The bioorthogonal chemical reporter strategy is emerging as a versatile method for the labeling of biomolecules, such as nucleic acids, lipids, carbohydrates, and proteins.1 In this approach, an abiotic chemical functionality (reporter) is incorporated into a target biomolecule and can then react with a complementary bioorthogonal functional group linked to one of a diverse set of probes.
The azide functional group, which is the most commonly employed reporter, can react in a Staudinger ligation with modified phosphines,2 in a copper(I)-catalyzed cycloaddition with terminal alkynes (CuAAC),3 or in a strain-promoted alkyne–azide cycloaddition (SPAAC).4 The last type of reaction5 is attractive because it does not require a cytotoxic metal catalyst and therefore provides unique opportunities for the labeling of cell-surface glycans4b, 6 and proteins7 of living cells, the decoration of polymeric nanostructures,8 the labeling of lipids,9 proteomics,10 and tissue reengineering.11
The first generation of cyclooctynes suffered from relatively slow reaction rates; however, it has been found that the rate of strain-promoted cycloaddition can be increased by appending electron-withdrawing groups adjacent to the triple bond. For example, reactions of difluorinated cyclooctynes, such as 1 (Figure 1), with azides proceed approximately 60 times faster than the corresponding reactions of unsubstituted derivatives.5a We have found that derivatives of the 4-dibenzocyclooctynol 2 react fast with azido-containing saccharides and amino acids and can be employed for the visualization of metabolically labeled glycans of living cells.12 Attractive features of dibenzocyclooctynols include easy synthetic access, nontoxicity, and the straightforward attachment of a variety of probes. Recently, we introduced the more polar azacyclooctyne 3,13 which exhibits a higher rate of reaction. Despite these advances, there is an urgent need for new and faster bioorthogonal reactions for labeling at low concentration.1b
Figure 1.
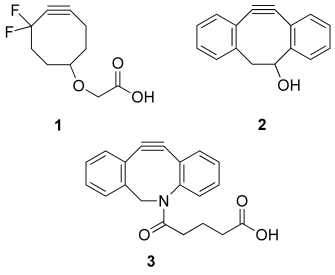
Ring-strained cyclooctynes for bioorthogonal cycloaddition reactions with azides.
We report herein a novel bioorthogonal reaction pair based on strain-promoted alkyne–nitrone cycloaddition (SPANC) to give N-alkylated isoxazolines with exceptionally fast reaction kinetics. The new methodology was used in a one-pot three-step protocol for the site-specific modification of peptides and proteins.
Nitrones 4 a–f were readily prepared by the condensation of appropriate aldehydes with N-methylhydroxylamine. Cycloaddition reactions of 4 a–f with cyclooctynol 2 in a mixture of acetonitrile and water gave the corresponding stable14 isoxazolines, in most cases in high yield (Table 1). We measured the rate constants of the cycloaddition reactions by 1H NMR or UV spectroscopy at 25 °C and found that the substituents on the nitrone greatly influenced the reaction kinetics. For example, the replacement of an N-methyl with a phenyl group (to give 4 c) led to a faster reaction,15 whereas nitrone 4 d, derived from a ketone, exhibited reaction kinetics that were too slow for accurate determination of the rate constant. Exceptionally high reaction rates were measured for the cycloaddition of 2 with α-carboxynitrones 4 e and 4 f. These reactions proceeded 18 and 32 times as fast, respectively, as the cycloaddition of 2 with benzyl azide (0.12 m−1 s−1).16 Also, we found that a high water content increased the reaction rate constants (e.g. 12.8 m−1 s−1 for a derivative of 2 in acetonitrile/water (1:9); see the Supporting Information).17 Finally, we determined a rate constant for the cycloaddition of azacyclooctyne 3 with 4 f. As expected,13 a further enhancement of the reaction rate (39 m−1 s−1) was observed when 3 was used in place of the carbon analogue 2.
Table 1.
