Abstract
Acute and chronic exposure to psychostimulants results in altered function of G-protein-coupled receptors in the forebrain. It is believed that neuroadaptations in G-protein signaling contribute to behavioral sensitivity to psychostimulants that persists over a prolonged drug-free period. Proteins termed activators of G-protein signaling (AGS) have been characterized as potent modulators of both receptor-dependent and receptor-independent G-protein signaling. Nevertheless, the regulation of AGS gene and protein expression by psychostimulants remains poorly understood. In the present study, we investigated amphetamine (AMPH)-induced changes in expression patterns of several forebrain-enriched AGS proteins. A single exposure to AMPH (2.5 mg/kg i.p.) selectively induced gene expression of AGS1, but not Rhes or AGS3 proteins, in the rat prefrontal cortex (PFC) as measured 3h later. Induction of AGS1 mRNA in the PFC by acute AMPH was transient and dose-dependent. Even repeated treatment with AMPH for 5 days did not produce lasting changes in AGS1 mRNA and protein levels in the PFC as measured three weeks post treatment. However, at this time point, a low dose AMPH challenge (1 mg/kg, i.p.) induced a robust behavioral response and up-regulated AGS1 expression in the PFC selectively in animals with an AMPH history. The effects of AMPH on AGS1 expression in the PFC were blocked by a D2, but not D1, dopamine receptor antagonist and partially by a glucocorticoid receptor antagonist. Collectively, the present study suggests that (1) AGS1 represents a regulator of G-protein signaling that is rapidly inducible by AMPH in the frontal cortex, (2) AGS1 regulation in the PFC parallels behavioral activation by acute AMPH in drug-naïve animals and hypersensitivity to AMPH challenge in sensitized animals, and (3) D2 dopamine and glucocorticoid receptors regulate AMPH effects on AGS1 in the PFC. Changes in AGS1 levels in the PFC may result in abnormal receptor-to-G-protein coupling that alters cortical sensitivity to psychostimulants.
Keywords: amphetamine, G-protein, dopamine receptor, glucocorticoid receptor, frontal cortex
Introduction
Psychostimulants exert their actions on the brain primarily through activation of D1 and D2 dopamine receptors located in the striatum and the prefrontal cortex (PFC). Activation of these receptors induces an array of signaling cascades followed by changes in expression of responsive genes. It is widely accepted that altered expression of some of these genes might trigger more enduring neuroadaptations leading to drug addiction. However, there is only limited evidence that acute or repeated exposure to psychostimulants produces changes in dopamine receptor levels in the forebrain (for reviews, see Pierce and Kalivas, 1997, Vanderschuren and Kalivas, 2000). In contrast, several studies have described psychostimulant-induced changes that take place downstream from G-protein-coupled dopamine receptors (GPCRs). These changes include altered G-protein coupling (Alttoa et al., 2007, Bailey et al., 2008), hyperactivity of adenylyl cyclase (Borgkvist and Fisone, 2007), changes in phosphorylation cascades (Borgkvist and Fisone, 2007, Zhai et al., 2008), and altered function of GPCR-associated ion channels (Kobayashi et al., 2004). Therefore, studying changes in D1 and D2 receptor-driven G-protein signaling is central to understanding psychostimulant-induced neural adaptations.
Two general classes of accessory proteins involved in signaling through heterotrimeric G-proteins have been identified to date. The first class, termed “regulators of G-protein signaling” (RGS), accelerate the GTPase activity of Gα-GTP, thus decreasing the efficiency and duration of receptor-to-G-protein signaling (for review, see Ross and Wilkie, 2000). The second class directly influences Gα-protein nucleotide exchange or Gα-Gβγ subunit interactions, thus altering G-protein signaling independently of receptor activation state. The latter class of proteins was termed “activators of G-protein signaling” (AGS; for review, see Blumer et al., 2005). The regulation of RGS gene and protein expression by psychostimulants has been investigated for over a decade (Burchett et al., 1999, Taymans et al., 2003, Schwendt et al., 2006). As a result, altered levels of several RGS proteins have been linked to abnormal D2 receptor-mediated signaling and behaviors (Rahman et al., 2003, Cabrera-Vera et al., 2004). In contrast, analogous mechanisms regulating the expression and function of AGS proteins remain largely unknown, possibly because of the diverse character of the AGS protein family. Unlike RGS proteins, members of the AGS protein family were identified in a functional screen for receptor-independent activators of G-protein signaling, rather than on the basis of sequence homology (Blumer et al., 2005). For example, among the AGS proteins enriched in rodent brain, AGS3 is a mid-size protein with modular domain structure containing multiple G-protein regulatory motifs also found in RGS12 and RGS14 proteins (Kimple at el., 2001). On the other hand, AGS1 (DexRas1, RasD1) is a small protein of the Ras superfamily, homologous to Rhes (RasD2; Blumer et al., 2005, Cismowski, 2006). Despite structural diversity, both AGS proteins share the ability to influence efficiency of Gαi/o and Gβγ signaling. One mechanism of action observed in neuronal and non-neuronal cell cultures is the ability of AGS proteins to directly activate Gαi- or Gβγ-dependent signaling independently of the receptor activation (Cismowski, 2006). In addition to this tonic effect on G-protein-dependent signaling, AGS proteins can interfere with GPCR-activated signaling by competing for the same G-protein substrates (Cismowski, 2006). Interestingly, the latter mechanism of action has been implicated in inhibition of D2 receptor signaling by AGS1 and AGS3 proteins (Nguyen and Watts, 2005, Webb et al., 2005). With regard to drug addiction, the ability of AGS3 in the nucleus accumbens (NAc) to interfere with Gαi-coupled receptor signaling has been implicated in facilitating cocaine, heroin and alcohol seeking (Bowers et al., 2004, Yao et al., 2005, Bowers et al., 2008). The effects of AGS3 on drug-seeking are likely related to an increased expression of this protein in the NAc and the PFC induced by cocaine or alcohol treatment (Bowers et al., 2004, 2008). In addition to changes in AGS3 levels, our preliminary findings indicate that acute amphetamine (AMPH) administration can upregulate AGS1 mRNA in the PFC (Schwendt et al. 2005). Despite the emerging evidence that levels of some AGS proteins in the brain are regulated by drugs of abuse, changes in expression patterns of individual AGS proteins or the identity of regulatory factors that control expression of AGS proteins have not yet been characterized.
Therefore, the present study was designed to examine changes in the expression of forebrain-enriched AGS proteins (AGS1, AGS1 homolog – Rhes, and AGS3) after acute or chronic exposure to amphetamine (AMPH). Since the expression of many psychostimulant-responsive genes is dependent on activation of dopamine and/or glucocorticoid receptors, this study further investigates the role of these receptors in the regulation of basal and AMPH-induced levels of AGS proteins across the forebrain. It is widely accepted that changes in receptor signaling (possibly as a consequence of altered levels of AGS proteins) occurring within these heavily dopamine-innervated brain regions represent key neuroadaptations underlying addictive behaviors.
Experimental Procedures
Animals
Adult male Sprague Dawley rats (225-275g) (Charles River Laboratories, Wilmington, MA, USA) were pair-housed in clear plastic cages and maintained on a 12 h light/dark cycle with food and water available ad libitum. All animals were habituated to their environment and handling for 5 days prior to drug administration to minimize any stress-induced behaviors. Every animal procedure in this study was approved by the Institutional Animal Care and Use Committee and in strict accordance with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press 1996).
