Abstract
The role of thyroid hormones in testis structure and function has been fairly well studied in laboratory rodents. However, there are no comprehensive data in the literature for mice regarding the effects of transiently induced neonatal hypo- and hyperthyroidism on testis and spermatogonial cell development from birth to adulthood. Our goals were to evaluate the effects of propylthiouracil (PTU) and triidothyronine (T3) on Sertoli cell proliferation/differentiation and to correlate these events with the evolution of the spermatogenic process, tubular lumen formation, blood vessel volume density, and size and number of different spermatogonial types. Although Sertoli cell maturation was accelerated or delayed, respectively, in T3- and PTU-treated mice, the pace of the germ cell maturation was only slightly altered before puberty and the period of Sertoli cell proliferation was apparently not affected by the treatments. However, compared with controls, the total number of Sertoli cells per testis from 10 days of age to adulthood was significantly increased and decreased in PTU- and T3-treated mice, respectively. In comparison to all other spermatogonia, type A2 was the largest cell in all ages and groups investigated. The PTU-treated mice had a significantly increased total number of undifferentiated spermatogonia as well as volume and percentage of vessels/capillaries, probably due to the higher number of Sertoli cells, particularly at 10 days of age. Taken together, our results suggest that neonatal hypothyroidism may be a valuable tool for studying spermatogonial biology as well as a means for providing more spermatogonial stem cells that could potentially be used for spermatogonial transplantation, thereby optimizing the efficiency of this technique when young mice are used as donors.
Keywords: C57BL/6J mice, propylthiouracil (PTU), Sertoli cell, spermatogonia, triidothyronine (T3), testis development
Introduction
Sertoli cells are the first somatic element to differentiate in the testis and their precursors express the sex-determining gene Sry in the short arm of the Y chromosome (Karl & Capel, 1998; Capel, 2000; DiNapoli & Capel, 2008). Therefore, Sertoli cells play a central role in testis differentiation, which occurs around 11.5 days post-coitus in mice (Capel, 2000). Sertoli cells proliferate more actively before birth and, in rodents, proliferation extends to 2 (mice) to 3 weeks (rats) after birth (Steinberger & Steinberger, 1971; Orth, 1982; Vergouwen et al. 1991; Joyce et al. 1993). Thereafter, the number of Sertoli cells per testis is considered stable throughout the life of the animal (França et al. 2005).
During early development, the primordial germ cells colonize the bipotential gonads and become gonocytes (Ross & Capel, 2005). In the testis of newborn mice, these gonocytes directly give rise to tyrosine kinase receptor (c-kit)-positive differentiating spermatogonia as well as Neurogenin (Ngn3)-positive undifferentiated spermatogonia (Yoshida et al. 2006). In laboratory rodents, stem cell functions reside in a small subset of spermatogonia designated undifferentiated spermatogonia (Aund), which are classified as Asingle (isolated single cells), Apaired (chains of 2 cells) and Aaligned (chains of 4, 8, 16 or occasionally 32 cells) (De Rooij & Russell, 2000). Aaligned chains transform into differentiated type A1 spermatogonia, followed by several mitotic divisions that give rise to A2, A3, A4, intermediate (In) and type B (B) spermatogonia as well as primary pre-leptotene spermatocytes, which initiates the first meiotic prophase (De Rooij & Russell, 2000; De Rooij & Grootegoed, 1998). The spermatogonial cells are located in the basal compartment of the seminiferous epithelium, in direct contact with Sertoli cells and the basement membrane. Particularly for the Asingle spermatogonia, this surrounding microenvironment is denominated the spermatogonial stem cell niche and spermatogonial stem cell self-renewal is maintained by the glial cell line-derived neurotrophic factor (GDNF), produced by Sertoli cells (Hofmann, 2008).
The different spermatogonial types can be characterized in toto by different methodologies, such as whole-mount (De Rooij & Russell, 2000), and by light and transmission electron microscopy based on the presence and distribution of heterochromatin (Chiarini-Garcia & Russell, 2001, 2002). It has been suggested that undifferentiated spermatogonia – probably including Asingle or stem cells – are preferentially located in particular regions of the seminiferous epithelium adjacent to the intertubular compartment (Chiarini-Garcia et al. 2001; Ogawa et al. 2005), more specifically close to the blood vessels (Shetty & Meistrich, 2007; Yoshida et al. 2007).
