Abstract
Experimental evidence indicates that lymph nodes in humans undergo alterations during ageing. This is clinically important because of the crucial role of these organs in the immune system and their lymph reabsorption and drainage function. Although some age-related changes in lymph node histoarchitecture have been described, they are seldom taken into account in traditional depictions of lymph nodes. Recently introduced clinical procedures, such as intranodal vaccination or lymph node transplantation, have demonstrated the need for an accurate knowledge of these degenerative processes. In this study, superficial inguinal lymph nodes were obtained from 41 deceased patients between 17 and 98 years old. To minimize immunological influences, such as chronic diseases, specimens were only obtained from forensic pathology autopsies. An immunohistochemical analysis was carried out, on the basis of which lymph node degeneration was scored according to the numbers of lymphocytes and high endothelial venules, and degree of fibrosis and lipomatosis. We observed an age-dependent tendency towards the replacement of areas populated with diverse immune cells by connective tissue. Paradoxically, these changes were also detected in some of the nodes from younger age groups. In conclusion, lymph nodes can display degenerative changes that are mainly age-related and often diverge from the common description found in textbooks. These alterations should be taken into account when dealing with lymph nodes diagnostically and therapeutically in clinical practice.
Keywords: age-dependent degeneration, ageing, immune senescence, lymph node
Introduction
Human lymph nodes are secondary lymphoid organs. They are known for displaying a distinct histoarchitectural pattern composed of a cortex with primary and/or secondary lymph follicles, a paracortex and a central region named the medulla. All compartments consist of specific subsets of T- and B-lymphocytes, dendritic cells, macrophages and stroma cells (Willard-Mack, 2006; Hoorweg & Cupedo, 2008).
Lymph nodes have been the object of histological characterization (Gray, 1995; Janeway, 2005; Willard-Mack, 2006; Hoorweg & Cupedo, 2008) and some publications analyse lymph node alterations according to different age groups (Denz, 1947; Luscieti et al. 1980). Although some of these descriptions present a solid method, they are outdated, as they are purely descriptive and provide no semi-quantitative methods of lymph node evaluation.
Recently, various research groups have focused on novel therapeutic approaches involving lymph nodes. Due to this, structural effects on immunity and function have gained new importance and questions around age-dependent alterations have been aroused. Senti et al. successfully investigated intranodal vaccination in humans as a very effective substitute for the common method of allergen desensitization (Senti et al. 2008). Classical desensitization in allergic subjects takes several years and demands a large number of injections causing compliance problems. The new approach appears to reach similar results in a much shorter time period with a total of only three intranodal injections (Senti et al. 2008; Martinez-Gomez et al. 2009). Tammela et al. (2007) performed lymph node transplantation in mice to analyse the capacity of tumour filtration in transplanted nodes. Our group (Blum et al. 2010; Hadamitzky et al. 2009) conducts research on lymphoedema, e.g. after oncological surgery, and is developing concepts to prevent it. These include lymph node transplantation to restore a sufficient lymphatic flow in rats and pigs. Research on processes of immunosenescence (Kovaiou et al. 2007; Fietta, 2008) also includes the study of the age-related altering of lymph nodes as an integral part of the immune system (Denz, 1947; Luscieti et al. 1980; Pan et al. 2008). Their degeneration may play a role in the observed age-related weakness of immune responses and impairment of inhibition of cancer metastasis.
It seems important to obtain a detailed insight into distinct anatomical changes within the human lymph node throughout life. Age-related degeneration can have a strong impact on the outcome of these new therapeutic strategies, making an age-dependent modulation of these research concepts necessary.
In this study, superficial inguinal human lymph nodes from different donors aged between 17 and 98 years are compared. The objective was to identify any changes in the histoarchitecture of lymph nodes, taking the stereotypical healthy lymph node (Alberts, 1994; Robbins, 1994; Gray, 1995; Földi, 2003; Janeway, 2005) commonly described in textbooks as the normal reference (Fig. 1). T- and B-cells are discriminated by means of immunohistochemistry. Correlation of architectural changes and age are analysed and quantified.
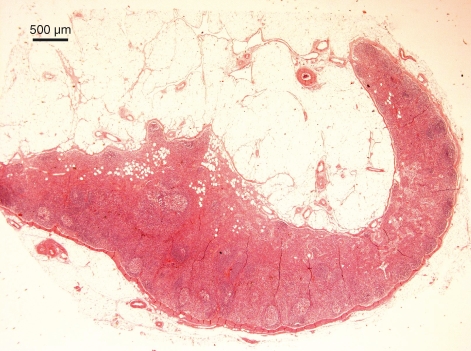
Fig. 1.
