Abstract
Aims:
Mechanical ventilation causes lung injury in premature infants. Hypothermia may protect against and hyperthermia may augment lung injury. We tested the effects of hypo- and hyperthermia on ventilation induced acute lung injury in preterm lambs.
Methods:
Twin sheep fetuses at 128 d GA (term 150 d) were surgically delivered and randomized to unventilated control (UVC), normothermia (38-39 °C) without lung injury (NTNI), or to 1 of 3 injurious ventilation groups: hypothermic (33-34 °C, LT), normothermic (38-39 °C, NT) or hyperthermic (40-41 °C, HT). NT, LT and HT groups had 15 min of injurious ventilation (PEEP 0 cmH2O, VT escalation to 15 mL/kg) following delivery and prior to surfactant. The animals were then gently ventilated (PEEP 5 cmH2O, VT 7.5 mL/kg) for 2 h 45 min. NTNI lambs received surfactant at birth prior to gentle ventilation. The lambs were then euthanized, and bronchoalveolar lavage (BAL) fluid and lung tissue were used to evaluate lung injury, inflammatory cell counts, inflammatory markers and cytokine mRNA.
Results:
Target temperatures were achieved by 15 min of age and maintained for 3 h. All ventilated groups had increased BAL protein, lung inflammation and increased cytokine mRNA. HT animals developed acidosis, premature death, pneumothoraces, impaired lung function and increased inflammatory mRNA expression. LT animals remained clinically stable without pneumothoraces or death, had improved ventilatory efficiency and trended toward lower inflammatory mRNA expression than NT animals.
Conclusion:
Hyperthermia exacerbated ventilator induced lung injury, while hypothermia may protect against lung injury in the preterm lamb.
Introduction
Preterm lungs are highly susceptible to ventilator induced injury (1, 2), and most preterm infants require intubation for ventilatory support immediately following delivery (3). Initially, high pressures are required to inflate surfactant deficient lungs to achieve gas exchange (4). Premature lungs have decreased matrix proteins and the compliant chest wall does not limit overexpansion (5). Volutrauma, atelectatrauma and excessive oxygen exposure are factors involved in the pathogenesis of ventilator induced lung injury (6). Body temperature during resuscitation also may affect lung injury. Preterm infants have only a limited ability to self-regulate core body temperature (7) and often experience large temperature variations after birth, primarily due to environmental factors. Despite emphasis on temperature regulation by the International Liaison Committee on Resuscitation (ILCOR) (8), both high and low body temperatures occur as a result of maternal fever, and the large surface area to body mass ratio of the wet preterm infant exposed to low temperatures or to radiant warmers, plastic bags and heat blankets (9-11).
Body temperature can modulate injury. Hypothermia is used clinically to decrease neurological injury following birth-related hypoxia-ischemia (12, 13). Hypothermia also protects adult animals from acute lung injury caused by wood smoke inhalation or endotoxin exposure (14-16). These combined observations suggest that hypothermia may protect the preterm infant from ventilator induced injury. Hyperthermia, on the other hand, accelerates lethal pulmonary vascular injury and increases circulating inflammatory cells in adult sepsis models in animals (17). Hyperthermia increased inflammatory and cytokine responses in a rat model of ventilator induced injury (18).
Previously, we evaluated high and low body temperatures in near-term newborn lambs that were gently ventilated from birth (19). These mature lambs had minimal ventilator induced lung injury and inflammation that was not affected by moderate hyper- or hypothermia. To date, there is no experimental information about the effects of high or low body temperature on the preterm lung, which has increased susceptibility to injury (5). In this current study, we used escalating tidal volumes and no positive end expiratory pressure (PEEP) to cause lung injury (4) in newborn preterm lambs and asked if temperature modulated lung injury/inflammation. We hypothesized that hyperthermia would increase and hypothermia would attenuate ventilator induced acute stretch injury to the lungs of newborn preterm lambs when compared to normothermia.