The period of Sertoli cell mitotic activity is extended by approximately 2 weeks in laboratory rodents made transiently hypothyroidic by reversible goitrogen 6-propyl-2-thiouracil (PTU) treatment during the neonatal period, leading to a significant increase in testis size, number of Sertoli cells per testis and sperm production in adult animals (Cooke et al. 1991, 2005; Van Haaster et al. 1992; Joyce et al. 1993; Holsberger & Cooke, 2005; Holsberger et al. 2005a,b;). In contrast, hyperthyroidism induced by triidothyronine (T3) accelerates Sertoli cell differentiation/maturation, resulting in a lower number of Sertoli cells per testis and lower sperm production (Van Haaster et al. 1993; Holsberger & Cooke, 2005). According to Hess et al. (1993), the larger Sertoli cell population stemming from the hypothyroidism is followed by an increased number of germ cells in the adult testis but this study did not investigate the number of the different spermatogonial types. Thus, the main objective of the present study was to evaluate the effects of neonatal hypo- and hyperthyroidism on testis development, Sertoli cell proliferation/differentiation and the correlation of these events with the evolution of spermatogenesis and sperm production. Moreover, the number and volume of undifferentiated (Asingle, Apaired and Aaligned) and differentiated (type A1–4, intermediate and type B spermatogonia) spermatogonia were evaluated for the first time in mice, along with volume density and volume of the testis vascular components from birth to adulthood.
Materials and methods
Animals and treatments
The C57BL/6J mice were bred in our colony and housed at 22 °C with a 12L : 12D photoperiod and given standard rodent food ad libitum. A total of 128 mice were used (control, n = 53; PTU-treated mice, n = 47; T3-treated mice, n = 28). Hypothyroidism was induced as described by Joyce et al. (1993) and the pups received PTU through the mother’s milk from birth to 20 days of age. To make hyperthyroid pups, T3 (Sigma) was dissolved in 0.025 N sodium hydroxide and diluted in physiological saline. T3 was administered by daily subcutaneous injections of 100 μg kg−1 body weight from day 1 to day 15 (Van Haaster et al. 1993). Testes were removed from mice at 1, 5, 10, 15, 20, 28 and 100 days of age (day of birth = 1). At least four animals were used for each age and treatment. All procedures and protocols followed approved guidelines for the ethical treatment of animals (Ethics Committee in Animal Experimentation from the Federal University of Minas Gerais - CETEA/UFMG).
Tissue preparation
The mice were killed at 1 and 5 days of age by decapitation and the testes were fixed by immersion in 5% (v/v) glutaraldehyde in 0.05 m sodium cacodylate buffer. Testes from mice of 10–100 days of age were perfused-fixed following the procedure described by Sprando (1990). After being separated from the epididymis and weighed, the testes were prepared for high-resolution light microscopy evaluations (Chiarini-Garcia & Russell, 2001) and stereological analysis. Briefly, the testes were cut into 0.5-mm slabs by perpendicular sectioning. The slabs were then cut into approximately 2-mm (length × width) portions and embedded such that cross-sections of seminiferous tubules would be obtained on sectioning. After an overnight fixation of diced slabs, the tissues were washed in three changes of buffer, post-fixed in an osmium-ferrocyanide mixture, dehydrated, embedded in Araldite resin, sectioned at approximately 1 μm thickness and stained with toluidine blue. From the knowledge of the body weight and testicular weight, the gonadosomatic index (testes mass divided by body weight) was estimated for all mice investigated.
Evolution of spermatogenesis and lumen formation
The specific basis for identifying the gonocytes and spermatogonia (undifferentiated and differentiated) followed those described by Vergouwen et al. (1991) and Chiarini-Garcia & Russell (2001), respectively. The most advanced germ cell type present in the seminiferous cords/tubules was investigated through a careful evaluation of all histological sections obtained from the mice killed from 5 to 28 days of age. This evaluation was not performed in newborn and adult mice, as these animals, respectively, presented only gonocytes (see below) or full spermatogenesis. The presence of lumen was evaluated from the analysis of 100 seminiferous cord/tubule cross-sections for each mouse – also killed from 5 to 28 days of age.
