Abstract
The National Research Council has outlined the need for non-mammalian toxicological models to test the potential health effects of a large number of chemicals while also reducing the use of traditional animal models. The nematode Caenorhabditis elegans is an attractive alternative model because of its well-characterized and evolutionarily-conserved biology, low cost, and ability to be used in high-throughput screening. A high-throughput method is described for quantifying the reproductive capacity of C. elegans exposed to chemicals for 48 h from the last larval stage (L4) to adulthood using a COPAS Biosort. Initially, the effects of exposure conditions that could influence reproduction were defined. Concentrations of DMSO vehicle ≤ 1% did not affect reproduction. Previous studies indicated that C. elegans may be influenced by exposure to low pH conditions. At pHs greater than 4.5, C. elegans reproduction was not affected, however below this pH there was a significant decrease in the number of offspring. Cadmium chloride was chosen as a model toxicant to verify that automated measurements were comparable to those of traditional observational studies. EC50 values for cadmium for automated measurements (176-192 μM) were comparable to those previously reported for a 72-h exposure using manual counting (151 μM). The toxicity of seven test toxicants on C. elegans reproduction was highly correlative with rodent lethality suggesting that this assay may be useful in predicting the potential toxicity of chemicals in other organisms.
Keywords: Caenorhabditis elegans, high-throughput screening, alternative toxicological models, cadmium
Introduction
The National Research Council and government agencies including the National Institutes of Health and the U.S. Environmental Protection Agency have defined a need for reliable high-throughput screening (HTS) methods to evaluate the potential human health impacts of the large number of chemicals in production (National Research Council, 2000; Dix et al., 2007). Although rodent-based assays have been the traditional model for toxicological studies, many agencies are now recognizing the advantages of in vivo whole organism studies using invertebrate species. These advantages include rapid and inexpensive testing, as well as a lack of animal welfare issues. Although in vitro cell-based HTS assays are commonly used, whole organism testing allows researchers to observe phenotypes that are well-characterized and biologically relevant.
The nematode Caenorhabditis elegans, a popular model organism for genetic and developmental biology research, is now being recognized as an attractive invertebrate model for high-throughput toxicological studies. C. elegans has a rapid and well-characterized life cycle and can be cultured in multi-well plates, making them amenable to HTS. There is also a high degree of conservation between C. elegans and mammalian species in processes controlling development, neurobiology, and stress responses (Kaletta and Hengartner, 2006). For these reasons, several pharmaceutical companies are using C. elegans as part of their drug discovery process (Artal-Sanz et al., 2006).
One easily quantifiable phenotype in C. elegans is reproduction. C. elegans develop from embryo to gravid adult through four distinct larval stages, termed L1-L4, in about three days at 20°C (Wood, 1988). At the L4 stage, germ cells within C. elegans hermaphrodites mature to sperm, while in the adult stage germ cells mature to oocytes. Following internal fertilization, the developing embryos are released by muscle contractions of the vulva, which are regulated by specific neurons that release serotonin, acetylcholine, or neuropeptides (Trent et al., 1983; Weinshenker et al., 1995; Bany et al., 2003).
Reproduction and egg-laying are affected by a number of environmental conditions including salt concentration (Horvitz et al., 1982) and food availability (Trent et al., 1983). Exposure to ethanol (Dhawan et al., 1999), metals (Anderson et al., 2001), Enterobacteriaceae (Sicard et al., 2007), anthelmintic agents (Kim et al., 2001), and nicotinic agonists (Kim et al., 2001; Bull et al., 2007) also affect reproduction. Because such a wide variety of toxicants impact C. elegans reproduction, it is a promising endpoint for HTS. Egg-laying and reproduction have been measured in low throughput by placing one to several nematodes on the surface of an agar plate or in liquid media. After exposure, the number of offspring was manually counted with the aid of a microscope. Automated tracking systems and image analysis have also been used to monitor the frequency of egg-laying behavior on agar surfaces over several minutes to hours (Kim et al., 2001; Davies et al., 2003; Geng et al., 2005). A more high-throughput, but indirect approach to quantify reproduction, measures chitinase, which is released during embryo hatching (Kaletta and Hengartner, 2006).
In this report, an automated HTS method is described that directly quantifies the number of C. elegans offspring after exposure to potential toxicants. In this assay, a COPAS Biosort (Pulak, 2006) is used to load L4 hermaphrodite nematodes into each well of a 96-well plate containing test chemicals. Following a 48-h incubation, the number of offspring in each well is quantified using the Biosort. In the presence of the test chemicals, there were toxicant and concentration dependent decreases in the level of reproduction. These decreases could be the result of reducing the number of sperm or oocytes, disrupting germ cell maturation, affecting egg-laying behavior, or increasing embryonic or larval lethality after egg-laying. The strength of this assay was evaluated by comparing the toxicity of several different classes of chemicals in the C. elegans assay to toxicity measures in mice and rodents.
Methods
Nematode culture
The Bristol N2 (wild-type) and CB5584 mIs12[myo-2::GFP, pes-10::GFP, F22B7.9::GFP] (referred to as myo-2::GFP) strains of C. elegans were obtained from the Caenorhabditis Genetic Center (Minneapolis, MN) and maintained at 20°C on K-agar plates (2% bacto-agar, 0.25% bacto-peptone, 51 mM sodium chloride, 32 mM potassium chloride, 13 μM cholesterol) seeded with E. coli OP50 (Williams and Dusenbery, 1988). Age-synchronized adult nematodes were prepared as previously described (Khanna et al., 1997).
