Abstract
IL-7 is required for B cell development in mouse and is a key regulator of T cell development and peripheral T cell homeostasis in mouse and human. Recently, we found that IL-7 is expressed in rabbit bone marrow and in vitro, is required for differentiation of lymphoid progenitors to B and T lineage cells. Herein, we report the identification of a novel rabbit IL-7 isoform, IL-7II. Recombinant IL-7II (rIL-7II) binds lymphocytes via the IL-7R and induces phosphorylation of STAT5. Further, rIL-7II supports proliferation and differentiation of BM progenitor cells into B and T lineage cells. IL7-II is generated by alternative splicing, with an 11 amino acid insertion encoded by a separate exon, exon 2b. Exon 2b is conserved in other lagomorphs, in Perissodactyla, Artiodactyla, and Carnivora, but is absent in mouse and human.
Keywords: IL-7, Alternative splicing, Isoform, Lymphopoiesis, Flow cytometry
1. INTRODUCTION
Resident stromal cells in bone marrow (BM) and thymus secrete IL-7, which promotes the survival, proliferation, and differentiation of B and T cell precursors (1–4). In mouse, IL-7 can bind to lymphocyte precursors through either of two heterodimeric receptors which are composed of the high affinity IL-7Rα chain and common γ chain (5–7), or the high affinity IL-7Rα chain and the thymic stromal lymphopoetin receptor (TSLPR) (8, 9). IL-7R signaling activates three distinct cascades including the JAK/STAT, phosphoinositide-3-kinase (PI3K), and MAPK/ERK pathways (10).
The role of IL-7/IL-7R interaction in lymphopoiesis is evident in human patients with mutations in either the IL-7Rα or γc chains. These patients lack all T cell subsets, yet B cell development remains unperturbed (11, 12). While these data suggest that IL-7 may not be required for B lymphopoiesis, recent in vitro data suggest that B cell production in the BM of adults is dependent on IL-7 (13).
IL-7Rα−/− and IL-7−/− mice have a block in γδ T cell development (14, 15), and produce new B cells only for a few months after birth. A similar arrest of B lymphopoiesis is observed in normal rabbits whereby B lymphopoiesis begins to wane approximately 1 month after birth and arrests by 4 months of age (16). The similarity in B lymphopoiesis arrest between IL-7Rα−/− and IL-7−/− mice and adult rabbits led to the investigation of a possible role of IL-7 in rabbit B lymphopoiesis.
Kalis et al cloned rabbit IL-7 cDNA and showed that recombinant IL-7(rIL-7) binds to a population of BM lymphoid progenitors (LPs) that can differentiate into either B or T cell precursors (17). Further, by in vitro LP differentiation on OP9 stromal cells the authors demonstrated that IL-7 is required for both B cell and T cell development. Assuming that IL-7 is also required in vivo for B cell development, one explanation for the observed arrest in B lymphopoiesis in adult rabbits would be that the level of IL-7 declines with age. By northern blot analysis, Kalis et al found an increase in IL-7 transcripts in adult rabbit BM, indicating that limited IL-7 expression is not the cause for the arrest of B lymphopoiesis (17).
During our previous studies, we identified cDNA that potentially encoded an isoform of IL-7. Examples of alternate isoforms that regulate and modulate cytokine activity abound in the literature (reviewed in 23), and in fact, several shorter human and equine IL-7 isoforms exist (18, 19). In this study, we characterized the isoform of IL-7 encoded by the recently identified cDNA and showed that it is generated by alternative splicing and like IL-7, the IL-7II isoform binds to the IL-7R and leads to activation of the JAK/STAT pathway. Further, we show in vitro, that IL-7II does not inhibit lymphopoiesis, but instead, promotes both B and T lymphopoiesis.
2. MATERIALS AND METHODS
2.1. Animals and DNA
Rabbits were maintained at Loyola University Chicago, Maywood, IL. The studies were approved by the Institutional Animal Care and Use Committee of Loyola University Chicago. Whole blood for extraction of DNA from horse, cow, pig, zebra, leopard and giraffe was obtained from animals housed at the Brookfield Zoo (Brookfield, IL.). Genomic DNA from cottontail, pika, and jackrabbit were as described previously (20). All other genomic DNA was obtained by phenol chloroform extraction method. cDNA of jackrabbit was obtained from spleen samples collected at Centro de Investigacao em Biodiversidade e Recursos Geneticos in Portugal.
2.2. Antibodies and flow cytometry
To generate mAb against IL-7, spleen cells were obtained from BALB/C mice immunized with IL-7 produced in E. coli. Hybridomas were generated (21) and those secreting antibody specific for IL-7 were identified by ELISA using microtiter plates coated with IL-7. One clone, 5H5, reacted with recombinant IL-7 (rIL-7) and IL-7II (rIL-7II) by ELISA and by western blot, was selected for use in this study. Both rIL-7 and rIL-7II proteins used for mAb screening were derived from E. coli as described previously (17). B lineage cells arising from co-cultures were identified using anti-CD79a (BD Pharmingen, clone HM47) and anti-IgM (BD Pharmingen, clone 367). Cytoplasmic staining with anti-CD79a and anti-IgM (clone 367) was performed after fixing and permeabilizing the cells using Cytofix/perm kit (BD Pharmingen) according to the manufacturers’ instructions. T cells from co-cultures were identified using either anti-CD3 (Spring Valley, clone MC1-B), followed by a biotinylated goat-anti-mouse Ig antibody and detected with fluorochrome labeled streptavidin. B cells from primary tissues were identified using anti-IgM (BD Pharmingen, clone 367) and T cells were identified using anti-CD43 (BD Pharmingen, clone L11/35) Analysis by flow cytometry was performed on a FACSCanto flow cytometer (BD Biosciences); only cells that fell within a lymphocyte FSC versus SSC gate were analyzed. Acquired flow cytometry data were analyzed using the Flowjo software (Treestar inc).