Experimental Design
In all experiments, rats were randomly assigned to appropriate experimental groups and received intraperitoneal (i.p.) injections of experimental drugs (as specified below). Following injections, behavioral activity of the rats was recorded in locomotor activity chambers as previously described (Schwendt et al., 2006). In Experiment 1, rats (n = 6 / group) were injected with either physiological saline (SAL) or 2.5 mg/kg, i.p. AMPH (D-amphetamine sulfate; Sigma, St. Louis, MO, USA). This dose of AMPH has been shown to reliably stimulate horizontal and vertical activity in rats (Wang and McGinty, 1995b). Three hours after the injection, rats were anesthetized with equithesin (10 ml/kg i.p.) and decapitated. In Experiment 2, rats (n = 6 / group) received an i.p. injection of either SAL or 2.5 mg/kg of AMPH and were decapitated 1, 3, 6 or 24 h after the injection. Additional rats (n = 6 / group) were injected with SAL or 1, 2.5, 5 or 7.5 mg/kg, i.p. of AMPH and decapitated 3 h after injection. In Experiment 3, rats (n = 8 / group) were injected with SAL or AMPH (5 mg/kg i.p.) daily for five days. After three weeks of abstinence (in their home cages) SAL- and AMPH-treated rats were challenged with SAL or AMPH (1 mg/kg i.p.) and their behavior recorded. Three hours after the injection, all groups of rats were anesthetized and decapitated. In Experiment 4, rats (n = 6 / group) were pretreated with SAL, the D1 receptor antagonist, SCH23390 (Tocris Bioscience, Ellisville, MI, USA; 0.1 mg/kg, i.p.), or the D2 receptor antagonist eticlopride (Sigma-Aldrich, St. Louis, MO, USA; 0.5 mg/kg, i.p.). The doses of the dopamine receptor antagonists were based on previous studies (Wang and McGinty, 1995a, Wang and McGinty, 1996, Schwendt et al., 2006). Ten min after the first injection, AMPH (2.5 mg/kg i.p.) or SAL was administered. One or 3 h after the second injection, all groups of rats were anesthetized with equithesin and decapitated. Additional rats (n = 4 / group) were injected with SAL, AMPH (2.5 mg/kg i.p.) or quinpirole (1 mg/kg i.p.) and decapitated three hours later. In Experiment 5, rats (n = 5 - 7 / group) were pretreated with the glucocorticoid receptor antagonist, RU486 (Tocris Bioscience, Ellisville, MI, USA; 25 mg/kg s.c.) or vehicle (20% DMSO/sesame oil; 0.5 ml/kg, s.c.). The dose of RU486 used was based on the previously published data (De Vries et al., 1996, Morsink et al., 2007) as well as on a pilot dose-response study. Forty-five minutes later, rats were injected either with SAL or AMPH (2.5 mg/kg i.p.). Three hours later, all rats were anesthetized and decapitated.
At the end of each experiment, one hemisphere was frozen in isopentane on dry ice and stored at -80°C until processed to hemisections used for in situ hybridization analysis. Brain regions for immunoblotting analysis were dissected from chilled 1 mm (PFC) or 2 mm (striatum) -thick coronal slices obtained from the contralateral hemisphere using a Precision Brain Slicer (Braintree Scientific, MA, USA). Tissue samples containing the PFC were obtained from brain slices ∼ 3.7-2.7 mm anterior to bregma (Paxinos and Watson, 2007) using a 2 mm tissue puncher. Tissue samples containing the dorsal striatum (dSTR) and NAc were dissected from brain slices ∼ 2.7-0.7 mm anterior to bregma (Paxinos and Watson, 2007) using 2 and 3 mm tissue punchers, respectively. All samples were quickly frozen on dry ice and stored at 80°C until processed.
Behavioral activity
To measure behavioral activity, rats were placed in automated photocell beam activity chambers (Accuscan Instruments, Columbus, OH, USA) and horizontal (total distance traveled) as well as vertical activity (rearing) was recorded as described previously (Schwendt et al., 2006). All rats were adapted to the environment for 3 days before the start of the experiment.
In Situ Hybridization Histochemistry
Quantitative in situ hybridization histochemistry was performed as previously described (Gonzalez-Nicolini and McGinty, 2002, Schwendt et al., 2006). Briefly, 12 μm brain sections obtained by cryostat sectioning were mounted onto silane-coated SuperFrost slides (VWR International, West Chester, PA, USA) and pretreated in a series of steps that fixed and defatted the tissue and blocked nonspecific hybridization. A synthetic cDNA oligodeoxynucleotide probes (48-mers) complementary to the rat sequences of AGS1 (bases 322 - 369; AF239157), AGS3 (bases 416 – 463; AF107723) and Rhes (1209 – 1256; AF134409) were end-labeled with [α35S]-dATP (1250 Ci/mmol; GE Healthcare, Piscataway, NJ, USA) using terminal deoxynucleotidyl transferase (Roche Diagnostics Corporation Indianapolis, IN, USA). Slides were incubated with 5×105 cpm/25 μl hybridization buffer/section overnight at 37°C. After incubation, slides were washed and air-dried before being placed into a film cassette, along with 14C standards (ARC, St Louis, MO, USA) and Kodak Biomax film for 18-21 days. Quantitation of each mRNA hybridization signal in selected brain areas was performed using NIH Image 1.62 software (http://rsb.info.nih.gov/nih-image).
Immunoblotting
Samples of total tissue protein were obtained as previously described (Schwendt et al., 2006). Twenty-five micrograms of total protein were resolved using SDS-PAGE and transferred to a PVDF membrane (BioRad, Hercules, CA, USA). The membrane was blocked with 5% milk/TBS/0.1% Tween-20 and probed with rabbit antiserum against AGS1 (obtained from Stephen Lanier, Ph.D.) characterized by Vaidyanathan and colleagues (2004). After the incubation with anti-rabbit HRP-conjugated secondary antibody at 1:5000 (Jackson Immuno Research, West Grove, PA, USA), immunoreactive bands on the membranes were detected by ECL+ chemiluminescence reagents on an X-ray film (GE Healthcare, Piscataway, NJ, USA). Integrated density of the bands was measured with Gel-Pro 3.1 software (Media Cybernetics, Silver Spring, MD, USA). Equal loading and transfer of proteins were confirmed by re-probing of the same membranes for calnexin, an independent protein that was not altered by any experimental treatment, using a rabbit anti-calnexin antiserum (Stressgen, Victoria, BC, Canada).
Statistical Analysis
Behavioral data (total distance traveled and rearing) were recorded over a given period of time and analyzed by a one-way or two-way ANOVA. AGS1 mRNA levels, assessed by in situ hybridization, were measured as the mean integrated density from three adjacent brain sections. Since these values are strongly correlated and cannot be treated independently, a nested repeated measures ANOVA was applied to analyze the data (mixed model SAS 9.1; SAS Institute Inc., Cary, NC, USA). Changes in mRNA levels were expressed as the percentage of the SAL-treated animals. Immunoblotting data, represented by band density values, were normalized for the density of calnexin immunoreactivity within the same sample, analyzed by a one-way or two-way ANOVA and expressed as the percentage of the SAL-treated animals within the same time point or treatment. Sigma Stat (SPSS, Chicago, IL, USA) software was used for statistical analysis unless otherwise noted.
Results
Experiment 1: Acute amphetamine upregulates AGS1 gene expression in the rat frontal cortex
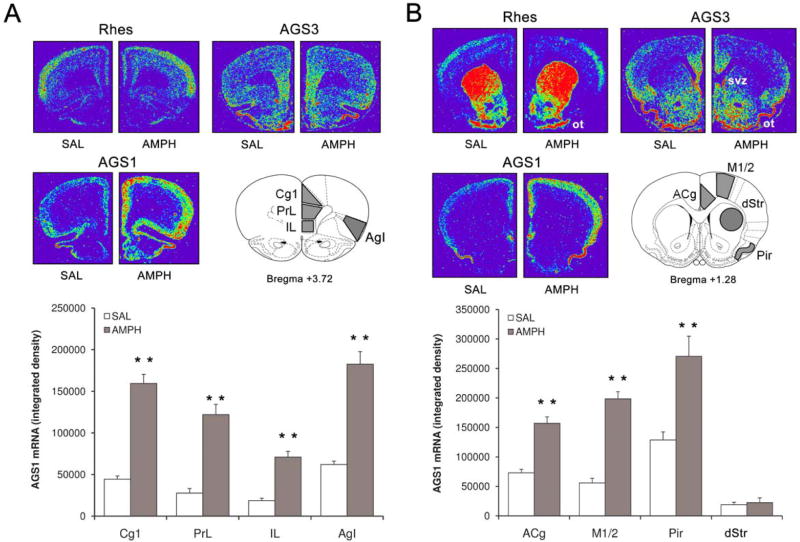
In the initial experiment, basal gene expression of AGS proteins and the effects of AMPH were analyzed using in situ hybridization histochemistry. Analysis of AGS1, Rhes and AGS3 mRNA signals in control (SAL-treated) rats using a set of rat forebrain coronal sections (+3.7/+1.2 mm relative to bregma; Paxinos and Watson, 2007) revealed diverse, non-overlapping distribution of each mRNA (Fig. 1 A and B). AGS1 mRNA is highly expressed throughout the frontal cortex (Fig.1A and B, lower left panels). Areas with the highest AGS1 mRNA concentrations included piriform cortex, insular cortex, medial PFC and anterior cingulate cortex (Fig.1A), while striatal sub-regions displayed very little AGS1 mRNA signal (Fig.1B). Detailed analysis of AGS1 expression within the cortex revealed higher expression in more superficial cortical layers, when compared to deeper layers (data not shown). In contrast to AGS1, Rhes mRNA is specifically enriched in the striatum and olfactory tubercle, with lower concentration found in the superficial layers of the frontal cortex (Fig. 1 A and B, top left panels; also see Vargiu et al. 2001). As described previously by Taymans and colleagues (2006), AGS3 mRNA is present in low to moderate levels across the frontal cortex and striatum, specifically enriched in the subventricular zone. In addition, our hybridization experiment reveals that relatively high AGS3 mRNA signal can be found in the medial PFC, anterior cingulate cortex, pirifiorm cortex, NAc and olfactory tubercle (Fig.1A and B, top right panels).