Rate constants for the cycloaddition of dibenzocyclooctynol 2 with nitrones 4 a–f.[a]
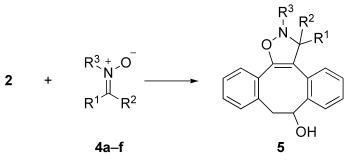 | ||||||
|---|---|---|---|---|---|---|
| 4 | R1 | R2 | R3 | k[b] [m−1 s−1] | k′ | Yield [%] |
| a | H | Ph | Me | 1.3×10−2 | 1 | 95 |
| b | H | CH2CH2Ph | Me | 3.2×10−2 | 3 | 80 |
| c | H | Ph | Ph | >0.2[c] | >17 | 89 |
| d | Me | CH2CH2CO2Et | Me | <1×10−3 | <0.1 | 33 |
| e | H | CO2Et | Me | 3.9 | 330 | 92 |
| f | H | C(O)NHBn | Me | 2.2 | 180 | 93 |
The nitrone substrates (except 4 d) were formed as pure Z isomers. Isoxazoline 5 was formed as a mixture of regio- and diastereoisomers. See the Supporting Information for reaction conditions.
Method A: The rate constant was determined by 1H NMR spectroscopy in CD3CN/D2O (3:1); [2 a]=18 mm, [4 a–f]=16.4 mm. Method B: The rate constant was determined18 by UV spectroscopy in CH3CN/H2O (3:1); [2 a]=0.33 mm, [4 a–f]=0.30 mm. These reactions were too fast for monitoring by NMR spectroscopy.
The reaction was too fast for accurate determination of the rate constant by NMR spectroscopy. Determination by UV spectroscopy was not possible owing to overlapping absorptions. Bn=benzyl.
Next, the challenge was to find a strategy for the incorporation of nitrones into biomolecules. We first focused our attention on metabolic labeling with monosaccharide derivatives bearing a nitrone moiety.19 Unfortunately, the incubation of Jurkat cells in the presence of nitrones 6–9 (10, 20, 50, and 100 μm; Figure 2), followed by labeling with dibenzocyclooctyne–biotin and staining with an avidin–fluorescein isothiocyanate (FITC) conjugate, led to no detectable fluorescence labeling of the cells.12 Presumably, either the biosynthetic glycosylation machinery does not accept nitrone modifications, or nitrones undergo intracellular hydrolysis in acidic compartments.
Figure 2.
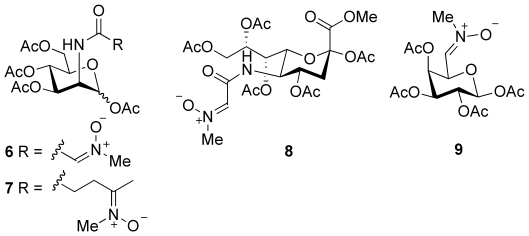
Nitrone derivatives of D-mannosamine (compounds 6 and 7), sialic acid (compound 8), and D-galactose (compound 9) for metabolic cell-surface labeling.
Fortunately, SPANC could be employed for efficient peptide and protein modification by implementing a one-pot three-step procedure. Thus, the N-terminal serine residue of model peptide 10 was oxidized20 with sodium periodate (1.1 equiv) to rapidly generate aldehyde 11, which was first treated with p-methoxybenzenethiol (6.6 equiv, 30 min), and then with N-methylhydroxylamine (2.2 equiv), p-anisidine (5 equiv), and 2 (2.2 equiv) to give the desired isoxazoline 13 via nitrone 12 (Scheme 1). We found that treatment with p-MeOC6H4SH was essential to avoid the conversion of N-methylhydroxylamine into nitrosomethane dimer ((MeNO)2) by oxidation with iodate (IO3−) formed in the previous step.21 Furthermore, the rate of nitrone formation was greatly enhanced by the addition of p-anisidine, probably by a similar mechanism to that described for the formation of oximes from aldehydes and hydroxylamines.22
Scheme 1.
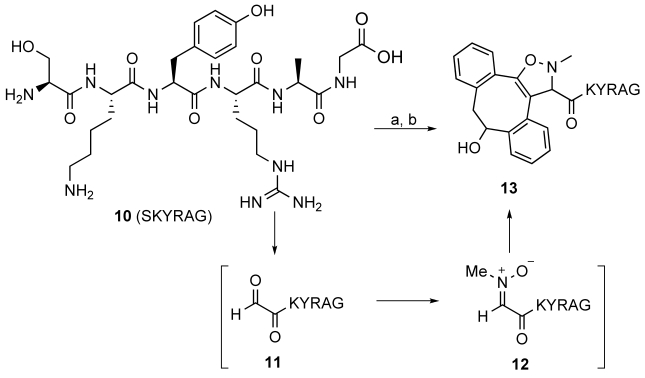
One-pot N-terminal conjugation of a hexapeptide by SPANC: a) 1. NaIO4, NH4OAc buffer, pH 6.8, room temperature, 1 h; 2. p-MeOC6H4SH, room temperature, 1 h; then p-MeOC6H4NH2, MeHNOH⋅HCl, room temperature, 20 min; b) 2, room temperature, 1 h.