Methods
Delivery of Lambs
The animal studies were performed in Western Australia with animal use approval from Cincinnati Children's Hospital and The University of Western Australia. 128 day gestational age (GA) twin fetuses (term is 150 d GA) from Merino ewes were randomized prior to delivery to one of 4 ventilation groups with different core temperature targets and ventilation strategies. Normothermic lambs (target 38-39 °C core temperature- physiologic normothermia for the lamb) without deliberate lung injury (NTNI) were used as the ventilation reference group for the 3 injurious ventilation groups: hypothermic (33-34 °C, LT), normothermic (38-39 °C, NT) or hyperthermic (40-41 °C, HT). A fifth group was euthanized at delivery as unventilated controls (UVC). Ewes were anesthetized prior to surgical delivery with an IV injection of Ketamine (10 mg/kg; Parnell Labs, NSW, Australia) and Medetomidine (Domitor®, 0.1 mg/kg; Pfizer Animal Health, NSW, Australia) and spinal anesthesia (2% Lidocaine; 60 mg). Lambs were exposed sequentially through an abdominal incision. Following delivery of the head, each lamb received local anesthesia with lidocaine (2%; 1 mL) prior to tracheostomy and insertion of a 4.5 cm internal diameter tracheal tube to a depth of 8 cm. Cord blood samples were collected. Following delivery, each lamb was weighed. The hypothermic animals were briefly (30 s) submerged in a cold-water bath prior to placement on the resuscitation bed and application of cold packs around the head and body. Normothermic lambs were maintained using radiant warmers and plastic wrap (Neowrap, Fisher & Paykel Healthcare, Auckland, NZ). Hyperthermia was achieved using plastic wrap with radiant warmers and supplemental heat lamps.
Ventilation and Monitoring
Each lamb was suctioned to remove lung fluid and ventilated with a volume controlled, pressure limited, time-cycled infant ventilator (Draeger, Babylog 8000+). Inspired gas was heated and humidified to 37 °C at the airway (MR850 Humidifier, Fisher & Paykel Healthcare, Auckland, NZ). The initial fractional inspired oxygen (FiO2) of 0.4 was adjusted to target oxygen saturations of 90-98%. Ventilators were set to deliver 50 breaths/min with an inspiratory time of 0.5 s.
The NTNI lambs were surfactant treated (Curosurf®, 100 mg/kg; Chiesi Pharmaceuticals, Parma, Italy) immediately after delivery and prior to initiation of ventilation with a positive end-expiratory pressure (PEEP) of 5 cmH2O and with gradual increases in tidal volume (VT) to 7.5 mL/kg by 15 min of age. NT, LT and HT groups received 15 minutes of injurious ventilation with a PEEP of 0 cmH2O and an escalating VT to 15 mL/kg by 15 min (20). The maximum peak inspiratory pressure (PIP) was limited to 50 cmH2O. Intratracheal surfactant was not delivered until 15 min. From 15 min to 3 h, all groups received gentle mechanical ventilation using a PEEP of 5 cmH2O and VT of 7.5 mL/kg (maximum PIP limit 40 cmH2O) with VT adjusted to target a PaCO2 of 50-60 mmHg. Rectal and esophageal temperature probes were placed for core body temperature monitoring. Esophageal temperature did not differ by more than ± 0.2 °C from rectal temperature. Continuous temperature monitoring permitted minute-to-minute ambient temperature adjustments.
Umbilical venous and arterial catheters were placed for continuous sedation and anesthesia using propofol (Repose®, 0.1 mg/kg/min; Norbrook Laboratories Ltd., Victoria, Australia) and remifentanil (Ultiva®, 0.05 μg/kg/min; GlaxoSmithKline, NSW, Australia). Peripheral oxygen saturation (SpO2) and pulse rate were monitored continuously using a transcutaneous monitor (OxiMax N-65; Nellcor, Boulder, CO). Temperature and physiologic data were recorded and arterial blood gas values were measured at 15, 30, 45, 60, 90, 120, 150 and 180 minutes. pH measurements were corrected for body temperature. At 3 h, each ventilated lamb was euthanized by IV pentobarbital injection (100 mg/kg) and re-weighed. UVC fetuses were similarly delivered, euthanized and weighed without receiving ventilation. The Ventilation Efficiency Index (VEI) and Oxygenation Index (OI) were calculated for each animal as described previously (21, 22).
Tissue Sampling
Postmortem, the lungs were removed. Bronchoalveolar lavage (BAL) fluid was pooled from 3 saline lavages of the left lung. BAL fluid was used for cell counts, cytospin preparations and additional assays. Tissue from the right lower lobe was snap frozen in liquid nitrogen. The right upper lobe was airway fixed with 10% formalin at 30 cmH2O, sequentially dehydrated in ethanol and embedded in paraffin for lung injury scoring.
Cell Counts and Scoring of Lung Injury
BAL cytospin slides were used for the blinded differential cell count of 200 cells per slide (23). Lung injury scoring was performed using a modified Acute Lung Injury (ALI) scoring system- 10 high-powered fields from each animal were qualitatively scored (0, 1+, 2+) for congestion/edema, hemorrhage and inflammatory cell infiltration with each component averaged for a total injury score ranging from 0-6 (24, 25). Representative histological sections were imaged using an Olympus BX-40 microscope with Pixera Studio Pro software.
