Abstract
Heat shock proteins (HSPs) are highly regulated proteins that are involved in normal cellular activity and are up regulated when the cell is exposed to stress such as heat or excess reactive oxygen species (ROS) production. HSPs are molecular chaperones that mediate the proper folding of proteins and promote recovery of the native conformations of proteins lost due to stress. Improperly folded or denatured proteins tend to aggregate and accumulate in cells. A number of neurodegenerative diseases such as Parkinson disease (PD) and Alzheimer disease (AD) have been called “protein misfolding disorders” due their characteristic pathology. Until now the exact mechanism(s) of AD progression and pathogenesis largely remains unknown. Reasoning that stress is present in brain in AD, we tested the suggestion that HSP levels would be increased in amnestic mild cognitive impairment (aMCI), a transition stage between normal aging and AD. Accordingly, in the present study we measured the levels of HSPs in hippocampus, inferior parietal lobule (IPL) and cerebellum of subjects with aMCI. The results of show a general induction of HSPs and decreased levels of Thioredoxin 1 in aMCI brain suggesting that alteration in the chaperone protein systems might contribute to the pathogenesis and progression of AD. The results also are consistent with the notion that targeting HSP could be a therapeutic approach to delay the progression of aMCI to AD.
Keywords: Mild cognitive impairment, heat shock proteins, thioredoxin, hippocampus, cerebellum, inferior parietal lobule
Introduction
Amnestic mild cognitive impairment (aMCI) is a term used to define the transitional phase between normal aging and early Alzheimer disease (AD). aMCI, reportedly, represent a high-risk stage for the development of AD and about 10–15% patients convert to AD yearly (Petersen, 2003; Visser et al., 2006). aMCI individuals share similar pathology with AD including temporal lobe atrophy, low CSF Aβ levels, and the accumulation of neurofibrillary tangles in the hippocampus and entorhinal cortex (Chertkow et al., 2001; Morris and Cummings, 2005; Petersen et al., 1999). However, the severity of the pathology is reduced in aMCI compared to AD. Further aMCI brain showed increased levels of oxidative stress as indicated by elevated level of oxidative markers of proteins, lipids, carbohydrates, and nucleic acid compared to age-matched controls (Butterfield et al., 2006; Butterfield et al., 2007; Keller et al., 2005; Reed et al., 2008). In recent years, several studies support the functional importance of oxidative stress as a critical event in mediating neurodegeneration and AD pathogenesis (Butterfield and Lauderback, 2002; Markesbery, 1997; Perry et al., 1998).
Heat shock proteins (HSPs) are up-regulated in response to cellular stress to protect the cell from a variety of stresses (Kelly and Yenari, 2002; Yenari, 2002). In the central nervous system (CNS) HSP synthesis is induced after hyperthermia, by alterations to intracellular redox environment and by exposure to heavy metals, aminoacid analogs or cytotoxic drugs, among other stresses (Calabrese et al., 2002a; Calabrese et al., 2002b; Mosser and Morimoto, 2004). HSPs are produced as a part of the heat shock response and are one of the most highly expressed classes of cellular proteins. HSPs account for roughly 1–2% of total protein in unstressed cells, but the concentration increases to 4–6% of cellular proteins when proteins are heated or under stress (Garrido et al., 2001). Heat-shock proteins act as chaperones, binding and regulating protein conformation. HSPs also escort protein across membranes or through organelles, or alter enzyme activity. Under conditions of cell stress HSPs prevent protein misfolding and oligomerization and transfer misfolded proteins to the proteasome for degradation (Becker and Craig, 1994). HSP synthesis is tightly regulated at the transcriptional level by heat shock factors (HSF). Denatured proteins activate HSFs within the cytosol by dissociating other HSPs that are normally bound to HSF. Active HSF enter the nucleus and binds to the heat shock elements within the promoters of different heat shock genes leading to transcription and synthesis of HSPs (Calabrese et al., 2007).
In mammalian cells, the stress response involves the induction of 4 major members of HSP families named according to their molecular weight: HSP 27, HSP 60, HSP 70 and HSP 90. Recent studies propose that HSPs are part of an extended network of stress response and cytoprotection, called the vitagene system, which includes the thioredoxin (Trx) system (Calabrese et al., 2004; Calabrese et al., 2006a; Calabrese et al., 2007; Mancuso et al., 2007). Thioredoxin-1 is involved in a variety of redox-dependent pathways such as supplying reducing equivalents for ribonucleotide reductase and peptide methionine sulfoxide reductase. Several lines of evidence suggest that manipulation of the cellular stress response may offer strategies to protect brain cells from the damage encountered following cerebral ischemia or during the progression of neurodegenerative diseases (Butterfield et al., 2002; Halliwell, 2001; Perluigi et al., 2006).
Given that oxidative stress is present in aMCI brain (Butterfield and Lauderback, 2002; Butterfield et al., 2006; Butterfield et al., 2007; Keller et al., 2005; Markesbery, 1997; Reed et al., 2008) and that oxidative stress is known to induce HSPs, in the present study we measured changes in the protein levels of HSPs and Trx-1, two major constituents of cellular stress response machinery in different regions of aMCI brain.