To examine whether the one-pot three-step protocol was suitable for protein modification, we selected the chemokine interleukin-8 (IL-8),23 as this prototypical protein has an N-terminal serine residue and a relatively low molecular weight (72 amino acids, MW=8382 Da), which facilitates direct analysis of chemical modification by mass spectrometry. Current labeling methods of IL-8, for example, for the installment of a radiolabel for scintigraphic imaging of infections,24 are based on random reactions of side-chain lysine amino groups with no control over the number of reactions that take place or the sites of reaction.
Thus, IL-8 in NH4OAc buffer (2 mm, pH 6.9) was subjected to oxidation with NaIO4 (1.1 equiv, 1 h), followed by treatment with p-MeOC6H4SH (6.6 equiv, 2 h), then N-methylhydroxylamine (10 equiv) and p-anisidine (10 equiv), and finally cyclooctynol 2 (25 equiv, 21 mm). After 24 h, mass spectrometric analysis showed the presence of a single protein with a mass corresponding to the isoxazoline conjugate 15 (MW=8599 Da; Scheme 2). The one-pot three-step SPANC protocol was also successfully employed to PEGylate25, 26 IL-8 by using the PEG2000-modified dibenzocyclooctyne 16 (PEG=poly(ethylene glycol)). Quantitative formation of PEG-modified IL-8 17 was observed by HPLC analysis (Figure 3).
Scheme 2.
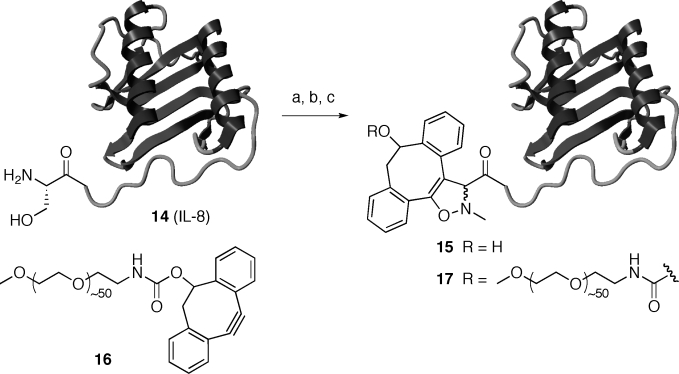
One-pot N-terminal functionalization of IL-8 by SPANC: a) 1. NaIO4, NH4OAc buffer, pH 6.9, room temperature, 1 h; 2. p-MeOC6H4SH, room temperature, 2 h; b) p-MeOC6H4NH2, MeNHOH⋅HCl, room temperature, 20 min; c) cyclooctynol 2 or PEG–cyclooctyne 16, room temperature, 20 h.
Figure 3.
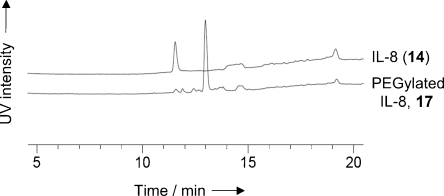
HPLC traces of IL-8 (14) and crude PEGylated IL-8, 17.
We have shown that 1,3-dipolar cycloadditions of cyclooctynes with nitrones that contain ester or amide α substituents exhibit much faster kinetics than similar reactions with azides.27 The new methodology was successfully employed for the site-specific modification of a peptide and a protein by implementing a one-pot three-step protocol. Besides serine or threonine oxidation, a variety of methods have been described for the installment of carbonyl groups in proteins,28 and it is to be expected that SPANC is compatible with these approaches. Furthermore, metal-free click reactions have found entry into materials science.11 SPANC will provide an additional tool for the preparation of increasingly complex materials by simple and flexible chemical manipulations. Finally, we anticipate that SPANC will offer an attractive alternative to the well-established oxime ligation29 because the synthesis of nitrones is simple,19 the isoxazoline products are stable,14 and the combination of a functionalized nitrone (R3 is a functional group, Table 1) with a cyclooctyne conjugate (such as 16) will make it possible to introduce two different functionalities in a single process.