Testis stereology
The diameter of the seminiferous cords/tubules was measured at 400 × magnification using an ocular micrometer calibrated with a stage micrometer. Between 10 and 20 tubule profiles that were either round or nearly round were chosen randomly and measured for each animal. The volume densities of testicular tissue components were determined by light microscopy using a 441-intersection grid placed in the ocular of the light microscope. A total of 30 (1–15 days of age), 40 (20 days) and 80 (28 and 100 days) randomly chosen fields (13 230, 17 640 and 35 280 points, respectively) were scored for each animal at 1000 × magnification. Artifacts were rarely seen and were not considered in the total number of points used to obtain volume densities. The testicular components were classified as one of the following: gonocytes, undifferentiated spermatogonia (Aund), differentiated spermatogonia [(A1, A2, A3 and A4), In and B] and Sertoli cells. The vessels (capillary, artery, vein and lymphatic vessel) were also evaluated. The volume of each component of the testis was determined as the product of the volume density and testis volume. For subsequent stereological calculations, the specific gravity of testis tissue was considered to be 1.0 (França & Godinho, 2003). To obtain a more precise measure of testis volume, the testis capsule was excluded from the testis weight. The Sertoli cell nuclear volume was obtained from the knowledge of the mean nuclear diameter and 40 evident nuclei were measured for each animal. Nuclear volume was expressed in μm3 using the formula 4/3Πr3, where R = nuclear diameter/2.
Cell counts and cell numbers
Individual volumes of the gonocytes and different types of spermatogonia (undifferentiated and differentiated) were obtained from the nucleus volume as well as the proportion between nucleus and cytoplasm. As these germ cell nuclei are usually round or ovoid, their nucleus volume was obtained from the knowledge of the mean nuclear diameter. For each cell type, 30–40 nuclei had their diameter measured for each animal. Germ cell nuclear volume was expressed in μm3 and obtained as mentioned above. To estimate individual germ cell volume, a 441-point square lattice was placed over the sectioned material at 1000 × magnification. A total of 20 cells of each germ cell type were evaluated for each animal. The number of germ cells per testis was estimated from the individual germ cell volume and the volume occupied by the particular germ cell in the testis parenchyma. Because, from 10 days of age, the germ cell associations that compose each of the XII stages of the epithelium cycle could already be characterized, the total number of undifferentiated spermatogonia was divided by 8.7 days, which is the duration of one cycle of the seminiferous epithelium in C57BL/6J mice, according to unpublished data from our laboratory. This divisor was utilized for all undifferentiated spermatogonia of all groups investigated from 10 to 100 days of age and took into account that these spermatogonia are present in all stages of the seminiferous epithelium cycle (De Rooij, 1998). For the other cell types (A1 to B), the duration of a particular stage in which these cells are located was used as a divisor. The total number of Sertoli cells per testis was determined as follows: total number of Sertoli cells per testis = total volume of Sertoli cell nucleus in the testicular parenchyma (μL)/Sertoli cell nuclear volume (μm3).
In order to estimate the meiotic index (number of newly-formed round spermatids per primary pachytene spermatocyte), Sertoli cell efficiency (number of spermatids per Sertoli cell) and spermatogenic efficiency (daily sperm production per testis) in 100-day-old mice, pachytene spermatocytes, round spermatids and Sertoli cell nucleoli present in stage VII (Russell et al. 1990) were counted in 10 randomly chosen round or nearly round seminiferous tubule cross-sections for each animal. These counts were corrected for section thickness and for nucleus or nucleolus diameter, based on the procedure described by Abercrombie (1946) and modified by Amann & Almquist (1962). For this purpose, the diameters of 10 nuclei (from pachytene spermatocytes and spermatids) or nucleoli (from Sertoli cells) were measured per animal. The cell ratios were obtained from the corrected counts obtained in stage VII. Spermatogenic efficiency was obtained from the following formula: Daily Sperm Production (DSP) = [total number of Sertoli cells per testis × ratio of round spermatids to Sertoli cells in stage VII × stage VII relative frequency (%)]/stage VII duration (in days) (França, 1992).