Reproduction assay
Using a COPAS Biosort (Union Biometrica Inc., Somerville, MA, USA), five L4 nematodes were added to each well of a 96-well plate, containing a final volume of 50 μl K-medium (51 mM NaCl, 32 mM KCl), E. coli, and test chemical. Stock solutions of test chemicals were prepared in K-medium or DMSO (final concentration ≤ 1% DMSO; see below) depending on the chemical’s aqueous solubility. The bacterial concentration was measured by determining the optical density at 550 nm immediately before nematode addition. Nematodes were incubated for 48 h, after which adults and offspring were aspirated using a Biosort. The Biosort records up to four attributes for each individual nematode: TOF, which relates to nematode length; EXT, which corresponds to the optical density; and two fluorescence measurements. TOF and EXT measurements are related to the age and size of the nematode; both increase as C. elegans develop. The TOF, EXT, and level of green fluorescence of individual C. elegans were recorded along with the total number of nematodes sampled per well.
Each treatment group consisted of six exposure wells (i.e. total of thirty parents per treatment condition) followed by two rinse wells to minimize carry-over of offspring between treatment groups. Total reproductive counts (i.e., number of non-adult nematodes) were used as the endpoint of the assay. Each experiment was replicated three times. Preliminary experiments were performed on untreated nematodes to determine the optimum number of parents per well and exposure duration to minimize count variability and carry-over between wells (data not shown).
Concentration-response curves
The reproduction assay was used to test the effects of eight chemicals on wild-type and myo-2::GFP strains of C. elegans. The relationship between chemical concentration and reproductive counts was modeled using a four parameter sigmoidal growth model (Copeland, 2000). The model, also known as the Morgan-Mercer-Flodin model (Ratkowsky, 1983), was parameterized as follows:
| {1} |
Here, v is taken to be the reproductive count at concentration [S]. VA and VB are the reproductive counts at the lower and upper asymptotes of the curve, respectively. [S]50 is the effective concentration producing 50% of the maximal reproductive count, which we will refer to as the EC50. h is the Hill coefficient. All parameters, including EC50s and confidence intervals were estimated using likelihood methods assuming constant variance in reproductive counts across concentration levels.
For each experiment, the benchmark concentration (BMC), which is the concentration corresponding to a specified excess risk relative to the control response was calculated as previously described (Crump, 2002). The BMC method has been suggested as an alternative to the traditional no observable adverse effect level (NOAEL) (Sand et al., 2008). The BMC was defined as the concentration that reduced the reproductive count by 10% relative to the untreated or control group. The control group was assumed to be normally distributed with mean and standard deviation estimated from the likelihood fit to equation {1}, and an adverse effect to be the first percentile of counts in the controls. The corresponding lower limit of a one-sided 95% confidence interval on the BMC was referred to as the BMCL, and was calculated using bootstrap methods. Technical details are provided in Supplemental Table 1.
Effects of pH
Two acids (ascorbic and acetic) were chosen to test the effects of pH on wild-type and myo-2::GFP reproduction. The pH was measured using an Orion 911600 semi-micro pH electrode (Thermo Scientific, Beverly, MA, USA) and a Pinnacle series M 540P pH meter (NovaAnalytics Corp, Woburn, MA, USA). After a 48-h exposure at the indicated pH, the number of nematodes was counted using the Biosort.
All calculations of the relationship between pH and reproductive counts were performed as described above for chemical exposures except that v in Equation {1} is the reproductive count at pH [S]. The pH producing 50% of the maximal reproductive count is referred to as the pH50 and the benchmark pH (BMpH) as the pH that decreased the reproductive count by 10% relative to the control group. Additional details are provided in Supplemental Table 2.
Results
Reproduction of untreated C. elegans
In the C. elegans reproduction assay, nematodes are exposed to chemicals from their last larval (L4) through the adult stage, and then their offspring are counted using a COPAS Biosort. Two strains of C. elegans that are routinely used in toxicological assays in our laboratory were examined: wild-type and myo-2::GFP. The myo-2::GFP strain, which has a fluorescently-labeled pharynx, is being used in the development of additional toxicological HTS assays, including motion tracking assays and assays in which discriminating the anterior-posterior orientation is required (Boyd et al., 2010).
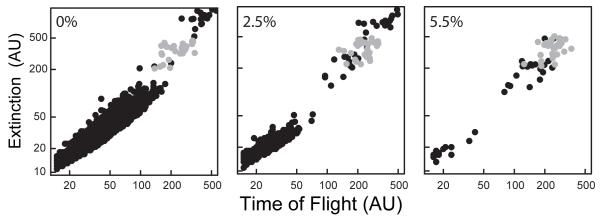
At the beginning of each experiment, five L4 animals were loaded into each of six wells of a 96-well plate (i.e., 30 L4s total per treatment group). The average number of offspring recovered after 48 h for untreated animals ranged from 33 - 43 per parent. Figure 1 presents a typical distribution of an untreated, wild-type population of nematodes after a 48 h incubation, at which time adults and offspring were recovered.