Supplemental material
References
- 1a.Prescher JA, Bertozzi CR. Nat. Chem. Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 1b.Sletten EM, Bertozzi CR. Angew. Chem. 2009;121:7108–7133. doi: 10.1002/anie.200900942. Angew. Chem. Int. Ed. 2009, 48, 6974-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxon E, Bertozzi CR. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 3a.Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;41:2596–2599. [Google Scholar]
- 3b.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. 2002;114:2708–2711. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. Angew. Chem. Int. Ed. 2002, 41, 2596-2599. [DOI] [PubMed] [Google Scholar]
- 4a.Blomquist AT, Liu LH. J. Am. Chem. Soc. 1953;75:2153–2154. [Google Scholar]
- 4b.Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 5a.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc. Natl. Acad. Sci. USA. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b.Lutz JF. Angew. Chem. 2008;120:2212–2214. Angew. Chem. Int. Ed. 2008, 47, 2182-2184. [Google Scholar]
- 5c.Becer CR, Hoogenboom R, Schubert US. Angew. Chem. 2009;121:4998–5006. doi: 10.1002/anie.200900755. Angew. Chem. Int. Ed. 2009, 48, 4900-4908. [DOI] [PubMed] [Google Scholar]
- 5d.Kalia J, Raines TR. Curr. Org. Chem. 2010;14:138–147. doi: 10.2174/138527210790069839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem. Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 7a.Link AJ, Vink MKS, Agard NJ, Prescher JA, Bertozzi CR, Tirrell DA. Proc. Natl. Acad. Sci. USA. 2006;103:10180–10185. doi: 10.1073/pnas.0601167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7b.Tanrikulu IC, Schmitt E, Mechulam Y, Goddard WA, III, Tirrell DA. Proc. Natl. Acad. Sci. USA. 2009;106:15285–15290. doi: 10.1073/pnas.0905735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Lallana E, Fernandez-Megia E, Riguera R. J. Am. Chem. Soc. 2009;131:5748–5750. doi: 10.1021/ja8100243. [DOI] [PubMed] [Google Scholar]
- 8b.Kele P, Mezö G, Achats F, Wolfbeis OS. Angew. Chem. 2009;121:350–353. doi: 10.1002/anie.200804514. Angew. Chem. Int. Ed. 2009, 48, 344-347. [DOI] [PubMed] [Google Scholar]
- 9a.Fernández-Suárez M, Baruah H, Martínez-Hernández L, Xie KT, Baskin JM, Bertozzi CR, Ting AY. Nat. Biotechnol. 2007;25:1483–1487. doi: 10.1038/nbt1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b.Neef AB, Schultz C. Angew. Chem. 2009;121:1526–1529. doi: 10.1002/anie.200805507. Angew. Chem. Int. Ed. 2009, 48, 1498-1500. [DOI] [PubMed] [Google Scholar]
- 10.Nessen MA, Kramer G, Back JW, Baskin JM, Smeenk LEJ, de Koning LJ, van Maarseveen JH, de Jong L, Bertozzi CR, Hiemstra H, de Koster CG. J. Proteome Res. 2009;8:3702–3711. doi: 10.1021/pr900257z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson JT, Krishnamurthy VR, Cui W, Qu Z, Chaikof EL. J. Am. Chem. Soc. 2009;131:18228–18229. doi: 10.1021/ja908887v. [DOI] [PubMed] [Google Scholar]
- 12.Ning X, Guo J, Wolfert MA, Boons G.-J. Angew. Chem. 2008;120:2285–2287. doi: 10.1002/anie.200705456. Angew. Chem. Int. Ed. 2008, 47, 2253-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debets MF, van Berkel SS, Schoffelen S, Rutjes FPJT, van Hest JCM, van Delft FL. Chem. Commun. 2010;46:97–99. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]
- 14. For example, isoxazoline 5 f was recovered unchanged after 24 h at 60 °C in CHCl3 or after being stirred at pH 3 or pH 9 in a THF/water mixture.
