman et al., 1996). In the palate, Tbx1 is strongly expressed in the palatal epithelium and mesenchyme, but the underlying role(s) of Tbx1 in palate development is unknown (Zoupa et al., 2006). Tbx1 has cell-autonomous effects in the pharyngeal endoderm where it is expressed. Specific deletion of Tbx1 from the pharyngeal endoderm leads to fourth pharyngeal arch anomalies, cleft palate and abnormal facial musculature development (Arnold et al., 2006; Zhang et al., 2005). However, Tbx1 has a non-cell autonomous role in guiding neural crest migration during cardiac morphogenesis (Vitelli et al., 2002). Thus, despite previous investigations, the etiology of the cleft palate in Tbx1-/- mice is still unclear.
We have hypothesized that Tbx1 is necessary for normal palatal elongation and elevation. In this study, we demonstrate that cleft palate in Tbx1-/- mice is associated with failed palatal elevation, likely due to decreased palatal width, increased tongue height, and palatal-oral fusions. This may be due, in part, to diminished hyaluronic acid production, decreased palatal Fgf8 expression, increased Fgf10 expression, and altered proliferation/apoptosis. These results suggest that Tbx1 is a key transcription factor necessary for normal palate development.
Results
Tbx1-/- mice display a primary defect in palate elongation and elevation
To better understand the role of Tbx1 in palate development, we examined the palate phenotype in Tbx1-/- mice. The Tbx1 null embryos all exhibit a cleft palate (N=20/20). Palatal shelves are formed in Tbx1-/- embryos, but the shelves appear shortened, and are unable to elongate and elevate (figure 1 C, D). Additionally, the palatal shelves of null embryos were found to have posterior fusions to the oral cavity in 50% (N=10/20 embryos) (figure 1 D). The etiology of the palatal defect from E13.5-E15.5 was failure of elevation of the palate shelves (N=20/20).
Figure 1. Tbx1-/- mice have cleft palates and palatal-oral fusion.
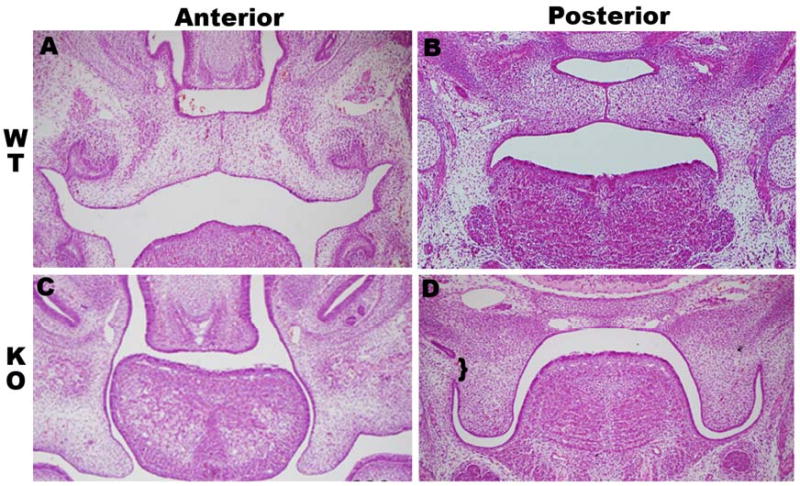
(A+B) Coronal H & E section through E14.5 wild type palate shelves undergoing fusion anteriorly (A) and posteriorly (B). (C) Coronal sections through anterior palate of Tbx1 -/- demonstrating the lack of palatal shelf elevation. (D) Posterior palate sections in Tbx1 -/- mice showing palatal-oral fusion (bracket).
In figure 2 the width of the palate shelves and the height of the tongue musculature were measured to determine if shortened palate shelves or tongue obstruction were potential mechanisms of cleft palate formation. In the E14.5 wild type mice we observed normal palate shelf elevation and tongue down-growth (figure 2 A, B, C). In the Tbx1-/- mice we observed failed palate shelf elevation, shortened palate shelves and increased tongue musculature height (figure 2 D, E, F). Measurements from the anterior, middle and posterior palate shelves and tongue musculature demonstrated significant differences in the Tbx1-/- mice. The palate shelves of the Tbx1-/- mice were significantly shorter in the middle and posterior palate (figure 2 G). The height of the tongue musculature in the Tbx1-/- mice was significantly greater in the anterior and middle tongue (figure 2 H). We measured E13.5 palate shelf length and tongue height and did not detect a statistical difference (data not shown).