Cytokines and Inflammatory Mediators
RNA was recovered from lung tissue using a modified Chomczynski and Sacchi Method. mRNA expression was measured for IL-1β, IL-6, MCP-1, HSP-70, Egr-1 and TLR-4 using 10 μg of RNA with sheep-specific radiolabeled riboprobes (Promega Riboprobe system) by RNA protection assays (26, 27). Band density was detected using PhosphorImager on ImageQuant software.
Statistics
Values are expressed as mean ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA with Newman-Keuls post-test for group comparisons. Selective two-tailed t-tests were used for two group comparisons. Statistical significance was accepted at p < 0.05.
Results
Description of Animals
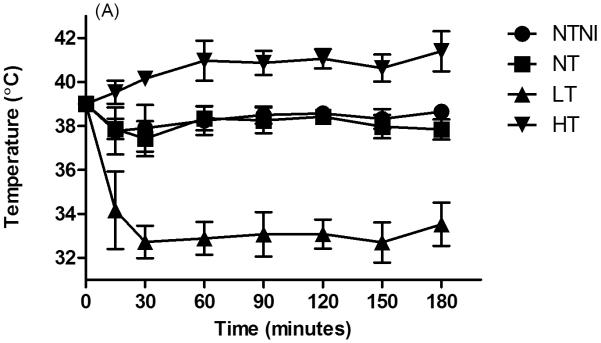
Of the 39 animals randomized, 1 died shortly after birth because of equipment failure and was removed from the analysis. The remaining 38 animals were included in the analysis. Lamb numbers, birth weights and cord blood pH values are given in Table 1. The low cord pH values resulted from elevated PaCO2 values secondary to maternal anesthesia and dorsal recumbancy positioning for delivery of the lamb. Target core temperatures were achieved by 15 min of age and successfully maintained for the duration of the experimental period (Figure 1A).
Table 1.
Characteristics of Animals and Initial Ventilation Variables
| Group | Unventilated Control |
Normothermia No Injury |
Normothermia Injury |
Hypothermia Injury |
Hyperthermia Injury |
|---|---|---|---|---|---|
| Abbreviation | UVC | NTNI | NT | LT | HT |
| N per group | 7 | 8 | 7 | 8 | 8 |
| Birth Wt (kg) | 3.2±0.2 | 2.7±0.2 | 3.0±0.3 | 2.6±0.3 | 2.8±0.3 |
| Cord pH | 7.1±0.1 | 7.2±0.1 | 7.2±0.1 | 7.2±0.1 | 7.2±0.1 |
| 5 min VT | --- | 5.3±0.1 | 7.3±1.0+ | 8.0±0.3+ | 7.1±1.0+ |
| PIP | --- | 33±6 | 38±3 | 39±8 | 38±2 |
| 10 min VT | --- | 7.8±0.3 | 10.3±1.2+ | 11.8±2.1+ | 9.9±1.4+ |
| PIP | --- | 34±3 | 44±6+ | 46±6+ | 47±3+ |
| 15 min VT | --- | 7.9±0.2 | 14.5±1.9+ | 15.2±2.0+ | 12.3±3.3+ |
| PIP | --- | 31±3 | 48±5+ | 47±6+ | 50±1+ |
VT is ventilatory tidal volume, expressed as VT/kg; PIP is peak inspiratory pressure, in cmH2O. Data expressed as mean ± SD.
p < 0.05 vs. NTNI.
Figure 1.
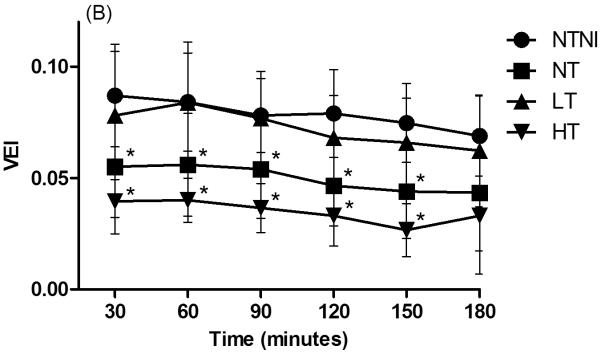
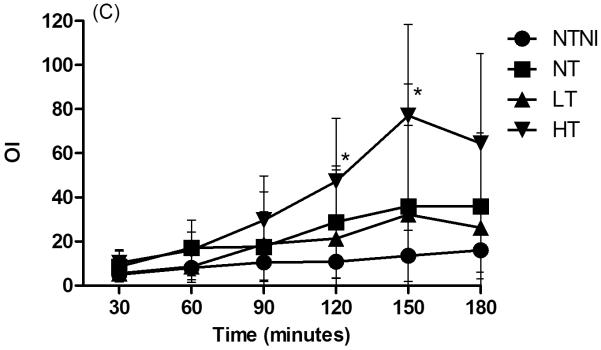
Physiologic Data for animals. (A): Core temperature by 30-minute intervals. NTNI and NT animals were maintained at physiologic normothermia for the lamb. High Temperature (HT) and Low Temperature (LT) were achieved by 15 min of age. For each time point, HT and LT groups had temperatures that were different from normothermia groups (p < 0.05). (B): Ventilation Efficiency Index (VEI), presented in mL·mmHg·min/kg. Decreases in VEI values correspond to poorer ventilation. (C): Oxygenation Index (OI). Increasing OI values denote poorer oxygenation. Graph points represent mean values. Error bars denote standard deviation from the mean. *p < 0.05 vs. NTNI.
