Abstract
One widely accepted metabolic activation pathway of the prototypic carcinogenic polycyclic aromatic hydrocarbon (PAH) benzo[a]pyrene (BaP) proceeds through the “bay region diol epoxide” BaP-(7R,8S)-diol-(9S,10R)-epoxide (2). However, few studies have addressed the analysis of human urinary metabolites of BaP which result from this pathway. Phenanthrene (Phe) is structurally related to BaP, but human exposure to Phe is far greater and its metabolites can be readily detected in urine. Thus, Phe metabolites have been proposed as biomarkers of PAH exposure and metabolic activation. Phe-tetraols in particular could be biomarkers of the diol epoxide pathway. While BaP-tetraols and Phe-tetraols have been previously quantified in human urine, no published studies have determined their enantiomeric composition. This is important because different enantiomers would result from the bay region diol epoxide and “reverse” diol epoxide pathways, the latter being associated with weak mutagenicity and carcinogenicity. We addressed this problem using chiral HPLC to separate the enantiomers of BaP-7,8,9,10-tetraol and Phe-1,2,3,4-tetraol. Urine samples from smokers were subjected to solid-phase extraction, chiral HPLC, and GC-NICI-MS/MS analysis for silylated Phe-1,2,2,4-tetraols. The results demonstrated that >96% of Phe-1,2,3,4-tetraol in smokers’ urine was Phe-(1S,2R,3S,4R)-tetraol (12), resulting from the “reverse” diol epoxide pathway, whereas less than 4% resulted from the “bay region diol epoxide” pathway of Phe metabolism. Urine from creosote workers was similarly analyzed for BaP-7,8,9,10-tetraol enantiomers. In contrast to the results of the Phe-tetraol analyses, 78% of BaP-7,8,9,10-tetraol in these human urine samples was BaP-(7R,8S,9R,10S)-tetraol (3) resulting from the “bay region diol epoxide” pathway of BaP metabolism. These results provide further support for the bay region diol epoxide pathway of BaP metabolism in humans and demonstrate differences in BaP and Phe metabolism which may be important when considering Phe-tetraols as biomarkers of PAH metabolic activation.
Keywords: phenanthrene metabolites, benzo[a]pyrene metabolites, PAH tetraols, human urine
Introduction
Polycyclic aromatic hydrocarbons (PAH), which are formed in the incomplete combustion of organic matter, are commonly found in polluted air and water, tobacco products and their smoke, broiled foods, and occupational environments involving coke production from coal, or other processes which generate soots and tars (1–5). Many PAH are potent carcinogens and are implicated as causes of cancers of the skin and lung in occupationally exposed individuals (3,6–9). PAH are also believed to be among the major causative agents for lung cancer in smokers (10). The most thoroughly studied of all PAH, and often considered a prototype, is benzo[a]pyrene (BaP, Scheme 1), rated as a human carcinogen by the International Agency for Research on Cancer (6). A number of PAH, including BaP, are “reasonably anticipated to be human carcinogens” according to the U.S. Department of Health and Human Services (3).
Scheme 1.
Tetraol formation from BaP and Phe bay region diol epoxides (2 and 8) and reverse diol epoxides (5 and 11).
PAH require metabolic activation to exert their carcinogenic effects (11). One widely accepted metabolic activation pathway for BaP proceeds by initial cytochrome P450-catalyzed oxidation of its 7,8 bond followed by epoxide hydrolase-catalyzed hydration yielding the proximate carcinogen BaP-(7R,8R)-diol (1, Scheme 1) (12–16). Diol 1, which is more carcinogenic than BaP on mouse skin, undergoes cytochrome P450-catalyzed oxidation to give BaP-(7R,8S)-diol-(9S,10R)-epoxide (2), also known in the literature as BPDE. Diol epoxide 2 is highly carcinogenic in newborn mice and reacts easily with DNA producing adducts which have been extensively characterized in vitro and in laboratory animals treated with BaP (17,18). Diol epoxide 2 is considered to be one major ultimate carcinogen of BaP. Reaction of diol epoxide 2 with H2O produces predominantly BaP-(7R,8S,9R,10S)-tetraol (3). An analogous pathway of BaP metabolism gives BaP-(9R,10R)-diol (4), which could be further metabolized to diol epoxide 5 and tetraol 6. While diol 4 is an established metabolite of BaP in vitro, much less is known about its conversion to diol epoxide 5 and tetraol 6 (13).
Many studies demonstrate large inter-individual differences in the metabolism of PAH, leading to the logical hypothesis that those people who metabolically activate PAH more extensively should be at higher risk for cancer (19–27). A vast literature has investigated this hypothesis, but its validity still remains relatively unsettled (28–35). Our approach to this question has been to use phenanthrene (Phe, Scheme 1) as a model compound (36). Phe is the simplest PAH with a bay region, a feature often associated with carcinogenicity, although Phe is not generally considered carcinogenic. The metabolism of Phe to diol epoxides follows pathways that are quite analogous to those of BaP, as shown in Scheme 1 (13,37). We have proposed that Phe-tetraol (9 and/or 12, Scheme 1) is a practical biomarker of PAH metabolic activation by the diol epoxide pathway, and that its levels may be used to evaluate lung cancer susceptibility in smokers (36). Phe-tetraol is readily measured in human urine by GC-MS/MS and is present in quantities 10,000 times greater than those of BaP-tetraols. All humans have Phe-tetraol in their urine, and levels are higher in smokers than in non-smokers (38,39). Thus, Phe-tetraol could be a biomarker of PAH exposure plus metabolic activation.
In previous studies in which we quantified Phe-tetraol, no attempt was made to distinguish its enantiomers 9 and 12. Studies of Phe metabolism in vitro have shown that the formation of diols 7 and 10 is stereoselective, producing mainly the (R,R)-enantiomers shown in Scheme 1 (37,40). Similar stereoselectivity has been observed in the metabolism of BaP to diols (Scheme 1) (13). Metabolic epoxidation of Phe diols 7 and 10 followed by hydrolysis gives mainly the tetraols arising from trans- ring opening of the anti-diol epoxides, as shown in Scheme 1, based on analysis of Phe-tetraol in human urine (36). Only minor amounts of tetraols resulting from the syn diol epoxides or from cis- ring opening of the anti-diol epoxides were observed. Thus, it becomes evident, as shown in Scheme 1, that the amounts of Phe-tetraol 9 in human urine would reflect the “bay region diol epoxide” pathway of Phe metabolism, proceeding through diol 7 and diol epoxide 8, whereas the amounts of Phe-tetraol 12 would reflect the “reverse bay region diol epoxide” pathway proceeding through 10 and 11. In a recent study carried out with human hepatocytes, we obtained data indicating that the latter pathway may predominate (41). This could impact the interpretation of our biomarker data. Therefore, in this study, we investigated levels of the tetraol enantiomers 9 and 12 in human urine.
As this study progressed, we realized that there had been no similar investigations of BaP-tetraol enantiomers 3 and 6 in human urine. Other than racemic tetraols (42,43), there are to our knowledge no reports in the literature on human urinary BaP metabolites relevant to the widely accepted bay region diol epoxide pathway. The only in vivo evidence for the existence of this pathway of BaP metabolic activation in humans is based on analyses of diol epoxide adducts to globin, albumin, and DNA, and some of these have produced mixed results with regard to detectability (44–46). Therefore, we extended our investigation to determine the levels of BaP-tetraol enantiomers in the urine of creosote workers who were exposed to relatively high amounts of PAH.