Results
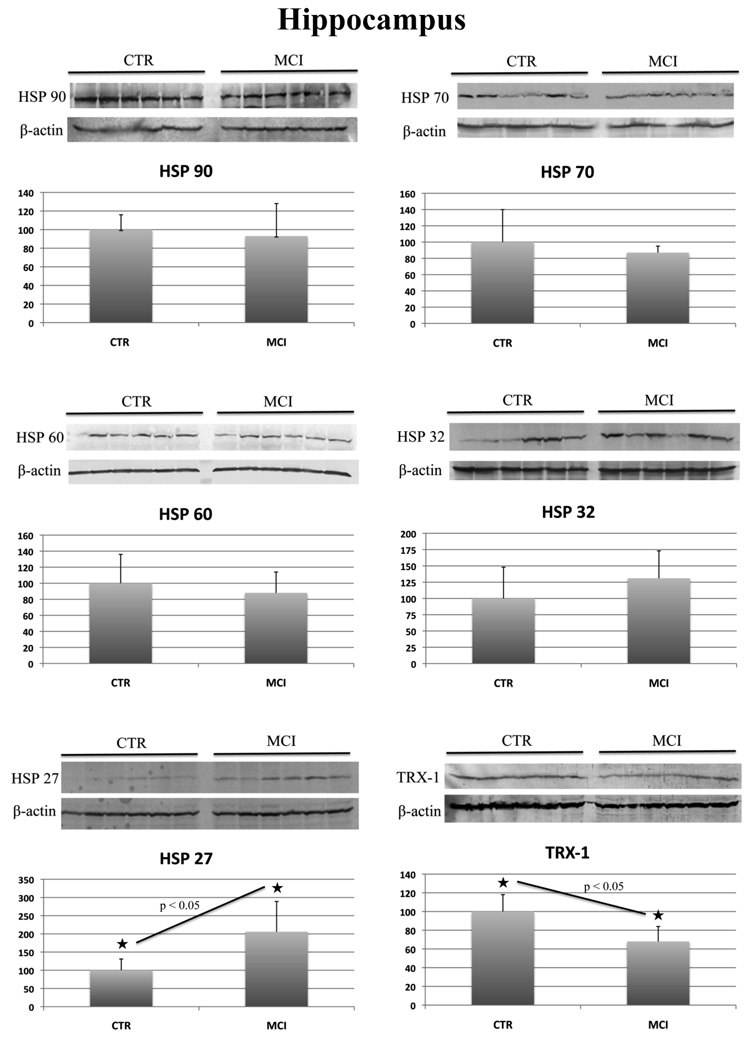
aMCI samples and age-matched control samples from hippocampus, IPL and cerebellum were homogenized, and analyzed separately by Western blot methods to determine the levels of HSPs and Trx-1. HSPs levels measured in the current study were HSP 27, 32, 60, 70 and 90. Table 2 summarizes the protein level results in all three brain regions. The expression values of each protein, in control and aMCI samples, have been normalized with β-actin levels, used as a loading control. The aMCI results are expressed as a percentage of the control values, arbitrarily set as 100%. As can be seen in Table 2 in hippocampus we found a significant (p value<0.05) increase of HSP 27 (200%) (Figure 1) in aMCI compared to control. Further, the levels of HSP 32 showed an increase of 130% (Figure 1) in aMCI compared to control, but the difference was not statistically significant (p value=0.16). The protein levels of Trx-1 were found significantly decreased by 32% in aMCI hippocampus compared to the control samples.
Table 2.
Summary of levels for HSPs and Trx-1 in hippocampus, IPL and cerebellum in aMCI compared to CTR (Taken as 100%) [Bold entries indicate statistically significant results]
| Inferior Parietal Lobule | Hippocampus | Cerebellum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CTR | MCI | p value |
CTR | MCI | p value |
CTR | MCI | p value |
|
| Hsp90 | 100±28 | 111±16 | 0.21 | 100±16 | 93±35 | 0.65 | 100±22 | 92±24 | 0.28 |
| Hsp70 | 100±27 | 157±54 | 0.047 | 100±40 | 87±8 | 0.47 | 100±12 | 104±18 | 0.83 |
| Hsp60 | 100±44 | 102±26 | 0.93 | 100±36 | 88±26 | 0.52 | 100±28 | 91±31 | 0.45 |
| Hsp32 | 100±42 | 130±26 | 0.16 | 100±48 | 131±42 | 0.33 | 100±17 | 121±30 | 0.16 |
| Hsp27 | 100±39 | 158±32 | 0.027 | 100±31 | 206±83 | 0.017 | 100±19 | 125±28 | 0.10 |
| Trx-1 | 100±34 | 104±32 | 0.84 | 100±18 | 68±16 | 0.018 | 100±26 | 41±15 | 0.0007 |
Figure 1.
HSP 27, 32, 60, 70, 90 and Trx-1 protein levels in control and aMCI hippocampus. For each protein the Western blot image (above) and the relative histogram (below) are shown. An equal amount (50 ug) of CTR (lane 1–6) and MCI (lane 7–12) samples were loaded onto a 8–16% SDS-PAGE gel, blotted onto nitrocellulose membrane and probed with specific antibodies. The immunoblots were then probed with β-actin, scanned and analyzed by image software. Densitometric values shown in the histogram are given as percentage of CTR, set as 100%, and are the product of the band value of the protein studied normalized with β-actin. n=6.