Figure 1. Acute AMPH upregulates AGS1 (but not AGS3 or Rhes) mRNA expression in the rat frontal cortex.
Top panels: Representative pseudocolor images of coronal hemisections at the level of PFC (A) and Str (B) illustrate Rhes, AGS3 and AGS1 mRNA hybridization signal in SAL- and AMPH-treated rats. Outlines of the coronal sections at the level of PFC (A) and Str (B) obtained from the rat brain atlas (Paxinos and Watson, 2007) depict brain areas analyzed in this study. Bottom: Quantitative analysis of AGS1 mRNA hybridization signal (integrated density) at the level of PFC (A) and Str (B) 3 hrs after single SAL or AMPH (2.5 mg/kg) treatment. Bars represent mean integrated density (+/- SEM), n = 6, **p<0.01 vs. SAL. ACg, anterior cingulate cortex; AgI, agranular insular cortex; Cg1, cingulate cortex area1; dStr, dorsal striatum; IL, infralimbic cortex; M1/2, primary and secondary motor cortex; Ot, olfactory tubercle; Pir, piriform cortex; PrL, dorsal prelimbic cortex; Svz, subventricular zone.
A single administration of AMPH (2.5 mg/kg i.p.) markedly increased AGS1 mRNA levels throughout the frontal cortex as detected 3h later (Fig.1A and B, lower left panels). AMPH treatment did not alter levels of Rhes or AGS3 in any forebrain region studied (Fig.1A and B, top panels). In case of AGS1, quantitative analysis of mRNA density revealed that the most prominent increase (relative to control SAL levels) occurred in the subregions of medial PFC, specifically in the prelimbic and cingulate 1 cortices (Fig.1A and B, bar graphs). Considering the role of medial PFC in mediating the effects of psychostimulants, the following experiments focused on characterizing the regulation of AGS1 expression in the PFC.
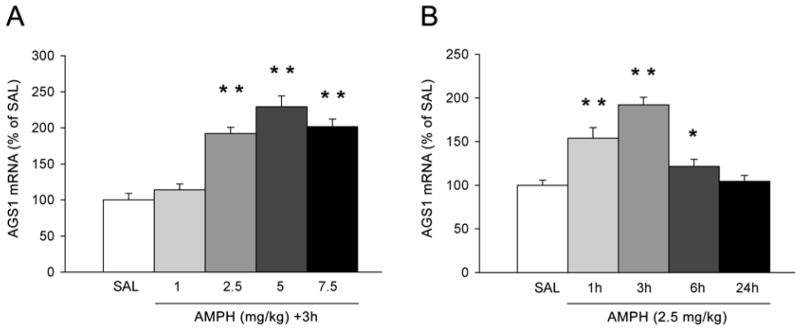
Experiment 2: Upregulation of AGS1 mRNA in the PFC by acute AMPH is transient and dose-dependent
The dose of AMPH used in the preceding studies produced a robust increase in cortical AGS1 mRNA density. However, it was possible that this dose (2.5 mg/kg of AMPH) was insufficient to stimulate AGS1 mRNA levels in the striatum. Therefore, a dose-dependent profile of AGS1 gene expression in both forebrain areas was characterized. In the cortex, we focused on the medial PFC region corresponding to the combined areas of prelimbic and cingulate 1 cortices measured in the previous experiment.
As depicted in Figure 2A, AMPH doses equal to and higher than 2.5-mg/kg i.p. induced AGS1 mRNA levels in the PFC as measured 3h later. However, there was no significant difference in AGS1 induction between AMPH doses of 2.5, 5 and 7.5 mg/kg, which indicates a possible ‘ceiling’ effect. In contrast to the PFC, none of the doses used induced AGS1 mRNA in the striatum (data not shown). In a follow up study, the temporal profile of AMPH-induced AGS1 mRNA levels was investigated in the PFC using 2.5 mg/kg dose of AMPH. At this dose, AGS1 mRNA density was already increased at 1h; reached maximal levels at 3h, and returned back to basal levels between 6h and 24h after AMPH injection (Fig. 2B).
Figure 2. Dose- and time-dependent upregulation of AGS1 mRNA levels by acute AMPH in the rat frontal cortex.
Quantitative analysis of AGS1 mRNA hybridization signal (integrated density) in the rat PFC as measured 3 hrs after SAL or AMPH (1 - 7.5 mg/kg) treatment (A), or 1 – 24 hrs after treatment with SAL or 2.5 mg/kg AMPH (B). Bars represent mean integrated density (+/- SEM) expressed as per cent of SAL, n = 6, *p<0.05 and **p<0.01 vs. SAL.
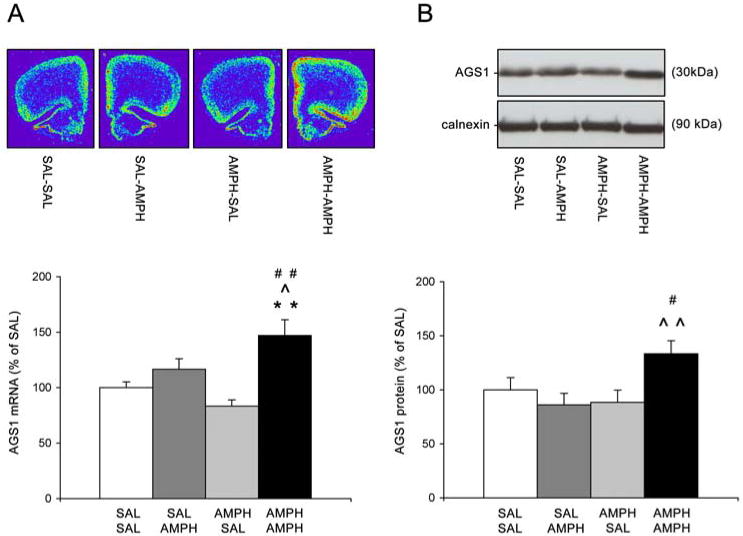
Experiment 3: Repeated treatment with AMPH results in lasting sensitization of AMPH-induced behavioral responses and AGS1 expression in the PFC
Repeated treatment with AMPH (5 mg/kg i.p.) for 5 consecutive days resulted in the development of behavioral sensitization as evidenced by a typical augmentation of initial stereotyped behaviors and increased post-stereotypy locomotion (data not shown), as described previously by (Wolf and Jeziorski, 1993). Further, a challenge AMPH injection (1 mg/kg, i.p.) administered 21 days after the cessation of repeated AMPH treatment, elicited significantly greater behavioral activity (horizontal activity and rearing) in AMPH-pretreated rats than in SAL-pretreated rats (Table 1; AMPH-AMPH vs. SAL-AMPH groups, p<0.05). AGS1 mRNA and protein levels in the PFC were analyzed 3h after SAL or AMPH challenge. Quantitative analysis of hybridization signals revealed that a low dose of AMPH (administered as a challenge) produced a significant augmentation in AGS1 mRNA (Fig.3A) and protein (Fig.3B) levels in the PFC of rats previously treated with repeated AMPH when compared to rats with no AMPH pretreatment history (AMPH-AMPH vs. SAL-AMPH groups, p<0.05). Furthermore, 1 mg/kg AMPH challenge given to rats previously treated with SAL was not sufficient to increase AGS1 mRNA and protein at all (Fig. 3A and 3B; SAL-AMPH vs. SAL-SAL groups). This finding corresponds with the dose-dependent regulation of AGS1 expression after acute AMPH exposure (Fig 2A). Repeated AMPH or SAL treatment followed by SAL challenge did not alter AGS1 mRNA and protein levels in the PFC.
Table 1.