Materials and Methods
Chemicals, Enzymes, and Chromatography Supplies
Racemic BaP-7,8,9,10-tetraol (3 + 6) and BaP-(7R,8R)-diol (1) were obtained from the National Cancer Institute Chemical Carcinogen Reference Standard Repository. Racemic Phe-1,2,3,4-tetraol (9 + 12) was kindly supplied by Drs. D. Jerina and H. Yagi, National Institutes of Health, Bethesda, MD. Phe-(1R,2R)-diol (7) and Phe-(3R,4R)-diol (10) were prepared by incubation of Phe with cytochrome P450 1A1 and co-factors, followed by HPLC purification (47). Phe-(1R,2S)-diol-(3S,4R)-epoxide (8) was prepared by oxidation of 7 with m-chloroperbenzoic acid(47). Purities of these standards were >95%, as determined by HPLC analysis. bis-Trimethylsilyltrifluoroacetamide (BSTFA) was purchased from Regis Technologies. β-Glucuronidase and arylsulfatase (from Helix pomatia) were obtained from Roche Diagnostics Corp., Indianapolis, IN. Strata-X polymeric SPE cartridges (200 mg/6 mL, #8B-S10-FCH) were obtained from Phenomenex. Bond Elute phenylboronic acid SPE cartridges (100 mg/1 mL, # 12102018) were from Varian and Oasis MAX SPE cartridges (60 mg/1 mL/# 186000378) were from Waters. An Astec Cyclobond II (250 × 4.6 mm, 5μm) chiral HPLC column was obtained from Sigma-Aldrich and a (R,R) Whelk-O 5/100 Kromasil #780201 (250 × 4.6 mm, 5μm) Pirkle chiral HPLC column was procured from Regis.
Urine Samples
Urine samples from smokers were obtained from ongoing studies at the University of Minnesota Tobacco Use Research Center. Urine samples from creosote workers were kindly provided by Mary Wolff, Mt. Sinai Medical Center, New York. Analysis of these samples for 1-hydroxypyrene showed levels which were several hundred times higher than in smokers or non-smokers.
Human Hepatocyte Incubations
These have been previously described (41). Incubation mixtures of racemic 7 or 10 from that study were analyzed for enantiomeric tetraols 9 and 12. Using the same conditions as those described (41), Phe-(1R,2R)-diol (7) or Phe-(3R,4R)-diol (10) were also incubated with hepatocytes, and the resulting mixtures analyzed for tetraols 9 and 12.
Analysis of Phe-tetraol Enantiomers 9 and 12 in Smokers’ Urine
This was carried out using a modification of a previously described method (41). A 0.5 mL aliquot of urine was added to a 1.5 mL polypropylene tube containing 0.5 mL H2O, 0.15 mL of 2.5 M NaOAc buffer, pH 5, β-glucuronidase (3,500 units) and arylsulfatase (28,000 units), and the mixture was incubated overnight with shaking at 37 °C. After incubation, the sample was partially purified by loading on a Strata-X polymeric sorbent cartridge that had been activated with 5 mL of CH3OH and 5 mL of H2O. The cartridge was washed twice with 1 mL of H2O, then 5 mL of 1% NH4OH in 10% CH3OH, and the analyte was eluted with 5 mL of 50% CH3OH, collected in an 8 mL silanized vial, and the solvents removed on a Speedvac. The residue was dissolved in 500 μL of H2O with sonication and loaded on a phenylboronic acid cartridge that had been activated with 1 mL of CH3OH and 1 mL of H2O. The cartridge was washed with 100 μL of H2O and placed under vacuum overnight. It was then washed twice with 1 mL of acetone (that had been dried with Na2SO4) and Phe-tetraols were eluted with 1 mL of 80% CH3OH in H2O. This fraction was collected in a 2 mL silanized vial and the solvent removed on a Speedvac. The residue was dissolved in 100 μL of CH3OH with sonication, transferred to an insert vial, and concentrated to dryness. The residue was dissolved using sonication in 50 μL of 10% isopropanol in H2O containing 500 ng of 2,7-dihydroxynaphthalene as a UV marker, 500 pg [D10]Phe-tetraol 9 [prepared from metabolically formed [D10]Phe-1,2-diol (7) and containing small amounts of [D10]Phe-tetraol 12] as enantiomer markers, and injected onto the Cyclobond II chiral HPLC column. The column was held at 23 °C, eluted with 0.6 mL/min H2O, and monitored by UV detection at 230.9 nm. One quarter of a min to two min fractions were collected in silanized vials over the entire 10 to 24 min portion of the chromatogram. Each vial contained 1 pmol of cis,anti-Phe-tetraol as a derivatization monitor. The UV marker eluted at 18 min and the Phe-tetraol enantiomers at 16 and 19 min (Figure 1). Fractions were dried on a Speedvac, and the residue was dissolved in 100 μL of CH3OH with sonication, transferred to an insert vial, concentrated to dryness, and dissolved in 10 μL of BSTFA and 10 μL of acetonitrile. The purpose of the acetonitrile was to break the bonding between residual Cyclobond II stationary phase bleed and Phe-tetraol analyte so that TMS-derivatization by BSTFA could proceed. The samples were heated to 60 °C for 60 min with periodic mixing and 2 μL was analyzed by GC-NICI-MS/MS.
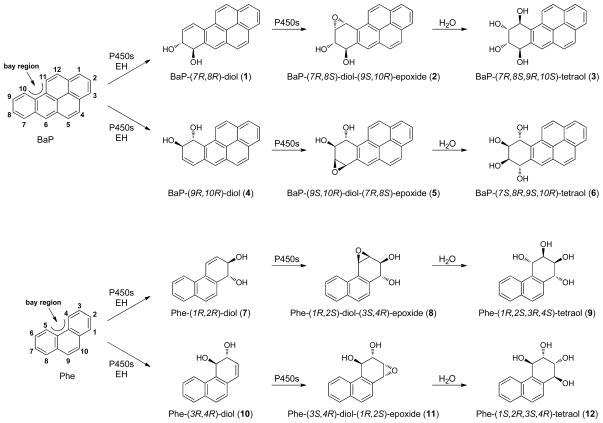
Figure 1.
Separation of standard Phe-tetraols 9 and 12 on a Cyclobond II HPLC column. 2,7-Dihydroxynaphthalene was added as a UV marker. For the analysis of urine, the UV peaks corresponding to 9 and 12 cannot be seen; fractions encompassing the correct retention times for Phe-tetraols 9 and 12 were collected using 2,7-dihydroxynaphthalene as a marker (see Materials and Methods for details).
Analysis of BaP-tetraol Enantiomers in Creosote Workers’ Urine
This was performed using a modification of the method described above for Phe-tetraol enantiomers. A 1.0 mL aliquot of urine was added to a 1.5 mL polypropylene tube containing 0.15 mL of 2.5 M NaOAc buffer, pH 5, β-glucuronidase (3,500 units) and arylsulfatase (28,000 units), and the mixture was incubated overnight with shaking at 37 °C. After incubation, the sample was partially purified by loading onto a Strata-X cartridge as described for the Phe-tetraols. The cartridge was washed twice with 1 mL of H2O, 5 mL of 1% NH4OH in 50% CH3OH, then 0.5 ml CH3OH, and the analyte was eluted using 2 mL of CH3OH. The analyte fraction was collected in a 4 mL silanized vial, and the solvents were removed on a Speedvac. The sample was dissolved in 750 μL of 30% CH3OH in H2O with sonication and loaded onto a phenylboronic acid cartridge as described for Phe-tetraols. The cartridge was washed with 100 μL of 30% CH3OH in H2O and placed under vacuum overnight. The BaP-tetraols were recovered from the cartridge and transferred to an insert vial as described for Phe-tetraols. The residue was dissolved with the aid of sonication in 50 μL of isopropanol containing 500 ng of 2,7-dihydroxynaphthalene as UV marker, and injected onto the Pirkle chiral HPLC column. The column was eluted with 0.5 mL/min isopropanol and the eluant was monitored by UV at 230.9 nm. The two enantiomers were collected in 4 mL silanized vials, each vial containing 1 pmol of cis,anti-Phe-tetraol as derivatization standard. The UV marker eluted at 7 min and the two BaP-tetraol enantiomers at 10 and 21 min. Fractions were collected over the entire chromatogram encompassing the two enantiomers. Fractions were dried on a Speedvac, and the residue was dissolved in 100 μL of CH3OH with sonication, transferred to an insert vial, concentrated to dryness, and dissolved in 10 μL of BSTFA. The samples were heated to 60 °C for 60 min with periodic mixing and 2 μL was analyzed by GC-NICI-MS/MS.