Lung Physiology
Tidal volume in the NTNI control group increased to 7.9 mL/kg at 15 min (Table 1). The three lung injury groups (LT, NT, HT) received tidal volume escalation toward the target of 15 mL/kg by 15 min. As anticipated by experimental design, both tidal volumes and PIP were significantly higher with injurious ventilation compared to the NTNI group at 15 min. In the HT group, the mean tidal volume was less than 15 mL/kg because the maximum PIP limit of 50 cmH2O was reached in some lambs.
After 3 h of ventilation or prior to death, the hyperthermic lambs had higher pulse rates and significant acidosis while LT animals had lower pulse rates but maintained normal acid-base status (Table 2). Compared to the NTNI group, HT lambs had increased PaCO2 values and required higher PIP values to achieve similar tidal volumes, suggesting more lung injury. In contrast, LT animals had PaCO2 values and PIP requirements that were similar to the NTNI group.
Table 2.
Physiologic Data and Lung Injury and Inflammation at 3 h or prior to Death
| Group | UVC | NTNI | NT | LT | HT | |
|---|---|---|---|---|---|---|
| pH | --- | 7.27±0.05 | 7.20±0.14 | 7.22±0.1 | 6.98±0.22+ | |
| PaCO2 | (mmHg) | --- | 58±7 | 75±34 | 58±11 | 113±44+ |
| PIP | (cmH2O) | --- | 26±6.9 | 34±7.5 | 28±5.8 | 38±3.2+ |
| VT/kg | (mL/kg) | --- | 7.2±1.6 | 7.8±1.9 | 6.0±1.4 | 7.1±1.8 |
| Pulse Rate | (bpm) | --- | 167±15 | 174±29 | 118±21+ | 209±44+ |
| Lung Injury and Inflammatory Responses | ||||||
| Pneumothorax | N | --- | 0 | 2 | 0 | 6 |
| Death < 3 h | N | --- | 0 | 0 | 0 | 5 |
| PIE at autopsy | N | 0 | 1 | 5 | 2 | 7 |
| ALI score | (0-6) | 0.6±0.4 | 1.6±1.3 | 4.2±0.3*+ | 1.4±0.9 | 3.2±0.7* |
| Inflammatory Cells in BAL | ×105/kg | 1.9±4 | 147±130* | 247±157* | 115±46* | 146±60* |
| Protein in BAL | mg/kg | 25±12 | 60±31* | 107±24*+ | 94±21*+ | 86±20* |
UVC= unventilated control group, NTNI= normothermic positive control group, NT= normothermic injury group, LT= hypothermic injury group, HT= hyperthermic injury group. bpm= beats per minute, PIE= pulmonary interstitial emphysema, ALI= acute lung injury, BAL= bronchoalveolar lavage fluid. (N) represents the number of affected animals per group. Data presented as mean ± SD.
p < 0.05 vs. UVC
p < 0.05 vs. NTNI.
The VEI tended to decrease in all ventilated groups with time (Fig. 1B). HT lambs had the lowest VEI values followed by NT animals, whereas LT and NTNI animals had the highest VEI values. Compared to the NTNI group, both NT and HT animals had significantly lower VEI values at 5 of 6 time points. The OI increased in all ventilated groups with time, consistent with injury progression (Fig. 1C). Compared to NTNI animals, HT animals had significantly higher OI values at 120 and 150 minutes and demonstrated large increases in OI beyond 60 minutes of ventilation. The increase in VEI and decrease in OI values for the HT group at 3 h represents data from the 3 surviving lambs, which accounts for the lack of statistical significance at this time point.