2.3. Purification of recombinant IL-7 and IL-7II
Recombinant IL-7 and IL-7II were purified from mammalian cells as follows. A cDNA encoding the ORF of IL-7II was PCR-amplified with sense primer 5′ATGTTCCATGTTTCTTTTAGGTATATCTTTGGAATT 3′ and anti-sense primer 5′ CATCATCACCATCACCATTGA 3′. This cDNA and also IL-7 cDNA were cloned with Myc and 6X His tags into pMEPURO vector, a generous gift of Dr. M. Iwashima (Loyola University Chicago). DNA sequence analysis confirmed the identity of the IL-7 and IL-7II clones and both were introduced into Chinese Hamster Ovarian (CHO) cells by transfection. Stable cell lines were generated by selection on puromycin (2μg/ml). Secretion and quantification of rIL-7 and rIL-7II was determined in a dot-blot assay using anti-Myc mAb (clone 9B11, Cell Signaling Biotechnology, Boston), anti-6x-His Tag mAb (clone G-18, Santa Cruz Biotechnology, Santa Cruz) and 5H5 anti-IL-7 mAb. rIL-7 and rIL-7II supernatants from CHO transfectants were used in OP9 and OP9-DL1 co-culture experiments. Purified rIL7 and rIL-7II from these cells were also used in the STAT5 phosphorylation assays. E. coli-purified rIL-7 and rIL-7II (17) were used for binding assays. Binding of rIL-7 to cells was determined using purified E. coli-derived protein followed by anti-IL-7 mAb, clone 5H5, and fluorochrome labeled goat anti-mouse Ig; in some experiments biotinylated E. coli-derived rIL-7 (bio-rIL-7 or bio-IL-7II) was used as indicated. Cells were analyzed by flow cytometry on the FACScanto (BD Biosciences, San Diego) using FlowJo software (Treestar, inc).
2.4. Semi-quantitative RT-PCR
For semi-quantitative RT-PCR, IL-7 and IL-7II were PCR-amplified using primers designed to anneal within exons 2 and 3 that would span across exon 2b: sense primer = 5′ ATGGCAAAGAATATGAGAATGTTCT 3′ and antisense primer = 5′ TAAAAAAGTTAGATTCATTATTCAGGC 3′. The resulting products were 150 bp (IL-7) and 183 bp (IL-7II). To establish that the two IL-7 isoforms PCR-amplified equally and were specific, pGEMT plasmids containing the cDNAs of either IL-7 alone or IL-7II alone were amplified singly and then together in varying ratios. No PCR bias of either isoform was found using IL-7:IL-7II ratios of 0.1, 0.2, 2 and 10. For the purposes of normalization, the GAPDH house-keeping gene was simultaneously PCR-amplified using the 5′ CATCACTGCCACCCAGAAGA 3′ and 5′ CAAGGTCATCCCCGAGCTGAA 3′ primer set. The thermocycler was paused after 30, 32 and 34 cycles and at each time point a 15μl aliquot of the PCR product was removed and resolved on a 5% polyacrylamide gel. Visualization was done on a Typhoon imaging system (GE Healthcare) and quantification was performed with the ImageQuant software (GE Healthcare).
2.5. Cloning and Expression of rabbit IL-7Rα chain
PCR primers for IL-7Rα chain were designed to regions conserved in the mouse and human IL-7Rα chain gene, spanning the transmembrane region: 5′ ATGACAATTCTAGGTA 3′ and 5′ TTTCAAAGGACCTGA 3′. The IL-7Rα chain was RT-PCR-amplified from 55D1 cells (a rabbit B cell line), subcloned in the pMEPURO vector, sequenced (Genebank accession number: GU35590) and transfected into CHO cells. Stable transfectants were selected with puromycin (2μg/ml). Expression of IL-7Rα in the transfectants was determined by binding to IL-7 and IL-7II.
2.6. Induction and detection of STAT5-phosphorylation
55D1 B cells (106) were seeded in a 6 well plate and incubated with medium alone, IL-7 (30 ng/ml), or IL-7II (30 ng/ml) for 15 minutes at 37°C. Cells were immediately fixed and permeabilized (Cytofix/Perm, BD Biosciences Pharmingen, San Diego), stained with 20μl of PE anti-phospho-STAT5 mAb (clone Py694, BD Biosciences Phamingen, San Diego) and analyzed by flow cytometry using a FACScanto II (BD Biosciences, San Diego) and Flow-Jo software.
2.7. OP9 and OP9-DL1 co-cultures
OP9 GFP or OP9 delta-like 1+ (DL1) stromal cells (22, 23), kindly provided by Juan Carlos Zuniga-Pfluecker (University of Toronto, Toronto, Ontario, Canada), were seeded in TC24 or TC96-well plates at 1 × 104 or 3 × 103 cells per well, respectively, in α-MEM medium (GIBCO-BRL, Grand Island, NY), supplemented with 20% FCS and 50% IL-7 supernatant, Cell growth was observed weekly and cells were fed up to two times per week.
2.8. Competitive inhibition assay
Spleen cells (106) were incubated with bio-rIL-7 (50ng) or with a mixture of: i) ice-cold bio-rIL-7 (50ng) and unlabeled rIL-7II (unl-rIL-7II) (250ng), ii) bio-rIL-7(50ng) and unl-IL-7II (2.5μg); controls were i) rIL-7-bio (50ng) and unl-IL-7 (250ng), ii)rIL-7-bio (50ng) and unl-IL-7 (2.5μg). Binding was then detected with PE-conjugated streptavidin and analyzed by flow cytometry.
3. Results
3.1. Cloning of rabbit IL-7II
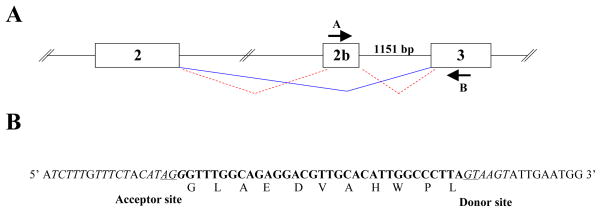
During previous cloning and sequence analysis of rabbit IL-7 (17), we identified an IL-7-encoding cDNA clone that contained an insertion of 33 nucleotides located between the predicted exons 2 and 3 (Fig. 1A). We reasoned that this clone might represent the sequence of a new IL-7 open reading frame (ORF) possibly generated by alternative splicing. To investigate this possibility, we attempted to PCR-amplify the genomic DNA located between the putative new exon and exon 3, using primers from the 33bp sequence and exon 3, (primers A and B, Fig. 1A). We obtained a PCR product which by nucleotide sequence corresponded to an 1151 bp intronic sequence that contained a consensus splice donor site, adjacent to the 33bp exon (Fig. 1B). We searched for nucleotide sequences upstream of the presumed 33bp exon using the genomic rabbit database (National Center for Biotechnology Information, NCBI). This analysis revealed the presence of a consensus splice acceptor site, 5′ of the 33bp sequence (Fig. 1B). The identification of consensus upstream and downstream acceptor and donor splice sites strongly indicates that the 33 bp insertion is encoded by a separate exon which we designate exon 2b. We conclude that this ORF produces an in-frame 11 amino acid insertion resulting in a newly identified IL-7 isoform, generated by alternative splicing. Consistent with this idea, exon 2b contains exonic sequence enhancers (ESE) (24), which could serve as binding sites for a class of specific serine/arginine-rich (SR) proteins that recruit the splice machinery directly to the transcript. We identify two potential binding sites for the SR complexes SF2/ASF and SC35: CAGAGGA, AGGACGT and GGACGTTG, GGCCCTTA respectively. Based on these observations, we conclude that alternative splicing generates the second IL-7 transcript, designated IL-7II.