Human lymph node histology as traditionally depicted in textbooks. Under the subcapsular sinus of this J-shaped lymph node are active follicles with germinal centres separated by medullary cords. Haematoxylin and eosin staining.
Materials and methods
A total of 41 superficial inguinal lymph nodes were obtained from 41 subjects during forensic pathological autopsies. The deceased patients were between 17 and 98 years old (mean 54 ± 21.6 years). None presented a diagnosis of chronic disease such as cancer or autoimmune inflammation (Table 1). Donors were divided into different age groups encompassing periods of 15 years, starting with young donors aged 16–30 years and ending with a group aged 76 years and more (Table 2).
Table 1.
Causes of death of the donors (n = 41).
| Causes of death | No. of subjects |
|---|---|
| Multiple trauma including cerebral trauma | 15 |
| Myocardial infarction | 11 |
| Intoxication | 4 |
| Hanging | 3 |
| Pulmonary failure/pulmonary embolism | 3 |
| Gastrointestinal bleeding | 2 |
| Cerebral haemorrhage | 1 |
| Third-grade burn | 1 |
| Multiple organ failure after colon resection | 1 |
Table 2.
Age groups of the donors (n = 41).
| Age group | 16–30years | 31–45years | 46–60years | 61–75years | 76+years |
|---|---|---|---|---|---|
| Total no. | 6 | 8 | 10 | 9 | 8 |
Lymph nodes were fixed in 4% buffered formalin and embedded in paraffin. Cross-sections were made centrally with the largest radius possible in lymph nodes of <0.5 cm in size. Lymph nodes with a larger diameter were cut at three levels dividing the organ into approximately one-quarter of its original size. In this case, sections were analysed simultaneously. Slides with cross-sections of 7 μm were stained with haematoxylin and eosin and Masson-Goldner to ascertain general aspects such as the number of high endothelial venules (HEVs) or occurrence of fibrosis, respectively (Fig. 2). In addition, immunohistochemical staining was performed using antibodies directed against CD3 (monoclonal mouse anti-human CD3; DakoCytomation, Glostrup, Denmark) and CD20 (monoclonal mouse anti-human CD20cy; DakoCytomation) to discriminate T- and B-cells, respectively. Based on the T- and B-cell distribution, amount of paracortical HEVs, presence of fibrous connective tissue and extension of fatty tissue, a score was created to ascertain the degree of lymph node degeneration (Table 3, Fig. 3). The score was attained by summing the degree of each of the four parameters, thereby resulting in a minimum of 0 (no degeneration) and a maximum of 8. A score lower than 3 was seen as minimal degeneration and scores between 3 and 5 were classified as average degeneration. Degeneration was considered extensive for scores greater than 5.
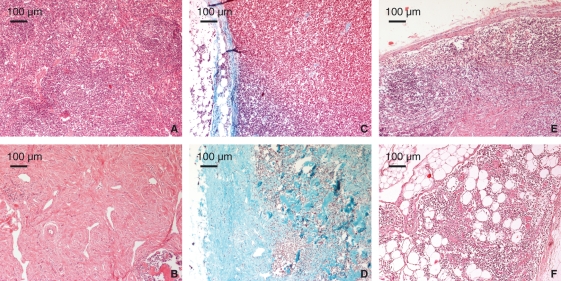
Fig. 2.
Histological examples of degeneration score. The upper row shows minimal scores (0) for low lymphocyte loss (A), low fibrosis (C) and low lipomatosis (E). The lower row shows examples of higher degeneration score (2) for lymphocyte depletion (B), advanced fibrosis (D) and advanced lipomatosis (F). Haematoxylin and eosin and Masson-Goldner staining.
Table 3.
Lymph node degeneration score based on immunohistochemistry.
| Degeneration score |
|||
|---|---|---|---|
| 0 | 1 | 2 | |
| Loss of lymphocytes | The larger part of the lymph node (except medullary cords) exhibits lymphocytes | Several lymphocyte-filled areas are represented | Only few diffuse lymphocytes |
| Loss of HEVs | More than 10 HEVs in a 20× magnification field of the paracortex (average of three fields) | Between 10 and 3 HEVs in a 20× magnification field of the paracortex (average of three fields) | Less than 3 HEVs in a 20× magnification field of the paracortex (average of three fields) |
| Degree of fibrosis | Normal architecture with typical trabeculae, medullary cords and hilus | Increased intranodal fibrous connective tissue | Most of the lymph node is composed of fibrous connective tissue |
| Degree of lipomatosis | No significant amount of fatty tissue | Less than 50% of the lymph node is substituted by fatty tissue | Most of the lymph node is composed of fatty tissue |
A higher score corresponds to a higher degree of degeneration.