Figure 2. Tbx1 -/- mice have shortened palates and greater tongue height.
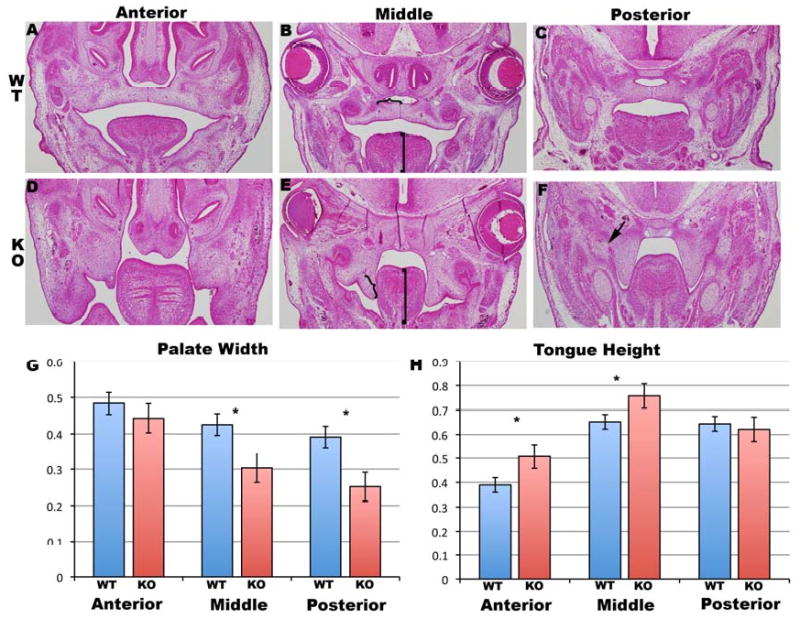
(A+B+C) Coronal H & E sections through E14.5 wild type palate shelves in the anterior (A), middle(B) and posterior(C) palate region. (D, E, F) Coronal sections through location matched (anterior, middle, posterior) palate regions in the Tbx1-/- mice. Measurements of palate width and tongue height were compared as demonstrated by the brackets on the palate (}) and tongue (]) in B and E. Statistical significance was denoted with * over the bar graphs in G and H. Note the posterior palatal-oral fusion in the in F (black arrow).
In vitro palate fusion assays were carried out to determine if proper palate fusion could occur in the Tbx1-/- mice. Using a filter culture technique in which the palatal shelves are isolated and artificially apposed, we found that the Tbx1-/- palatal shelves were capable of undergoing fusion when compared to controls (N=4/4) (figure 3 A, B). Using the roller bottle technique allowed us to study the palatal shelves in situ, with the tongue removed, identifying whether the palate shelves can properly elongate and fuse. In the roller bottle palate cultures we found that Tbx1-/- palatal shelves were unable to elongate and fuse compared to controls (N=4/4) (figure 3 C, D).
Figure 3. Tbx1-/- palatal shelves fail to develop in culture but are capable of fusion.

Coronal sections with H&E staining of wild type palate (A) filter culture after 48-hour incubation. Note full fusion and confluence of mesenchyme. Filter cultured Tbx1-/-(B) palate shelves also demonstrating fusion. Wild type roller culture palate shelves demonstrating fusion(C) but Tbx1-/-(D) roller culture palate shelves that fail to fuse due to the inability of the palate shelves to appose. PS=palate shelf
To determine if poor hydrostatic turgor of Tbx1-/- palate shelves contributed to poor palate shelf elongation and failed elevation we used alcian blue staining in the Tbx1-/- mice to detect hyaluronic acid. Coronal sections of E13.5 wild-type mice demonstrated abundant hyaluronic acid staining in the anterior and posterior palate shelves (figure 4 A, C). However, there was diminished intensity of hyaluronic acid staining in the E13.5 anterior and posterior palate shelves of the Tbx1-/- mice (figure 4 B, D). The E13.5 wild-type and Tbx1-/- coronal sections were also treated with hyaluronidase, demonstrating no alcian blue staining in the palate, confirming that the blue staining represents hyaluronic acid (data not shown).
Figure 4. Tbx1-/- palate shelves have diminished hyaluronic acid staining.