Analysis of Phe-diol Enantiomers in Smokers’ Urine
The method was similar to that used for the analysis of tetraols. Urine samples were worked up as described above for tetraols, and after the tetraols were eluted from the Strata-X cartridge using 5 mL of 50% CH3OH, the cartridge was washed with 3 mL of CH3OH to elute Phe-1,2-diol and Phe-3,4-diol. The solvents were removed on a Speedvac, and the sample was dissolved in 1 mL of H2O with sonication and loaded onto a Oasis MAX cartridge that had been activated using 1 mL of CH3OH and 1 mL of 3% NH4OH in H2O. The cartridge was washed with 1 mL of 3% NH4OH in H2O, 1 mL of H2O, and the Phe-diols were eluted with 1 mL of CH3OH into a 2 mL silanized vial and the solvent removed on the Speedvac. The residue was dissolved in 100 μL of CH3OH with sonication, transferred to an insert vial, and concentrated to dryness. For Phe-1,2-diols, the residue was dissolved using sonication in 50 μL of 10% isopropanol in hexane containing 500 ng of 2,7-dihydroxynaphthalene as UV marker, 100 pmol [13C6]Phe(1R,2R)-diol as enantiomer marker, and injected onto the Pirkle chiral HPLC column. The column was eluted with 1 mL/min of 10% isopropanol in hexane and the eluant was monitored by UV at 230.9 nm. Fractions were collected from 8 to 14 min in 2 mL silanized vials, each vial containing 236 pmol of Phe-9,10-diol as derivatization monitor. The UV marker eluted at 15 min and the two Phe-1,2-diol enantiomers at 10 and 11 min. The Phe-3,4-diols were analyzed the same way except the residue was dissolved in 50% isopropanol in hexane, 94 pmol [13C6]Phe(3R,4R)-diol was used as enantiomer marker, the column was eluted with 50% isopropanol in hexane, fractions were collected from 6 to 11 min, each vial contained 79 pmol of Phe-1,2-diol as derivatization standard, the UV marker eluted at 4 min and the two Phe-3,4-diol enantiomers eluted at 8 and 9 min. Fractions were dried on a Speedvac, and the residue was dissolved in 100 μL of methanol with sonication, transferred to an insert vial, concentrated to dryness, dissolved in 10 μL of BSTFA, heated to 60 °C for 60 min with periodic mixing, and 2 μL was analyzed by GC-NICI-MS/MS.
GC-NICI-MS/MS Analysis
This was carried out with a TSQ Quantum instrument (Thermo Electron). All analytes were detected as their trimethylsilyl derivatives. For detection of tetraols, a 0.25 mm I.D. × 0.15 μm film thickness × 30 m DB17-MS column (Agilent) with a 0.53 mm I.D. × 3 m deactivated fused silica pre-column was used, and for detection of diols, a 0.18 mm I.D. × 20 m, 0.18-μm film thickness, DB-5 MS UI column (Agilent) was used. The oven temperature program was 80 °C for 1 min (except for Phe-3,4-diol, 3 min was used), then 80 to 200 °C at 35 °C/min, then 200 to 215 °C at 3 °C/min, then 215 to 320 °C at 35 °C/min, then hold for 3 min. The carrier gas was He at a flow rate of 1.0 mL/min, and the injection port (except for analysis of Phe-3,4-diol) and MS transfer line were kept at 250°C and 320°C, respectively. For the analysis of Phe-3,4-diol, a programmed temperature vaporization injector with baffled inlet liner was used. The injector was operated in the splitless mode, the evaporation temperature was 80 °C for 2 min, then 80 to 260 °C at 5 °C/sec, then hold for 1 min. The NICI-MS conditions were as follows: CI gas, methane at 2.0 mL/min except for Phe-3,4-diol which required argon at 3.3 mL/min; source temperature, 200 °C; emission current, 100 μA for diols and 500 μA for tetraols. Selected ion monitoring (SIM) with electron energy of −150 eV for Phe-1,2-diol and −100 eV for Phe-3,4-diol was used to detect the diols at m/z 193. Selected reaction monitoring (SRM) with a collision energy of 12 eV, electron energy of −100 eV for Phe-tetraols and −150 eV for BaP-tetraols, and Ar collision gas at 1.0 mTorr was used to detect Phe-tetraols, [D10]Phe-tetraols, and BaP-tetraols at m/z 372 → 210, m/z 382 → 220, and m/z 446 → 255, respectively.
Results
The first step was to separate and characterize Phe-tetraol enantiomers 9 and 12. Racemic trans,anti-Phe-1,2,3,4-tetraol was separated into its component enantiomers on a Cyclobond II chiral HPLC column. This is a -cyclodextrin bonded to silica, in which glucopyranose units form a truncated cone, and enantiomers are separated by forming inclusion complexes. Baseline separation was achieved as illustrated in Figure 1. The large peak eluting between the two enantiomers is 2,7-dihydroxynaphthalene, which was added as a UV marker to facilitate collection of the appropriate fractions containing each tetraol enantiomer.
The next step was to assign structures, e.g. 9 or 12, to the separated tetraols. This was accomplished by isolating Phe-(1R,2R)-diol (7) and Phe-(3R,4R)-diol (10) from incubation mixtures of human P450 1A1, Phe, and co-factors, conditions which are known to produce these diols stereoselectively (37,40,48). Oxidation of 7 with m-chloroperbenzoic acid selectively produces anti-diol epoxide 8 (48), which upon hydrolysis yields tetraol 9, from trans- ring opening, as the major product (36). Similarly, epoxidation and hydrolysis of diol 10 gives tetraol 12. Analysis of each of these tetraols on the Cyclobond II column gave essentially one peak, each of which co-eluted with one of the two peaks shown in Figure 1. Thus, we were able to assign the first eluting tetraol peak in Figure 1 as tetraol 9 and the second eluting peak as tetraol 12. These tetraols were also separable on a Pirkle HPLC column, and had the same order of elution.
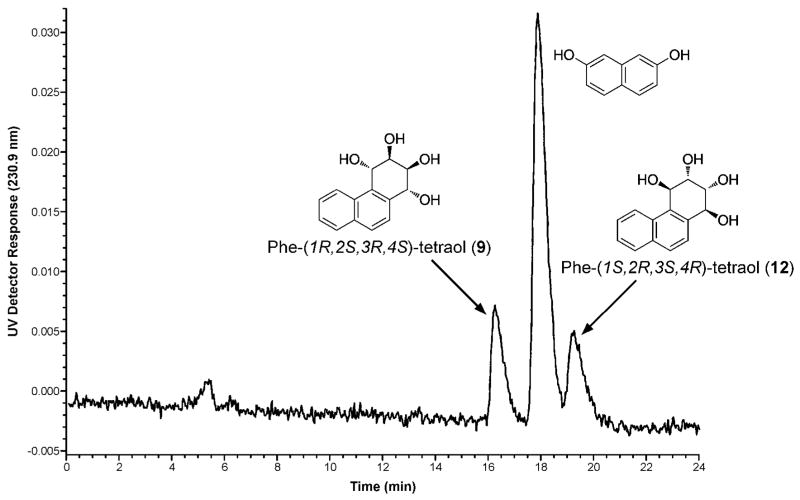
Fifteen urine samples from smokers were then analyzed for tetraols 9 and 12. The first step in the analysis was treatment with β-glucuronidase and aryl sulfatase, to produce the unconjugated tetraols. The urine was then partially purified by sequential solid-phase extractions using Strata-X reverse phase and phenyl boronic acid cartridges. The phenyl boronic acid cartridge binds tetraols due to their adjacent hydroxyl groups. The appropriate fraction from the phenyl boronic acid cartridge was injected on the Cyclobond II HPLC column and fractions containing each enantiomer were collected and analyzed for tetraols 9 and 12 by GC-MS/MS. Typical chromatograms are illustrated in Figure 2A–D. Clear peaks were observed for the enantiomeric tetraols, the corresponding internal standard, and for cis,anti-Phe-tetraol (resulting from cis- ring opening of anti-Phe-1,2-diol-3,4-epoxide), which was used as an injection standard to monitor silylation efficiency.