Figure 1. Scatter plots illustrating concentration-dependent decreases in C. elegans reproduction.
Extinction (log10 scale) plotted against time of flight (log10 scale) for untreated (left panel), 2.5% DMSO (center panel), and 5.5% DMSO (right panel) wild-type nematodes. C. elegans L4s (gray circles) were loaded at t=0 h. After 48 h, the nematodes were recovered as adults and their offspring (black circles). Each point corresponds to a single nematode. (AU, arbitrary units)
Effect of DMSO on C. elegans reproduction
DMSO is commonly used as a solvent in many chemical exposure studies. Therefore it was critical to determine the maximum concentration of DMSO that will not affect C. elegans reproduction. Increasing concentrations of DMSO up to 5.5 % led to decreased numbers of offspring (Fig. 1), but were not lethal to the parents based on visual observations. The numbers of offspring decreased to 17 - 29 per parent at 2.5% DMSO, while only 1 - 3 offspring were recovered per parent at 5.5% DMSO (Fig. 1).
The concentration of a test chemical leading to a 50% reduction in C. elegans reproduction was calculated as the EC50, with corresponding 95% confidence intervals. The concentration leading to no effect, defined as less than a 10% decrease in reproduction, was calculated as the benchmark concentration (BMC) with corresponding lower limits (BMCL).
Increasing the DMSO concentration caused the number of offspring to sharply decrease for both wild-type and myo-2::GFP C. elegans, with EC50s ranging from 2 - 2.5% DMSO (Fig. 2, Table 1). BMCs ranged from 1.27 – 1.71% DMSO for wild-type and from 1.08 – 2.04% for myo-2::GFP nematodes. Based on these observations, DMSO concentrations ≤ 1% were selected for subsequent studies in which this solvent was used as a vehicle.
Figure 2. Effect of DMSO on C. elegans reproduction.
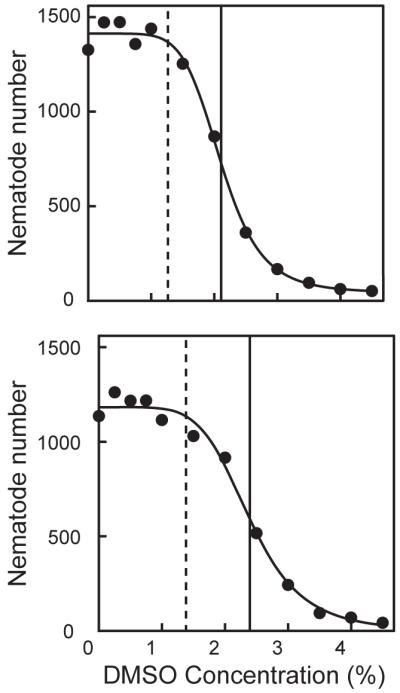
Total number of offspring plotted against DMSO concentration (%) of wild-type (upper panel) and myo-2::GFP (lower panel) strains for one representative experiment. The solid, black vertical lines represent a 50% reduction in the total number of offspring (EC50). The dashed, black vertical lines represent the BMC, below which the reproductive rate is not significantly affected by DMSO.
Table 1.
Effect of DMSO on C. elegans reproduction
| Strain | EC50 | 95% CI | BMC | 95% BMCL (lower limit) |
|---|---|---|---|---|
| wild-type | 2.11 | (2.04, 2.18) | 1.27 | 1.07 |
| 2.21 | (2.11, 2.32) | 1.71 | 1.48 | |
| 2.39 | (2.23, 2.56) | 1.62 | 1.35 | |
| myo-2::GFP | 2.01 | (1.81, 2.20) | 1.08 | 0.79 |
| 2.52 | (2.22, 2.83) | 2.04 | 1.55 | |
| 2.39 | (2.30, 2.49) | 1.38 | 1.20 |
Values expressed as % DMSO
Effect of pH
Previous studies demonstrated that exposing C. elegans to low pH leads to decreased survival and movement (Khanna et al., 1997; Cole et al., 2004). Many chemicals are known to decrease the pH of exposure solutions, which could potentially confound the observed effects. Therefore, the pH range that does not affect C. elegans reproduction was determined. Figure 3 presents representative experiments showing fitted Hill functions for the effects of pH on reproductive counts for wild-type nematodes treated with ascorbic acid and myo-2::GFP nematodes treated with acetic acid. Calculated BMpH values ranged from 3.73 – 4.47 for wild-type nematodes treated with ascorbic acid and from 4.51 – 4.65 for myo-2::GFP nematodes treated with acetic acid (Table 2). For each acid, BMpHs and pH50s were similar suggesting that there was a critical pH range below which reproduction would be severely inhibited. Based on these results, all reproduction assays were performed at pH > 4.5 to prevent confounding effects on chemical toxicity.
Figure 3. Effects of pH on C. elegans reproduction.