Figure 1. Rabbit IL-7II and structure of exon 2b.
A) Schematic of proposed IL-7 mRNA splicing between exons 2, 2b and 3. Lines indicate splicing for IL-7 (solid line) and IL-7II (dashed lines). Arrows indicate primer sites used for PCR-amplification from genomic DNA. Primer A = 5′ GGCAGAGGACGTTGCACATTGG 3′; and Primer B = 5′ CTTATTATCATCACATGAATGTT 3′. B) The 33bp insertion sequence (boldface), flanking intronic DNA of the proposed exon 2b and the amino acid sequence for the 33bp insertion are shown. Underlined nucleotides indicate invariant residues of the splice acceptor and donor sites.
Identifying a novel IL-7 splice variant in adult rabbit BM, opened the possibility that the observed lymphopoiesis arrest in adult rabbits might correlate with a decline in IL-7II expression. In experiments described below, we tested this possibility and also characterized this novel IL-7 isoform.
3.2. IL-7II expression in lymphoid and non-lymphoid tissues from young and adult rabbits
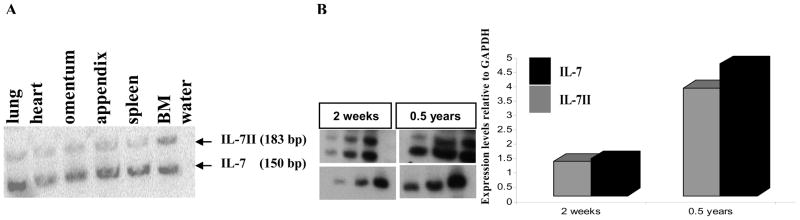
In humans, expression of IL-7 isoforms in various lymphoid and non-lymphoid tissues and novel roles for IL-7 have been reported (25, 26). To investigate in which tissues rabbit IL-7 isoforms were expressed, by RT-PCR using primers that amplify both IL-7 isoforms, we found that IL-7 and IL-7II are expressed in both lymphoid and non-lymphoid tissues (Fig. 2A). After determining that both isoforms were transcribed in BM of each of ten rabbits tested, we asked if changes in the expression of IL-7 and IL-7II from birth to adulthood correlate with the waning and arrest of B cell development. Using BM cDNA templates from a young and an adult rabbit, we found similar relative levels of IL-7 and IL-7II expression in the young and adult rabbit, but in the adult rabbit the expression of both isoforms was increased (Fig 2B). These results confirm and extend our previous findings showing that the decline and arrest of B lymphopoiesis that occurs in rabbits beginning a few weeks after birth is not due to limited expression of IL-7 (17) and further that the arrest does not appear to be due to altered expression of IL-7II relative to the expression of IL-7.
Figure 2. RT-PCR analysis of IL-7 and IL-7II expression in lymphoid and non-lymphoid tissues.
Using exon 2 and exon 3 primers (see Material and Methods), RT-PCR was performed on lymphoid and non-lymphoid tissues B) semi-quantitative RT-PCR was performed on BM samples from a young rabbit (2 weeks) and an adult rabbit, (0.5 years). PCR products were resolved on 5% polyacrylamide gels and intensity of ethidium bromide-stained bands (left) were quantified relative to GAPDH bands (right). Similar experiments were performed with BM from other young (10 days, 2 weeks) and adult (7 months, 11 months, 1 year and 3.8 years) rabbits and the expression of neither IL-7 nor IL-7II increased in age.
3.3. rIL-7II binding to BM lymphocyte progenitors and secondary lymphocytes
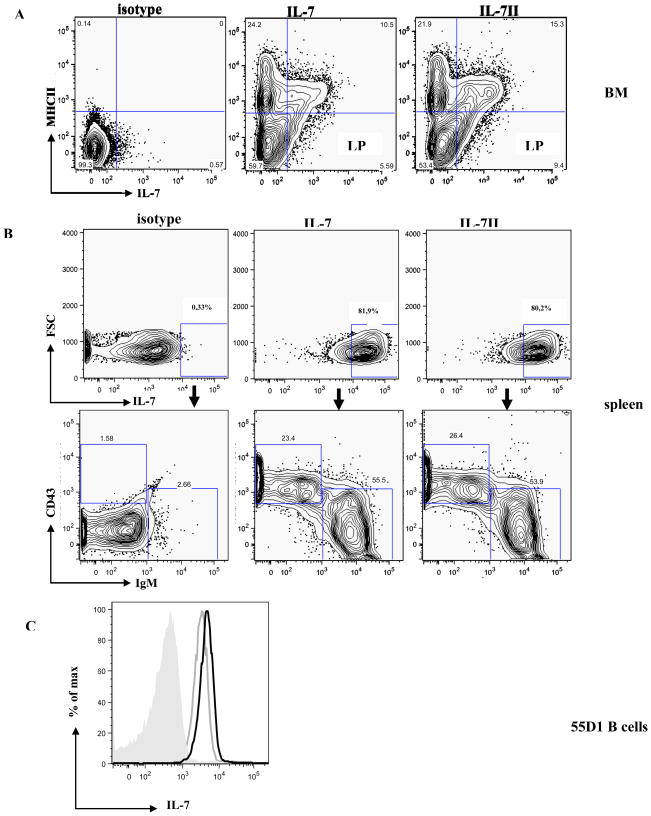
Although we did not find an increase in IL-7II expression relative to IL-7 in adult rabbit BM, we considered the possibility that IL-7II plays an inhibitory role in lymphopoeisis. To test this and to further characterize IL-7II, we investigated if IL-7II binds to a BM lymphocyte progenitor (MHCII−,IL-7R+) as shown for IL-7(17). By flow cytometry we find that rIL-7II indeed binds these BM early lymphocyte progenitor cells (Fig. 3A), indicating a possible a role in lymphopoiesis.
Figure 3. Flow cytometric analysis of rIL-7 and rIL-7II binding to B and T lineage cells.
A) BM cells (106) were stained with FITC-anti-MHCII, isotype controls (left), bio-rIL-7 (200ng) (middle) or bio-rIL-7II (200ng) (right), detected with streptavidin-APC; LP = lymphoid progenitor.