Assessment of behavioral sensitization to AMPH (Experiment 3).
| Behavior | Experimental Groups | |||
|---|---|---|---|---|
| SAL-SAL | SAL-AMPH | AMPH-SAL | AMPH-AMPH | |
| Total distance traveled (Horizontal activity) | 4107 ± 765 | 16812 ± 4046* | 3956 ± 499 | 26597 ± 3363** ˆ |
| Vertical activity (Rearing) | 402 ± 125 | 1730 ± 569* | 373 ± 94 | 3073 ± 594** ˆ |
Behavioral data are expressed as total distance traveled (cm) or vertical activity (beam breaks) measured over 3h following the SAL or AMPH (1 mg/kg i.p.) challenge administered to rats pretreated with repeated SAL or AMPH. Mean (± SEM), n = 6-8,
p<0.05,
p<0.01 vs. SAL-SAL,
p<0.05 vs. SAL-AMPH. SAL, saline; AMPH, amphetamine
Figure 3. Pretreatment with repeated AMPH augments up-regulation of AGS1 mRNA and protein in the PFC by acute AMPH challenge.
Top: Representative pseudocolor images of coronal hemisections illustrate AGS1 mRNA hybridization signal in each treatment group (A). Representative Western blot images depict immunoreactivity of AGS1 protein and control protein calnexin in each treatment group (B). Bottom: Quantitative analysis of AGS1 mRNA hybridization signal (integrated density) (A) or AGS1 immunoreactivity (B) in the PFC 3 hrs after a challenge with SAL or AMPH (1 mg/kg). Preceding the challenge, animals received repeated SAL or AMPH (5 mg/kg) injections for 5 days followed by 21 days of abstinence. Bars represent mean integrated density of AGS1 mRNA or mean AGS1 immunoreactivity (+/- SEM) expressed as per cent of SAL, n = 8, **p<0.01 vs. SAL-SAL, ˆp<0.05, ˆˆp<0.01 vs. SAL-AMPH, #p<0.05, ##p<0.01 vs. AMPH-SAL.
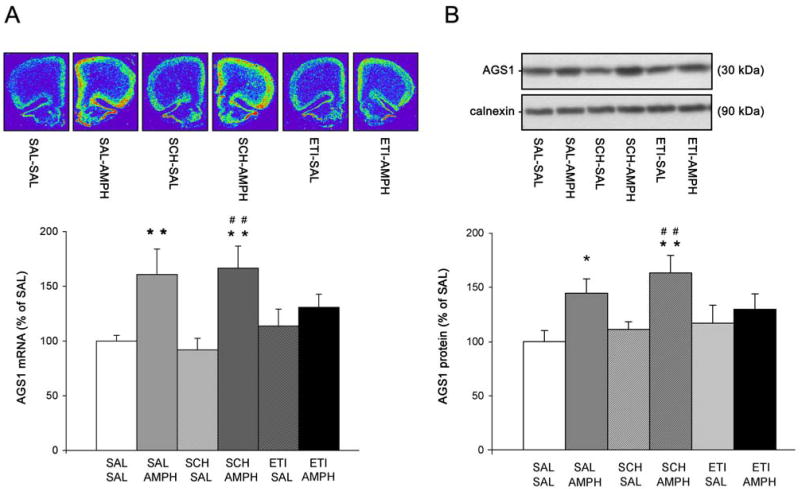
Experiment 4: Inhibition of D2, but not D1, dopamine receptors blocks AMPH-induced increase in AGS1 mRNA and protein levels in the PFC
AMPH is an indirect dopamine agonist exerting its effects on gene and protein expression in the brain through the activation of D1 and D2 dopamine receptors. Therefore in this study, the effect of D1 or D2 receptor blockade on AMPH-induced AGS1 mRNA and protein expression in the PFC was assessed. Doses of the D1 and D2 receptor antagonists used in this study have been previously shown to inhibit AMPH-induced behavioral activity, as well as gene expression of several activity-regulated genes in the striatum and frontal cortex induced by acute AMPH or cocaine (Wang and McGinty, 1995a, Wang and McGinty, 1996, Alonso et al. 1998, Schwendt et al. 2006). In accordance, pretreatment with either D1 (SCH23390 - 0.1 mg/kg i.p.), or D2 (eticlopride - 0.5 mg/kg i.p.) receptor antagonists completely blocked AMPH-induced horizontal and vertical activity (Table 2). In contrast, blockade of D1 receptors had no effect on the increase of AGS1 mRNA or protein levels elicited by acute AMPH (Fig.4A and 4B respectively). On the other hand, systemic administration of the D2 receptor antagonist eticlopride (0.5 mg/kg i.p.) blocked the induction of AGS1 mRNA and protein levels in AMPH-treated rats when compared to their SAL-treated counterparts (Fig.4; ETI-SAL vs. ETI-AMPH groups). There was no effect of either receptor antagonist on basal levels of AGS1 mRNA and protein in the PFC. In contrast to AGS1, there was no change in Rhes or AGS3 mRNA levels observed in any of the treatment groups (data not shown).
Table 2.
Regulation of AMPH-induced behaviors by systemic blockade of D1 or D2 receptors (Experiment 4).
| Behavior | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| SAL-SAL | SAL-AMPH | SCH-SAL | SCH-AMPH | ETI-SAL | ETI-AMPH | |
| Total distance traveled (Horizontal activity) | 809 ± 330 | 15438 ± 4012** | 238 ± 90 | 388 ± 147ˆˆ | 736 ± 642 | 2421 ± 1181ˆˆ |
| Vertical activity (Rearing) | 74 ± 32 | 1976 ± 629** | 25 ± 4.7 | 78 ± 49ˆˆ | 31 ± 6.9 | 83 ± 57ˆˆ |
Behavioral data are expressed as total distance traveled (cm) or vertical activity (beam breaks) measured over 1h following the SAL or AMPH challenge administered to rats pretreated with SCH23390 (0.1 mg/kg i.p.) or eticlopride (0.5 mg/kg i.p.). Mean (± SEM), n = 6,
p<0.01 vs. SAL-SAL;
p<0.01 vs. SAL-AMPH; SAL, saline; AMPH, amphetamine; SCH, SCH23390; ETI, eticlopride.
Figure 4. Inhibition of D2 but not D1 dopamine receptors blocks AMPH-induced AGS1 mRNA and protein in the PFC.
Top: Representative pseudocolor images of coronal hemisections illustrate AGS1 mRNA hybridization signal in each treatment group (A). Representative Western blot images depict immunoreactivity of AGS1 protein and control protein calnexin in each treatment group (B). Bottom: Quantitative analysis of AGS1 mRNA hybridization signal (integrated density) (A) or AGS1 immunoreactivity (B) in the PFC 1-3 hrs after single SAL or AMPH (2.5 mg/kg) treatment. Animals received a single dose of D1 (SCH23390, 0.1 mg/kg) or D2 (eticlopride, 0.5 mg/kg) receptor antagonists 15 mins prior to SAL or AMPH treatment. Bars represent mean integrated density of AGS1 mRNA or mean AGS1 immunoreactivity (+/- SEM), n = 6, *p<0.05, **p<0.01 vs. SAL-SAL, ##p<0.01 vs. SCH-SAL.
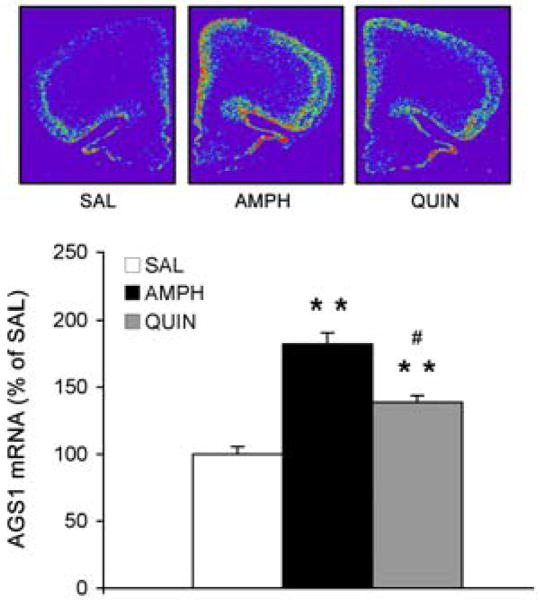
To further characterize the role of D2 receptors in regulating AGS1 expression in the PFC, direct and indirect stimulation of D2 receptors was compared. Systemic administration of the D2 receptor agonist, quinpirole (1 mg/kg), elevated AGS1 mRNA levels in the PFC (138% of SAL, p<0.01). Interestingly, direct D2 receptor activation resulted in significantly less induction of AGS1 mRNA levels when compared to the AMPH-induced AGS1 increase (Fig. 5; QUIN vs. AMPH groups, p<0.05).