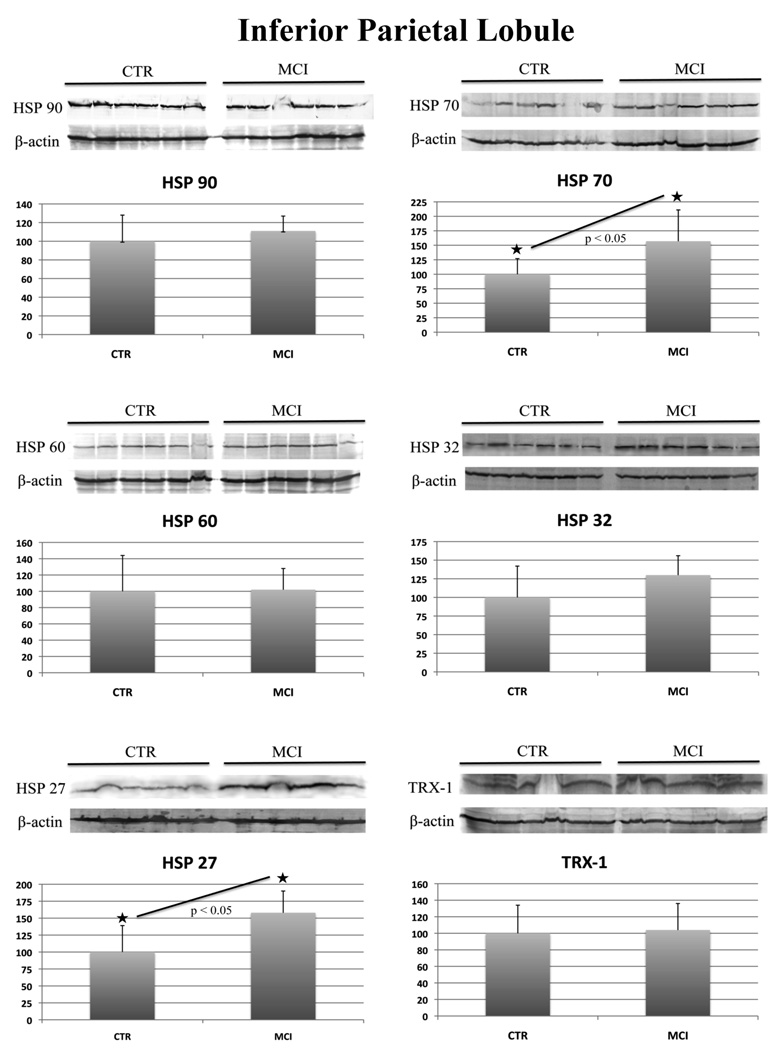
In aMCI IPL compared to control we found a 160% increase of HSP 27 and HSP 70 that was statistically significant (Figure 2). Like aMCI hippocampus, aMCI IPL have increased HSP 32 levels but the value is not significant (Figure 2).
Figure 2.
HSP 27, 32, 60, 70, 90 and Trx-1 protein levels in control and aMCI IPL. For each protein the Western blot image (above) and the relative histogram (below) are shown. An equal amount (50 ug) of CTR (lane 1–6) and MCI (lane 7–12) samples were loaded onto a 8–16% SDS-PAGE gel, blotted onto nitrocellulose membrane and probed with specific antibodies. Immunoblots were later on probed with β-actin, scanned by densitometry and all values were normalized to β-actin. Densitometric values shown in the histogram are given as percentage of CTR, set as 100%, and are the product of the band value of the protein studied normalized with β-actin. n=6.
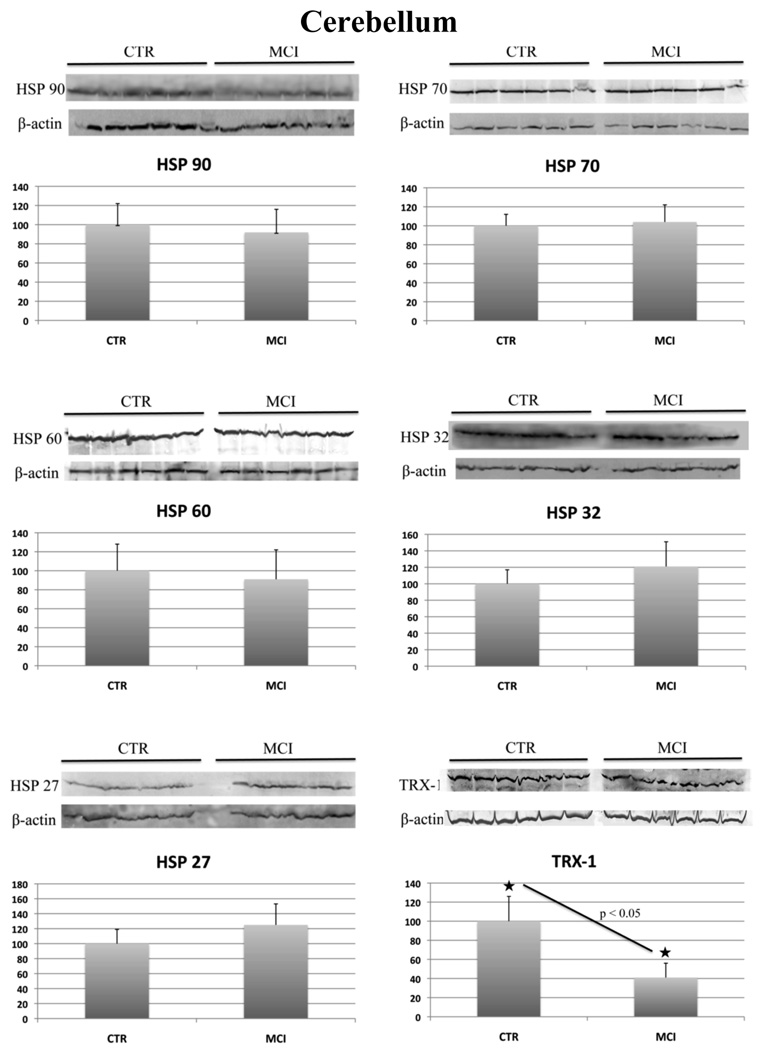
In aMCI cerebellum we did not observe any changes in the levels of any of the heat shock proteins analyzed. HSP 32 protein levels were found elevated in all the three brains regions but did not show a significant difference compared to respective age-matched controls. In contrast, the levels of Trx-1 were found decreased by 41% in aMCI cerebellum compared to that of controls (Figure 3).