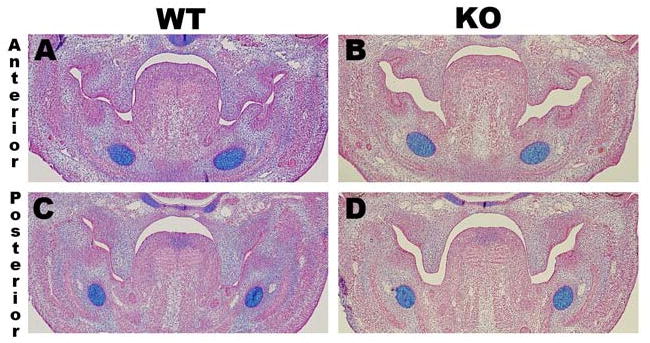
Coronal sections of E13.5 wild-type(A) anterior palate shelves showing diffuse alcian blue staining (arrow) as well as diffuse staining in the posterior palate shelves (C). In contrast, there is diminished alcian blue staining in the E13.5 Tbx1-/- palate shelves in the anterior (B) and posterior (D) regions. Treatment with hyaluronidase removed all alcian blue staining from the palate, indicating that the blue staining represents hyaluronic acid (data not shown).
Abnormal palatal elongation and elevation is associated with alterations in proliferation and apoptosis
To assess if cell proliferation was altered in Tbx1-/- embryos, we examined proliferation rates using phosphohistone-H3 immunohistochemistry. Cell proliferation was elevated at E12.5 and E13.5 in Tbx1-/- palatal shelves (p=0.001) (figure 5 A, C, D). To determine if alterations in programmed cell death were associated with the Tbx1-/- phenotype, we assessed apoptosis using TUNEL staining on Tbx1-/- palatal shelves (figure 5 B, E). Palatal shelf apoptosis was normal at E12.5 but elevated in E13.5 Tbx1-/- embryos (p=0.02) (figure 5 F).
Figure 5. Alteration of proliferation and apoptosis in Tbx1-/- palates.
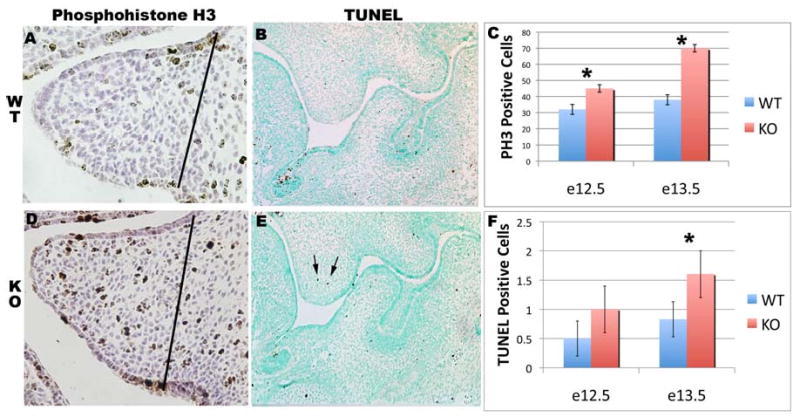
Coronal sections through wild type (A) and Tbx1-/-(D) palate shelves at E13.5 stained with phosphohistone H3. (C) Graphical representation of palate proliferation at E12.5 and E13.5. Coronal sections through wild type (B) and Tbx1 -/-(E) E13.5 palate shelves stained with TUNEL kit (arrow=TUNEL positive cell). (F) Graphical representation of palate apoptosis at E12.5 and E13.5. The dotted lines indicates the area of the palate counted. (*=Statistical significance)
Tbx1-/- palates display discrete abnormalities in Fgf8 and Fgf10
To determine targets of Tbx1 signaling we investigated changes in candidate growth factors necessary during palate development (figure 6). Using in situ hybridization, we evaluated the expression patterns of Fgf10, Fgf8, Shh, and Tgfb3 in Tbx1-/- mice. Fgf8 expression was greatly decreased in the absence of Tbx1 (figure 6 A, B). Fgf10 expression was increased in the midline of the tongue and the nasal aspect of the palate shelves of Tbx1-/- mice (figure 6 C, D). Shh expression was unchanged in the Tbx1-/- palate shelves (figure G, H). Tgfb3 is diminished in the mandible and skull base (figure 6H astericks) but still present in the medial edge epithelium (MEE) (figure 6F arrows).
Figure 6. Diminished expression of Fgf8 and increased expression of Fgf10 in Tbx1-/- palate shelves.