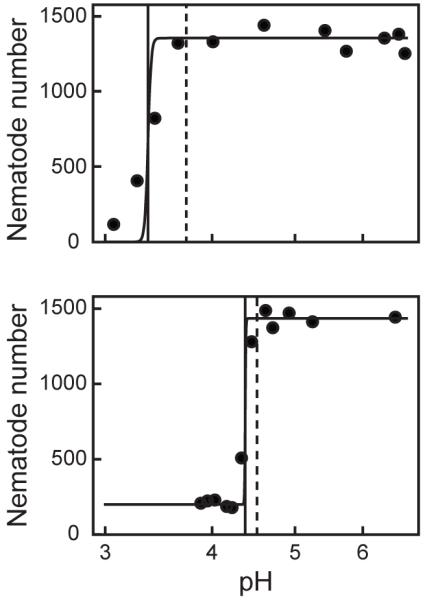
Total number of offspring plotted against pH for one representative experiment. Increasing concentrations of ascorbic acid (upper panel) or acetic acid (lower panel) caused a decrease in the number of wild-type and myo-2::GFP offspring, respectively. The solid, black vertical lines represent a 50% reduction in the total number of offspring (pH50). The dashed, black vertical lines represent the BMpH, above which the reproductive rate is not significantly affected by pH.
Table 2.
Effect of pH on C. elegans reproduction
| Acid | Strain | pH50 | 95% CI | BMpH | 95 % BMpH (upper limit) |
|---|---|---|---|---|---|
| Ascorbic acid | wild-type | 3.70 | (3.62, 3.78) | 4.26 | 4.53 |
| 3.37 | (3.34, 3.40) | 3.73 | 3.83 | ||
| 3.81 | (3.70, 3.93) | 4.47 | 4.82 | ||
| Acetic acid | myo2::GFP | 4.41 | (4.37, 4.45) | 4.55 | 4.64 |
| 4.45 | (4.43, 4.47) | 4.65 | 4.70 | ||
| 4.37 | (4.36, 4.38) | 4.51 | 4.54 |
Effects of toxicants on C. elegans reproduction
Cadmium chloride was used as a model toxicant to evaluate whether results obtained from the Biosort were comparable to those made by manual counting of offspring. The number of offspring measured after 48-h cadmium exposures decreased in a concentration-dependent manner (Fig. 4) with EC50s ranging from 176 – 192 μM for wild-type C. elegans (Table 3). These results are in good agreement with a previous report of an EC50 of 151 μM cadmium, measured by manual counting (Anderson et al., 2001). The myo-2::GFP strain was slightly more sensitive than the wild-type strain to cadmium, with EC50s ranging from 113 – 120 μM (Table 3). Although the myo-2::GFP strain was more sensitive to cadmium, the shapes of the concentration-response curves were similar with a sharp drop in reproduction observed between 100 and 200 μM cadmium (Fig. 4).
Figure 4. Effect of cadmium on C. elegans reproduction.
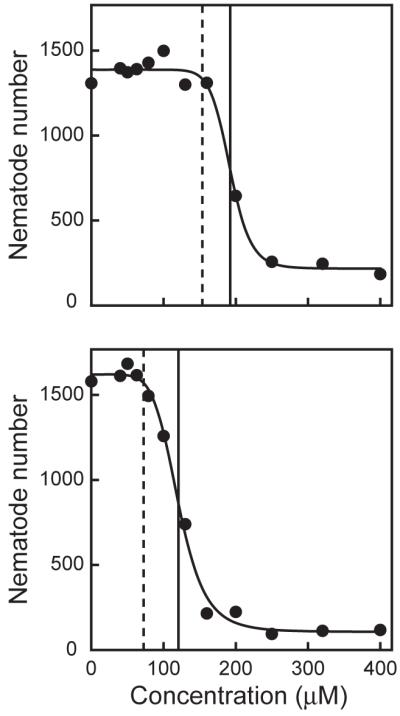
Total number of offspring plotted against cadmium concentration (μM) of wild-type (upper panel) and myo-2::GFP (lower panel) strains for one representative experiment. The solid, black vertical lines represent a 50% reduction in the total number of offspring (EC50). The dashed, black vertical lines represent the BMC, below which the reproductive rate is not significantly affected by cadmium.
Table 3.