HEV, high endothelial venule.
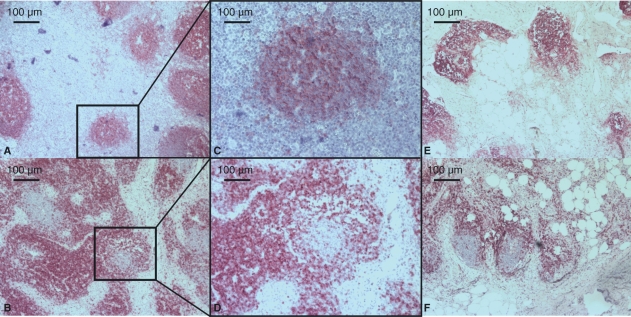
Fig. 3.
Upper row: CD20 staining (B-cells). Lower row: CD3 staining (T-cells). (A–D) Different sections of a lymph node with a low degeneration score (32-year-old male, fall from high building, score 1). (E and F) Different sections of a lymph node with a high degeneration score and high lipomatosis (77-year-old female, brain trauma, score 6). Note that B- and T-lymphocytes are found at the typical site in spite of degeneration.
Results
The macroscopic characteristics of human lymph nodes did not differ between low and high degeneration scores. No significant correlation was found between lymph node size, colour and consistency, and degeneration features.
Immunohistochemical analysis for B- and T-cells showed cortical and paracortical distribution, respectively, which was independent of degeneration grades (Fig. 3). The interface between B- and T-lymphocytes, an architectural feature with functional relevance, was fully preserved in lymph nodes undergoing focal degeneration but only in areas with normal appearance. In lymph nodes undergoing severe diffuse (generalized) degeneration, lymphocyte paucity rendered interfaces unclear and B- and T- zones, although still present, were not well demarcated.
A broad paracortex rich in T-cells was found in most lymph nodes. Small primary follicles were usually present within the cortex. Surprisingly, only two specimens presented germinal follicles as signs of an ongoing immunological reaction. Although lymph nodes with a large central area composed of fat or fibrous connective tissue usually had only small amounts of lymphocytes in their rim, some specimens were very heterogeneous. It was therefore possible to observe fully functional areas along with degenerative portions all under the same organ capsule (Fig. 4). In those cases, the score was attributed according to the proportion occupied by the functioning lymph node in comparison to the degenerated area (Table 3).
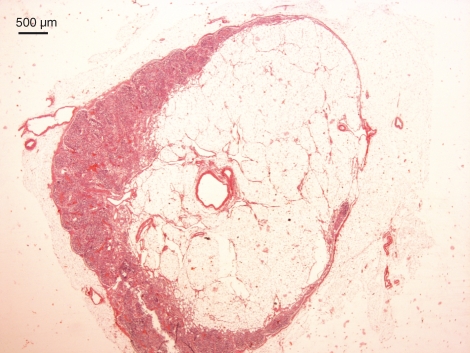
Fig. 4.
Human lymph node presenting degenerative features. Lipomatosis is intracapsular. Haematoxylin and eosin staining.
The results suggested a tendency to lymphocyte loss with ageing without total disappearance (Table 4). Diffuse degeneration affected B- and T-cells equally.
Table 4.
Lymphocyte loss according to age group.
| Score for loss of lymphocytes | 16–30years | 31–45years | 46–60years | 61–75years | 76+years |
|---|---|---|---|---|---|
| 0 | 2 | 2 | 0 | 2 | 0 |
| 1 | 4 | 5 | 10 | 5 | 6 |
| 2 | 0 | 1 | 0 | 2 | 2 |
A higher score corresponds to fewer lymphocytes as a sign of increasing degeneration (n = 41).
Analysis of the loss of HEVs as a sign of disconnection from the vascular system and therefore lower immunological function revealed only high numbers of HEVs in four lymph nodes in the age group 16–30 years (n = 6), whereas nodes from the age group 31–45 years were evenly distributed through the middle and high loss categories (scores 1 and 2 of degeneration). In the age groups 46–60, 61–75 and 76+ years, the great majority only had very few HEVs (Table 5).