B) rIL-7-binding spleen cells (106) stained with PE-anti-IgM mAb and FITC-anti-CD43 mAb; left = isotype control; middle = IL-7, and right = IL-7II.
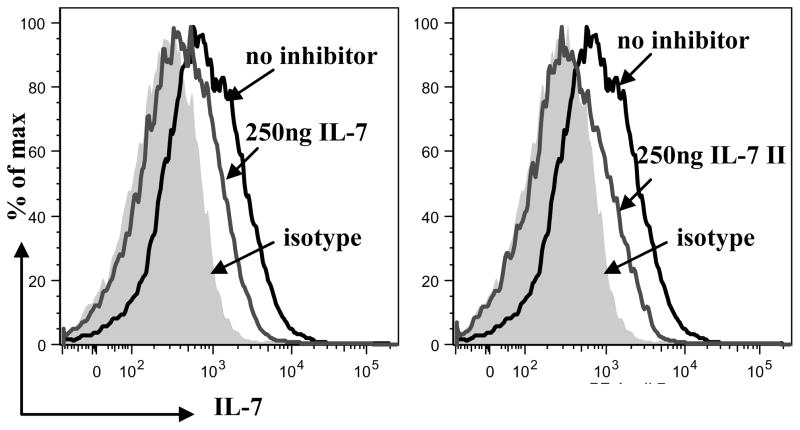
C) 55D1 B cells (106) incubated with rIL-7 (800ng) and rIL-7II (800ng) followed by anti-IL-7 mAb and analyzed by flow cytometry (grey shaded peak= isotype control, grey line = IL-7 and solid line = IL-7II).
In mice and humans, lymphopoiesis in the BM follows several regulated developmental stages, characterized by cell-surface markers, and IL-7 is crucial at several of these stages (27). Signals from IL-7 binding to the IL-7R are important in differentiation, survival and proliferation of both B and T cells (1, 4, 28). After finding that rIL-7II binds an early BM lymphocyte progenitor, we tested binding to mature B and T lymphocytes. We used flow cytometry and found that rIL-7 and rIL-7II bind splenic rabbit T lymphocytes (Fig 3B), but unlike mice and humans, the IL-7 isoforms also bind splenic B cells (Fig 3B) and a rabbit B cell line, 55D1 (Fig 3C), indicating a possible homoestatic role for IL-7 and IL-7II, in both B and T cells.
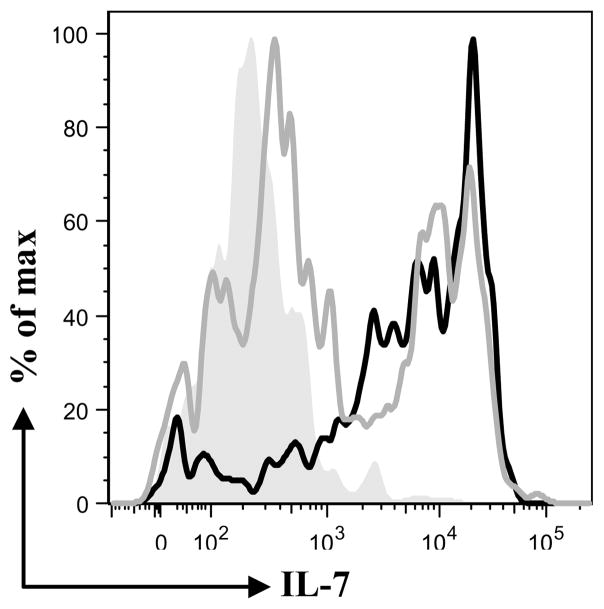
After finding that both IL-7 isoforms bind the same population of lymphocytes, we determined if binding was via the same receptor. Because the expression of the IL-7Rα chain is sufficient to bind IL-7 (5), we cloned rabbit IL-7Rα chain from 55D1 B cells and tested if rIL-7II binds IL-7Rα-expressing-CHO transfectants. By flow cytometry we found that, both rIL-7 and rIL-7II bound to these IL-7Rα-expressing-CHO cells (Fig. 4). We conclude that IL-7II binds the canonical IL-7R.
Figure 4. Flow cytometric analysis of rIL-7 and rIL-7II binding to IL-7Rα-expressing CHO cells.
IL-7Rα-expressing-CHO cells were incubated with rIL-7 and rIL-7II and analyzed by flow cytometry. (grey shaded peak= untransfected cells, grey line = IL-7 and solid line = IL-7II).
Our data indicate that rIL-7 and rIL-7II not only bind the same population of lymphocytes but also presumably via the canonical IL-7R. These findings led us to test if binding to this receptor was competitive. By flow cytometry, we found that that the binding of rIL-7II to the IL-7R inhibited binding of rIL-7 (Fig. 5), indicating competition of the IL-7 isoforms for the same receptor.
Figure 5. Flow cytometric analysis of rIL-7II inhibition of rIL-7 binding to B cells.
Binding of bio-rIL-7 (50ng) to splenocytes was inhibited by unl-rIL-7II (250 ng) (left panel) and by unl-rIL-7 (250 ng) (right panel). Biotinylated IL-7 was identified with streptavidin-PE. For simplicity purposes, only the inhibition analyses using 250ng unl-IL-7II and 250ng unl-IL-7 are shown).