Figure 2.
Chromatograms obtained upon GC-MS/MS analysis of tetra-trimethylsilyl derivatives of (A) Phe-tetraol 9; (B) internal standard [D10]Phe-tetraol 9; (C) Phe-tetraol 12; and (D) internal standard [D10]Phe-tetraol 12, which were isolated from the urine of smokers by solid phase extraction and chiral HPLC as described in the text. A second internal standard, cis,anti-Phetetraol, was added to each sample to monitor silylation efficiency. (Internal standards [D10]Phe-tetraol 9 and [D10]Phe-tetraol 12 were prepared from metabolically formed [D10]Phe-1,2-diol, which was mostly the (R,R)-enantiomer. Therefore, [D10]Phe-tetraol is predominantly 9 with smaller amounts of 12, as seen in panels B and D).
The results are summarized in Table 1. In each case, 93% or more of Phe-1,2,3,4-tetraol was the second eluting peak from the Cyclobond II column, e.g. tetraol 12, and 7% or less was tetraol 9. These results indicated that most of Phe-1,2,3,4- tetraol in smokers’ urine results from hydrolysis of the reverse diol epoxide 11, while less than 7% originates from the bay region diol epoxide 8 (Scheme 1). However, this conclusion would only be valid if the formation of Phe-diols 7 and 10 in humans were stereoselective, as shown in Scheme 1 and indicated in previous in vitro studies (37,40). Since there were no data in the literature on the stereochemistry of Phe-diols 7 and 10 in human urine, we addressed this topic.
Table 1.
Enantiomeric composition of Phe-tetraols in the urine of 15 smokers
| Urine sample | Percent composition |
|
|---|---|---|
| Phe-(1R,2S,3R,4S)-tetraol (9) | Phe-(1S,2R,3S,4R)-tetraol (12) | |
| 1 | 4.0 | 96.0 |
| 2 | 7.0 | 93.0 |
| 3 | 3.8 | 96.2 |
| 4 | 2.0 | 98.0 |
| 5 | 4.2 | 95.8 |
| 6 | 0.2 | 99.8 |
| 7 | 4.0 | 96.0 |
| 8 | 2.5 | 97.5 |
| 9 | 1.8 | 98.2 |
| 10 | 4.8 | 95.2 |
| 11 | 5.8 | 94.2 |
| 12 | 5.7 | 94.3 |
| 13 | 2.9 | 97.1 |
| 14 | 0.5 | 99.5 |
| 15 | 2.0 | 96.6 |
| Mean | 3.4 ± 2.0 | 96.5 ± 1.9 |
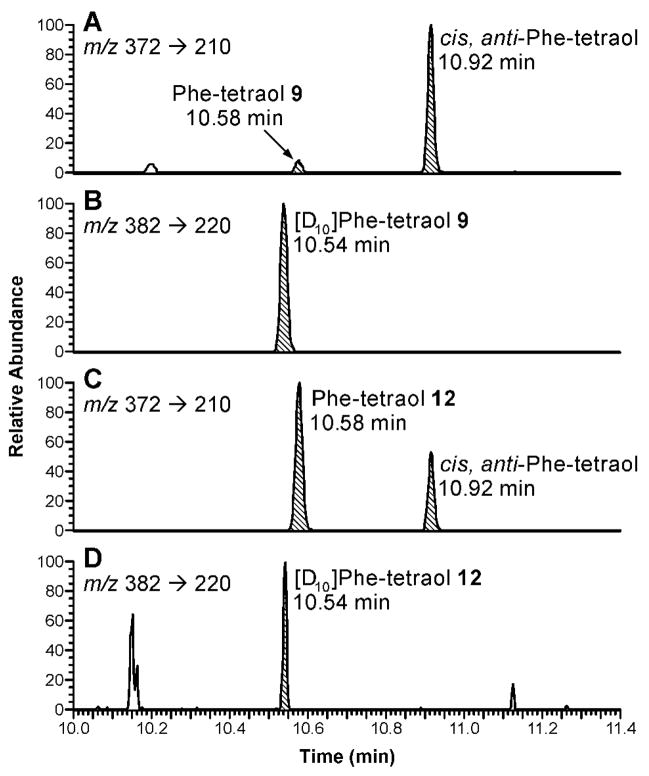
Enantiomers of Phe-1,2-diol and Phe-3,4-diol were separated on a Pirkle column, as shown in Figure 3A,B. In each case, the (R,R)-diol, 7 and 10 (Scheme 1), eluted first, as established by analyzing the diols formed by in vitro metabolism of Phe with human P4501A1 and co-factors. For analysis of the Phe diols in human urine, the samples were treated with β-glucuronidase and aryl sulfatase, then partially purified by solid-phase extraction, followed by injection of the appropriate fraction on the Pirkle column. The fractions containing the diols were collected and analyzed by GC-MS/MS. The results of this study, carried out on urine samples from 4 smokers, showed that greater than 96% of both Phe-1,2-diol and Phe-3,4-diol were the (R,R)-enantiomers, eg. compounds 7 and 10 of Scheme 1.
Figure 3.
Separation of (A) enantiomers of Phe-1,2-diol and (B) enantiomers of Phe-3,4-diol on a Pirkle HPLC column. For the analysis of urine, the UV peaks corresponding to the diols cannot be seen; they were collected based on retention times.
Although these results demonstrated that the Phe-diols were formed stereoselectively in smokers, it was still possible that metabolism of these diols to quinones followed by reduction could lead to biological racemization, possibly confounding the results. We investigated this possibility by analyzing the enantiomeric tetraols formed in incubations of each enantiomeric or racemic diol with human hepatocytes. The results showed that tetraol 9 was formed from diol 7, and tetraol 12 from diol 10. Furthermore, the enantiomeric tetraols 9 and 12 were also formed selectively from the racemic diols (data not shown).