Table 5.
High endothelial venule (HEV) loss according to age group.
| Score for loss of HEVs | 16–30years | 31–45years | 46–60years | 61–75years | 76+years |
|---|---|---|---|---|---|
| 0 | 4 | 0 | 0 | 1 | 0 |
| 1 | 1 | 4 | 4 | 2 | 3 |
| 2 | 1 | 4 | 6 | 6 | 5 |
A higher score corresponds to fewer HEVs as a sign of increasing degeneration (n = 41).
Fibrosis and lipomatosis were registered separately for the degeneration score as their occurrence seems to be independent of each another. Both degeneration patterns (in mild and strong form, scores 1 and 2) appeared simultaneously in n = 16 (39%) of the nodes. In n = 18 (43.9%) only fibrosis was present and in n = 3 (7.3%) only lipomatosis was present. These degenerative features had no significant preference for medullary or subcapsular location. An analysis of the degree of fibrosis showed a high prevalence in the age groups 61–75 and 76+ years, namely three nodes from age group 61–75 years in grade 1 and four in grade 2 (n = 9), and six nodes from age group 76+ years in fibrosis grade 2 (n = 8). Surprisingly, three nodes from age group 16–30 years (n = 6) presented a fibrosis of grade 1 (mild) and two presented a fibrosis of grade 2 (severe). Most lymph nodes in the age groups 31–45 and 46–60 years presented only little or mild fibrosis. Therefore, the occurrence of lymph node fibrosis is not only determined by age, as it was often possible to see this type of alteration in young adults. As for lipomatosis, it was rare in the age group 16–30 years, which had the lowest score (grade 0) in all except one case of mild (grade 1) lipomatosis. In the other age groups its occurrence was constant, with grade 1 lipomatosis (substitution of up to 50% of the lymph node through fatty tissue) in two lymph nodes of age group 31–45 years (n = 6), four nodes of age group 46–60 years (n = 10), four nodes of age group 61–75 years (n = 9) and three nodes of age group 76+ years (n = 8). Grade 2 lipomatosis was rare, with most cases in the age group 31–45 years (two cases).
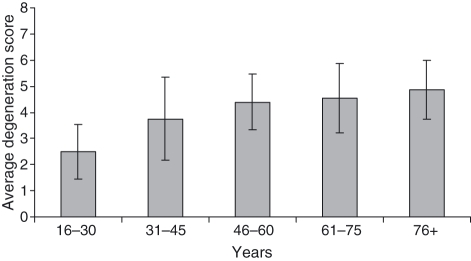
The degeneration score was calculated for each individual. Analysis of the average scores in each age group clearly showed an increasing tendency for lymph node degeneration with age (Fig. 5).
Fig. 5.
Average degeneration score and SD according to each age group. A higher score corresponds to an increase in degenerative features.
Discussion
Although degenerative changes within lymph nodes associated with ageing have been described in the past (Luscieti et al. 1980; Junt et al. 2008; Pan et al. 2008), none of the existing publications provide clear criteria to quantify and qualify these changes. Research on lymph node transplantation (Tammela et al. 2007; Blum et al. 2010; Hadamitzky et al. 2009; Lin et al. 2009), intranodal vaccination (Senti et al. 2008; Martinez-Gomez et al. 2009) and immunosenescence (Kovaiou et al. 2007; Fietta, 2008) makes such an assessment fundamental. Furthermore, degenerative processes within the lymph node not only have numerous implications for the above topics but also challenge the widespread notions of lymph nodal internal architecture throughout life. Lymph nodes composed of a well-defined cortex containing primary and/or secondary follicles, a T-cell-rich paracortex and a typical medulla as widely described in the literature (Alberts, 1994; Robbins, 1994; Gray, 1995; Földi, 2003; Janeway, 2005) were seldom seen in our specimens.