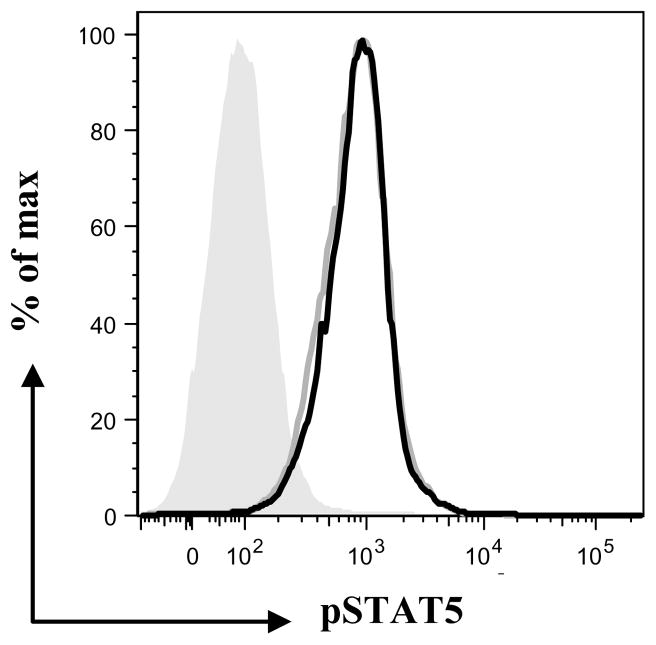
Though IL-7 isoforms in other species have been identified (18, 19), only recently was the functionality of the human IL-7 isoforms investigated (25, 26). To further characterize IL-7II, we investigated if recombinant proteins were biologically active. To determine this we made use of the fact that IL-7 binding to the IL-7R activates the JAK/STAT pathway by cross-linking the extracellular domains of the IL-7Rα and γ chains and bringing together the intracellular tyrosine kinases, JAK1 and JAK3, which leads to mutual phosphorylation (27). We expected that if IL-7II binds the IL-7R, the JAK/STAT pathway would also be activated and STAT5, a key regulator downstream of the JAK/STAT pathway would become phosphorylated. We incubated 55D1 B cells with IL-7 and IL-7II and tested for phosphorylation of STAT5 by flow cytometry using anti-phosphyorylated STAT5 mAb. Binding of both isoforms led to STAT5 phosphorylation (Fig. 6), demonstrating that IL-7II, as well as IL-7, activates B cells through the JAK/STAT pathway, presumably by binding the IL-7R.
Figure 6. Flow cytometric analysis of rIL-7 and rIL-7II activation of B cells via the STAT/JAK pathway.
55D1B cells (106) were incubated with rIL-7 (30ng/ml) or rIL-7II (30ng/ml), fixed, permeabilized, stained with PE anti-phosphorylated STAT5 antibody and analyzed by flow cytometry, (grey shaded population = unstimulated cells, grey line = IL-7 and solid line = IL-7II).
3.4. Function of IL-7II
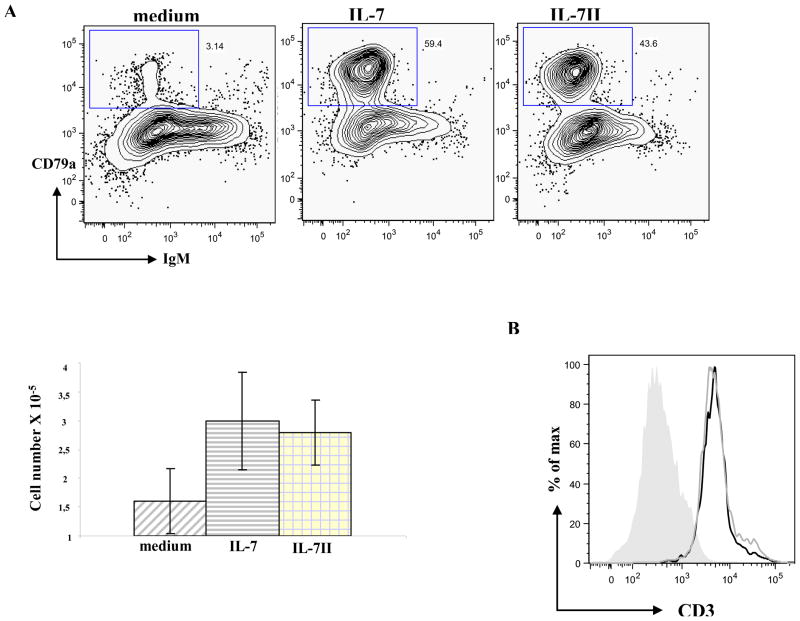
We have previously reported that IL-7 is required for the differentiation of BM progenitor cells in vitro and that the waning of B lymphopoiesis in adult rabbits is not due to limited IL-7 expression (17). In the current study we confirmed the previous finding that IL-7 expression increases with age and we find that IL-7II expression also increases with age. After determining that rIL-7II binds BM early lymphoid progenitors and is functionally active, we tested what role IL-7II might play in lymphopoiesis. To pursue this, we employed the murine OP9 and OP9 DL1 stromal cell line co-culture systems. OP9 and OP9 DL1 stromal cell lines have been shown to promote differentiation of B and T lineage cells, respectively, from hematopoietic stem cells (HSCs) of mice, humans and rabbits (17, 22, 23). We co-cultured total BM cells from adult rabbits with either OP9 or OP9 DL1 stromal cells in medium alone, or containing either rIL-7 or rIL-7II. After one week, cells arising from the co-cultures were examined by flow cytometry and we identified similar levels of proB cells (CD79a+ IgM−) from the OP9 co-cultures (Fig 7A) with IL-7II as with IL-7, and more than medium alone, indicating that IL-7II supports differentiation of HSCs into B-lineage cells. Further we find that the cultures with either rIL-7 or rIL-7II had two-fold more cell growth than the medium control (Fig. 7A, bottom). Similarly, IL-7II supported development of T-lineage cells (CD3+) in the OP9 DL1 co-cultures, indicating that IL-7II also supports development of T lineage cells (Fig 7B). These results indicate that in vitro, IL-7II like IL-7 supports differentiation and proliferation of BM progenitors into B and T cell lineages.
Figure 7. Flow cytometric analysis of B- and T-lineage cells obtained from BM cells co-cultured with OP9 and OP9 DL1 stromal cells in the presence of IL-7 and IL-7II.
A) Cells arising from adult rabbit BM cells co-cultured with OP9 stromal cells in medium alone (left panel), IL-7 (middle panel) or IL-7II (right panel) were cytoplasmically-stained with PE-anti-CD79a and APC-anti-IgM and analyzed by flow cytometry (upper). To determine the extent of proliferation, cells arising from the different culture conditions were enumerated by Trypan blue exclusion. The data shown in the lower panel are the means ± SEM of duplicate wells of each condition. Similar results were obtained from 2 independent experiments.
B) Cells arising from adult rabbit BM cells co-cultured with OP9-DL1 stromal cells in medium alone (grey shaded population), IL-7 (grey line) or IL-7II (solid line) were stained with anti-CD3 and analyzed by flow cytometry.