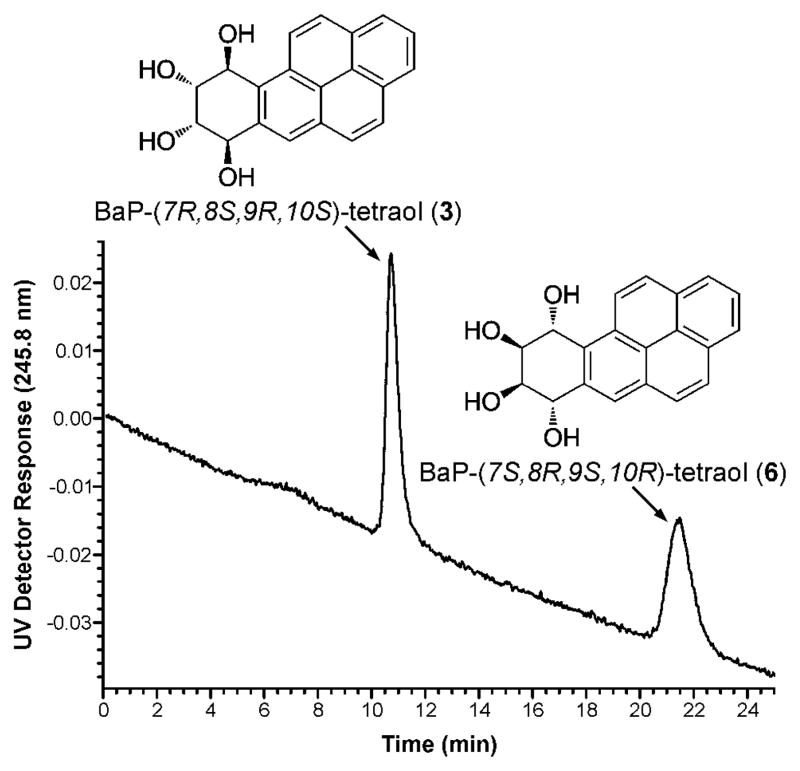
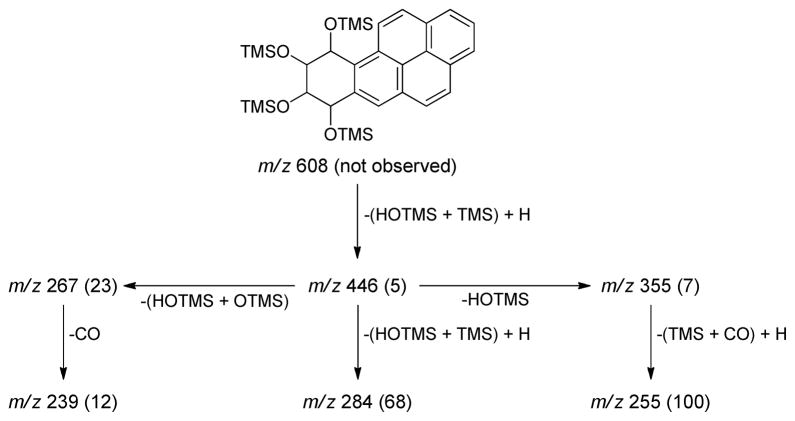
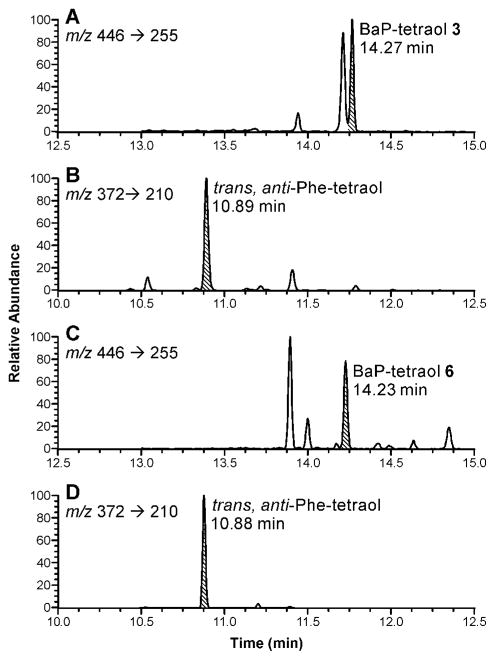
In the next phase of this study, we extended this research to BaP. We analyzed urine samples from creosote workers who were exposed to high levels of PAH, thus facilitating the analysis of BaP-tetraols in urine. BaP-tetraols 3 and 6 were separated on the Pirkle column as illustrated in Figure 4. Based on comparison of their CD spectra to published data (49), BaP-tetraol 3 eluted first and BaP-tetraol 6 eluted second. This was confirmed by preparing tetraol 3 from BaP-(7R,8R)-diol (1), by m-chloroperbenzoic acid oxidation to 2 followed by hydrolysis. Urine samples from four creosote workers were analyzed using a method similar to that employed for the Phe-tetraols. After partial purification and collection from the Pirkle column, the appropriate fractions were silylated and the tetra-trimethylsilyl derivatives of the BaP-tetraols were analyzed by GC-MS/MS. Fragments and their relative intensities in a daughter ion spectrum of m/z 446 are illustrated in Scheme 2; little or no molecular ion, m/z 608, was observed. We monitored the transition m/z 446 → m/z 255. The chromatograms shown in Figure 5A,C have clear peaks eluting at the correct retention times for BaP-tetraols 3 and 6, respectively. These retention times were confirmed by co-injection with racemic BaP-7,8,9,10-tetraol. The relative intensities of the daughter ion peaks of the isolated BaP-tetraols 3 and 6 were similar to those of standard racemic BaP-7,8,9,10-tetraol (Scheme 2). BaP-tetraol 3 comprised 78 ± 1.7% and BaP-tetraol 6 22 ± 1.7% of BaP-7,8,9,10-tetraol, indicating that BaP-7,8,9,10-tetraol in the urine of creosote workers results mainly from hydrolysis of the bay region diol epoxide BaP-(7R,8S)-diol-(9S,10R) epoxide (2).
Figure 4.
Separation of BaP-tetraols 3 and 6 on a Pirkle HPLC column. For the analysis of urine, the UV peaks corresponding to 3 and 6 cannot be seen; fractions encompassing their retention times were collected.
Scheme 2.
Fragmentation pattern and relative intensities (in parentheses) in the daughter ion spectrum of m/z 446 of racemic tetra-trimethylsilyl-BaP-tetraol. TMS = (CH3)3Si
Figure 5.
Chromatograms obtained upon GC-MS/MS analysis of urine samples from creosote workers for BaP-7,8,9,10-tetraols. The shaded peaks are tetra-trimethylsilyl derivatives of (A) BaP-tetraol 3; (B, D) internal standard racemic trans, anti-Phe-tetraol added upon collection of the BaP-tetraol enantiomers to monitor silylation efficiency; (C) BaP-tetraol 6.
Discussion
We present the first data on BaP-tetraol and Phe-tetraol enantiomer composition in human urine. The BaP-tetraol results are fully consistent with the view that BaP is metabolically activated in humans to bay region diol epoxide 2 as an ultimate carcinogen. A vast amount of in vitro data using human tissues and P450 enzymes provide strong support for this hypothesis (12–16,50–56), but there are relatively few studies of BaP metabolites in humans. Pertinent to the diol epoxide pathway, only racemic tetraols have been previously analyzed (42,43). The only other studies of BaP metabolites in human urine quantified 3-hydroxyBaP as a biomarker of exposure (57,58). Consistent with our results and the bay region diol epoxide hypothesis, a number of studies using structure specific methods have characterized DNA adducts of BaP resulting from this pathway, although some have reported negative results (44,46). BaP-tetraols have also been identified in hydrolysates of globin and albumin (44,45,59–62). Our results are consistent with in vitro studies which indicate that BaP-7,8-diol is converted to a diol epoxide more extensively than is BaP-9,10-diol (63).
The results reported here leave no doubt that Phe-tetraol in human urine is predominantly comprised of enantiomer 12 originating from metabolism of Phe-(3R,4R)-diol 10, with far smaller amounts of enantiomer 9 derived from metabolism of Phe-(1R,2R)-diol 7. Thus, in contrast to BaP, angular ring Phe-diol metabolism in humans proceeds mainly through the reverse diol epoxide 11, as opposed to the bay region diol epoxide 8. These results are consistent with those of our recent study of human hepatocyte metabolism of Phe diols, which demonstrated 2.5–29-fold more conversion of Phe-3,4-diol than Phe-1,2-diol to tetraols (41). That study also demonstrated more extensive glutathione conjugation of the reverse diol epoxide than of the bay region diol epoxide.
A number of investigations have examined the further metabolism in vitro of PAH bay region diols analogous to Phe-3,4-diol (13). These studies mainly used rat liver microsomal preparations. As mentioned above, there was limited conversion to tetraols resulting from reverse diol epoxides in the metabolism of the bay region 9,10-diol of BaP, and similar results were obtained with benzo[e]pyrene-9,10-diol. But studies of the metabolism of the bay region diols of benz[a]anthracene and chrysene found moderate and high conversion, respectively, to tetraols (13). Corresponding investigations of Phe diol metabolism do not seem to have been reported. Based on the available literature, it would appear that the extent of metabolism of bay region diols to tetraols via reverse diol epoxides is quite dependent on the structure of the parent PAH.