Figure 3.
HSP 27, 32, 60, 70, 90 and Trx-1 protein levels in control and aMCI cerebellum. For each protein the Western blot image (above) and the relative histogram (below) are shown. An equal amount (50 ug) of CTR (lane 1–6) and MCI (lane 7–12) samples were loaded onto a 8–16% SDS-PAGE gel, blotted onto nitrocellulose membrane and probed with specific antibodies. Immunoblots were later on probed with β-actin, scanned by densitometry and all values were normalized to β-actin. Densitometric values showed in the histogram are given as percentage of CTR, set as 100%, and are the product of the band value of the protein studied normalized with β-actin. n=6.
Discussion
This is the first study that reports the protein levels of the main component of the HSP family and thioredoxin-1 in three different brain regions of aMCI hippocampus, IPL and cerebellum. Investigating the levels of HSP and Trx in aMCI may help to understand HSPs involvement in aMCI pathogenesis and progression to AD.
Like AD, brain from aMCI subjects show increased levels of oxidative stress markers and (Butterfield and Lauderback, 2002; Butterfield et al., 2006; Butterfield et al., 2007; Keller et al., 2005; Markesbery, 1997; Reed et al., 2008). The accumulation of oxidative stress markers that occur in aMCI brain are consistent with the notion that oxidative stress could be an early event in the conversion of aMCI subjects to AD (Muchowski and Wacker, 2005; Sultana and Butterfield, 2009). The alterations of protein structure by oxidative damage generally impair function and lead to the formation of protein aggregates in the cytosol.
HSPs appear to be up-regulated in several neurodegenerative diseases acting to protect brain cells against free radical injury, oxidative stress and misfolded proteins. (Calabrese et al., 2004; Kalmar and Greensmith, 2009). In AD, HSPs expression is associated with Aβ deposition and neurofibrillary tangles, and recent findings suggest that HSPs prevent the accumulation of Aβ (Abdul et al., 2006; Evans et al., 2006; Shimura et al., 2004; Sultana et al., 2005). In view of the increased oxidative stress parameters observed in aMCI, we expected to see increased levels of all of the stress induced heat shock proteins. However, we observed that only the levels of HSP 70 in IPL and HSP 27 in IPL and hippocampus are increased in aMCI compared to controls with no effects on the other members of HSPs measured in the current study. Although, HSP 32 showed increased levels in all the three aMCI regions studied, these increases were not statistically significant. Our results suggest that not all the HSPs protein levels are affected in similar way in different regions of the brain in this early stage of the AD.
HSP 27 and HSP 70, among all HSPs, are the most strongly induced after stresses such as oxidative stress, anticancer drugs, or irradiation (Lanneau et al., 2008). HSP 27 is an ATP-independent chaperone that protects the cells from protein aggregation. The affinity of HSP 27 for proteins to be chaperoned is modulated by its phosphorylation and oligomerization status (Shimura et al., 2004). In AD brain, increased expression of HSP 27 associated with NFTs has been found, especially in proliferating and degenerating astrocytes and hippocampal neurons (Bjorkdahl et al., 2008; Charette and Landry, 2000; Renkawek et al., 1993; Renkawek et al., 1994). Our findings reporting increased levels of HSP 27 in both hippocampus and IPL of aMCI are consistent with the increase oxidative stress in these two aMCI regions (Butterfield et al., 2006; Butterfield et al., 2007; Keller et al., 2005). The increased oxidative stress and consequent oxidative damage of proteins and other cellular component could easily explain the increased levels of HSP 27. However, the lack of a broad increase of all the HSPs analyzed in aMCI suggests that other factors may influence HSP 27 induction. Increased HSP 27 levels might be favored by the occurrence of hyperphosphorylated tau and consequent formation of NFTs. Several studies showed that HSP 27 directly binds to hyperphosphorylated tau, thereby protecting against cell death (Bjorkdahl et al., 2008). Moreover, HSP 27 facilitates the degradation of hyperphosphorylated tau without ubiquitination (Shimura et al., 2004). Hyperphosphorylated tau and NFT are present in aMCI hippocampus and IPL, and increased levels of HSP 27 are consistent with increased risk for conversion to AD (Markesbery et al., 2006). HSP 27 also has a direct function in blocking apoptosis and several mechanisms have been proposed to account for its negative regulation of programmed cell death (Garrido et al., 2001). HSP 27 specifically interacts with released cytochrome c from the mitochondria present in the cytosol and inhibits the activation of procaspase-9, an initiator caspase, in apoptosis (Parcellier et al., 2003). Further, HSP 27 inhibits Daxx, a Fas-binding protein that activates apoptosis (Charette and Landry, 2000). Recently our laboratory showed evidence for an increased activation of apoptosis by the activation of the p53 pathway in aMCI brain (Cenini et al., 2008a; Cenini et al., 2008b). HSP 27, as a negative regulator of apoptosis, might increase as a consequence of the activation of the p53 apoptotic pathway. Consequently elevated level of HSP 27 in MCI can be considered as a process triggered early in the progression of AD. Interestingly, the up regulation of clusterin, a versatile chaperone protein and functional homologue of HSP 27, is gaining recently remarkable attention as a valuable neuroprotective response during AD progression (Bertram and Tanzi; Nuutinen et al., 2009). Clusterin has high affinity for Aβ peptides and oligomers and is involved in prevention of Aβ aggregation, Aβ clearance and in the suppression of apoptotic signals (Calero et al., 2005; Trougakos and Gonos, 2006; Yerbury and Wilson), supporting the functional role proposed for HSP 27.