Indicators of Lung Injury and Inflammation
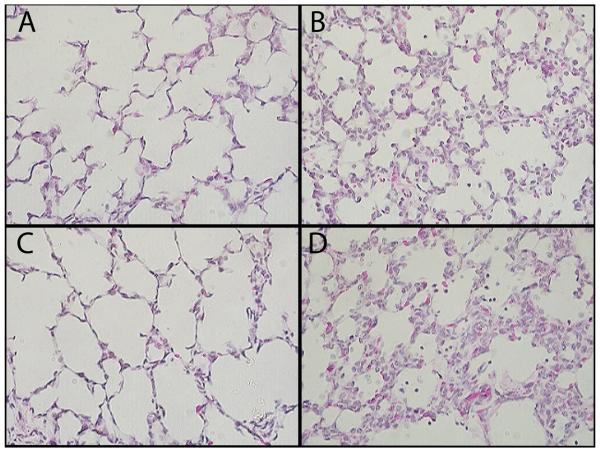
All lambs in the low and normal temperature groups survived for the 3 h ventilation, but 5/8 HT lambs died between 2 and 3 hours (Table 2). Clinically-diagnosed pneumothorax requiring chest tube placement developed in 6/8 HT animals and 2/7 NT animals. At autopsy, pleural blebs and/or pulmonary interstitial emphysema (PIE) was observed in 7/8 HT lambs, 5/7 NT lambs, 2/8 LT lambs and 1/8 NTNI lambs. ALI scoring on histology sections demonstrated prominent inflammatory cell infiltration and hemorrhage in both HT and NT groups. Representative histological sections from each ventilation group are shown in Figure 2. All ventilation groups had increased inflammatory cells in BAL fluid when compared to the unventilated control group (Table 2). Protein concentrations in BAL were increased in all ventilated groups relative to the UVC controls. When compared to the NTNI group, all injury ventilation groups had increased BAL protein.
Figure 2.
Representative Histologic Sections using hematolylin and eosin staining at 40x magnification. (A): NTNI lung tissue (B): NT lung tissue (C): LT lung tissue (D): HT lung tissue. NT and HT sections show increased congestion with inflammatory cells and erythrocytes visible within alveoli.
Cytokine and Acute Phase mRNA Expression
Representative pro-inflammatory cytokines were measured based on prior experiments demonstrating lung injury responses in preterm lambs: Egr-1, TLR-4, MCP-1, HSP-70, IL-1β and IL-6 (Table 3). Egr-1 was increased in LT and HT groups when compared to unventilated controls, but increased only in HT animals when compared to the NTNI group. Both TLR-4 and IL-6 were increased in HT animals when compared to both UVC and NTNI groups. MCP-1 was increased in all injury ventilation groups compared to the UVC group, and increased in both NT and HT groups when compared to the NTNI group. HSP-70 decreased with ventilation but increased when ventilation was combined with hyperthermia. IL-1β was increased in NT and HT groups only when compared to both UVC and NTNI animals. Compared to NTNI lambs, HT animals had significantly increased mRNA expression in all 6 tested markers. NT lambs trended to toward increased inflammatory marker expression over NTNI lambs, with statistical significance achieved for MCP-1 and IL-1β. LT animals had cytokine levels comparable to the NTNI group for all 6 measured markers. When compared by t-test, LT animals had only MCP-1 levels elevated over NTNI animals with lower TLR-4, HSP-70, IL-1β and IL-6 expression than HT animals (p < 0.05).
Table 3.
Lung Cytokine and Acute Phase mRNA Response
| Group | UVC | NTNI | NT | LT | HT | |
|---|---|---|---|---|---|---|
| Egr-1 | mRNA (fold increase) |
1.0±0.5 | 1.8±1.6 | 2.1±1.0 | 2.8±1.3* | 3.7±1.1*+ |
| TLR-4 | 1.0±0.5 | 1.0±0.5 | 1.3±0.4 | 1.2±0.4 | 2.4±0.5*+ | |
| MCP-1 | 1.0±0.3 | 16±6 | 39±19*+ | 28±12* | 45±21*+ | |
| HSP-70 | 1.0±0.2 | 0.6±0.3* | 0.5±0.1* | 0.3±0.03* | 1.7±0.4*+ | |
| IL-1β | 1.0±0.1 | 5±4 | 13±5*+ | 9±6 | 18±8*+ | |
| IL-6 | 1.0±0.2 | 7±8 | 17±8 | 9±5 | 40±33*+ | |
Lung cytokine and inflammatory marker mRNA expression in lung tissue. mRNA measurements were compared to unventilated control (UVC) values, which were normalized to a value of 1.0. Data is presented as fold increase/decrease relative to UVC ± SD.
p < 0.05 vs. UVC
p < 0.05 vs. NTNI.