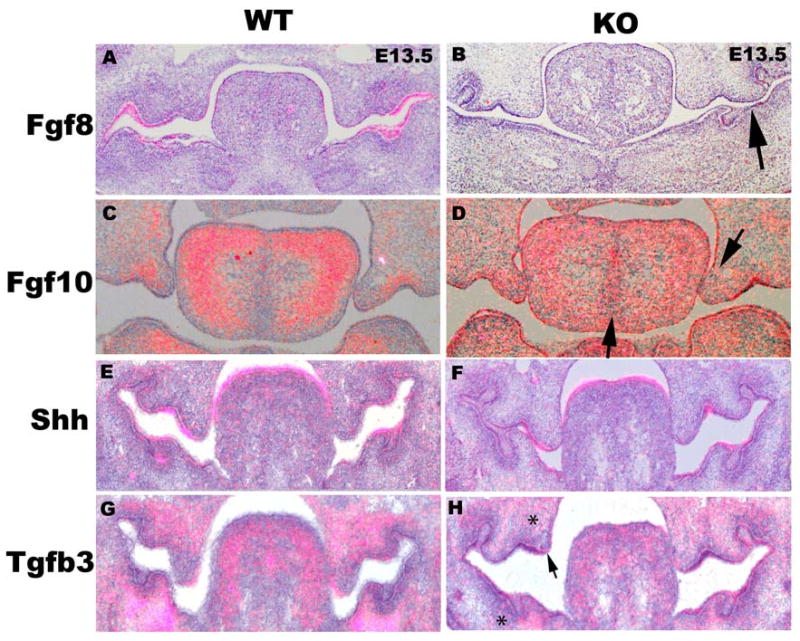
Coronal sections of situ hybridization with an Fgf8 probe on E13.5 wild type (A) and Tbx1-/-(B) mice palates (note diminished Fgf8 expression (arrow)). There is increased Fgf10 expression in the midline of the tongue and the nasal side of the Tbx1-/- palate shelves (C, D). There is no change in gene expression comparing wild type mice with E13.5 Tbx1-/- mice for Shh (E, F), but there is decreased Tgfb3 expression in the mandibular and skull base mesenchyme (*) but maintained MEE expression (arrow)(G, H).
Discussion
Without Tbx1, normal palate development fails to occur. In this paper we show that Tbx1 is necessary for palatal elongation and elevation. To support this, we identified phenotypic changes in palate shape, and behavior. In addition we identified intrinsic differences in palate composition and growth factor expression that explain, in part, the Tbx1-/- palate phenotype.
Tbx1 Palate Shelves are Incapable of Elongation and Elevation
Palatal development is dependent on coordination of palatal elongation, elevation, and fusion. Morphologically we demonstrate shortened palatal shelves that fail to elongate and elevate characterize the Tbx1-/- cleft palate phenotype (figure 1). Furthermore palatal-oral fusions occur in 50% of mice, tethering the posterior palate (figure 1D, 2F). The presence of palatal-oral fusions suggests that Tbx1 acts to maintain epithelial integrity in the posterior palate. Palatal-oral adhesions leading to cleft palate formation have been demonstrated in Fgf10, Jagged2 and Irf6 knock-out mice, all of which have defective epithelial development but, unlike the Tbx1-/- mice, maintain epithelial integrity (Ingraham et al., 2006; Jiang et al., 1998; Rice et al., 2004; Richardson et al., 2009; Richardson et al., 2006). Irf6 and Jagged2 were found to work together to maintain peridermal cell viability, preventing palatal-oral adhesions. However, areas of fusion between the palate shelves and the tongue and oral cavity have been found in the Irf6-/- and Fgf10-/- mice (Alappat et al., 2005; Richardson et al., 2009). The palatal-oral fusions in Tbx1-/- animals may directly prevent normal palatal elevation, therefore contributing to cleft palate formation. However, all of the Tbx1-/- mice have a cleft palate but the presence of the palatal-oral fusions was detected in only 50% of Tbx1-/- mice and that palate fusion typically occurs from anterior to posterior, suggests that palatal-oral fusions alone do not lead to cleft palate formation in the Tbx1-/- mice.