HSP 70 is the major heat-inducible chaperone protein that exerts a cytoprotective effect under a number of different conditions. HSP 70 prevents protein aggregation, assists in the refolding of damaged proteins, and chaperones nascent polypeptides along ribosomes (Calabrese et al., 2006a). Moreover, HSP 70 is also involved in the ubiquitin-proteasome pathway. HSP 70 shows low expression levels in brain under physiological conditions, but it is strongly induced during oxidative stress (Calabrese et al., 2000; Calabrese et al., 2002a). In AD, in which misfolded brain proteins accumulate, HSP 70 synthesis is increased in various cell populations of the CNS and in several brain regions including the IPL, hippocampus and cerebellum. Recently, HSP 70 was identified as a protector against intracellular Aβ accumulation: its overexpression rescued neurons from Aβ-mediated toxicity (Evans et al., 2006; Magrane et al., 2004). To explain this phenomenon, it has been proposed that HSP 70 acts to attenuate the cytotoxicity of Aβ by binding amyloidogenic peptides and restoring the balance between aggregation, folding and degradation (Evans et al., 2006). Interestingly, previous redox proteomics studies from our laboratory identified HSP 70 as oxidatively modified by carbonylation in aMCI IPL (Sultana et al., 2009). HSP 70 carbonylation may inactivate its function, leading to the accumulation of toxic oligomeric assemblies of Aβ peptides and subsequent oxidative damage. The current results show increased HSP 70 levels in IPL and correlates with HSP 70 carbonylation in the same region. Hence, we propose that the inactivation of HSP 70 by oxidation may lead to an induction of HSP 70 expression in attempt to preserve its cytoprotective capacity to defend against oxidative stress.
Trx-1 expression levels represent a valuable tool to understand the capability of the cell to counteract oxidative stress conditions in aMCI. Thioredoxin is a multifunctional and ubiquitous protein characterized by the presence of a redox-active disulfide/dithiol within its conserved active site, essential to maintain the redox homeostatic state of the cells. Trx scavenges ROS, regulates transcription factors and modifies the structure of proteins by reducing disulfides (Calabrese et al., 2006b; Lovell et al., 2000). Trx and GSH act together to form a powerful system controlling redox regulation of gene expression, signal transduction, cell proliferation, protection against oxidative stress, anti-apoptotic functions, growth factor and co-cytokine effects, as well as a variety of redox-dependent pathways such as supplying reducing equivalents (Nordberg and Arner, 2001). Previous data demonstrate a general decrease in Trx levels in AD brain regions: a significant decrease in the levels of Trx-1 were reported for amygdala, SMTG and hippocampus (Lovell et al., 2000), which is consistent with the presence of high levels of Aβ in these regions. Furthermore, neuronal culture studies also show that Trx-1 acts as a radical scavenger inhibiting neuronal injury induced by Aβ (Lovell et al., 2000). Our current results of decreased Trx-1 levels in aMCI hippocampus, mirroring the situation in AD, are consonant with the notion that oxidative stress is an early event in the progression of the AD. The hippocampus in aMCI, presents a large amount of oxidative damage, NFT, and Aβ deposition. The decrease in Trx-1 levels in aMCI hippocampus conceivably could be due to the general increase of oxidatively modified proteins and decrease levels of redox molecules in this an arguably earliest form of AD, that might be instrumental in the progression of aMCI to AD. However, it is interesting to note that the levels of Trx-1 are also significantly lower in aMCI cerebellum, a region that is devoid of AD pathology and oxidative stress (Keller et al., 2005; Markesbery, 2009). At present we have no explanation for this observation, but we speculate that the different cell types between hippocampus and cerebellum may somehow contribute to these results.
HSP 32 is also known as heme oxygenase 1 (HO-1), but differs from the other chaperone molecule by protecting brain cells from oxidative stress by degrading toxic heme into free iron, carbon monoxide (CO) and biliverdin (Errico et al., 2010). Biliverdin is then reduced into bilirubin (BR), a linear tetrapyrrole with antioxidant properties (Calabrese et al., 2007). Although, HSP 32 was not statistically significantly increased in all the regions of the brain in aMCI studied increased levels were found, compared to age-matched controls. This result suggest that increased HSP 32 levels in AD may be initiated in aMCI that lead to a significant increase in the levels of HO-1 associated with NFTs, Aβ deposition and oxidative damage in AD (Takeda et al., 2000).