We also observed enantiomeric preference in the composition of Phe-diols in human urine, with Phe-(1R,2R)-diol (7) and Phe-(3R,4R)-diol (10) predominating. These results are consistent with in vitro metabolism studies of Phe, using both rat and human enzymes (37,40). Phe-diols have been previously analyzed in human urine, but their stereochemistry was not reported. Those studies found higher levels of Phe-1,2-diol than Phe-3,4-diol (64,65). It is not clear whether that observation results from preferential formation of Phe-1,2-diol or preferential metabolism, as indicated here, of Phe-3,4-diol.
Our results raise some questions about the utility of Phe metabolism as a surrogate for BaP metabolism, as we have previously proposed. Although many features of their metabolic transformations are similar in terms of enzyme involvement and stereochemistry, the tetraol end products of the diol epoxide pathway result predominantly from different intermediate diol epoxides. Thus, measurement of racemic Phe-tetraol in urine, as we have reported in previous studies, does not reflect mainly the carcinogenic bay region diol epoxide pathway but rather the reverse diol epoxide pathway, which is generally associated with weak mutagenicity and carcinogenicity (12,47,66). Nevertheless, the same enzymes are involved in both pathways, so a higher level of racemic Phe-tetraol is still likely to reflect a higher level of bay region diol epoxide formation from carcinogenic PAH such as BaP. This question needs to be addressed in more detail. We are currently developing a method for analysis specifically of Phe-tetraol 9 in human urine. Although it represents only about 3% of racemic Phe-tetraol, the levels of racemic Phe-tetraol are approximately 10,000 times as great as those of racemic BaP-tetraol in most samples. Therefore, its concentration should still be 300 times greater than that of racemic BaP-tetraol in samples from smokers and others only moderately exposed to PAH, and may be a more practical biomarker than BaP-tetraol.
In summary, the results of this study provide the first analysis of human urinary PAH tetraol stereochemistry. We confirm the widely held view that BaP is metabolized by the bay region diol epoxide pathway in humans. In contrast, Phe is metabolized predominantly via the reverse diol epoxide pathway. These results provide some important new insights relative to the development of biomarkers to assess PAH metabolism in humans.
Acknowledgments
This study was supported by grant no. CA-92025 from the National Cancer Institute. We thank Dr. Silvia Balbo for carrying out the hepatocyte incubations and Bob Carlson for editorial assistance.
References
- 1.Brandt HC, Watson WP. Monitoring human occupational and environmental exposures to polycyclic aromatic compounds. Ann Occup Hyg. 2003;47:349–378. doi: 10.1093/annhyg/meg052. [DOI] [PubMed] [Google Scholar]
- 2.Luch A. Polycyclic aromatic hydrocarbon-induced carcinogenesis-an introduction. In: Luch A, editor. The carcinogenic effects of polycyclic aromatic hydrocarbons. Imperial College Press; London: 2005. pp. 1–18. [Google Scholar]
- 3.U.S. Department of Health and Human Services. Report on Carcinogens. 11. Research Triangle Park; N.C: 2004. pp. III-220–III-222. [Google Scholar]
- 4.Ding YS, Ashley DL, Watson CH. Determination of 10 carcinogenic polycyclic aromatic hydrocarbons in mainstream cigarette smoke. J Agric Food Chem. 2007;55:5966–5973. doi: 10.1021/jf070649o. [DOI] [PubMed] [Google Scholar]
- 5.Stepanov I, Villalta PW, Knezevich A, Jensen J, Hatsukami D, Hecht SS. Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography-mass spectrometry. Chem Res Toxicol. 2009 doi: 10.1021/tx900281u. epub ahead of print 27-Oct-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol. 2005;6:931–932. doi: 10.1016/s1470-2045(05)70458-7. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Vol. 32. IARC; Lyon, FR: 1983. Polynuclear Aromatic Compounds, Part 1, Chemical, Environmental, and Experimental Data; pp. 33–91. [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Vol. 34. IARC; Lyon, FR: 1984. Polynuclear Aromatic Compounds, Part 3. Industrial Exposures in Aluminum Production, Coal Gasification, Coke Production, and Iron and Steel Founding; pp. 65–131. [Google Scholar]
- 9.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Vol. 35. IARC; Lyon, France: 1985. Polynuclear Aromatic Compounds, Part 4. Bitumens, Coal-Tars and Derived Products, Shale Oils and Soots; pp. 83–241. [PubMed] [Google Scholar]
- 10.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 11.Luch A, Baird WM. Metabolic activation and detoxification of polycyclic aromatic hydrocarbons. In: Luch A, editor. The carcinogenic effects of polycyclic aromatic hydrocarbons. Imperial College Press; London: 2005. pp. 19–96. [Google Scholar]
- 12.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G.H.A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 13.Thakker DR, Yagi H, Levin W, Wood AW, Conney AH, Jerina DM. Polycyclic aromatic hydrocarbons: metabolic activation to ultimate carcinogens. In: Anders MW, editor. Bioactivation of Foreign Compounds. Academic Press, Inc; New York: 1985. pp. 177–242. [Google Scholar]
- 14.Cooper CS, Grover PL, Sims P. The metabolism and activation of benzo[a]pyrene. Prog Drug Metab. 1983;7:295–396. [Google Scholar]
- 15.Dipple A, Moschel RC, Bigger CAH. Polynuclear aromatic hydrocarbons. In: Searle CE, editor. Chemical Carcinogens, Second Edition, ACS Monograph 182. Vol. 1. American Chemical Society; Washington, D.C: 1984. pp. 41–163. [Google Scholar]
- 16.Harvey RG. Polycyclic Aromatic Hydrocarbons: Chemistry and Carcinogenicity. Cambridge University Press; Cambridge, England: 1991. [Google Scholar]
- 17.Geacintov NE, Cosman M, Hingerty BE, Amin S, Broyde S, Patel DJ. NMR solution structures of stereoisomeric covalent polycyclic aromatic carcinogen - DNA adducts: principles, patterns, and diversity. Chem Res Toxicol. 1997;10:112–146. doi: 10.1021/tx9601418. [DOI] [PubMed] [Google Scholar]
- 18.Szeliga J, Dipple A. DNA adduct formation by polycyclic aromatic hydrocarbon dihydrodiol epoxides. Chem Res Toxicol. 1998;11:1–11. doi: 10.1021/tx970142f. [DOI] [PubMed] [Google Scholar]
- 19.Welch RM, Harrison YE, Conney AH, Poppers PJ, Finster M. Cigarette smoking: stimulatory effect on metabolism of 3,4-benzpyrene by enzymes in human placenta. Science. 1968;160:541–542. doi: 10.1126/science.160.3827.541. [DOI] [PubMed] [Google Scholar]
- 20.Kellermann G, Shaw CR, Luyten-Kellerman M. Aryl hydrocarbon hydroxylase inducibility and bronchogenic carcinoma. N Engl J Med. 1973;289:934–937. doi: 10.1056/NEJM197311012891802. [DOI] [PubMed] [Google Scholar]