In the Tbx1-/- mice there are quantifiable differences in the palate width and tongue height. Palate shelf width in the E14.5 Tbx1-/- mice was significantly shorter in the middle and posterior regions (figure 2 G). Tongue musculature height in the Tbx1-/- mice was significantly greater in the anterior and middle regions (figure 2 H). The lack of palatal lengthening and palatal-oral fusions discussed above suggests two separate mechanisms that might contribute to inhibition of palate elevation in the Tbx1 -/- mice. In addition, lack of tongue down-growth in the Tbx1 -/- mice results in lingual interference with proper palate elevation providing a third mechanism contributing to the cleft palate phenotype. This data suggests that not only is palate development affected by Tbx1 deletion, but that tongue development is aberrant as well. The failure of tongue down growth causing cleft palate formation has been demonstrated in other mouse models (Huang et al., 2008). In Tbx2 deficient mice cleft palate also occurs due to deficient palatal shelves. At e14.0 the Tbx2-/- palate shelves were statistically smaller, leading to cleft palate(Zirzow et al., 2009). The Tbx2-/- palate phenotype is similar to the Tbx1-/- phenotype shown in figure 2, however the tongue shape was not assessed in the Tbx2-/- mutants.
Palate Culture Assays Demonstrate Shortened Palate Shelves in Tbx1-/- mice
Use of two palate culture techniques demonstrated that Tbx1-/- palate shelves are capable of fusion, and the primary pathology is failed palatal elongation. There were marked differences in palate culture assay when we compared filter and roller bottle assays (figure 3). The filter culture assay showed that the Tbx1-/- palate shelves are capable of undergoing fusion, despite there being delayed apoptosis in the Tbx1-/- palate shelves (figure 5). However, the roller culture assay demonstrated that the Tbx1-/- palate shelves fail to elongate and fuse, even when palatal-oral fusions were not a restriction. These data suggest that the primary pathology present in the Tbx1-/- mice palates is due to failed palatal elongation and elevation. This is similar to the Tbx2-/- mice, the palatal shelves were able to fuse in apposition, however the roller bottle technique was not used to identify primary palatal insufficiency(Zirzow et al., 2009).
Diminished Hyaluronic Acid in Tbx1-/- Palate Shelf May Impair Elevation
Elevation of the palate shelves is proposed to occur due to the proliferative and hydrostatic forces within the palate. Hydration of extracellular matrix components, including hyaluronan and chondroitin sulfate proteoglycans, has been suggested to provide an elevating turgor pressure within the palatal shelves (Morris-Wiman and Brinkley, 1992). In figure 4 we show that the E13.5 Tbx1-/- palatal shelves have diminished hyaluronic acid (B, D) compared to the wild-type palate shelves (A, C). Decreased hyaluronic acid content of the Tbx1-/- palate shelves likely contributes to their decreased width and failed elevation. This provides a potential mechanism contributing to cleft palate formation in the Tbx1-/- mice and is, to our knowledge, the first demonstration of altered extracellular matrix in the Tbx1 mutant palate.
Abnormal Proliferation and Apoptosis in the Tbx1-/- Palate shelves
To identify the underlying defect in palate development we examined if alterations in proliferation or apoptosis may be an etiologic factor leading to cleft palate formation. In figure 5 we demonstrate an increase in proliferation at E12.5 and E13.5 in the Tbx1-/- palate shelves. In the palate, proliferation is mostly confined to the neural crest derived mesenchyme, suggesting that the absence of Tbx1 in the palate induces the neural crest to proliferate (Dudas et al., 2007; Ito et al., 2003). This data is contrary to the observed decrease in cell proliferation during cardiac development in Tbx1 nulls, suggesting that Tbx1 may act on the cranial neural crest in a context dependent manner that is different than its role during palatogenesis (Vitelli et al., 2002). In Fgf10-/- mice, there was decreased palatal proliferation at E12.5 and E13.5, suggesting that Fgf10 may induce cellular proliferation in the palate (Rice et al., 2004). Increased Fgf10 expression (discussed below) in the Tbx1-/- palate shelves may explain the increased cellular proliferation observed in figure 5. In the Tbx2-/- palate shelves there is also increased cellular proliferation at E12.5, suggesting that cellular proliferation alone does not account for alterations in palatal size (Zirzow et al., 2009).
In the Tbx1-/- palatal shelves, there is a significant increase in apoptosis in day E13.5. The premature increase in apoptosis at E13.5 may allow for epithelial break down in the lateral palate shelves and oral cavity leading to aberrant fusion demonstrated in figures 1D and 2F. In the Tbx2-/- mutants increased apoptosis was identified at e11.5 and e12.5, demonstrating a similar pattern of cellular changes seen in the Tbx1-/- mutant mice (Zirzow et al., 2009).