Effects of toxicants on C. elegans reproduction
| Chemical | Strain | EC50 | 95% CI | BMC | 95% BMCL (lower limit) |
|---|---|---|---|---|---|
| Cadmium | wild-type | 186 | (170, 201) | 169 | 138 |
| 176 | (158, 194) | 132 | 95 | ||
| 192 | (186, 198) | 154 | 137 | ||
| myo-2::GFP | 113 | (109, 117) | 81 | 72 | |
| 119 | (112, 125) | 89 | 77 | ||
| 120 | (116, 125) | 72 | 63 | ||
| Diquat | myo-2::GFP | 756 | (655, 858) | 62 | 26 |
| 797 | (686, 907) | 54 | 21 | ||
| 683 | (492, 875) | 55 | 11 | ||
| Paraquat | wild-type | 1677 | (1075, 2279) | 294 | 64 |
| 1739 | (1473, 2005) | 975 | 601 | ||
| 2346 | (2005, 2687) | 463 | 232 | ||
| Parathion | myo-2::GFP | 2.17 | (2.01, 2.32) | 1.31 | 1.02 |
| 1.22 | (1.07, 1.36) | 0.43 | 0.26 | ||
| 1.14 | (1.08, 1.20) | 0.69 | 0.56 | ||
| EMS | wild-type | 4656 | (4188, 5124) | 3766 | 2908 |
| 4666 | (4347, 4986) | 4115 | 3320 | ||
| 4768 | (4539, 4997) | 4028 | 3465 | ||
| Caffeine | myo-2::GFP | 8903 | (7584, 10223) | 1845 | 1024 |
| 12650 | (11278, 14021) | 3431 | 2063 | ||
| 8739 | (7160, 10318) | 2091 | 1058 | ||
| Methadone | wild-type | 207 | (192, 221) | 63 | 40 |
| 270 | (255, 285) | 57 | 36 | ||
| 380 | (340, 419) | 56 | 27 |
Values expressed as μM
The effects on wild-type and myo-2::GFP C. elegans reproduction were examined with six additional chemicals including three pesticides diquat, paraquat, and parathion; the mutagen ethyl methanesulfonate (EMS); and two drugs caffeine and methadone (Table 3; Suppl. Fig. 1). The organophosphate pesticide, parathion, was at least two orders of magnitude more toxic than any chemical tested with EC50s between 1.14 – 2.17 μM. Although the EC50s for methadone were slightly higher than those for cadmium (207 – 380 μM vs. 176 – 192 μM), the BMCs for methadone were less than half of cadmium (56 - 63 μM vs. 132 - 169 μM) indicating that C. elegans is more sensitive to lower concentrations of methadone. A similar but more dramatic trend was observed after diquat exposure in which the EC50s ranged from 683 – 797 μM, while the BMC was similar to methadone (54 – 62 μM). Paraquat was less toxic as judged by the EC50s (1,677 – 2,346 μM) or BMCs (294 – 975 μM). Ethyl methanesulfonate exhibited low toxicity to C. elegans with EC50s and BMCs between 4.66 – 4.77 and 3.77 – 4.12 mM, respectively. Caffeine was the least toxic of the chemicals tested as calculated by the EC50 of 8.74 – 12.65 mM; however, C. elegans reproduction was affected by lower concentrations of caffeine than EMS as measured by the BMC (1.84 – 3.43 mM).
Discussion
The C. elegans reproduction assay described in this report used the COPAS Biosort to count the number of offspring produced between the L4 larval stage and adulthood. This period was chosen to coincide with the developmental stage when the number of germ cells reached its maximum, but before the embryonic membrane became impermeable (Anderson, 1995). Before testing chemical toxicity, preliminary experiments focused on optimizing the duration of the chemical exposure, the amount of bacterial food, and number of animals to be used. An exposure time of 48 h was chosen to maintain a relatively low number of offspring of 33-43 per untreated parent. This exposure time avoided overcrowding in the wells and allowed nematode counts to remain within the sampling limits of the Biosort. Chemical exposures were started at the L4 stage to maximize the chance of observing potential chemical effects on fertility, while avoiding potential effects on parental growth. When the offspring were sampled, adults had been producing embryos for approximately 36 h and still had several days of embryo production remaining.
Other studies have quantified the effects of a variety of chemicals on C. elegans reproduction (Dhawan et al., 1999; Anderson et al., 2001; Bull et al., 2007; Sicard et al., 2007). Traditionally, one to five parents are treated for 2 to 3 day incubation periods. At the end of the incubation, or every 24 h, the number of offspring is manually determined by visual observation. These methods can be tedious and imprecise. In addition, when nematodes are cultured in liquid medium, obtaining exact counts for moving larvae can be difficult. To determine the effects of hundreds to thousands of chemicals on reproduction an automated high-throughput system would be required. The chitinase assay, which indirectly measures reproduction by estimating the numbers of hatching embryos, may be applicable to HTS (Kaletta and Hengartner, 2006). This assay, however, gives only a snapshot of the number of embryos at a specific developmental stage, but does not include hatched larvae or less developed embryos. The reproduction assay described in this report directly measures the number of offspring in a rapid, automated fashion. Because the Biosort measures the size of each nematode, the population size distribution of offspring after parental exposure can also be quantified, which could provide further insight into the chemical’s effects on growth and development (Boyd et al., 2009; Smith et al., 2009).
Because many organic chemicals have limited aqueous solubility, many chemical libraries such as the NTP’s 1408 (Xia et al., 2008) and the EPA’s ToxCast 320 (Dix et al., 2007) are prepared in 100% DMSO. For this reason, a concentration of DMSO that would not significantly affect C. elegans reproduction was determined. C. elegans reproduction was unaffected at DMSO concentrations below 1% DMSO, which confirmed that this solvent could be used as a vehicle in future C. elegans HTS.
Cadmium chloride was used to determine if Biosort data accurately reflect the effects of chemicals on C. elegans reproduction. Biosort EC50 values were compared to a previously published reproduction EC50 made by microscopic observations. An average EC50 of 185 μM for wild-type nematodes agreed with the previously reported value of 151 μM (Anderson et al., 2001). In addition, these values are comparable to those observed in other C. elegans toxicological assays. For example, an EC50 of 122 μM cadmium for C. elegans feeding was previously reported (Boyd et al., 2007).