Discussion
We successfully targeted body temperature and ventilated preterm lambs for a 3 h experimental period. The 3 h study duration was chosen based on prior studies demonstrating significant cytokine and inflammatory mediator mRNA responses, as well as changes in physiologic variables, in lambs of similar gestational age ventilated for this time period (4, 20, 22). Ventilation of preterm lambs led to lung inflammation, protein leak and pro-inflammatory cytokine mRNA induction, regardless of ventilation mode or temperature. The indicators of gross lung injury for ventilated animals were increased pneumothoraces, PIE and pulmonary hemorrhage. Clinically, pneumothoraces occurred in 75% of the HT lambs and 25% of the NT lambs but did not occur in either the LT or NTNI groups. Similarly, PIE at autopsy was most frequent and severe in the HT lambs, and was least often observed in LT and NTNI animals. HT lambs had qualitatively increased mRNA expression of all tested inflammatory markers when compared to NTNI animals. LT lambs, on the other hand, had inflammatory marker profiles similar to the NTNI group.
Observational studies demonstrate both hypo- and hyperthermia occur in premature infants as a result of perinatal factors and delivery room interventions. In one large clinical experience, nearly 11% of very low birth weight infants were hyperthermic (>37 °C) and 46.7% were hypothermic (<36 °C) upon arrival in the NICU (11). Our goal was to test clinically relevant temperature ranges. Hypothermia to 33-34 °C was chosen because it is the currently accepted target temperature for cooling infants following hypoxic-ischemic birth injury. The 40-41 °C hyperthermia target was chosen as it is just 2 °C above the normal body temperature of 38-39 °C for sheep. Historically, neonatal hypothermia was associated with increased mortality (28). However, these outcomes were observed in the setting of prolonged, variable and uncontrolled hypothermia. The controlled hypothermia with maintenance of acid-base status and hemodynamic stability is a very different scenario.
Hyperthermic lambs had severe lung injury with the highest mortality of all ventilated groups, impaired gas exchange and function and increased inflammatory cytokine expression. Similar physiologic deterioration is seen in hyperthermic rats ventilated with high pressures (18). Hyperthermic rats showed increased pro-inflammatory cytokine levels in BAL and plasma. These findings may result from multiple underlying mechanisms which in combination magnify the injury response. Burn researchers reported changes in macrophage cytokine release in response to hyperthermic injury (29). In ICU patients with fever, 38 genes involved in immunity and inflammation, including TLRs and HSPs, were altered, regardless of the infectious nature of hyperthermia (30). Secreted HSPs can signal through TLR-4, further amplifying inflammation (31, 32). Additionally, hyperthermia combined with lung injury may result in cell dysfunction and death (33).
The hypothermic lambs maintained a normal acid-base status despite decreased pulse rates. These lambs did not have pulmonary hemorrhage, hemodynamic instability or other adverse outcomes. The injury score on lung sections was similar to the no injury group. They required lower tidal volumes and inspiratory pressures to achieve adequate minute ventilation, suggesting lower CO2 production. Reduced ventilatory requirements with hypothermia may be one contributing mechanism that protects the lung against ventilator induced injury. Similar to our findings of lower BAL inflammatory cell counts in hypothermic animals compared to normothermic animals, Chu et al showed that hypothermia decreased pulmonary neutrophil-mediated inflammation, resulting in a decreased cell mediated inflammatory response (34). Hypothermia also decreased the pro-inflammatory cytokine response as demonstrated by attenuated IL-1β and IL-6 increases when compared to other injury groups. In support, Lim et al showed that hypothermia decreased cytokine production from stimulated alveolar macrophages (35).
The lung physiology measurements for the hypothermic animals more closely resembled the lung physiology profiles for NTNI animals, despite the fact that they received injurious ventilation. The VEI is an overall assessment of ventilation that includes the variables rate, pressure and PaCO2. A decreasing VEI indicates more respiratory failure. While both NT and HT animals had lower ventilation efficiency indices compared to NTNI lambs, the VEI of LT animals closely mirrored that for NTNI lambs. Some of the changes in hypothermic lambs may be attributed to the fact that cold animals are in a low-flow pulmonary hemodynamic state with increased shunting across a PDA (36). Lung inflammation and injury occurred in normothermic, gently ventilated lambs (NTNI). Similar degrees of injury were previously seen in lambs ventilated with a tidal volume of 8 mL/kg without surfactant, suggesting prophylactic surfactant does not eliminate the lung and airway injury from initiation of ventilation in the preterm (20). Perhaps, if we studied hypothermic preterm animals exposed only to gentle ventilation (a LTNI group), we might see lung injury profiles even more comparable to the NTNI group. While this was not seen in our previously published study of gently ventilated near-term lambs exposed to mild temperature variations (19), the preterm lambs in this study had significantly more lung injury and inflammation. The combination of moderate hypothermia and gentle ventilation may be more protective in the preterm. Limitations of this study are the small group sizes, which likely contributes to the lack of statistical significance observed for injury scoring and inflammatory marker expression, as well as the individual lamb variability in injury response. However, clearly diverging trends were evident in both HT and LT groups. We did not evaluate systemic and more prolonged effects of high and low body temperatures, particularly in the LT animals to assess safety of whole body controlled hypothermia in the preterm. However, whole body cooling following birth hypoxia-ischemia suggests controlled hypothermia is safe and preserves end-organ function in term infants (37). Such findings are promising and may translate to the preterm population.