Altered Fgf8 and Fgf10 Expression in Tbx1-/- Palate Shelves
To identify the targets of Tbx1 function in the palate shelves we evaluated the Tbx1-/- palate shelves for altered expression of Shh, Tgfb3, Fgf8, and Fgf10. In the Tbx1-/- mice we observed increased palatal and tongue mesenchymal Fgf10 expression (figure 6 C, D) but marked down-regulation of Fgf8 in the palate epithelium (figure 6 A, B). Tbx1 acts upstream of Fgf10 and Fgf8 in heart development, and others have shown that Fgf8 and Fgf10 are required for normal palatal development (Alappat et al., 2005; Brown et al., 2004; Rice et al., 2004). Conditional deletion of Tbx1 from the pharyngeal epithelium led to a loss of Fgf8 expression and was concomitant with aberrant pharyngeal pouch development and outgrowth (Arnold et al., 2006). Investigation of Tbx1+/-; Fgf10+/-; Fgf8+/- mice showed a possible functional redundancy between Fgf10 and Fgf8 during pharyngeal arch development. Loss of Fgf8 expression in pharyngeal arch development leads to increases in apoptosis, suggesting that Tbx1 may provide a cell-protective effect in the palate epithelium (Abu-Issa et al., 2002). Diminished Fgf8 expression in the palatal epithelium of Tbx1-/- mice may explain the increase in apoptosis we observed (figure 5), similar to the findings in pharyngeal arch development. Notably, loss of Fgf8 signaling in the MEE in the Tbx1-/- mice occurs in the same region as the palatal-oral fusions. Expression of Tgfb3 in the Tbx1-/- mice is still present in the MEE but reduced in the mandibular, and skull base mesenchyme. Loss of Fgf10 expression has been shown to alter the location of Tgfb3 signaling in the posterior palate MEE but the increased Fgf10 expression seen in the Tbx1-/- palatal shelves has not altered Tgfb3 palatal expression(Alappat et al., 2005).
Conclusion
Our data demonstrates that Tbx1 is a key transcriptional mediator of palatal development. The finding of palatal-oral fusions, increased tongue height, decreased palatal width and impaired palatal elevation all cause physical impediments to normal palatal elevation and apposition, leading to cleft palate formation. The lack of normal hyaluronic acid in the Tbx1-/- palate shelves likely alters the normal palate length and turgor pressure necessary for palatal elevation and elongation. Absence of Tbx1 leads to aberrant palatal proliferation and apoptosis that may be explained by decreased Fgf8 expression and increased Fgf10 expression. The palatal development and fusion assay clearly shows that the Tbx1-/- palatal shelves fail to elongate and elevate but are capable of fusion. These data taken together provide important insight into the role of Tbx1 in palatogenesis and suggest several key processes that will require further elucidation.
Materials and Methods
Mice were maintained in a mixed background, and a 0600 to 1800 light-dark cycle was used. Noon of the day of observation of a vaginal plug was defined as E0.5. The Tbx1 null mice were courtesy of Dr. Bernice Morrow. All procedures and animal experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Palate and Tongue measurements
The E13.5 and E14.5 mice heads were fixed, embedded in paraffin, and were sectioned in a coronal plane (N=3 of wild type and Tbx1 knockout). They were then hematoxylin and eosin stained. Digital photographs of the sections were taken using a Nikon e800 microscope with a Olympus digital capture system. The photographs were taken at 4× with a digital caliper placed on image with the Olympus software. Standardization of the anterior, middle and posterior palate images were performed. Using Adobe Photoshop CS3 the palate shelf width was measured using the lasso tool to outline the area of interest. Prior to measuring, we standardized the length using the Olympus digital caliper. The width of the palate and height of the tongue musculature were measured as demonstrated in figure 2 B and D. Specifically, the lateral extent of the palate shelf was determined by drawing a perpendicular line from the “hinge” region to the opposite palatal surface. The medial aspect of the palate shelf was defined as the level of the medial edge epithelium or the fusion plane of the palate. To account for palate orientation, all measurements were performed with the palate in a horizontal position, by rotating the un-elevated Tbx1-/- palate shelf images to a horizontal plane. The height of the tongue was measured from the digastric muscles inferiorly to the most superior aspect of the mid-tongue epithelium in the midline. Statistical analysis of the measurements was performed using Excel and a paired student t-test. Statistical significance was determined if p<0.05.