- 15a.Huisgen R, Seidl H, Bruning I. Chem. Ber. 1969;102:1102. [Google Scholar]
- 15b.Dolbier WR, Jr., Wicks GE, Burkholder CR. J. Org. Chem. 1987;52:2196–2201. [Google Scholar]
- 16. We accurately redetermined a rate constant of 0.12 m−1 s−1 for the reaction of 2 with benzyl azide.
- 17a.Gholami MR, Yangjeh AH. J. Chem. Res. Synop. 1999;3:226–227. [Google Scholar]
- 17b.Butler RN, Coyne AG, Cunningham WJ, Burke LA. J. Chem. Soc. Perkin Trans. 2. 2002:1807–1815. [Google Scholar]
- 18.Poloukhtine AA, Mbua NE, Wolfert MA, Boons GJ, Popik VV. J. Am. Chem. Soc. 2009;131:15769–15776. doi: 10.1021/ja9054096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merino P. In: Science of Synthesis, Vol. 27. Padwa A, editor. Stuttgart: Thieme; 2004. pp. 511–580. [Google Scholar]
- 20.Geoghegan KF, Stroh JG. Bioconjugate Chem. 1992;3:138–146. doi: 10.1021/bc00014a008. [DOI] [PubMed] [Google Scholar]
- 21.Emery T, Neilands JB. J. Am. Chem. Soc. 1960;82:4903–4904. [Google Scholar]
- 22.Dirksen A, Hackeng TM, Dawson PE. Angew. Chem. 2006;118:7743–7746. doi: 10.1002/anie.200602877. Angew. Chem. Int. Ed. 2006, 45, 7581-7584. [DOI] [PubMed] [Google Scholar]
- 23.Baggiolini M, Clark-Lewis I. FEBS Lett. 1991;307:97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- 24.van der Laken CJ, Boerman OC, Oyen WJG, van de Ven MTP, van der Meer JWM, Corstens FHM. J. Nucl. Med. 2000;41:463–469. [PubMed] [Google Scholar]
- 25a.Carrico IS. Chem. Soc. Rev. 2008;37:1423–1431. doi: 10.1039/b703364h. [DOI] [PubMed] [Google Scholar]
- 25b.Hackenberger CPR, Schwarzer D. Angew. Chem. 2008;120:10182–10228. Angew. Chem. Int. Ed. 2008, 47, 10030-10074. [Google Scholar]
- 26.Dondoni A, Massi A, Nanni P, Roda A. Chem. Eur. J. 2009;15:11444–11449. doi: 10.1002/chem.200901746. [DOI] [PubMed] [Google Scholar]
- 27.McKay CS, Moran J, Pezacki JP. Chem. Commun. 2010;46:931–933. doi: 10.1039/b921630h. During the preparation of this manuscript, the cycloaddition of nitrones with nonfunctionalized dibenzocyclooctyne was reported: [DOI] [PubMed] [Google Scholar]
- 28a.Gilmore JM, Scheck RA, Esser-Kahn AP, Joshi NS, Francis MB. Angew. Chem. 2006;118:5433–5437. doi: 10.1002/anie.200600368. Angew. Chem. Int. Ed. 2006, 45, 5307-5311. [DOI] [PubMed] [Google Scholar]
- 28b.Carrico IS, Carlson BL, Bertozzi CR. Nat. Chem. Biol. 2007;3:321–322. doi: 10.1038/nchembio878. [DOI] [PubMed] [Google Scholar]
- 28c.Zeng Y, Ramya TNC, Dirksen A, Dawson PE, Paulson JC. Nat. Methods. 2009;6:207–209. doi: 10.1038/nmeth.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28d.Ebisu K, Tateno H, Kuroiwa H, Kawakami K, Ikeuchi M, Hirabayashi J, Sisido M, Taki M. ChemBioChem. 2009;10:2460–2464. doi: 10.1002/cbic.200900430. [DOI] [PubMed] [Google Scholar]
- 29a.Dawson PE, Kent SBH. Annu. Rev. Biochem. 2000;69:923–960. doi: 10.1146/annurev.biochem.69.1.923. [DOI] [PubMed] [Google Scholar]
- 29b.Borgia JA, Fields GB. Trends Biotechnol. 2002;18:243–251. doi: 10.1016/s0167-7799(00)01445-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