Our study gives a broad picture on HSP response in aMCI brain. HSP induction is one of the first effective responses of the cell to stress and is an important tool for the protection of the brain from degeneration and progression to disease. aMCI brain demonstrates oxidative stress and AD hallmarks, NFTs and Aβ deposition, but in a milder status compared to AD. The brain’s capability to protect and counteract the increase of damage and subsequent neurodegeneration process is given by the ability to activate cytoprotective elements like HSPs. Our findings show a general low induction of HSPs in this early stage of AD, with the exceptions of HSP 27 and 70, coupled with a reduction of Trx-1 levels. During aMCI, this low induction of HSPs promotes oxidative modification of proteins altering functional activity of the cell. Moreover, the increase of oxidative stress and the weak cell stress response to increased oxidative damage might represent a favorable condition for the progression from aMCI to AD, playing a key role in neurodegenerative processes. Previous studies from our laboratory investigated with success the ability of ferulic acid ethyl ester (FAEE), a phenolic compound that acts as a strong HSP inducer, to potentiate cell stress response (Joshi et al., 2006; Perluigi et al., 2006; Sultana et al., 2005), providing protection to the cell from oxidative stress and Aβ toxicity. Our results suggest that alterations in chaperone protein systems might be a mechanism in the pathogenesis and progression of AD, and that developing a therapeutic approach to induce HSP levels could be a potential strategy to treat or delay the onset of AD.
Experimental procedures
Materials
Unless otherwise stated, chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nitrocellulose membranes and electrophoresis transfer system Trans-blot semi-dry Transfer Cell were obtained from Bio-Rad (Hercules, CA, USA), while anti-HSP 27, anti-HSP 60 and anti-HSP 70 mouse monoclonal antibodies were purchased from Assay Designs, Inc. (Ann Arbor, MI, USA). Anti-HSP 32 mouse monoclonal antibody was obtained from Calbiochem (San Diego, CA, USA), while anti-HSP90 mouse monoclonal antiboby was obtained from AbCam (Cambridge, MA, USA). Anti-Trx-1 was purchased from Millipore (Temecula, CA, USA), anti-β actin rabbit polyclonal antibody, anti-mouse and anti rabbit IgG alkaline phosphatase secondary antibody were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-mouse and anti-rabbit IgG horseradish peroxidase conjugate secondary antibody and ECL plus Western blot detection reagents were obtained from GE Healthcare Bio-Sciences corp. (Piscataway, NJ, USA).
Subjects
Frozen hippocampus, inferior parietal lobule (IPL), and cerebellum samples from aMCI and age-matched controls (6 Controls and 6 aMCI for each brain region) were obtained from the University of Kentucky Rapid Autopsy Program of the Alzheimer’s Disease Clinical Center (UK ADC) with an average PMI of 3 h for aMCI patients and control subjects. All the subjects were longitudinally followed and underwent annual neuropsychological testing, and neurological and physical examinations. Control subjects were without history of dementia or other neurological disorders and with intact activities of daily living (ADLs), they underwent annual mental status testing and semi-annual physical and neurological exams as part of the UK ADC normal volunteer longitudinal aging study. aMCI patients met the criteria described by Petersen (Petersen, 2003), which include: a memory complaint supported by an informant, objective memory test impairment (age- and education-adjusted), general normal global intellectual function, intact ADLs, Clinical Dementia Rating score of 0.0 to 0.5, no dementia, and a clinical evaluation that revealed no other cause for memory decline. The control subjects showed no significant histopathological alterations and the average Braak score was 1.33±0.52. The aMCI patients had an average Braak score of 3.8±1.0. The demographic parameters of controls and aMCI subjects are provided in Table 1.
Table 1.
Demographic information of the control and aMCI.
| Age (years) | Sex | Brain Weight (g) |
PMI (hrs) | Braak | |
|---|---|---|---|---|---|
| Control 1 | 93 | Female | 1080 | 2.75 | 2 |
| Control 2 | 74 | Male | 1140 | 4.00 | 1 |
| Control 3 | 86 | Female | 1150 | 1.75 | 1 |
| Control 4 | 76 | Female | 1315 | 2.00 | 1 |
| Control 5 | 79 | Male | 1240 | 1.75 | 2 |
| Control 6 | 86 | Female | 1300 | 3.75 | 1 |
| Average | 82 ± 7.2 | 1204 ± 95 | 2.67 ± 1.01 | 1.33 ± 0.52 | |
| MCI 1 | 99 | Female | 930 | 2.00 | 5 |
| MCI 2 | 88 | Female | 1080 | 2.25 | 5 |
| MCI 3 | 87 | Male | 1200 | 3.50 | 4 |
| MCI 4 | 87 | Male | 1170 | 2.25 | 3 |
| MCI 5 | 91 | Female | 1155 | 5.00 | 3 |
| MCI 6 | 82 | Female | 1075 | 3.00 | 3 |
| Average | 89 ± 5.7 | 1102 ± 98 | 3.0 ± 1.1 | 3.8 ± 1.0 |
Sample preparation
The brain tissues (hippocampus, IPL and cerebellum) from control and aMCI were sonicated in Media 1 lysis buffer (pH 7.4) containing 320 mM Sucrose, 1% of 990 mM Tris-HCl (pH=8.8), 0.098 mM MgCl2, 0.076 mM EDTA, as well as proteinase inhibitors leupeptin (0.5 m g/mL), pepstatin (0.7 μg/mL), aprotinin (0.5 mg/mL) and PMSF (40 μg/mL). Homogenates were centrifuged at 14,000 × g for 10 min to remove debris. Protein concentration in the supernatant was determined by the Pierce BCA method (Pierce, Rockford, IL, USA).