- 21.Harris CC, Autrup H, Connor R, Barrett LA, McDowell EM, Trump BF. Interindividual variation in binding of benzo[a]pyrene to DNA in cultured human bronchi. Science. 1976;194:1067–1069. doi: 10.1126/science.982061. [DOI] [PubMed] [Google Scholar]
- 22.Sabadie N, Richter-Reichhelm HB, Saracci R, Mohr U, Bartsch H. Inter-individual differences in oxidative benzo(a)pyrene metabolism by normal and tumorous surgical lung specimens from 105 lung cancer patients. Int J Cancer. 1981;27:417–425. doi: 10.1002/ijc.2910270402. [DOI] [PubMed] [Google Scholar]
- 23.Nowak D, Schmidt-Preuss U, Jorres R, Liebke F, Rudiger HW. Formation of DNA adducts and water-soluble metabolites of benzo[a]pyrene in human monocytes is genetically controlled. Int J Cancer. 1988;41:169–173. doi: 10.1002/ijc.2910410202. [DOI] [PubMed] [Google Scholar]
- 24.McLemore TL, Adelberg S, Liu MC, McMahon NA, Yu SJ, Hubbard WC, Czerwinski M, Wood TG, Storeng R, Lubet RA, Eggleston JC, Boyd MR, Hines RN. Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J Natl Cancer Inst. 1990;82:1333–1339. doi: 10.1093/jnci/82.16.1333. [DOI] [PubMed] [Google Scholar]
- 25.Kiyohara C, Nakanishi Y, Inutsuka S, Takayama K, Hara N, Motohiro A, Tanaka K, Kono S, Hirohata T. The relationship between CYP1A1 aryl hydrocarbon hydroxylase activity and lung cancer in a Japanese population. Pharmacogenetics. 1998;8:315–323. doi: 10.1097/00008571-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Nebert DW. Drug-metabolizing enzymes, polymorphisms and interindividual response to environmental toxicants. Clin Chem Lab Med. 2000;38:857–861. doi: 10.1515/CCLM.2000.124. [DOI] [PubMed] [Google Scholar]
- 27.Alexandrov K, Cascorbi I, Rojas M, Bouvier G, Kriek E, Bartsch H. CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers’ lung: comparison with aromatic/hydrophobic adduct formation. Carcinogenesis. 2002;23:1969–1977. doi: 10.1093/carcin/23.12.1969. [DOI] [PubMed] [Google Scholar]
- 28.Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers & Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- 29.Smith GB, Harper PA, Wong JM, Lam MS, Reid KR, Petsikas D, Massey TE. Human lung microsomal cytochrome P4501A1 (CYP1A1) activities: impact of smoking status and CYP1A1, aryl hydrocarbon receptor, and glutathione S- transferase M1 genetic polymorphisms. Cancer Epidemiol Biomarkers & Prev. 2001;10:839–853. [PubMed] [Google Scholar]
- 30.Lee WJ, Brennan P, Boffetta P, London SJ, Benhamou S, Rannug A, To-Figueras J, Ingelman-Sundberg M, Shields P, Gaspari L, Taioli E. Microsomal epoxide hydrolase polymorphisms and lung cancer risk: a quantitative review. Biomarkers. 2002;7:230–241. doi: 10.1080/13547500210121882. [DOI] [PubMed] [Google Scholar]
- 31.Benhamou S, Lee WJ, Alexandrie AK, Boffetta P, Bouchardy C, Butkiewicz D, Brockmoller J, Clapper ML, Daly A, Dolzan V, Ford J, Gaspari L, Haugen A, Hirvonen A, Husgafvel-Pursiainen K, Ingelman-Sundberg M, Kalina I, Kihara M, Kremers P, Le Marchand L, London SJ, Nazar-Stewart V, Onon-Kihara M, Rannug A, Romkes M, Ryberg D, Seidegard J, Shields P, Strange RC, Stucker I, To-Figueras J, Brennan P, Taioli E. Meta- and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis. 2002;23:1343–1350. doi: 10.1093/carcin/23.8.1343. [DOI] [PubMed] [Google Scholar]
- 32.Hung RJ, Boffetta P, Brockmoller J, Butkiewicz D, Cascorbi I, Clapper ML, Garte S, Haugen A, Hirvonen A, Anttila S, Kalina I, Le Marchand L, London SJ, Rannug A, Romkes M, Salagovic J, Schoket B, Gaspari L, Taioli E. CYP1A1 and GSTM1 genetic polymorphisms and lung cancer risk in Caucasian non-smokers: a pooled analysis. Carcinogenesis. 2003;24:875–882. doi: 10.1093/carcin/bgg026. [DOI] [PubMed] [Google Scholar]
- 33.Veglia F, Matullo G, Vineis P. Bulky DNA adducts and risk of cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003;12:157–160. [PubMed] [Google Scholar]
- 34.Raimondi S, Paracchini V, Autrup H, Barros-Dios JM, Benhamou S, Boffetta P, Cote ML, Dialyna IA, Dolzan V, Filiberti R, Garte S, Hirvonen A, Husgafvel-Pursiainen K, Imyanitov EN, Kalina I, Kang D, Kiyohara C, Kohno T, Kremers P, Lan Q, London S, Povey AC, Rannug A, Reszka E, Risch A, Romkes M, Schneider J, Seow A, Shields PG, Sobti RC, Sorensen M, Spinola M, Spitz MR, Strange RC, Stucker I, Sugimura H, To-Figueras J, Tokudome S, Yang P, Yuan JM, Warholm M, Taioli E. Meta- and pooled analysis of GSTT1 and lung cancer: a HuGE-GSEC review. Am J Epidemiol. 2006;164:1027–1042. doi: 10.1093/aje/kwj321. [DOI] [PubMed] [Google Scholar]
- 35.Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JP. Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. Am J Epidemiol. 2008;167:759–774. doi: 10.1093/aje/kwm383. [DOI] [PubMed] [Google Scholar]
- 36.Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: a potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol Biomarkers & Prev. 2003;12:1501–1508. [PubMed] [Google Scholar]
- 37.Shou M, Korzekwa KR, Krausz KW, Crespi CL, Gonzalez FJ, Gelboin HV. Regio- and stereo-selective metabolism of phenanthrene by twelve cDNA-expressed human, rodent, and rabbit cytochromes P-450. Cancer Lett. 1994;83:305–313. doi: 10.1016/0304-3835(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 38.Hecht SS, Chen M, Yoder A, Jensen J, Hatsukami D, Le C, Carmella SG. Longitudinal study of urinary phenanthrene metabolite ratios: effect of smoking on the diol epoxide pathway. Cancer Epidemiol Biomarkers & Prev. 2005;14:2969–2974. doi: 10.1158/1055-9965.EPI-05-0396. [DOI] [PubMed] [Google Scholar]
- 39.Hecht SS, Carmella SG, Yoder A, Chen M, Li Z, Le C, Jensen J, Hatsukami DK. Comparison of polymorphisms in genes involved in polycyclic aromatic hydrocarbon metabolism with urinary phenanthrene metabolite ratios in smokers. Cancer Epidemiol Biomarkers & Prev. 2006;15:1805–1811. doi: 10.1158/1055-9965.EPI-06-0173. [DOI] [PubMed] [Google Scholar]
- 40.Nordqvist M, Thakker DR, Vyas KP, Yagi H, Levin W, Ryan DE, Thomas PE, Conney AH, Jerina DM. Metabolism of chrysene and phenanthrene to bay-region diol epoxides by rat liver enzymes. Mol Pharmacol. 1981;19:168–178. [PubMed] [Google Scholar]
- 41.Hecht SS, Berg JZ, Hochalter JB. Preferential glutathione conjugation of a reverse diol epoxide compared to a bay region diol epoxide of phenanthrene in human hepatocytes: Relevance to molecular epidemiology studies of glutathione-S-transferase polymorphisms and cancer. Chem Res Toxicol. 2009;22:426–432. doi: 10.1021/tx800315m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson CD, Wu MT, Christiani DC, Santella RM, Carmella SG, Hecht SS. Determination of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in human urine by gas chromatography-negative ion chemical ionization-mass spectrometry. Chem Res Toxicol. 2000;13:271–280. doi: 10.1021/tx990202c. [DOI] [PubMed] [Google Scholar]
- 43.Weston A, Bowman ED, Carr P, Rothman N, Strickland PT. Detection of metabolites of polycyclic aromatic hydrocarbons in human urine. Carcinogenesis. 1993;14:1053–1055. doi: 10.1093/carcin/14.5.1053. [DOI] [PubMed] [Google Scholar]