One of the striking conclusions of this study is that lymph node degeneration is very prevalent. Degeneration causes other than age were minimized, as our specimens were exclusively obtained from forensic pathological autopsies. Probes can be seen as representative of an average individual immunological activity – no high activation through chronic diseases and no dormancy through immunological isolation (e.g. pathogen-free conditions as are common in experimental animals) or immune suppression (e.g. through corticosteroids during prolonged disease or as treatment). The generally low numbers of HEVs within our findings along with the overall presence of small primary follicles and the lack of high numbers of secondary follicles probably represent an immunological resting stage of the lymph node that confirms our choice of material in order to minimize prevalence of pathological conditions. All lymph nodes were taken from the same anatomical region to allow comparison. Superficial inguinal lymph nodes were chosen as they are of palpable size in all age groups for, until now, unknown reasons in contrast to other regions such as the axilla.
An increase of connective tissue replacing normal lymphoid structure was widely but not exclusively found within lymph nodes of elderly people. This was frequently associated with a reduction of lymphocytes. Lymphocytes as well as other immune cells are the prerequisite for appropriate lymph node function. Their partial absence might result in an inability to properly filter lymphatic fluid and perform adequate, rapid immune responses. As observations were static it is difficult to ascertain to what extent this process is reversible and dependent on immune challenge. It is nevertheless clinically established that age causes a certain loss of immune function (Pahlavani et al. 1987; Grewe, 2001) and the present findings contribute to the explanation of this phenomenon.
Lymph node degeneration should be seen as a combination of factors, whereas isolated degenerative features can be observed independent of age. A score resulting from the combination of isolated features could prove to be the adequate instrument to assess it. Degeneration is an internal process of each lymph node rather than a ubiquitous phenomenon happening simultaneously within every lymph node of an individual, which explains the heterogeneity of our findings.
The presented results, leading to a reformulation of historical concepts of the lymph node microanatomy, have a considerable impact on ongoing research. The dynamic process of lymph node degeneration can be understood as an underestimated source of clinical bias. With regard to the mentioned method of intranodal vaccination (Senti et al. 2008; Martinez-Gomez et al. 2009), it is possible that intranodal injections in degenerated areas allow for the extraordinarily low response observed in some subjects. Sonographic techniques allowing visual observation of the vaccine in the nodal sinus should be used to reduce the influence of lymph node degeneration in desensitization.
Another concept that is greatly influenced by these findings is the use of lymph node transplantation to enhance post-operative metastasis filtration (Tammela et al. 2007) or allow for better lymph transport and resorption after regeneration of the fragments in acquired lymphoedema (Becker et al. 2006; Blum et al. 2010; Hadamitzky et al. 2009; Lin et al. 2009). To guarantee that functional nodes are used it is probably necessary to perform histological examination and grading of the donor nodes before transplantation. The older the patient, the higher the probability that degenerated nodal tissue will be transplanted, potentially rendering the operative procedure redundant.
In addition, awareness of lymph node degeneration has consequences in tumour-staging procedures. Pathologists asked to assess risk profiles by analysing local lymphatic drainage excised during cancer surgery are often confronted with a small amount of lymph nodes in the probes (Dillman et al. 2009). When all located lymph nodes are free of cancer cells, it seems appropriate to regard these patients as a group with a better prognosis than those displaying lymph node metastases. However, many studies have already shown a negative influence of low lymph node numbers on long-term prognosis (Caplin et al. 1998; Swanson et al. 2003; Berberoglu, 2004; Bui et al. 2006). Among pathologists, these cases are retrospectively considered as understaged. Pan et al. (2008) have recently described so-called ‘transparent’ lymph nodes, which represent a lymph node degeneration stage beyond the regressive changes found in this study. These inactive lymph nodes cannot be detected macroscopically and therefore were not part of our pool. In all likelihood, in older patients, most of the lymph nodes in a region of interest have already reached total degeneration and are therefore macroscopically undetectable. These lymph nodes are unable to trap and control bypassing cancer cells. Under these circumstances, it might be inappropriate to generally classify patients with a low lymph node count without metastases as low-risk. Further studies correlating age, number of lymph node findings and prognosis should investigate the necessity of introducing a new term for pathological classification including the notions of lymph node degeneration and thereby identifying new high-risk groups.
Conclusion
Human lymph node degeneration, a concept not taken into account by classical textbooks, is very prevalent and its incidence increases with age. This study provides a score based on the degree of lymphocyte depletion, HEVs, lymph node fibrosis and lipomatosis to permit an assessment of the degree of degeneration. Degeneration grades must be considered when planning interventions to the lymph nodes, e.g. for transplantation or allergen desensitization. Interpretation of pathology findings should be revised in order to include lymph node degenerative features as a potential predictor of oncological prognosis.
Acknowledgments
The authors thank Andrea Herden and Karin Westermann for excellent technical assistance and Selma Nolte for polishing the English.