An EC50of 18 μM cadmium was previously reported for C. elegans reproduction using the Biosort (Boyd et al., 2007). This difference may be attributed to the differences in the solvents used in the two studies. The previous study was performed using K-medium containing 1% DMSO (final concentration). The current study however was performed using completely aqueous media. The ability of DMSO to affect membrane permeability in toxicological assays has been well documented. Thus, DMSO may have affected cadmium’s bioavailability by allowing it to easily pass through the nematode cuticle. An analogous phenomena has been observed in C. elegans mutants in which the cuticle is more permeable. These mutants are more sensitive to chemical toxicity compared to wild type animals (Watanabe et al., 2005; Partridge et al., 2008).
Several potential mechanisms by which a chemical could reduce reproductive performance include: (1) diminished fertility, due to a decrease in oocyte or sperm production; (2) inhibited egg-laying, through neurotoxic effects on vulval neurons and muscles; or (3) reduced offspring survival, by impairing hatching or increasing embryonic lethality. The set of chemicals examined in this report have the potential to affect reproduction via several of these mechanisms.
The DNA alkylating agent EMS has been used as an effective chemical mutagen to generate point mutants in countless numbers of reverse genetic studies (Rosenbluth et al., 1983). Parent nematodes are typically exposed to 50 mM EMS for several hours after which their offspring are screened for desired phenotypes. Although the exposure concentration used for creating mutants is much higher than the EC50 for reproduction (50 mM vs. 470 μM), the exposure duration is also much shorter (4 h vs. 48 h). EMS-induced DNA damage would be expected to lead to the production of non-viable sperm and oocytes, as well as decreased embryonic hatching and survival. This hypothesis is supported by the observation that hundreds of embryonic lethal and reproduction-deficient C. elegans mutants have been isolated during EMS screens.
The pro-oxidant herbicides paraquat and diquat cause toxicity via generation of intracellular superoxide anions. The production of reactive oxygen species can damage a variety of cell types including oocytes and sperm (McCarthy et al., 2004; Salinas et al., 2006). Because of this increase in oxidative stress, a range of paraquat exposure conditions have been used to investigate stress tolerance in C. elegans (Ishii et al., 1990; Hartman et al., 1995; Khare et al., 2009; Nemoto-Sasaki and Kasai, 2009). Increased time to first egg-laying (Hartman et al., 1995) and decreased fecundity (Khare et al., 2009) have been observed in paraquat-exposed C. elegans at concentrations similar to those used in the current study (100-300 μM).
Parathion was the most potent inhibitor of reproduction, followed by cadmium and methadone. Methadone and parathion are potent disruptors of the central nervous system in humans acting by binding to opioid receptors (Garrido and Troconiz, 1999) and inhibiting cholinesterase activity (Aardema et al., 2008), respectively. Cadmium-induced neurotoxicity is caused by a variety of mechanisms including oxidative neuropathy and disruption of neurotransmitter release (Méndez-Armenta and Ríos, 2007). Neurotoxic phenotypes are also observed in C. elegans exposed to cadmium. Cadmium inhibits C. elegans neuromuscular activity (i.e., movement) and causes ‘bagging’, a phenotype in which hermaphrodites fail to lay embryos and larvae hatch inside the parents (Freedman, unpublished observation). Egg-laying defects are generally caused by a failure of vulval function and have been observed following exposure to other neurotoxicants (Bany et al., 2003; Tokuoka et al., 2008). In addition to being a neurotoxicant, cadmium also increases germ cell apoptosis and decreases germ cell proliferation in C. elegans (Wang et al., 2008). These various modes of cadmium toxicity would lead to a decrease in C. elegans reproduction. Although parathion and methadone exposures have not been previously described in C. elegans, they may also directly affect C. elegans egg-laying.
One of the goals for the development of alternative test species is their eventual application in the prediction of human toxicological responses. If C. elegans toxicological data is to be used to predict human toxicity, then the predictive power relative to traditional models of toxicity must be evaluated. To evaluate the C. elegans assay, the average reproduction EC50s were listed in rank order from most to least toxic, as compared to previously reported mouse and rat LD50s (Table 4). In the three species, parathion was found to have the highest toxicity followed by cadmium and methadone. In rodents, EMS was the least toxic; however, in C. elegans, EMS was ranked sixth with caffeine being the least. The Spearman non-parametric correlation r-values were 0.8214 and 0.9286 when regressing C. elegans EC50s against mouse and rat LD50s, respectively, compared to an r-value of 0.8571 for mouse vs. rat LD50s, with corresponding p-values of 0.0341, 0.0067, and 0.0238. Thus, an excellent correlation was observed between C. elegans and mice or rat toxicity. These correlations were at levels equal to that observed between mice and rats. Overall, C. elegans reproduction appears to be an excellent predictor of rodent lethality; however, more chemicals need to be tested to verify these results.
Table 4.
Comparison of average C. elegans reproduction EC50s with rodent oral LD50s
| Chemical | C. elegans EC50a | Mouse LD50b | Rat LD50b |
|---|---|---|---|
| Parathion | 1.51 | 5 | 2 |
| Cadmium | 185 | 60 | 88 |
| Methadone | 286 | 70 | 86 |
| Diquat | 745 | 233 | 120 |
| Paraquat | 1,921 | 120 | 150 |
| EMS | 4,697 | 470 | 350c |
| Caffeine | 10,097 | 127 | 192 |
EC50s expressed as μM
Oral LD50s expressed in mg/kg (Lewis, 2004)
Intraperitoneal injection LD50 expressed as mg/kg (Lewis, 2004)
Conclusion
To assess the large number of chemicals that are lacking sufficient toxicity data, international agencies are exploring the use of high-throughput screening and testing using alternative model organisms. When using alternative model organisms it is necessary to consider the advantages and limitations of each system. The C. elegans reproduction assay is rapid, reproducible, and responses can be observed for a wide range of chemicals. In the future, C. elegans responses to additional toxicant exposures will need to be correlated with responses observed in other species and in vitro HTS assays.