Acknowledgments
This work was supported by grant NH12714 from the U.S. National Institute of Child Health, equipment and a grant from Fisher & Paykel Healthcare, Auckland, New Zealand, a Viertel Senior Medical Research Fellowship (JJP), and a NHFA/NHMRC Fellowship (GRP). Surfactant was provided by Chiesi Pharmaceuticals, Parma, Italy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
These sponsors had no involvement in the design, analysis or preparation of the study for publication.
References
- 1.Wada K, Jobe AH, Ikegami M. Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J Appl Physiol. 1997;83:1054–1061. doi: 10.1152/jappl.1997.83.4.1054. [DOI] [PubMed] [Google Scholar]
- 2.Bjorklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, Vilstrup CT. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res. 1997;42:348–355. doi: 10.1203/00006450-199709000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(147):e141–148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW, Jobe AH. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med. 2007;176:575–581. doi: 10.1164/rccm.200701-051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998;53:81–94. doi: 10.1016/s0378-3782(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 6.Clark RH, Gerstmann DR, Jobe AH, Moffitt ST, Slutsky AS, Yoder BA. Lung injury in neonates: Causes, strategies for prevention, and long-term consequences. J Pediatr. 2001;139:478–486. doi: 10.1067/mpd.2001.118201. [DOI] [PubMed] [Google Scholar]
- 7.Soll RF. Heat loss prevention in neonates. J Perinatol. 2008;28(Suppl 1):S57–59. doi: 10.1038/jp.2008.51. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain D. The international liaison committee on resuscitation (ilcor)-past and present: Compiled by the founding members of the international liaison committee on resuscitation. Resuscitation. 2005;67:157–161. doi: 10.1016/j.resuscitation.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Vohra S, Roberts RS, Zhang B, Janes M, Schmidt B. Heat loss prevention (help) in the delivery room: A randomized controlled trial of polyethylene occlusive skin wrapping in very preterm infants. J Pediatr. 2004;145:750–753. doi: 10.1016/j.jpeds.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Impey LW, Greenwood CE, Black RS, Yeh PS, Sheil O, Doyle P. The relationship between intrapartum maternal fever and neonatal acidosis as risk factors for neonatal encephalopathy. Am J Obstet Gynecol. 2008;198(49):e41–46. doi: 10.1016/j.ajog.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Laptook AR, Salhab W, Bhaskar B. Admission temperature of low birth weight infants: Predictors and associated morbidities. Pediatrics. 2007;119:e643–649. doi: 10.1542/peds.2006-0943. [DOI] [PubMed] [Google Scholar]
- 12.Schulzke SM, Rao S, Patole SK. A systematic review of cooling for neuroprotection in neonates with hypoxic ischemic encephalopathy - are we there yet? BMC Pediatr. 2007;7:30. doi: 10.1186/1471-2431-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 14.Huang PS, Tang GJ, Chen CH, Kou YR. Whole-body moderate hypothermia confers protection from wood smoke-induced acute lung injury in rats: The therapeutic window. Crit Care Med. 2006;34:1160–1167. doi: 10.1097/01.CCM.0000207342.50559.0F. [DOI] [PubMed] [Google Scholar]
- 15.Hong SB, Koh Y, Lee IC, Kim MJ, Kim WS, Kim DS, Kim WD, Lim CM. Induced hypothermia as a new approach to lung rest for the acutely injured lung. Crit Care Med. 2005;33:2049–2055. doi: 10.1097/01.ccm.0000178186.37167.53. [DOI] [PubMed] [Google Scholar]
- 16.Chin JY, Koh Y, Kim MJ, Kim HS, Kim WS, Kim DS, Kim WD, Lim CM. The effects of hypothermia on endotoxin-primed lung. Anesth Analg. 2007;104:1171–1178. doi: 10.1213/01.ane.0000260316.95836.1c. tables of contents. [DOI] [PubMed] [Google Scholar]