In Situ Hybridization
Evaluation of Fgf10, Fgf8, and Shh expression in both wild-type mice and Tbx1-/- mice was evaluated using in situ hybridization. After anesthetizing females using halothane, females were sacrificed using cervical dislocation. All complete embryos were fixed in 4% paraformaldehde (PFA) overnight and embedded in paraffin in preparation for in situ hybridization. Coronal sections were then prepared for in situ hybridization by rehydrating to PBS. Sections were treated with Proteinase K and refixed. After equilibration in tri-ethanolamine solution, sections are incubated in acetic anhydride to prevent nonspecific binding of the probe. Radioactive probe, both sense and antisense, was created using S35-dUTP. Sections were incubated overnight, followed by washings to remove unbound probe. Slides were then developed on Kodak Biomax film to check for signal. If signal was present, sections were exposed to emulsion and incubated for 10-20 days depending on the strength of the radiographic signal before developing. The radioactive signal was evaluated using darkfield microscopy. Each section was photographed using a Nikon e800 microscope with an Olympus camera. The brightfield and darkfield images were merged using Olympus software.
Palate Culture
For the filter culture of the palatal shelves, E13.5 Tbx1-/- and wild type embryos were dissected in cold PBS. The palatal shelves were removed and placed in 0.4 μM filter culture wells (Falcon, Franklin Lakes, NJ). The palatal cultures were incubated in 1 ml of serum-free BGJb media (Gibco, Grand Island, NY) at the air-media interface. The cultures were incubated at 37 degrees with 95% O2 5% CO2 for 72 hours with the media changed every 24 hours. The cultures were then fixed with 4% paraformaldehyde for 2 hours at 4 degrees. The palates were serially dehydrated using ethanol, embedded in paraffin and then sectioned and stained with hematoxylin and eosin.
For roller culture of the palatal shelves, E13.5 Tbx1-/- and +/+ embryos were dissected in cold PBS. The mandibles and brain tissue were dissected free from the midface of each embryo. The remaining midface was placed in a roller bottle with 3 ml of serum-free BGJb. The cultures were incubated for 72 hours at 37 degrees with 95% O2 and 5% C O2 with media changes every 24 hours. The palatal cultures were fixed, sectioned, and stained as above.
Apoptosis and Proliferation Assays
To assess apoptosis, E12.5 and E13.5 Tbx1-/- (N=3) and +/+ (N=3) embryos were fixed and sectioned as mentioned above. Each section was TUNEL stained according to Roche protocol (Mannheim, Germany). Each embryo was sectioned from the primary palate to the end of the secondary palate. Each palate shelf was analyzed separately at 40×, and the plane of the lateral nasal wall and hinge region were used to determine the lateral edge of the palate shelf to be counted (area counted indicated by dotted line in figure 5 A, B, D, E).
To assess proliferation, E12.5 and E13.5 Tbx1-/- (N=3) and +/+ (N=3) embryos were fixed and sectioned as mentioned above. Each section was stained for phosphohistone H3 using the Cell Signaling protocol (Danvers, MA). The sections were then reviewed under 40× magnification and the number of proliferative cells per palate were counted in and identical fashion as above. Cell counts for Tbx1-/- and wild-type littermates were compared and assessed for statistical significance by Student's T-test.
Alcian Blue Staining
To assess the content of hyaluronic acid in the palate shelves, E13.5 wild-type and Tbx1-/- were fixed in 4% paraformaldehyde, embedded in paraffin and coronally sectioned. The sections were then dewaxed and were stained for alcian blue at pH 2.5 using the Dako Artisian staining protocol (Carpinteria, CA). Hyaluronidase (Sigma-Aldrich, St. Louis, MO) was used (50mg in 100ml PBS) was used to treat the slides for 1 hour at 37 C followed by the Dako protocol to remove hyaluronan.