3.5. Conservation of IL-7 exon 2b in lagamorphs and other mammalian species
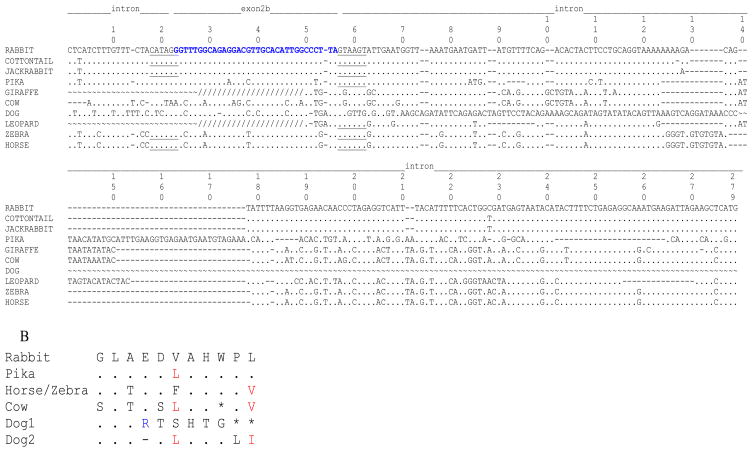
Isoforms of IL-7 have been reported in humans and horses (18, 19) but these isoforms did not have an exon2b. We searched for exon 2b in genomic DNA of other mammals by PCR, using exon 2b and exon 3 PCR primer pairs. The order LAGOMORPHA is divided into two families: Ochotonidae and Leporidae. The family Ochotonidae is restricted to the genus Ochotona (Pikas). The family Leporidae encompasses 11 genera that including: Oryctolagus (European rabbit, normally referred to as rabbit in the literature), Sylvilagus (Cottontail) and Lepus (Jackrabbit). These two families diverged around 40 million years ago (29, 30). A molecular supermatrix combining both nuclear and mitochondrial genes showed that rabbit, cottontail and jackrabbit diverged at ~12 million years ago (29). All lagomorphs (pika, cottontail and jackrabbit) carried IL-7 exon 2b (Fig. 8A). We found that the 5′ splice acceptor site in cottontail, jackrabbit and Pika were identical to the rabbit sequence. The exon 2b sequence in cottontail, rabbit and jackrabbit were identical and 91% homologous to the pika exon 2b sequence. It is interesting that from the three nucleotide differences observed, one of them resulted in an amino acid replacement (Fig. 8B). A full-length IL-7II ORF was obtained from jackrabbit splenic cDNA, indicating that IL-7 exon 2b splicing occurs in at least one other species. Analysis of sequence downstream of IL-7 exon 2b revealed that a stretch of 220 bp of intronic DNA was highly conserved between rabbit (Oryctolagus), cottontail (Sylvilagus) and jackrabbit (Lepus), and the splice donor site and immediate sequence downstream of exon 2b in pika was identical to the rabbit sequence, supporting the possibility that IL-7 exon 2b may be functional in pika (Fig. 8A).
Figure 8. Partial IL-7 nucleotide and amino acid sequences.
A) The partial IL-7 genomic sequence including exon 2b and downstream intronic DNA is shown. Genomic IL-7 sequences obtained by PCR (cottontail, jackrabbit, giraffe, leopard, zebra, and horse) or on the Ensembl database (cow, pika, dog,) were aligned with respect to rabbit sequence. IL-7 exon 2b (denoted) is in bold letters and splice donor and acceptor sites underlined. ~, no sequence; ., identity with rabbit sequence; -, deletion; /, sequence derived from exon 2b primer. Sequences upstream of the exon 2b primer were obtained from the Ensembl database or from PCR using an intronic primer that anneals immediately upstream of exon 2b.
B) Partial amino acid sequence of rabbit IL-7II, encoded by exon 2 b is shown, compared to pika, horse, zebra cow and dog. The second sequence for dog disregards the nt deletion reported in Ensembl; the affected codon is indicated by a dash. *, stop codon; ., identical amino acids.
IL-7 PCR products were also found in horse and zebra (Order Perissodactyla), leopard (Order Carnivora), and giraffe (Order Artiodactyla), but no PCR products were obtained from human (Order Primates), mouse (Order Rodentia), or cow (Order Artiodactyla) genomic DNA. For horse and zebra IL-7 exon 2b, the donor and acceptor splice sites were identical with rabbit, and the exon 2b ORF encoded amino acids that were 73% identical and 82% similar to rabbit (including conserved residues), indicating that exon 2b is highly conserved among members of (Lagomorpha and Perissodactyla) (Fig 8A).
We also searched for exon 2b using the publicly available genome databanks (Ensembl database; http://www.ensembl.org/index.html. We scanned the exon 2–3 intronic DNA sequence for remnants of exon 2b in the genomes of dog, cow, human, and mouse and found that dog genomic DNA carries an exon 2b-like region (79% identity to rabbit); however, it contains a deletion after the third codon that introduces two stop codons into the ORF. Similarly, an exon 2b-like region was found in cow; the nucleotide sequence was 73% identical to rabbit, but it also carried a stop codon within the ORF (Fig. 8B). No sequence was found in the murine or human IL-7 locus that resembled rabbit exon 2b at the amino acid level. We conclude that this exon 2b genomic region was present in the mammalian ancestry and then was maintained in some orders and disappeared in others.
4. DISCUSSION
We report the identification of a novel and functional rabbit IL-7 isoform, which we have designated IL-7II. IL-7II carries an insertion of 11 amino acids encoded by a newly identified exon, exon 2b, and is likely generated by alternative splicing. Consistent with this idea, exon 2b is not only flanked by complete splice acceptor and donor sites but also contains exonic sequence enhancers (ESE) (24).
We found that IL-7II is expressed in various lymphoid and non-lymphoid tissues. The finding that rabbit IL-7 isoforms are expressed in non-lymphoid tissues is consistent with recent findings in humans, in which some human IL-7 isoforms have been implicated in the differentiation of neural progenitor cells; a novel function for IL-7 (25). The expression of IL-7II in lymphoid tissues, especially in the BM implicates a possible role in lymphopoiesis, an idea supported by our finding that rIL-7II binds a BM lymphoid progenitor population.
We show that rIL-7 and rIL-7II bind peripheral T cells as does IL-7 in mice and humans; but unlike in mice and humans, we find that rIL-7 and rIL-7II also bind peripheral B cells. Both rIL-7 and rIL-7II bind to the IL-7Rα chain and the binding to B cells is competitive such that the interaction of one isoform inhibits binding of the other isoform. Not surprisingly, IL-7II, like IL-7 activated B cells through the JAK/STAT pathway, indicating that the IL-7II is not only transcribed but is also functionally active.