Supplementary Material
Acknowledgements
This work was supported in part by the National Toxicology Program, and by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (Z01ES102045 and Z01ES102046). Nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). The authors would like to thank Dr. Grace E. Kissling, Biostatistics Branch, NIEHS, for statistical advice.
Abbreviations
- TOF
time of flight (nematode length)
- EXT
extinction (optical density)
- EC50
half maximal effective concentration
- BMC
benchmark concentration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aardema H, Meertens J, Ligtenberg JJM, Peters-Polman OM, Tulleken JE, Zijlstra JG. Organophosphorus pesticide poisoning: cases and developments. Neth. J. Med. 2008;66:149–153. [PubMed] [Google Scholar]
- Anderson GL, Boyd WA, Williams PL. Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2001;20:833–838. [PubMed] [Google Scholar]
- Anderson P. Mutagenesis. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. Academic Press, Inc.; San Diego, CA: 1995. p. 58. [Google Scholar]
- Artal-Sanz M, de Jong L, Tavernarakis N. Caenorhabditis elegans: a versatile platform for drug discovery. Biotechnol. J. 2006;1:1405–1418. doi: 10.1002/biot.200600176. [DOI] [PubMed] [Google Scholar]
- Bany IA, Dong MQ, Koelle MR. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J. Neurosci. 2003;23:8060–8069. doi: 10.1523/JNEUROSCI.23-22-08060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WA, McBride SJ, Freedman JH. Effects of genetic mutations and chemical exposures on Caenorhabditis elegans feeding: evaluation of a novel, high-throughput screening assay. PLoS One. 2007;2:e1259. doi: 10.1371/journal.pone.0001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WA, Smith MV, Kissling GE, Freedman JH. Medium- and high-throughput screening of neurotoxicants using C. elegans. Neurotoxicol. Teratol. 2010;32:68–73. doi: 10.1016/j.ntt.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WA, Smith MV, Kissling GE, Rice JR, Snyder DW, Portier CJ, Freedman JH. Application of a mathematical model to describe the effects of chlorpyrifos on Caenorhabditis elegans development. PLoS One. 2009;4:e7024. doi: 10.1371/journal.pone.0007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull K, Cook A, Hopper NA, Harder A, Holden-Dye L, Walker RJ. Effects of the novel anthelmintic emodepside on the locomotion, egg-laying behaviour and development of Caenorhabditis elegans. Int. J. Parasitol. 2007;37:627–636. doi: 10.1016/j.ijpara.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Cole RD, Anderson GL, Williams PL. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol. Appl. Pharmacol. 2004;194:248–256. doi: 10.1016/j.taap.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Copeland RA. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis. Wiley-VCH Publishers; New York, NY: 2000. Chapter 5; pp. 109–145. [Google Scholar]
- Crump K. Critical issues in benchmark calculations from continuous data. Crit. Rev. Toxicol. 2002;32:133–153. doi: 10.1080/20024091064200. [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Dhawan R, Dusenbery DB, Williams PL. Comparison of lethality, reproduction, and behavior as toxicological endpoints in the nematode Caenorhabditis elegans. J. Toxicol. Environ. Health, Part A. 1999;58:451–462. doi: 10.1080/009841099157179. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol. Sci. 2007;95:5–12. doi: 10.1093/toxsci/kfl103. [DOI] [PubMed] [Google Scholar]
- Garrido MJ, Troconiz IF. Methadone: a review of its pharmacokinetic/pharmacodynamic properties. J. Pharmacol. Toxicol. Methods. 1999;42:61–66. doi: 10.1016/s1056-8719(00)00043-5. [DOI] [PubMed] [Google Scholar]
- Geng W, Cosman P, Palm M, Schafer WR. Caenorhabditis elegans egg-laying detection and behavior study using image analysis. EURASIP J. Appl. Si. Proc. 2005;2005:2229–2240. [Google Scholar]
- Hartman P, Childress E, Beyer T. Nematode development is inhibited by methyl viologen and high oxygen concentrations at a rate inversely proportional to life-span. J. Gerontol. A. Biol. Sci. Med. Sci. 1995;50:B322–B326. doi: 10.1093/gerona/50a.6.b322. [DOI] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- Ishii N, Takahashi K, Tomita S, Keino T, Honda S, Yoshino K, Suzuki K. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans. Mutat. Res. 1990;237:165–171. doi: 10.1016/0921-8734(90)90022-j. [DOI] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006;5:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Khanna N, Cressman CP, 3rd, Tatara CP, Williams PL. Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in aquatic media. Arch. Environ. Contam. Toxicol. 1997;32:110–114. doi: 10.1007/s002449900162. [DOI] [PubMed] [Google Scholar]