Western blot analysis
For Western blot analyses 50μg of protein were denaturated in sample buffer for 5 min at 100 °C, and proteins were separated on 8–16 % precast Criterion gels (Bio-Rad) by electrophoresis at 100 mA for 2 h into Bio-Rad apparatus. The proteins from the gels were then transferred to nitrocellulose membrane using the Transblot-BlotSD Semi-Dry Transfer Cell at 20 mA for 2 hr. Subsequently, the membranes were blocked at 4°C for 1 h with fresh blocking buffer made of 3% bovine serum albumin (BSA) in phosphate-buffered saline containing 0.01% (w/v) sodium azide and 0.2% (v/v) Tween 20 (PBST). The membranes were incubated with primary antibody in PBST for 2 hr with gentle rocking at room temperature. All the membranes were co-incubated with actin or Tubulin antibody to validate equal loading of the proteins. The membranes were then washed three times for 5 min with PBST followed by incubation with anti-mouse alkaline phosphatase or horseradish peroxidase conjugate secondary antibody (1:3000) in PBST for 2 hr at room temperature. Membranes were then washed three times in PBST for 5 min and developed using or 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium (BCIP/NBT) color developing reagent for alkaline phosphatase secondary antibody or ECL plus WB detection reagents for horseradish peroxidase conjugate secondary antibody. Blots were dried, scanned or in TIF format using Adobe Photoshop on a Canoscan 8800F (Canon) or STORM UV transilluminator (λex=470 nm, λem=618 nm, Molecular Dynamics, Sunnyvale, CA, USA) for chemiluminescence. The images has been quantified with Image Quant TL 1D version 7.0 software (GE Healthcare)
Statistical analysis
Two-tailed, Student’s t-tests were used to analyze differences in protein levels between aMCI sample and age-matched controls samples. A p-value of less than 0.05 was considered statistically significant
Acknowledgements
This work was supported by NIH grants to DAB [AG-05119; AG-10836], and FDD was supported by a fellowship from Istituto Pasteur – Fondazione Cenci Bolognetti
Abbreviations
- HSP
heat shock protein
- ROS
reactive oxygen species
- AD
Alzheimer disease
- aMCI
amnestic mild cognitive impairment
- IPL
inferior parietal lobule
- Trx
thioredoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul HM, Calabrese V, Calvani M, Butterfield DA. Acetyl-L-carnitine-induced up-regulation of heat shock proteins protects cortical neurons against amyloid-beta peptide 1–42-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. J Neurosci Res. 2006;84:398–408. doi: 10.1002/jnr.20877. [DOI] [PubMed] [Google Scholar]
- Becker J, Craig EA. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994;219:11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Alzheimer disease: New light on an old CLU. Nat Rev Neurol. 6:11–13. doi: 10.1038/nrneurol.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkdahl C, Sjogren MJ, Zhou X, Concha H, Avila J, Winblad B, Pei JJ. Small heat shock proteins Hsp27 or alphaB-crystallin and the protein components of neurofibrillary tangles: tau and neurofilaments. J Neurosci Res. 2008;86:1343–1352. doi: 10.1002/jnr.21589. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Drake J, Scapagnini G, Calabrese V. Vitamin E and neurodegenerative disorders associated with oxidative stress. Nutr Neurosci. 2002;5:229–239. doi: 10.1080/10284150290028954. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol Dis. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, Keller JN, Markesbery WR, Sultana R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007;1148:243–228. doi: 10.1016/j.brainres.2007.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Testa G, Ravagna A, Bates TE, Stella AM. HSP70 induction in the brain following ethanol administration in the rat: regulation by glutathione redox state. Biochem Biophys Res Commun. 2000;269:397–400. doi: 10.1006/bbrc.2000.2311. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Fariello RG, Giuffrida Stella AM, Abraham NG. Regional distribution of heme oxygenase, HSP70, and glutathione in brain: relevance for endogenous oxidant/antioxidant balance and stress tolerance. J Neurosci Res. 2002a;68:65–75. doi: 10.1002/jnr.10177. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Giuffrida Stella AM, Butterfield DA. Molecular chaperones and their roles in neural cell differentiation. Dev Neurosci. 2002b;24:1–13. doi: 10.1159/000064941. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Stella AM, Butterfield DA, Scapagnini G. Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid Redox Signal. 2004;6:895–913. doi: 10.1089/ars.2004.6.895. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Butterfield DA, Scapagnini G, Stella AM, Maines MD. Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: relevance to brain aging, neurodegenerative disorders, and longevity. Antioxid Redox Signal. 2006a;8:444–477. doi: 10.1089/ars.2006.8.444. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G, Mancuso C, Stella AM, Butterfield DA. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxid Redox Signal. 2006b;8:1975–1986. doi: 10.1089/ars.2006.8.1975. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Guagliano E, Sapienza M, Panebianco M, Calafato S, Puleo E, Pennisi G, Mancuso C, Butterfield DA, Stella AG. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem Res. 2007;32:757–773. doi: 10.1007/s11064-006-9203-y. [DOI] [PubMed] [Google Scholar]
- Calero M, Rostagno A, Frangione B, Ghiso J. Clusterin and Alzheimer’s disease. Subcell Biochem. 2005;38:273–298. [PubMed] [Google Scholar]