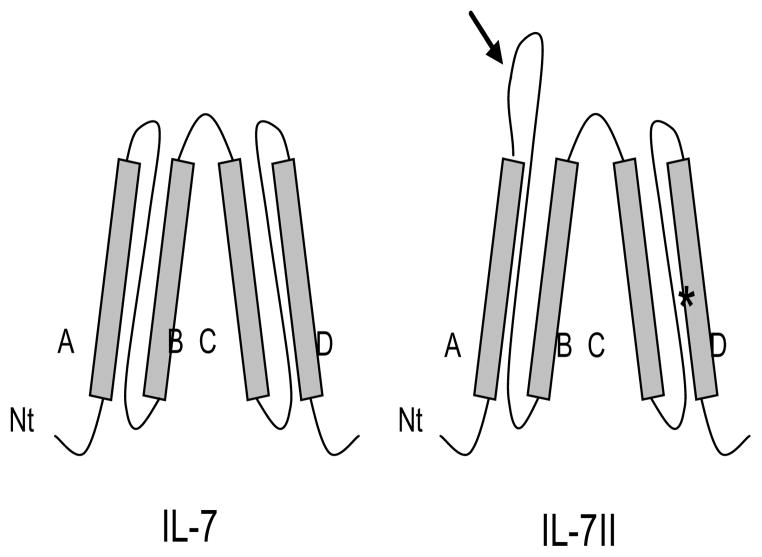
IL-7 binding to the IL-7R and subsequent signaling has been shown to be dependent on a tryptophan residue in helix D of the IL-7 protein (31). We speculate that the corresponding tryptophan residue in IL-7II also participates in IL-7R binding and signaling. Further, a second tryptophan residue is encoded by exon 2b (Figure 1A) and based on the rabbit IL-7 and IL-7II secondary structure model that we generated (Fig. 9), the second tryptophan residue is located within the loop region between helix A and alpha helix B (32). In the four-helical bundle cytokine family, of which IL-7 is a member, amino acids involved in receptor binding are commonly found either within helices A and D, or in the loop regions between helices A and B, and helices C and D (32). The location of the amino acid insertion likely exposes this domain to protein-protein interactions. Therefore, the insertion sequence in IL-7II may not only allow for protein-protein interactions, but the second tryptophan residue within this sequence may also play a role in binding and activation through the IL-7R or another receptor
Figure 9. Proposed secondary protein structure of IL-7 and IL-7II.
Proposed rabbit IL-7II protein structure based on modeling of human IL-7 (32). Shaded boxes indicate four alpha helical regions (A–D); curved lines indicate flexible loop regions; N, amino terminus; *, tryptophan residue required for IL-7 binding and activation of IL-7R; Arrow indicates 11 amino acid insertion in IL-7II including a tryptophan residue.
Because splice variants of some cytokines and growth factors can act as antagonists for homeostatic regulation of activity (33), we considered the possibility that the major function of IL-7II was to inhibit B lymphopoiesis since B cells are only generated in the BM of rabbits for the first four months of life. If IL-7II functions as an antagonist of IL-7 in lymphoiesis, we expected to find that either the expression of IL-7II increased with age, or the expression of IL-7 decreased with age. However, we found not only that the expression levels of both isoforms increased with age, but also that IL-7 was expressed at a higher level than IL-7II. We conclude that IL-7II probably does not function by serving as an antagonist of IL-7 to inhibit B lymphopoiesis.
We have previously reported that rIL-7 is required for B and T lymphopoiesis in vitro. Since we find that rIL-7II and rIL-7 competitively bind B cells, we reasoned that IL-7II could function in a manner similar to IL-7 in promoting B and T lymphopoiesis from progenitors. Using the murine OP9 and OP9-DL1 co-culture system, we find that rIL-7II like rIL-7, supports proliferation and differentiation of BM progenitors into cells of both B and T lineages. Although we find the functions of both isoforms to be similar in lymphopoiesis, it is possible that their roles differ in other cellular processes. In mouse, early B cell progenitors require IL-7 signaling not only for survival and proliferation, but also for initiating the B cell transcriptional program and for instigating chromatin accessibility prior to Ig gene rearrangements (34–36). It is not unlikely that IL-7II promotes VH distal gene rearrangements because rabbits utilize predominantly the D-proximal, VH gene segment (VH1) in VDJ gene rearrangements. Perhaps in rabbits, IL-7II functions to negate IL-7-induced VH accessibility, thereby restricting VH gene usage to VH1. It is also interesting that rabbit and jackrabbit share V(H)a alleles (trans-species polymorphism) which suggest that the divergence of the V(H)a lineages preceded the jackrabbit vs rabbit evolution split (37). Perhaps in rabbits and also in jackrabbits, IL-7II functions to negate IL-7-induced VH accessibility, thereby restricting VH gene usage to VH1.
IL-7II could also play a role in the diversification or survival of B cells in rabbits. While it is known that B cell immunity in rabbits as well as in chicken is established by massive B cell expansion and Ig gene diversification in organized lymphoid tissues within the gut associated lymphoid tissue (GALT) (38), the manner in which GALT develops and the factors that drive these processes are largely unknown. By RT-PCR analysis, B cells in the rabbit appendix were found to express the IL-7Rα gene (data not shown) and we also found that both IL-7 and IL-7II are expressed in GALT. Therefore, IL-7II may be involved in GALT development. Another possibility is that IL-7II contributes to B cell survival in adults in light of the arrest of B lymphopoiesis. The observation that mature B cells in rabbits express IL-7Rα gives credence to this possible role for IL-7II. In mice, IL-7 has been demonstrated to be a critical factor in both T cell and B cell development. On the other hand, in humans IL-7 preferentially regulates T cell development and survival (3, 5, 28). Recently, it has been reported that in humans, an isoform of IL-7 was shown to promote T cell survival (25). It is thus possible that in rabbits IL-7II could play a similar role, since we also report in this study that IL-7II binds T cells from secondary lymphoid tissues. Our, in vitro experiments revealed that primary rabbit B and T lymphocytes do not survive in the presence of IL-7 alone; presumably a second signal is required.