Figure 5. Direct stimulation of D2 receptors by quinpirole up-regulates AGS1 mRNA in the PFC.
Quantitative analysis of AGS1 mRNA hybridization signal (integrated density) in the PFC 3 hrs after a single treatment with SAL, AMPH (2.5 mg/kg), or quinpirole (QUIN, 1 mg/kg). Bars represent mean integrated density of AGS1 mRNA (+/- SEM), n = 4, **p<0.01 vs. SAL, #p<0.01 QUIN vs. AMPH.
Experiment 5: Glucocorticoid receptors co-regulate the AMPH-induced increase in AGS1 mRNA levels in the PFC
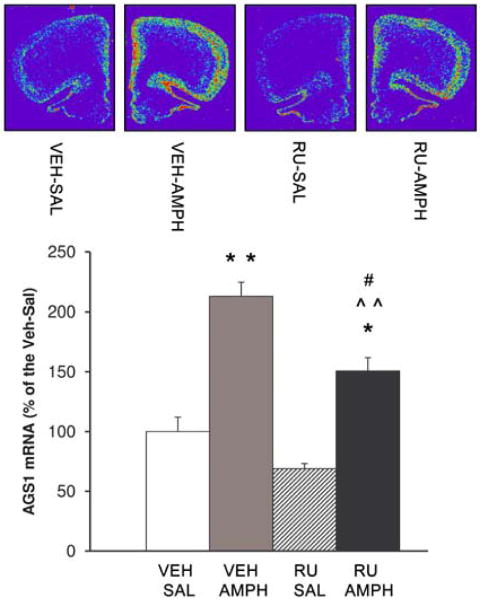
AGS1 gene expression in several cell lines is known to be regulated by glucocorticoids (Kemppainen and Behrend, 1998, Brogan et al., 2001). One of the consequences of acute AMPH exposure is activation of the hypothalamo-pituitary-adrenocortical (HPA) axis and elevation of glucocorticoids in the bloodstream and in the brain (Knych and Eisenberg, 1979, Swerdlow et al., 1993). The following study was designed to determine whether glucocorticoids (in addition to D2 receptors) regulate AMPH-induced AGS1 expression in the PFC. Pretreatment with the glucocorticoid receptor antagonist, RU486 (25 mg/kg s.c.) attenuated horizontal activity of AMPH-treated rats when compared to their vehicle-pretreated counterparts (Table 3; RU-AMPH vs. VEH-AMPH groups, p<0.05). However, RU486 pretreatment did not completely block AMPH-induced horizontal activity (Table 3; RU-AMPH vs. RU-SAL groups, p<0.01). Further, blockade of glucocorticoid receptors did not alter AMPH-induced vertical activity (rearing; Table 3). In agreement with the behavioral data, RU486 attenuated (but did not completely suppress) AMPH-induced AGS1 gene expression (Fig. 6; RU-AMPH vs. VEH-AMPH groups, p<0.05). Interestingly, there was a trend towards a decrease in baseline AGS1 mRNA levels in the PFC after RU-486 treatment (Fig. 6; RU486-SAL vs. VEH-SAL groups, p=0.55). Surprisingly, a higher dose of RU486 (50 mg/kg s.c.) produced augmentation rather than inhibition of AMPH-induced behaviors (data not shown), probably engaging non-glucocorticoid mechanisms of action; therefore, this dose was not used in the current study
Table 3.
Regulation of AMPH-induced behaviors by systemic blockade of glucocorticoid receptors (Experiment 5).
| Behavior | Experimental Groups | |||
|---|---|---|---|---|
| Veh-SAL | Veh-AMPH | RU486-SAL | RU486-AMPH | |
| Total distance traveled (Horizontal activity) | 2207 ± 1017 | 23634 ± 3987** | 3769 ± 402 | 13798 ± 2367* ˆ # |
| Rearing (Vertical activity) | 160 ± 81 | 2190 ± 394** | 246 ± 110 | 2957 ± 875** ˆˆ |
Behavioral data are expressed as total distance traveled (cm) or rearing (beam breaks) measured over 1h following the SAL or AMPH challenge administered to rats pretreated with RU486 (25 mg/kg s.c.) or vehicle. Mean (± SEM), n = 5-7,
p<0.05,
p<0.01 vs. Veh-SAL;
p<0.05,
p<0.01 vs. RU486-SAL;
p<0.05 vs. Veh-AMPH. SAL, saline; AMPH, amphetamine; Veh, vehicle.
Figure 6. Inhibition of glucocorticoid receptors with RU486 attenuates AMPH-induced AGS1 mRNA in the PFC.
Quantitative analysis of AGS1 mRNA hybridization signal (integrated density) in the PFC 3 hrs after SAL or AMPH (2.5 mg/kg) treatment. Animals received a single dose of RU486 (25 mg/kg) or vehicle 45 mins prior to SAL or AMPH treatment. Bars represent mean integrated density of AGS1 mRNA (+/- SEM), n = 5-7, *p<0.05, **p<0.01 vs. Veh-SAL, ˆˆp<0.01 vs. RU-SAL, #p<0.01 RU-AMPH vs. VEH-AMPH.
Discussion
This study demonstrates for the first time that AGS1 expression in the brain is induced by psychostimulants. The effects of acute AMPH exposure on gene expression were selective for AGS1 when compared to other brain-enriched AGS-like proteins. Further, we demonstrated that a history of repeated AMPH treatment augments AGS1 expression in the PFC in response to AMPH challenge. Finally, induction of AGS1 expression in the PFC by AMPH is regulated by D2 dopamine receptors, and less so, by glucocorticoid receptors. Collectively, these data suggest that AGS1 is a cortical regulator of G-protein signaling that is rapidly induced by psychostimulants and is co-regulated by D2 and glucocorticoid receptors.
Induction of AGS1 gene and protein expression by AMPH in the frontal cortex
Previously, several factors have been identified to modulate the levels of AGS1 mRNA in the rodent brain. Administration of the synthetic glucocorticoid analogue, dexamethasone, up-regulated AGS1 gene expression in pituitary cells and in the mouse brain (Kemppainen and Behrend, 1998, Brogan et al., 2001). Further, circadian rhythms regulated AGS1 expression in some (suprachiasmatic nucleus of the hypothalamus), but not in other (frontal cortex) parts of the mouse brain (Takahashi et al., 2003). And finally, repeated morphine treatment followed by acute (naloxone-precipitated) withdrawal increased AGS1 gene expression in the mouse frontal cortex (Ammon-Treiber et al., 2004). The present data demonstrate that psychostimulants such as AMPH can upregulate AGS1 mRNA and protein levels in the rodent brain as well. Acute exposure to AMPH elevated AGS1 mRNA levels across the frontal cortex with the most robust response detected in the PFC. Induction of AGS1 mRNA in the PFC by acute AMPH was a rapid, but relatively transient event as elevated levels of AGS1 mRNA returned back to baseline between 6-24 h after treatment. Even repeated AMPH treatment did not produce lasting changes in AGS1 expression in the PFC as measured 21 days later. However, a low dose AMPH challenge administered after a 21-day abstinence period rapidly increased AGS1 mRNA and protein levels in the PFC, but only in animals previously exposed to AMPH. Thus, changes in AGS1 expression in the PFC parallel transient behavioral activation following acute AMPH in drug-naïve animals, as well as enduring behavioral hypersensitivity to AMPH challenge in animals with an AMPH history. In general, these gene expression patterns are typical for a number of activity-regulated genes including transcription factors (like c-fos and zif/268) and effector genes (like arc, bdnf and homer1a) induced by psychostimulants in the forebrain (Graybiel et al., 1990, Daunais and McGinty, 1994, Tan et al., 2000, Le Foll et al., 2005, Ghesamzadeh et al. 2009). Interestingly, several members of the RGS protein family of G-protein regulators in the striatum and PFC are regulated by psychostimulants in an analogous manner (Burchett et al., 1999, Geurts et al. 2002, Taymans et al. 2003 and 2004, Schwendt et al., 2006, Schwendt and McGinty 2006). Despite similarities, the present study revealed several distinctive characteristics of psychostimulant-regulated AGS1 expression. First, AMPH upregulates AGS1 gene expression in the PFC, but not in the striatum. Typically, a robust induction of activity-regulated genes by psychostimulants occurs in forebrain regions receiving dense dopaminergic input; that is both in the PFC and striatum. Second, pre-exposure to AMPH sensitized responses of AGS1 mRNA in the PFC to a subsequent AMPH challenge. On the other hand, gene and protein expression of several activity regulated-genes in the PFC did not become sensitized following repeated psychostimulant treatment (Fujiyama et al. 2003, Nordquist et al 2008). These differences could stem from a specific modulation of psychostimulant-induced AGS1 expression in the PFC by dopamine and glucocorticoid receptors described in the present study.