- Cenini G, Sultana R, Memo M, Butterfield DA. Effects of oxidative and nitrosative stress in brain on p53 proapoptotic protein in amnestic mild cognitive impairment and Alzheimer disease. Free Radic Biol Med. 2008a;45:81–85. doi: 10.1016/j.freeradbiomed.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenini G, Sultana R, Memo M, Butterfield DA. Elevated levels of pro-apoptotic p53 and its oxidative modification by the lipid peroxidation product, HNE, in brain from subjects with amnestic mild cognitive impairment and Alzheimer’s disease. J Cell Mol Med. 2008b;12:987–994. doi: 10.1111/j.1582-4934.2008.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette SJ, Landry J. The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann N Y Acad Sci. 2000;926:126–131. doi: 10.1111/j.1749-6632.2000.tb05606.x. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bergman H, Schipper HM, Gauthier S, Bouchard R, Fontaine S, Clarfield AM. Assessment of suspected dementia. Can J Neurol Sci. 2001;28(Suppl 1):S28–S41. doi: 10.1017/s0317167100001189. [DOI] [PubMed] [Google Scholar]
- Evans CG, Wisen S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J Biol Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Joshi G, Perluigi M, Sultana R, Agrippino R, Calabrese V, Butterfield DA. In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane) dihydrochloride (AAPH) or Fe(2+)/H(2)O(2): insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem Int. 2006;48:318–327. doi: 10.1016/j.neuint.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Kelly S, Yenari MA. Neuroprotection: heat shock proteins. Curr Med Res Opin. 2002;18(Suppl 2):s55–s60. [PubMed] [Google Scholar]
- Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Gabbita SP, Markesbery WR. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer’s disease brain. Free Radic Biol Med. 2000;28:418–427. doi: 10.1016/s0891-5849(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Magrane J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso C, Bates TE, Butterfield DA, Calafato S, Cornelius C, De Lorenzo A, Dinkova Kostova AT, Calabrese V. Natural antioxidants in Alzheimer’s disease. Expert Opin Investig Drugs. 2007;16:1921–1931. doi: 10.1517/13543784.16.12.1921. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Neuropathologic Alterations in Mild Cognitive Impairment: A Review. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2010-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J Alzheimers Dis. 2005;7:235–239. doi: 10.3233/jad-2005-7306. discussion 255–62. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–2918. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Clusterin: a forgotten player in Alzheimer’s disease. Brain Res Rev. 2009;61:89–104. doi: 10.1016/j.brainresrev.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, Plenchette S, Khochbin S, Solary E, Garrido C. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perluigi M, Joshi G, Sultana R, Calabrese V, De Marco C, Coccia R, Cini C, Butterfield DA. In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1–42-induced oxidative stress. J Neurosci Res. 2006;84:418–426. doi: 10.1002/jnr.20879. [DOI] [PubMed] [Google Scholar]
- Perry G, Castellani RJ, Hirai K, Smith MA. Reactive Oxygen Species Mediate Cellular Damage in Alzheimer Disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment clinical trials. Nat Rev Drug Discov. 2003;2:646–653. doi: 10.1038/nrd1155. [DOI] [PubMed] [Google Scholar]
- Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol Dis. 2008;30:107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Bosman GJ, Gaestel M. Increased expression of heat-shock protein 27 kDa in Alzheimer disease: a preliminary study. Neuroreport. 1993;5:14–16. doi: 10.1097/00001756-199310000-00003. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Bosman GJ, de Jong WW. Expression of small heat-shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol. 1994;87:511–519. doi: 10.1007/BF00294178. [DOI] [PubMed] [Google Scholar]
- Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004;279:17957–17962. doi: 10.1074/jbc.M400351200. [DOI] [PubMed] [Google Scholar]
- Sultana R, Ravagna A, Mohmmad-Abdul H, Calabrese V, Butterfield DA. Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1–42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92:749–758. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J Bioenerg Biomembr. 2009 doi: 10.1007/s10863-009-9241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Newman SF, Pierce WM, Cini C, Coccia R, Butterfield A. Redox Proteomic Analysis of Carbonylated Brain Proteins in Mild Cognitive Impairment and Early Alzheimer’s Disease. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Perry G, Abraham NG, Dwyer BE, Kutty RK, Laitinen JT, Petersen RB, Smith MA. Overexpression of heme oxygenase in neuronal cells, the possible interaction with Tau. J Biol Chem. 2000;275:5395–5399. doi: 10.1074/jbc.275.8.5395. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic Res. 2006;40:1324–1334. doi: 10.1080/10715760600902310. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Kester A, Jolles J, Verhey F. Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology. 2006;67:1201–1207. doi: 10.1212/01.wnl.0000238517.59286.c5. [DOI] [PubMed] [Google Scholar]
- Yenari MA. Heat shock proteins and neuroprotection. Adv Exp Med Biol. 2002;513:281–299. doi: 10.1007/978-1-4615-0123-7_10. [DOI] [PubMed] [Google Scholar]
- Yerbury JJ, Wilson MR. Extracellular chaperones modulate the effects of Alzheimer’s patient cerebrospinal fluid on Abeta(1–42) toxicity and uptake. Cell Stress Chaperones. 15:115–121. doi: 10.1007/s12192-009-0122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]