- 17.Hasday JD, Garrison A, Singh IS, Standiford T, Ellis GS, Rao S, He JR, Rice P, Frank M, Goldblum SE, et al. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am J Pathol. 2003;162:2005–2017. doi: 10.1016/S0002-9440(10)64333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita Y, Oda S, Sadahiro T, Nakamura M, Oshima T, Otani S, Hirasawa H. The effects of body temperature control on cytokine production in a rat model of ventilator-induced lung injury. Cytokine. 2009;47:48–55. doi: 10.1016/j.cyto.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Ball MK, Jobe AH, Polglase GR, Kallapur SG, Cheah FC, Hillman NH, Pillow JJ. High and low body temperature during the initiation of ventilation for near-term lambs. Resuscitation. 2009;80:133–137. doi: 10.1016/j.resuscitation.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Polglase GR, Hillman NH, Pillow JJ, Cheah FC, Nitsos I, Moss TJ, Kramer BW, Ikegami M, Kallapur SG, Jobe AH. Positive end-expiratory pressure and tidal volume during initial ventilation of preterm lambs. Pediatr Res. 2008;64:517–522. doi: 10.1203/PDR.0b013e3181841363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notter RH, Egan EA, Kwong MS, Holm BA, Shapiro DL. Lung surfactant replacement in premature lambs with extracted lipids from bovine lung lavage: Effects of dose, dispersion technique, and gestational age. Pediatr Res. 1985;19:569–577. doi: 10.1203/00006450-198506000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Polglase GR, Hillman NH, Ball MK, Kramer BW, Kallapur SG, Jobe AH, Pillow JJ. Lung and systemic inflammation in preterm lambs on continuous positive airway pressure or conventional ventilation. Pediatr Res. 2009;65:67–71. doi: 10.1203/PDR.0b013e318189487e. [DOI] [PubMed] [Google Scholar]
- 23.Kramer BW, Kramer S, Ikegami M, Jobe AH. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2002;283:L452–459. doi: 10.1152/ajplung.00407.2001. [DOI] [PubMed] [Google Scholar]
- 24.Teke Z, Sacar M, Yenisey C, Atalay AO, Bicakci T, Erdem E. Activated protein c attenuates intestinal reperfusion-induced acute lung injury: An experimental study in a rat model. Am J Surg. 2008;195:861–873. doi: 10.1016/j.amjsurg.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Nishina K, Mikawa K, Takao Y, Maekawa N, Shiga M, Obara H. Ono-5046, an elastase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth Analg. 1997;84:1097–1103. doi: 10.1097/00000539-199705000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Kallapur SG, Kramer BW, Moss TJ, Newnham JP, Jobe AH, Ikegami M, Bachurski CJ. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L633–642. doi: 10.1152/ajplung.00344.2002. [DOI] [PubMed] [Google Scholar]
- 27.Hillman NH, Moss TJ, Nitsos I, Kramer BW, Bachurski CJ, Ikegami M, Jobe AH, Kallapur SG. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr Res. 2008;63:388–393. doi: 10.1203/PDR.0b013e3181647b3a. [DOI] [PubMed] [Google Scholar]
- 28.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The epicure study: Outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106:659–671. doi: 10.1542/peds.106.4.659. [DOI] [PubMed] [Google Scholar]
- 29.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 30.Sonna LA, Hawkins L, Lissauer ME, Maldeis P, Towns M, Johnson SB, Moore R, Singh IS, Cowan MJ, Hasday JD. Core temperature correlates with expression of selected stress and immunomodulatory genes in febrile patients with sepsis and noninfectious sirs. Cell Stress Chaperones. 2009 doi: 10.1007/s12192-009-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. Hsp70 as endogenous stimulus of the toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 32.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular hsp70: Role of toll-like receptor (tlr) 2 and tlr4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 33.Orgill DP, Porter SA, Taylor HO. Heat injury to cells in perfused systems. Ann N Y Acad Sci. 2005;1066:106–118. doi: 10.1196/annals.1363.026. [DOI] [PubMed] [Google Scholar]
- 34.Chu SJ, Perng WC, Hung CM, Chang DM, Lin SH, Huang KL. Effects of various body temperatures after lipopolysaccharide-induced lung injury in rats. Chest. 2005;128:327–336. doi: 10.1378/chest.128.1.327. [DOI] [PubMed] [Google Scholar]
- 35.Lim CM, Kim EK, Koh Y, Kim WS, Kim DS, Kim WD. Hypothermia inhibits cytokine release of alveolar macrophage and activation of nuclear factor kappab in endotoxemic lung. Intensive Care Med. 2004;30:1638–1644. doi: 10.1007/s00134-004-2336-z. [DOI] [PubMed] [Google Scholar]
- 36.Black PR, van Devanter S, Cohn LH. Effects of hypothermia on systemic and organ system metabolism and function. J Surg Res. 1976;20:49–63. doi: 10.1016/0022-4804(76)90083-4. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar S, Barks JD, Bhagat I, Donn SM. Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: Whole-body cooling versus selective head cooling. J Perinatol. 2009;29:558–563. doi: 10.1038/jp.2009.37. [DOI] [PubMed] [Google Scholar]