- Khare S, Gomez T, Linster CL, Clarke SG. Defective responses to oxidative stress in protein L-isoaspartyl repair-deficient Caenorhabditis elegans. Mech. Ageing Dev. 2009;130:670–680. doi: 10.1016/j.mad.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Poole DS, Waggoner LE, Kempf A, Ramirez DS, Treschow PA, Schafer WR. Genes affecting the activity of nicotinic receptors involved in Caenorhabditis elegans egg-laying behavior. Genetics. 2001;157:1599–1610. doi: 10.1093/genetics/157.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RJ. Sax’s Dangerous Properties of Industrial Materials. Jon Wiley & Sons; 2004. [Google Scholar]
- McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; Neuroprotection by water-soluble Coenzyme Q10. Toxicol. Appl. Pharmacol. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Méndez-Armenta M, Ríos C. Cadmium neurotoxicity. Environ. Toxicol. Phar. 2007;23:350–358. doi: 10.1016/j.etap.2006.11.009. [DOI] [PubMed] [Google Scholar]
- National Research Council . Scientific frontiers in developmental toxicology and risk assessment. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- Nemoto-Sasaki Y, Kasai K. Deletion of lec-10, a galectin-encoding gene, increases susceptibility to oxidative stress in Caenorhabditis elegans. Biol. Pharm. Bull. 2009;32:1973–1977. doi: 10.1248/bpb.32.1973. [DOI] [PubMed] [Google Scholar]
- Partridge FA, Tearle AW, Gravato-Nobre MJ, Schafer WR, Hodgkin J. The C. elegans glycosyltransferase BUS-8 has two distinct and essential roles in epidermal morphogenesis. Dev. Biol. 2008;317:549–559. doi: 10.1016/j.ydbio.2008.02.060. [DOI] [PubMed] [Google Scholar]
- Pulak R. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol. Biol. 2006;351:275–286. doi: 10.1385/1-59745-151-7:275. [DOI] [PubMed] [Google Scholar]
- Ratkowsky DA. Nonlinear Regression Modeling, A Unified Practical Approach. Marcel Dekker; New York, NY: 1983. Chapter 4; pp. 75–79. [Google Scholar]
- Rosenbluth RE, Cuddeford C, Baillie DL. Mutagenesis in Caenorhabditis elegans .1. a rapid eukaryotic mutagen test system using the reciprocal translocation, Eti(Iii-V) Mutat. Res. 1983;110:39–48. [Google Scholar]
- Salinas LS, Maldonado E, Navarro RE. Stress-induced germ cell apoptosis by a p53 independent pathway in Caenorhabditis elegans. Cell Death Differ. 2006;13:2129–2139. doi: 10.1038/sj.cdd.4401976. [DOI] [PubMed] [Google Scholar]
- Sand S, Victorin K, Filipsson A. The current state of knowledge on the use of the benchmark dose concept in risk assessment. J. Appl. Toxicol. 2008;28:405–421. doi: 10.1002/jat.1298. [DOI] [PubMed] [Google Scholar]
- Sicard M, Hering S, Schulte R, Gaudriault S, Schulenburg H. The effect of Photorhabdus luminescens (Enterobacteriaceae) on the survival, development, reproduction and behaviour of Caenorhabditis elegans (Nematoda: Rhabditidae) Environ. Microbiol. 2007;9:12–25. doi: 10.1111/j.1462-2920.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- Smith MV, Boyd WA, Kissling GE, Rice JR, Snyder DW, Portier CJ, Freedman JH. A discrete time model for the analysis of medium-throughput C. elegans growth data. PLoS One. 2009;4:e7018. doi: 10.1371/journal.pone.0007018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuoka SM, Saiardi A, Nurrish SJ. The mood stabilizer valproate inhibits both inositol- and diacylglycerol-signaling pathways in Caenorhabditis elegans. Mol. Biol. Cell. 2008;19:2241–2250. doi: 10.1091/mbc.E07-09-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent C, Tsung N, Horvitz HR. Egg-laying defective-mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Tang ML, Pei B, Xiao X, Wang J, Hang HY, Wu LJ. Cadmium-induced germline apoptosis in Caenorhabditis elegans: the roles of HUS1, p53, and MAPK signaling pathways. Toxicol. Sci. 2008;102:345–351. doi: 10.1093/toxsci/kfm220. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Mitani N, Ishii N, Miki K. A mutation in a cuticle collagen causes hypersensitivity to the endocrine disrupting chemical, bisphenol A, in Caenorhabditis elegans. Mutat. Res., Fundam. Mol. Mech. Mutagen. 2005;570:71–80. doi: 10.1016/j.mrfmmm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg-laying in C. elegans. J. Neurosci. 1995;15:6975–6985. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PL, Dusenbery DB. Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol. Ind. Health. 1988;4:469–478. doi: 10.1177/074823378800400406. [DOI] [PubMed] [Google Scholar]
- Wood WB. Introduction to C. elegans Biology. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. pp. 1–16. [Google Scholar]
- Xia M, Huang R, Witt KL, Southall N, Fostel J, Cho MH, Jadhav A, Smith CS, Inglese J, Portier CJ, Tice RR, Austin CP. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ. Health Perspect. 2008;116:284–291. doi: 10.1289/ehp.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.