The role of dopamine receptors in AMPH-regulated AGS1 expression in the PFC
In general, D1 dopamine receptor antagonists suppress psychostimulant-induced mRNA expression of activity-regulated genes in the PFC and striatum whereas the effects of D2 dopamine receptor blockade are less consistent and depend on the experimental conditions and the particular gene studied (Cole et al., 1992, Ruskin and Marshall, 1994, Wang and McGinty, 1995a, Fumagalli et al., 2006, Ghasemzadeh et al., 2009). Surprisingly, we found that a D1 receptor antagonist (SCH23390) failed to suppress AGS1 expression in the PFC induced by AMPH, even though the dose antagonist used (0.1mg/kg i.p.) was sufficient to completely block AMPH-induced behaviors. In contrast to D1 receptor inhibition, pretreatment with a D2 receptor antagonist (eticlopride) inhibited both AMPH-induced behaviors, as well as AMPH-induced AGS1 mRNA and protein expression in the PFC. In support of D2-dependent regulation of AGS1 expression, direct stimulation of D2 receptors by quinpirole increased the expression of AGS1 mRNA in the PFC. Although not typical, D2-dependent regulation of basal and stimulated gene expression in the PFC has been recently reported for several activity-regulated genes (Wang and McGinty, 1995a, Fumagalli et al., 2003, Ghasemzadeh et al., 2009). Even though these studies suggested the role of D2 receptors in mediating the effects of AMPH on AGS1 gene and protein expression, it remains to be established whether this regulation involves direct (cellular) and/or indirect (circuitry, systemic) mechanisms.
The role of glucocorticoid receptors in AMPH-regulated AGS1 expression in the PFC
It is well established that dopamine receptor agonists and AMPH activate the hypothalamic-pituitary-adrenal axis resulting in increased levels of glucocorticoids in the bloodstream and in the brain (Knych and Eisenberg, 1979, Borowsky and Kuhn, 1992, Swerdlow et al., 1993). Elevated levels of glucocorticoids upregulate AGS1 gene expression in the mouse brain (Brogan et al., 2001). This effect is likely to be mediated via direct binding of activated glucocorticoid receptors to the glucocorticoid-response element present in the promoter of the AGS1 gene (Kemppainen et al., 2003). In support of the functional significance of this interaction, we found that systemic administration of the glucocorticoid receptor antagonist RU486 partially inhibited AGS1 gene expression in the PFC as well as AMPH-induced locomotor behavior. The contribution of glucocorticoids to AMPH-induced up-regulation of AGS1 gene expression might also explain ‘hyperresponsivness’ of AGS1 expression in sensitized animals. Indeed, hypersecretion of corticosterone occurs in response to a challenge in rats sensitized to cocaine or AMPH (Schmidt et al., 1995, 1999) and administration of RU486 prevents the expression of AMPH-induced behavioral sensitization (De Vries et al., 1996). Interestingly, hyperresponsivness of gene expression in rats sensitized to AMPH as well as glucocorticoid-dependent regulation of gene expression have been previously described for RGS4, another cortically-enriched G-protein regulator (Ni et al., 1999, Schwendt and McGinty, 2006).
Possible cellular consequences of altered AGS1 levels
Cellular and signaling consequences of increased levels of AGS1 (like those seen after AMPH or quinpirole treatment) have been investigated in a number of studies. Overexpression of AGS1 in several cell lines resulted in Gαi-dependent suppression of adenylyl cyclase activity (Graham et al., 2004) and potentiation of ERK-signaling mediated by Gβγ (Graham et al., 2002). These effects are due to AGS1 acting as a tonic, receptor-independent activator of G-protein signaling. However, upon activation of Gαi-coupled GPCRs, AGS1 interferes with the ability of receptor to trigger Gβγ-dependent signaling events (Takesono et al., 2002, Nguyen and Watts, 2005, Thapliyal et al., 2008). In relation to the current study, Nguyen and Watts (2005) demonstrated that overexpression of AGS1 (in various cell lines) suppresses Gβγ-dependent cellular signaling activated by the D2 receptor agonist quinpirole. It remains unclear whether AGS1 displays functional selectivity only for certain Gαi-coupled receptors. Nevertheless, it is tempting to hypothesize that the D2 receptor-mediated increase in AGS1 levels in the PFC (as observed in the present study) represents a negative-feedback-response diminishing excessive D2 receptor activity. Interestingly, while behavioral sensitization to AMPH or cocaine wasn't accompanied by changes in a total number of D2 receptors in the PFC (Bonhomme et al., 1995, Zhang et al., 2000, Moro et al., 2007), D2-dependent behavioral responses and sensitivity of D2 receptors were found to be augmented in AMPH-sensitized animals (Karler et al., 1998, Vanderschuren et al., 1999, Seeman et al., 2007). However, the distribution of AGS1 and D2 receptor mRNAs in the PFC displays only partial overlap. While AGS1 mRNA is predominately present in superficial cortical layers, D2 receptor mRNA is enriched in the deeper layers of the PFC (Gaspar et al., 1995). This pattern suggests that AMPH-induced changes in AGS1-dependent cellular signaling would affect horizontal intracortical pathways (originating in more superficial PFC layers), rather than descending cortico-striatal connections (involving projection neurons residing in the deeper cortical layers). It is well established, that cellular adaptations within the cortico-striatal circuitry mediate persistent behaviors like behavioral sensitization and drug-seeking (Pierce and Kalivas, 1997, Kalivas et al., 2009). Nevertheless, recent evidence suggests that superficial layers of the primate PFC exhibit more pronounced changes in dendritic morphology following AMPH-sensitization than neurons in deeper layers (Selemon et al., 2007).
It should be noted, that AGS1 has been implicated in other cellular pathways not directly related to GPCR signaling. AGS1 was also identified as a binding partner of the adaptor protein termed ‘Carboxyl-terminal PDZ ligand of neuronal nitric oxide synthase’ (CAPON), which is involved in NMDA receptor-dependent activation of neuronal nitric oxide synthase (Fang et al., 2000). It remains to be determined whether AMPH-induced changes in AGS1 protein levels affect CAPON-dependent signaling in the postsynaptic density. However, in our experimental conditions, AMPH treatment did not alter CAPON mRNA levels in the PFC (data not shown).
Conclusions
In conclusion, the data presented in this report demonstrate for the first time that AGS1 expression in the frontal cortex is rapidly increased in response to acute AMPH treatment. Moreover, this up-regulation is augmented in rats sensitized to AMPH. Since the regulation of AGS1 expression depends on activity of D2 receptors and glucocorticoid receptors, AGS1 may be an important mediator of interactions between psychostimulant- and stress-induced neuroadaptations. Although, it is currently not clear what are the cellular targets affected by fluctuation of AGS1 levels in the PFC, likely candidates include D2-receptor- and ERK-activated signaling pathways. Future studies will address the role of AGS1 overexpression in the context of psychostimulant-induced neuroadaptations in the PFC related to persistence of drug-seeking and relapse.
Acknowledgments
The authors thank Adrian Gomez for excellent technical assistance. We also thank Dr. Joseph Blumer and Dr. Stephen Lanier for kindly providing AGS1 antiserum and for valuable suggestions during the preparation of this manuscript. This work was supported by the National Institutes of Health Grant R01 DA03982.