References
- Alberts B. Molecular Biology of the Cell. New York: Garland Publishing; 1994. [Google Scholar]
- Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313–315. doi: 10.1097/01.sla.0000201258.10304.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberoglu U. Prognostic significance of total lymph node number in patients with T1-4N0M0 colorectal cancer. Hepatogastroenterology. 2004;51:1689–1693. [PubMed] [Google Scholar]
- Blum KS, Hadamitzky C, Gratz KF, et al. Effects of autotransplanted lymph node fragments on the lymphatic system in the pig model. Breast Cancer Res Treat. 2010;120:59–66. doi: 10.1007/s10549-009-0367-4. [DOI] [PubMed] [Google Scholar]
- Bui L, Rempel E, Reeson D, et al. Lymph node counts, rates of positive lymph nodes, and patient survival for colon cancer surgery in Ontario, Canada: a population-based study. J Surg Oncol. 2006;93:439–445. doi: 10.1002/jso.20499. [DOI] [PubMed] [Google Scholar]
- Caplin S, Cerottini JP, Bosman FT, et al. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer. 1998;83:666–672. [PubMed] [Google Scholar]
- Denz FA. Age changes in lymph nodes. J Pathol Bacteriol. 1947;59:575–591. doi: 10.1002/path.1700590409. [DOI] [PubMed] [Google Scholar]
- Dillman RO, Aaron K, Heinemann FS, et al. Identification of 12 or more lymph nodes in resected colon cancer specimens as an indicator of quality performance. Cancer. 2009;115:1840–1848. doi: 10.1002/cncr.24185. [DOI] [PubMed] [Google Scholar]
- Fietta P. The immunosenescence. Riv Biol. 2008;101:247–263. [PubMed] [Google Scholar]
- Földi M. Textbook of Lymphology. Munich: Elsevier; 2003. [Google Scholar]
- Gray H. Gray’s Anatomy. Edinburgh: Churchill Livingstone; 1995. [Google Scholar]
- Grewe M. Chronological ageing and photoageing of dendritic cells. Clin Exp Dermatol. 2001;26:608–612. doi: 10.1046/j.1365-2230.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- Hadamitzky C, Blum KS, Pabst R. Regeneration of autotransplanted avascular lymph nodes in the rat is improved by platelet-rich plasma. J Vasc Res. 2009;46:389–396. doi: 10.1159/000194269. [DOI] [PubMed] [Google Scholar]
- Hoorweg K, Cupedo T. Development of human lymph nodes and Peyer’s patches. Semin Immunol. 2008;20:164–170. doi: 10.1016/j.smim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Janeway CA. Immunobiology. New York: Garland Science Publishing; 2005. [Google Scholar]
- Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8:764–775. doi: 10.1038/nri2414. [DOI] [PubMed] [Google Scholar]
- Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Rev Mol Med. 2007;9:1–17. doi: 10.1017/S1462399407000221. [DOI] [PubMed] [Google Scholar]
- Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg. 2009;123:1265–1275. doi: 10.1097/PRS.0b013e31819e6529. [DOI] [PubMed] [Google Scholar]
- Luscieti P, Hubschmid T, Cottier H, et al. Human lymph node morphology as a function of age and site. J Clin Pathol. 1980;33:454–461. doi: 10.1136/jcp.33.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gomez JM, Johansen P, Erdmann I, et al. Intralymphatic injections as a new administration route for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2009;150:59–65. doi: 10.1159/000210381. [DOI] [PubMed] [Google Scholar]
- Pahlavani MA, Richardson A, Cheung HT. Age-dependent changes of the mesenteric lymph node of Fischer F344 rats: morphological and histometric analysis. Mech Ageing Dev. 1987;39:137–146. doi: 10.1016/0047-6374(87)90005-4. [DOI] [PubMed] [Google Scholar]
- Pan WR, Suami H, Taylor GI. Senile changes in human lymph nodes. Lymphat Res Biol. 2008;6:77–83. doi: 10.1089/lrb.2007.1023. [DOI] [PubMed] [Google Scholar]
- Robbins SL. Pathologic Basis of Disease. Philadelphia: W.B. Saunders; 1994. [Google Scholar]
- Senti G, Prinz Vavricka BM, Erdmann I, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci USA. 2008;105:17908–17912. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RS, Compton CC, Stewart AK, et al. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Holopainen T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34:409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]