Acknowledgments
We gratefully acknowledge the technical assistance of Shi-Kang Zhai and Mae Kingzette. This work was supported by the National Institutes of Health Grant AI068390
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 2.Namen AE, Schmierer AE, March CJ, Overell RW, Park LS, Urdal DL, Mochizuki DY. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988;167:988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namen AE, Williams DE, Goodwin RG. Interleukin-7: a new hematopoietic growth factor. Prog Clin Biol Res. 1990;338:65–73. [PubMed] [Google Scholar]
- 4.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin RG, Friend D, Ziegler SF, Jerzy R, Falk BA, Gimpel S, Cosman D, Dower SK, March CJ, Namen AE, et al. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990;60:941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- 6.Lai SY, Molden J, Goldsmith MA. Shared gamma(c) subunit within the human interleukin-7 receptor complex. A molecular basis for the pathogenesis of X-linked severe combined immunodeficiency. J Clin Invest. 1997;99:169–177. doi: 10.1172/JCI119144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 8.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 9.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giliani S, Mori L, de Saint Basile G, Le Deist F, Rodriguez-Perez C, Forino C, Mazzolari E, Dupuis S, Elhasid R, Kessel A, Galambrun C, Gil J, Fischer A, Etzioni A, Notarangelo LD. Interleukin-7 receptor alpha (IL-7Ralpha) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol Rev. 2005;203:110–126. doi: 10.1111/j.0105-2896.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 11.Buckley RH. Primary immunodeficiency diseases due to defects in lymphocytes. N Engl J Med. 2000;343:1313–1324. doi: 10.1056/NEJM200011023431806. [DOI] [PubMed] [Google Scholar]
- 12.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 13.Parrish YK, Baez I, Milford TA, Benitez A, Galloway N, Rogerio JW, Sahakian E, Kagoda M, Huang G, Hao QL, Sevilla Y, Barsky LW, Zielinska E, Price MA, Wall NR, Dovat S, Payne KJ. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182:4255–4266. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(−/)− mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7 −/− mice. J Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- 16.Jasper PJ, Zhai SK, Kalis SL, Kingzette M, Knight KL. B lymphocyte development in rabbit: progenitor B cells and waning of B lymphopoiesis. J Immunol. 2003;171:6372–6380. doi: 10.4049/jimmunol.171.12.6372. [DOI] [PubMed] [Google Scholar]
- 17.Kalis SL, Zhai SK, Yam PC, Witte PL, Knight KL. Suppression of B lymphopoiesis at a lymphoid progenitor stage in adult rabbits. Int Immunol. 2007;19:801–811. doi: 10.1093/intimm/dxm048. [DOI] [PubMed] [Google Scholar]
- 18.Cook RF, Cook SJ, Even DL, Schaffer C, Issel CJ. Full-length and internally deleted forms of interleukin-7 are present in horse (Equus caballus) lymph node tissue. Vet Immunol Immunopathol. 2008;125:126–134. doi: 10.1016/j.vetimm.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Korte A, Moricke A, Beyermann B, Kochling J, Taube T, Kebelmann-Betzing C, Henze G, Seeger K. Extensive alternative splicing of interleukin-7 in malignant hematopoietic cells: implication of distinct isoforms in modulating IL-7 activity. J Interferon Cytokine Res. 1999;19:495–503. doi: 10.1089/107999099313947. [DOI] [PubMed] [Google Scholar]
- 20.Burnett RC, Hanly WC, Zhai SK, Knight KL. The IgA heavy-chain gene family in rabbit: cloning and sequence analysis of 13 C alpha genes. Embo J. 1989;8:4041–4047. doi: 10.1002/j.1460-2075.1989.tb08587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 22.Kitajima K, Tanaka M, Zheng J, Sakai-Ogawa E, Nakano T. In vitro differentiation of mouse embryonic stem cells to hematopoietic cells on an OP9 stromal cell monolayer. Methods Enzymol. 2003;365:72–83. doi: 10.1016/s0076-6879(03)65005-6. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 24.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31:3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moors M, Vudattu NK, Abel J, Kramer U, Rane L, Ulfig N, Ceccatelli S, Seyfert-Margolies V, Fritsche E, Maeurer MJ. Interleukin-7 (IL-7) and IL-7 splice variants affect differentiation of human neural progenitor cells. Genes Immun. 2009 doi: 10.1038/gene.2009.77. [DOI] [PubMed] [Google Scholar]
- 26.Vudattu NK, Magalhaes I, Hoehn H, Pan D, Maeurer MJ. Expression analysis and functional activity of interleukin-7 splice variants. Genes Immun. 2008 doi: 10.1038/gene.2008.90. [DOI] [PubMed] [Google Scholar]
- 27.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin RG, Lupton S, Schmierer A, Hjerrild KJ, Jerzy R, Clevenger W, Gillis S, Cosman D, Namen AE. Human interleukin 7: molecular cloning and growth factor activity on human and murine B-lineage cells. Proc Natl Acad Sci U S A. 1989;86:302–306. doi: 10.1073/pnas.86.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthee CA, van Vuuren BJ, Bell D, Robinson TJ. A molecular supermatrix of rabbits and hares (Leporidae) allows for the identification of five intercontinental exchanges during the Miocene. Systems Biology. 2004;53:433. doi: 10.1080/10635150490445715. [DOI] [PubMed] [Google Scholar]
- 30.matthee CA. A timetree of life. oxford Press; New York: 2008. [Google Scholar]
- 31.vanderSpek JC, Sutherland JA, Gill BM, Gorgun G, Foss FM, Murphy JR. Structure function analysis of interleukin 7: requirement for an aromatic ring at position 143 of helix D. Cytokine. 2002;17:227–233. doi: 10.1006/cyto.2002.1004. [DOI] [PubMed] [Google Scholar]
- 32.Mott HR, I, Campbell D. Four-helix bundle growth factors and their receptors: protein-protein interactions. Curr Opin Struct Biol. 1995;5:114–121. doi: 10.1016/0959-440x(95)80016-t. [DOI] [PubMed] [Google Scholar]
- 33.Atamas SP. Alternative splice variants of cytokines: making a list. Life Sci. 1997;61:1105–1112. doi: 10.1016/s0024-3205(97)00243-9. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury D, Sen R. Transient IL-7/IL-7R signaling provides a mechanism for feedback inhibition of immunoglobulin heavy chain gene rearrangements. Immunity. 2003;18:229–241. doi: 10.1016/s1074-7613(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury D, Sen R. Stepwise activation of the immunoglobulin mu heavy chain gene locus. Embo J. 2001;20:6394–6403. doi: 10.1093/emboj/20.22.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corcoran AE, Riddell A, Krooshoop D, Venkitaraman AR. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature. 1998;391:904–907. doi: 10.1038/36122. [DOI] [PubMed] [Google Scholar]
- 37.Esteves PJ, Lanning D, Ferrand N, Knight KL, Zhai SK, van der Loo W. The evolution of the immunoglobulin heavy chain variable region (IgVH) in Leporids: an unusual case of transspecies polymorphism. Immunogenetics. 2005;57:874–882. doi: 10.1007/s00251-005-0022-0. [DOI] [PubMed] [Google Scholar]
- 38.Weill JC, Reynaud CA. Early B-cell development in chickens, sheep and rabbits. Curr Opin Immunol. 1992;4:177–180. doi: 10.1016/0952-7915(92)90009-4. [DOI] [PubMed] [Google Scholar]