List of abbreviations
- AGS
activator of G-protein signaling
- AMPH
amphetamine
- ANOVA
analysis of variance
- ERK
extracellular signal-regulated kinase
- ETI
eticlopride
- GPCR
G-protein coupled receptor
- QUIN
quinpirole
- PFC
prefrontal cortex
- RGS
regulator of G-protein signaling
- SAL
saline
- SCH
SCH23390
- TBS
Tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso R, Voutsinos B, Fournier M, Labie C, Steinberg R, Souilhac J, Le Fur G, Soubrié P. Blockade of cannabinoid receptors by SR141716 selectively increases Fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience. 1998;91:607–620. doi: 10.1016/s0306-4522(98)00675-7. [DOI] [PubMed] [Google Scholar]
- Alttoa A, Eller M, Herm L, Rinken A, Harro J. Amphetamine-induced locomotion, behavioral sensitization to amphetamine, and striatal D2 receptor function in rats with high or low spontaneous exploratory activity: differences in the role of locus coeruleus. Brain Res. 2007;1131:138–148. doi: 10.1016/j.brainres.2006.10.075. [DOI] [PubMed] [Google Scholar]
- Ammon-Treiber S, Tischmeyer H, Riechert U, Hollt V. Gene expression of transcription factors in the rat brain after morphine withdrawal. Neurochem Res. 2004;29:1267–1273. doi: 10.1023/b:nere.0000023613.44988.9d. [DOI] [PubMed] [Google Scholar]
- Bailey A, Metaxas A, Yoo JH, McGee T, Kitchen I. Decrease of D2 receptor binding but increase in D2-stimulated G-protein activation, dopamine transporter binding and behavioural sensitization in brains of mice treated with a chronic escalating dose ‘binge’ cocaine administration paradigm. Eur J Neurosci. 2008;28:759–770. doi: 10.1111/j.1460-9568.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- Blumer JB, Cismowski MJ, Sato M, Lanier SM. AGS proteins: receptor-independent activators of G-protein signaling. Trends Pharmacol Sci. 2005;26:470–476. doi: 10.1016/j.tips.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Bonhomme N, Cador M, Stinus L, Le Moal M, Spampinato U. Short and long-term changes in dopamine and serotonin receptor binding sites in amphetamine-sensitized rats: a quantitative autoradiographic study. Brain Res. 1995;675:215–223. doi: 10.1016/0006-8993(95)00067-z. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Fisone G. Psychoactive drugs and regulation of the cAMP/PKA/DARPP-32 cascade in striatal medium spiny neurons. Neurosci Biobehav Rev. 2007;31:79–88. doi: 10.1016/j.neubiorev.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Kuhn CM. D1 and D2 dopamine receptors stimulate hypothalamo-pituitary-adrenal activity in rats. Neuropharmacology. 1992;31:671–678. doi: 10.1016/0028-3908(92)90145-f. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, Chou JK, Guillory AM, Chang SJ, Janak PH, Bonci A, Diamond I. Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proc Natl Acad Sci U S A. 2008;105:12533–12538. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogan MD, Behrend EN, Kemppainen RJ. Regulation of Dexras1 expression by endogenous steroids. Neuroendocrinology. 2001;74:244–250. doi: 10.1159/000054691. [DOI] [PubMed] [Google Scholar]
- Burchett SA, Bannon MJ, Granneman JG. RGS mRNA expression in rat striatum: modulation by dopamine receptors and effects of repeated amphetamine administration. J Neurochem. 1999;72:1529–1533. doi: 10.1046/j.1471-4159.1999.721529.x. [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Hernandez S, Earls LR, Medkova M, Sundgren-Andersson AK, Surmeier DJ, Hamm HE. RGS9-2 modulates D2 dopamine receptor-mediated Ca2+ channel inhibition in rat striatal cholinergic interneurons. Proc Natl Acad Sci U S A. 2004;101:16339–16344. doi: 10.1073/pnas.0407416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismowski MJ. Non-receptor activators of heterotrimeric G-protein signaling (AGS proteins) Semin Cell Dev Biol. 2006;17:334–344. doi: 10.1016/j.semcdb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Bhat RV, Patt C, Worley PF, Baraban JM. D1 dopamine receptor activation of multiple transcription factor genes in rat striatum. J Neurochem. 1992;58:1420–1426. doi: 10.1111/j.1471-4159.1992.tb11358.x. [DOI] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF. Acute and chronic cocaine administration differentially alters striatal opioid and nuclear transcription factor mRNAs. Synapse. 1994;18:35–45. doi: 10.1002/syn.890180106. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Tjon GH, Nestby P, Mulder AH, Vanderschuren LJ. Mifepristone prevents the expression of long-term behavioural sensitization to amphetamine. Eur J Pharmacol. 1996;307:R3–4. doi: 10.1016/0014-2999(96)00308-1. [DOI] [PubMed] [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Fujiyama K, Kajii Y, Hiraoka S, Nishikawa T. Differential regulation by stimulants of neocortical expression of mrt1, arc, and homer1a mRNA in the rats treated with repeated methamphetamine. Synapse. 2003;49:143–149. doi: 10.1002/syn.10220. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Frasca A, Di Pasquale L, Racagni G, Riva MA. Corticostriatal up-regulation of activity-regulated cytoskeletal-associated protein expression after repeated exposure to cocaine. Mol Pharmacol. 2006;70:1726–1734. doi: 10.1124/mol.106.026302. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Maragnoli ME, Gennarelli M, Perez J, Racagni G, Riva MA. Dopaminergic D2 receptor activation modulates FGF-2 gene expression in rat prefrontal cortex and hippocampus. J Neurosci Res. 2003;74:74–80. doi: 10.1002/jnr.10733. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Geurts M, Hermans E, Maloteaux JM. Opposite modulation of regulators of G protein signalling-2 RGS2 and RGS4 expression by dopamine receptors in the rat striatum. Neurosci Lett. 2002;333:146–150. doi: 10.1016/s0304-3940(02)01004-2. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Windham LK, Lake RW, Acker CJ, Kalivas PW. Cocaine activates Homer1 immediate early gene transcription in the mesocorticolimbic circuit: differential regulation by dopamine and glutamate signaling. Synapse. 2009;63:42–53. doi: 10.1002/syn.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Nicolini V, McGinty JF. Gene expression profile from the striatum of amphetamine-treated rats: a cDNA array and in situ hybridization histochemical study. Brain Res Gene Expr Patterns. 2002;1:193–198. doi: 10.1016/s1567-133x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Graham TE, Prossnitz ER, Dorin RI. Dexras1/AGS-1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinases. J Biol Chem. 2002;277:10876–10882. doi: 10.1074/jbc.M110397200. [DOI] [PubMed] [Google Scholar]
- Graham TE, Qiao Z, Dorin RI. Dexras1 inhibits adenylyl cyclase. Biochem Biophys Res Commun. 2004;316:307–312. doi: 10.1016/j.bbrc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56 1:169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karler R, Calder LD, Thai DK, Bedingfield JB. The role of dopamine in the mouse frontal cortex: a new hypothesis of behavioral sensitization to amphetamine and cocaine. Pharmacol Biochem Behav. 1998;61:435–443. doi: 10.1016/s0091-3057(98)00133-6. [DOI] [PubMed] [Google Scholar]
- Kemppainen RJ, Behrend EN. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem. 1998;273:3129–3131. doi: 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- Kemppainen RJ, Cox E, Behrend EN, Brogan MD, Ammons JM. Identification of a glucocorticoid response element in the 3′-flanking region of the human Dexras1 gene. Biochim Biophys Acta. 2003;1627:85–89. doi: 10.1016/s0167-4781(03)00079-4. [DOI] [PubMed] [Google Scholar]
- Kimple RJ, De Vries L, Tronchère H, Behe CI, Morris RA, Gist Farquhar M, Siderovski DP. RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor activity. J Biol Chem. 2001;276:29275–29281. doi: 10.1074/jbc.M103208200. [DOI] [PubMed] [Google Scholar]
- Knych ET, Eisenberg RM. Effect of amphetamine on plasma corticosterone in the conscious rat. Neuroendocrinology. 1979;29:110–118. doi: 10.1159/000122912. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Washiyama K, Ikeda K. Modulators of G protein-activated inwardly rectifying K+ channels: potentially therapeutic agents for addictive drug users. Ann N Y Acad Sci. 2004;1025:590–594. doi: 10.1196/annals.1316.073. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Moro H, Sato H, Ida I, Oshima A, Sakurai N, Shihara N, Horikawa Y, Mikuni M. Effects of SKF-38393, a dopamine D1 receptor agonist on expression of amphetamine-induced behavioral sensitization and expression of immediate early gene arc in prefrontal cortex of rats. Pharmacol Biochem Behav. 2007;87:56–64. doi: 10.1016/j.pbb.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Morsink MC, Van Gemert NG, Steenbergen PJ, Joels M, De Kloet ER, Datson NA. Rapid glucocorticoid effects on the expression of hippocampal neurotransmission-related genes. Brain Res. 2007;1150:14–20. doi: 10.1016/j.brainres.2007.02.083. [DOI] [PubMed] [Google Scholar]