Acknowledgments
The authors would like to thank Brian Schutte, Chin Chiang, Joey Barnett and Lance Prince for critically reading this manuscript. Expert technical assistance was performed by Kathleen Boyer and Yan Zhao. This work was supported by 1K08DE017953 and a Triological Career Development award to SG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–25. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- Aggarwal VS, Liao J, Bondarev A, Schimmang T, Lewandoski M, Locker J, Shanske A, Campione M, Morrow BE. Dissection of Tbx1 and Fgf interactions in mouse models of 22q11DS suggests functional redundancy. Hum Mol Genet. 2006;15:3219–28. doi: 10.1093/hmg/ddl399. [DOI] [PubMed] [Google Scholar]
- Alappat SR, Zhang Z, Suzuki K, Zhang X, Liu H, Jiang R, Yamada G, Chen Y. The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev Biol. 2005;277:102–13. doi: 10.1016/j.ydbio.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Arnold JS, Werling U, Braunstein EM, Liao J, Nowotschin S, Edelmann W, Hebert JM, Morrow BE. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006;133:977–87. doi: 10.1242/dev.02264. [DOI] [PubMed] [Google Scholar]
- Brown CB, Wenning JM, Lu MM, Epstein DJ, Meyers EN, Epstein JA. Cre-mediated excision of Fgf8 in the Tbx1 expression domain reveals a critical role for Fgf8 in cardiovascular development in the mouse. Dev Biol. 2004;267:190–202. doi: 10.1016/j.ydbio.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–75. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–90. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Dudas M, Li WY, Kim J, Yang A, Kaartinen V. Palatal fusion - where do the midline cells go? A review on cleft palate, a major human birth defect. Acta Histochem. 2007;109:1–14. doi: 10.1016/j.acthis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A. Molecular control of secondary palate development. Dev Biol. 2007;301:309–26. doi: 10.1016/j.ydbio.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Huang X, Goudy SL, Ketova T, Litingtung Y, Chiang C. Gli3-deficient mice exhibit cleft palate associated with abnormal tongue development. Dev Dyn. 2008;237:3079–87. doi: 10.1002/dvdy.21714. [DOI] [PubMed] [Google Scholar]
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, Murray JC, Schutte BC. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006 doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003;130:5269–80. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–91. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–57. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, Tokooya K, Jore BS, Lopez M, Pandita RK, Lia M, Carrion D, Xu H, Schorle H, Kobler JB, Scambler P, Wynshaw-Boris A, Skoultchi AI, Morrow BE, Kucherlapati R. TBX1 is responsible for cardiovascular defects in velo-cardiofacial/DiGeorge syndrome. Cell. 2001;104:619–29. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Morris-Wiman J, Brinkley L. An extracellular matrix infrastructure provides support for murine secondary palatal shelf remodelling. Anat Rec. 1992;234:575–86. doi: 10.1002/ar.1092340413. [DOI] [PubMed] [Google Scholar]
- Nawshad A. Palatal seam disintegration: To die or not to die? that is no longer the question. Dev Dyn. 2008 doi: 10.1002/dvdy.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R, Spencer-Dene B, Connor EC, Gritli-Linde A, McMahon AP, Dickson C, Thesleff I, Rice DP. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J Clin Invest. 2004;113:1692–700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Jiang R, Dixon MJ. Integration of IRF6 and Jagged2 signalling is essential for controlling palatal adhesion and fusion competence. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, Shore P, Whitmarsh A, Dixon MJ. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38:1329–34. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- Schutte BC, Murray JC. The many faces and factors of orofacial clefts. Hum Mol Genet. 1999;8:1853–9. doi: 10.1093/hmg/8.10.1853. [DOI] [PubMed] [Google Scholar]
- Trainor PA. Specification and patterning of neural crest cells during craniofacial development. Brain Behav Evol. 2005;66:266–80. doi: 10.1159/000088130. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Morishima M, Taddei I, Lindsay EA, Baldini A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum Mol Genet. 2002;11:915–22. doi: 10.1093/hmg/11.8.915. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cerrato F, Xu H, Vitelli F, Morishima M, Vincentz J, Furuta Y, Ma L, Martin JF, Baldini A, Lindsay E. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development. 2005;132:5307–15. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]
- Zirzow S, Ludtke TH, Brons JF, Petry M, Christoffels VM, Kispert A. Expression and requirement of T-box transcription factors Tbx2 and Tbx3 during secondary palate development in the mouse. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Zoupa M, Seppala M, Mitsiadis T, Cobourne MT. Tbx1 is expressed at multiple sites of epithelial-mesenchymal interaction during early development of the facial complex. Int J Dev Biol. 2006;50:504–10. doi: 10.1387/ijdb.052116mz. [DOI] [PubMed] [Google Scholar]