- 44.Boysen G, Hecht SS. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutation Res. 2003;543:17–30. doi: 10.1016/s1383-5742(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 45.Ragin AD, Crawford KE, Etheredge AA, Grainger J, Patterson DG., Jr A gas chromatography-isotope dilution high-resolution mass spectrometry method for quantification of isomeric benzo[a]pyrene diol epoxide hemoglobin adducts in humans. J Anal Toxicol. 2008;39:728–736. doi: 10.1093/jat/32.9.728. [DOI] [PubMed] [Google Scholar]
- 46.Beland FA, Churchwell MI, Von Tungeln LS, Chen S, Fu PP, Culp SJ, Schoket B, Gyorffy E, Minarovits J, Poirier MC, Bowman ED, Weston A, Doerge DR. High-performance liquid chromatography electrospray ionization tandem mass spectrometry for the detection and quantitation of benzo[a]pyrene-DNA adducts. Chem Res Toxicol. 2005;18:1306–1315. doi: 10.1021/tx050068y. [DOI] [PubMed] [Google Scholar]
- 47.Hecht SS, Villalta PW, Hochalter JB. Analysis of phenanthrene diol epoxide mercapturic acid detoxification products in human urine: relevance to molecular epidemiology studies of glutathione-S-transferase polymorphisms. Carcinogenesis. 2008;29:937–943. doi: 10.1093/carcin/bgn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whalen DL, Ross AM, Yagi H, Karle JM, Jerina DM. Stereoelectronic factors in the solvolysis of bay region diol epoxides of polycyclic aromatic hydrocarbons. J Am Chem Soc. 1978;100:5218–5221. [Google Scholar]
- 49.Weems HB, Yang SK. Chiral stationary phase high-performance liquid chromatographic resolution and absolute configuration of enantiomeric benzo[a]pyrene diol-epoxides and tetrols. Chirality. 1989;1:276–283. doi: 10.1002/chir.530010406. [DOI] [PubMed] [Google Scholar]
- 50.Jiang H, Shen YM, Quinn AM, Penning TM. Competing roles of cytochrome P450 1A1/1B1 and aldo-keto reductase 1A1 in the metabolic activation of (+/−)-7,8-dihydroxy-7,8-dihydro-benzo[a]pyrene in human bronchoalveolar cell extracts. Chem Res Toxicol. 2005;18:365–374. doi: 10.1021/tx0497245. [DOI] [PubMed] [Google Scholar]
- 51.Shimada T, Gillam EMJ, Oda Y, Tsumara F, Sutter TR, Guengerich FP, Inoue K. Metabolism of benzo[a]pyrene to trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by recombinant human cytochrome P450 1B1 and purified liver epoxide hydrolase. Chem Res Toxicol. 1999;12:623–629. doi: 10.1021/tx990028s. [DOI] [PubMed] [Google Scholar]
- 52.Kim JH, Stansbury KH, Walker NJ, Trush MA, Strickland PT, Sutter TR. Metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis. 1998;19:1847–1853. doi: 10.1093/carcin/19.10.1847. [DOI] [PubMed] [Google Scholar]
- 53.Staretz ME, Murphy SE, Nunes MG, Koehl W, Amin S, Koenig L, Guengerich FP, Hecht SS. Comparative metabolism of the tobacco smoke carcinogens benzo[a]pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and N′-nitrosonornicotine in human hepatic microsomes. Drug Metab Dispos. 1997;25:154–162. [PubMed] [Google Scholar]
- 54.Bauer E, Guo Z, Ueng YF, Bell LC, Zeldin D, Guengerich FP. Oxidation of benzo[a]pyrene by recombinant human cytochrome P450 enzymes. Chem Res Toxicol. 1995;8:136–142. doi: 10.1021/tx00043a018. [DOI] [PubMed] [Google Scholar]
- 55.Shou M, Korzekwa KR, Crespi CL, Gonzalez FJ, Gelboin HV. The role of 12 cDNA-expressed human, rodent, and rabbit cytochromes P450 in the metabolism of benzo[a]pyrene and benzo[a]pyrene trans-7,8-dihydrodiol. Mol Carcinogenesis. 1994;10:159–168. doi: 10.1002/mc.2940100307. [DOI] [PubMed] [Google Scholar]
- 56.Jacob J, Doehmer J, Grimmer G, Soballa V, Raab G, Seidel A, Greim H. Metabolism of phenanthrene, benz[a]anthracene, benz[a]pyrene, chrysene and benzo[c]phenanthrene by eight cDNA-expressed human and rate cytochromes P450. Polycyclic Aromatic Compounds. 1996;10:1–9. [Google Scholar]
- 57.Gündel J, Angerer J. High-performance liquid chromatographic method with fluorescence detection for the determination of 3-hydroxybenzo[a]pyrene and 3-hydroxybenz[a]anthracene in the urine of polycyclic aromatic hydrocarbon-exposed workers. J Chromatog B. 2000;738:47–55. doi: 10.1016/s0378-4347(99)00499-5. [DOI] [PubMed] [Google Scholar]
- 58.Forster K, Preuss R, Rossbach B, Bruning T, Angerer J, Simon P. 3-Hydroxybenzo[a]pyrene in the urine of workers with occupational exposure to polycyclic aromatic hydrocarbons in different industries. Occup Environ Med. 2008;65:224–229. doi: 10.1136/oem.2006.030809. [DOI] [PubMed] [Google Scholar]
- 59.Ozbal CC, Skipper PL, Yu MC, London SJ, Dasari RR, Tannenbaum SR. Quantification of (7S,8R)-dihydroxy-(9R,10S)-epoxy-7,8,9,10- tetrahydrobenzo[a]pyrene adducts in human serum albumin by laser- induced fluorescence: implications for the in vivo metabolism of benzo[a]pyrene. Cancer Epidemiol Biomarkers Prev. 2000;9:733–739. [PubMed] [Google Scholar]
- 60.Melikian AA, Sun P, Pierpont C, Coleman S, Hecht SS. Gas chromatography-mass spectrometric determination of benzo[a]pyrene and chrysene diol epoxide globin adducts in humans. Cancer Epidemiol Biomarkers & Prev. 1997;6:833–839. [PubMed] [Google Scholar]
- 61.Pastorelli R, Restano J, Guanci M, Maramonte M, Magagnotti C, Allevi R, Lauri D, Fanelli R, Airoldi L. Hemoglobin adducts of benzo[a]pyrene diolepoxide in newspaper vendors: association with traffic exhaust. Carcinogenesis. 1996;17:2389–2394. doi: 10.1093/carcin/17.11.2389. [DOI] [PubMed] [Google Scholar]
- 62.Pastorelli R, Guanci M, Cerri A, Minoia C, Carrer P, Negri E, Fanelli R, Airoldi L. Benzo(a)pyrene diolepoxide-haemoglobin and albumin adducts at low levels of benzo(a)pyrene exposure. Biomarkers. 2000;5:245–251. doi: 10.1080/135475000413791. [DOI] [PubMed] [Google Scholar]
- 63.Thakker DR, Yagi H, Lehr RE, Levin W, Buening M, Lu AY, Chang RL, Wood AW, Conney AH, Jerina DM. Metabolism of trans-9,10-dihydroxy-9,10-dihydrobenzo[a]pyrene occurs primarily by arylhydroxylation rather than formation of a diol epoxide. Mol Pharmacol. 1978;14:502–513. [PubMed] [Google Scholar]
- 64.Seidel A, Spickenheuer A, Straif K, Rihs HP, Marczynski B, Scherenberg M, Dettbarn G, Angerer J, Wilhelm M, Bruning T, Jacob J, Pesch B. New biomarkers of occupational exposure to polycyclic aromatic hydrocarbons. J Toxicol Environ Health A. 2008;71:734–745. doi: 10.1080/15287390801985265. [DOI] [PubMed] [Google Scholar]
- 65.Jacob J, Grimmer G, Dettbarn G. Profile of urinary phenanthrene metabolites in smokers and non-smokers. Biomarkers. 1999;4:319–327. doi: 10.1080/135475099230705. [DOI] [PubMed] [Google Scholar]
- 66.Glatt H, Wameling C, Elsberg S, Thomas H, Marquardt H, Hewer A, Phillips DH, Oesch F, Seidel A. Genotoxicity characteristics of reverse diol-epoxides of chrysene. Carcinogenesis. 1993;14:11–19. doi: 10.1093/carcin/14.1.11. [DOI] [PubMed] [Google Scholar]