- Nguyen CH, Watts VJ. Dexras1 blocks receptor-mediated heterologous sensitization of adenylyl cyclase 1. Biochem Biophys Res Commun. 2005;332:913–920. doi: 10.1016/j.bbrc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Ni YG, Gold SJ, Iredale PA, Terwilliger RZ, Duman RS, Nestler EJ. Region-specific regulation of RGS4 (Regulator of G-protein-signaling protein type 4) in brain by stress and glucocorticoids: in vivo and in vitro studies. J Neurosci. 1999;19:3674–3680. doi: 10.1523/JNEUROSCI.19-10-03674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist RE, Vanderschuren LJ, Jonker AJ, Bergsma M, de Vries TJ, Pennartz CM, Voorn P. Expression of amphetamine sensitization is associated with recruitment of a reactive neuronal population in the nucleus accumbens core. Psychopharmacology (Berl) 2008;198:113–126. doi: 10.1007/s00213-008-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. San Diego: Academic Press; 2007. [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Luedtke RR, Self D, Neve RL, Lester HA, Simon MI, Nestler EJ. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Marshall JF. Amphetamine- and cocaine-induced fos in the rat striatum depends on D2 dopamine receptor activation. Synapse. 1994;18:233–240. doi: 10.1002/syn.890180309. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Tilders FJ, Binnekade R, Schoffelmeer AN, De Vries TJ. Stressor- or drug-induced sensitization of the corticosterone response is not critically involved in the long-term expression of behavioural sensitization to amphetamine. Neuroscience. 1999;92:343–352. doi: 10.1016/s0306-4522(98)00725-8. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Tilders FJ, Janszen AW, Binnekade R, De Vries TJ, Schoffelmeer AN. Intermittent cocaine exposure causes delayed and long-lasting sensitization of cocaine-induced ACTH secretion in rats. Eur J Pharmacol. 1995;285:317–321. doi: 10.1016/0014-2999(95)00540-2. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Chandler LJ, Blumer JB, Lanier SM, McGinty JF. Expression of activator of G-protein signaling AGS1/DexRas1 in the rat brain increases during development and in response to acute amphetamin. Program and abstracts of the Society for Neuroscience 33rd Annual Meeting; Washington, DC. 2005. p. 342.2. [Google Scholar]
- Schwendt M, Gold SJ, McGinty JF. Acute amphetamine down-regulates RGS4 mRNA and protein expression in rat forebrain: distinct roles of D1 and D2 dopamine receptors. J Neurochem. 2006;96:1606–1615. doi: 10.1111/j.1471-4159.2006.03669.x. [DOI] [PubMed] [Google Scholar]
- Schwendt M, McGinty JF. Repeated administration of amphetamine and/or amphetamine challenge causes a decrease of RGS4 expression in rat dorsal striatum and prefrontal cortex. Program and abstracts of the Society for Neuroscience 34th Annual Meeting; Atlanta, GA. 2006. p. 690.610. [Google Scholar]
- Seeman P, McCormick PN, Kapur S. Increased dopamine D2(High) receptors in amphetamine-sensitized rats, measured by the agonist [(3)H](+)PHNO. Synapse. 2007;61:263–267. doi: 10.1002/syn.20367. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Begovic A, Goldman-Rakic PS, Castner SA. Amphetamine sensitization alters dendritic morphology in prefrontal cortical pyramidal neurons in the non-human primate. Neuropsychopharmacology. 2007;32:919–931. doi: 10.1038/sj.npp.1301179. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary-adrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav. 1993;45:629–637. doi: 10.1016/0091-3057(93)90518-x. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Umeda N, Tsutsumi Y, Fukumura R, Ohkaze H, Sujino M, van der Horst G, Yasui A, Inouye ST, Fujimori A, Ohhata T, Araki R, Abe M. Mouse dexamethasone-induced RAS protein 1 gene is expressed in a circadian rhythmic manner in the suprachiasmatic nucleus. Brain Res Mol Brain Res. 2003;110:1–6. doi: 10.1016/s0169-328x(02)00543-0. [DOI] [PubMed] [Google Scholar]
- Takesono A, Nowak MW, Cismowski M, Duzic E, Lanier SM. Activator of G-protein signaling 1 blocks GIRK channel activation by a G-protein-coupled receptor: apparent disruption of receptor signaling complexes. J Biol Chem. 2002;277:13827–13830. doi: 10.1074/jbc.M201064200. [DOI] [PubMed] [Google Scholar]
- Tan A, Moratalla R, Lyford GL, Worley P, Graybiel AM. The activity-regulated cytoskeletal-associated protein arc is expressed in different striosome-matrix patterns following exposure to amphetamine and cocaine. J Neurochem. 2000;74:2074–2078. doi: 10.1046/j.1471-4159.2000.0742074.x. [DOI] [PubMed] [Google Scholar]
- Taymans JM, Leysen JE, Langlois X. Striatal gene expression of RGS2 and RGS4 is specifically mediated by dopamine D1 and D2 receptors: clues for RGS2 and RGS4 functions. J Neurochem. 2003;84:1118–1127. doi: 10.1046/j.1471-4159.2003.01610.x. [DOI] [PubMed] [Google Scholar]
- Taymans JM, Kia HK, Claes R, Cruz C, Leysen J, Langlois X. Dopamine receptor-mediated regulation of RGS2 and RGS4 mRNA differentially depends on ascending dopamine projections and time. Eur J Neurosci. 2004;19:2249–2260. doi: 10.1111/j.0953-816X.2004.03336.x. [DOI] [PubMed] [Google Scholar]
- Thapliyal A, Bannister RA, Hanks C, Adams BA. The monomeric G proteins AGS1 and Rhes selectively influence Galphai-dependent signaling to modulate N-type (CaV2.2) calcium channels. Am J Physiol Cell Physiol. 2008;295:C1417–1426. doi: 10.1152/ajpcell.00341.2008. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan G, Cismowski MJ, Wang G, Vincent TS, Brown KD, Lanier SM. The Ras-related protein AGS1/RASD1 suppresses cell growth. Oncogene. 2004;23:5858–5863. doi: 10.1038/sj.onc.1207774. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schoffelmeer AN, Mulder AH, De Vries TJ. Dopaminergic mechanisms mediating the long-term expression of locomotor sensitization following pre-exposure to morphine or amphetamine. Psychopharmacology (Berl) 1999;143:244–253. doi: 10.1007/s002130050943. [DOI] [PubMed] [Google Scholar]
- Vargiu P, Morte B, Manzano J, Perez J, de Abajo R, Gregor Sutcliffe J, Bernal J. Thyroid hormone regulation of rhes, a novel Ras homolog gene expressed in the striatum. Brain Res Mol Brain Res. 2001;94:1–8. doi: 10.1016/s0169-328x(01)00140-1. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Differential effects of D1 and D2 dopamine receptor antagonists on acute amphetamine- or methamphetamine-induced up-regulation of zif/268 mRNA expression in rat forebrain. J Neurochem. 1995a;65:2706–2715. doi: 10.1046/j.1471-4159.1995.65062706.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alteration in zif/268 and preprodynorphin mRNA expression induced by amphetamine or methamphetamine in rat forebrain. J Pharmacol Exp Ther. 1995b;273:909–917. [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. D1 and D2 receptor regulation of preproenkephalin and preprodynorphin mRNA in rat striatum following acute injection of amphetamine or methamphetamine. Synapse. 1996;22:114–22. doi: 10.1002/(SICI)1098-2396(199602)22:2<114::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Webb CK, McCudden CR, Willard FS, Kimple RJ, Siderovski DP, Oxford GS. D2 dopamine receptor activation of potassium channels is selectively decoupled by Galpha-specific GoLoco motif peptides. J Neurochem. 2005;92:1408–1418. doi: 10.1111/j.1471-4159.2004.02997.x. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Jeziorski M. Coadministration of MK-801 with amphetamine, cocaine or morphine prevents rather than transiently masks the development of behavioral sensitization. Brain Res. 1993;613:291–294. doi: 10.1016/0006-8993(93)90913-8. [DOI] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Inoue Y, Diamond I. Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:8746–8751. doi: 10.1073/pnas.0503419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, Li Y, Wang X, Lu L. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cell Mol Neurobiol. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Tarazi FI, Baldessarini RJ. Dopamine D(4) receptors in rat forebrain: unchanged with amphetamine-induced behavioral sensitization. Neuroscience. 2000;97:211–213. doi: 10.1016/s0306-4522(00)00082-8. [DOI] [PubMed] [Google Scholar]