Statistical analysis
All data are presented as mean ± SEM and were analyzed using anova. Analysis was performed using the statistica 3.11 software program for Windows (StatSoft, Inc., Tulsa, OK, USA). The significance level was set at P < 0.05.
Results
Biometric data
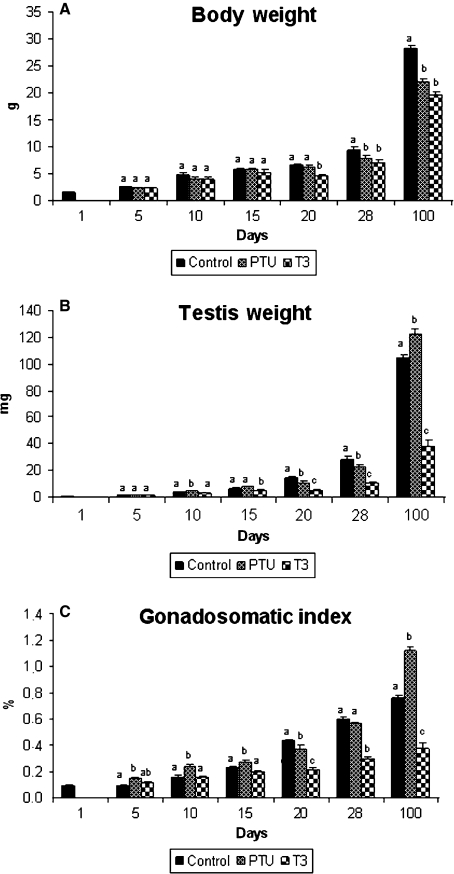
Body weight, testicular weight and gonadosomatic index for the control, PTU- and T3-treated mice are illustrated in Fig. 1. In comparison to the other two groups, body weight was significantly decreased in T3-treated mice at 20 days of age, whereas the body weight of the controls was greater at 28 and 100 days of age (P < 0.05) than that of mice subjected to either treatment. Compared with the controls, the testicular weight of PTU-treated animals was greater (∼ 30%; P < 0.05) at 10 and 100 days, whereas the mice treated with T3 always had lower testis weight (P < 0.05) from 15 days of age to adulthood. The gonadosomatic index was higher (P < 0.05) in PTU-treated mice between 5 and 15 days of age as well as in adult animals. Conversely, following the same trend observed for the testis weight, the gonadosomatic index was always lower (P < 0.05) in T3-treated mice evaluated from 20 to 100 days of age.
Fig. 1.
Body weight (A), testis weight (B) and gonadosomatic index (C) in controls, propylthiouracil PTU- and T3-treated animals killed at different ages after birth (mean + SEM). Different letters for the same age denote significant differences (P < 0.05).
Evolution of spermatogenesis, diameter of seminiferous cords/tubules and lumen formation
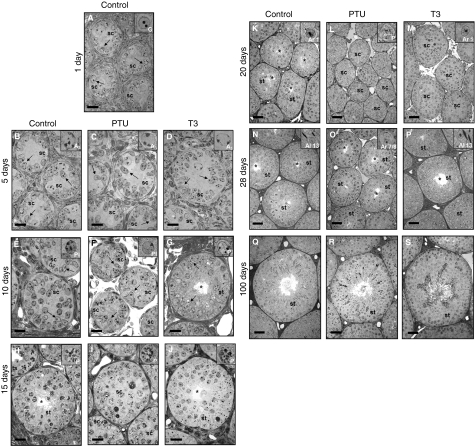
In all groups evaluated, gonocytes, differentiated A3 spermatogonia, pre-leptotene spermatocytes and pachytene spermatocytes were, respectively, the most advanced germ cell types in the seminiferous epithelium at 1, 5, 10 and 15 days of age (Figs 2 and 3). Although few different germ cell types were present at 10 days, it was already possible to observe the organization of these cells in the seminiferous epithelium, resembling the different cell associations, denominated stages. Except for the hypothyroidic mice, in which germ cells remained in the pachytene stage at 20 days of age, the most advanced germ cells in both control and T3-treated mice were newly-formed round spermatids. At 28 days, these cells were usually step 9/10 spermatids (one animal presented step 13 spermatids), step 7/8 spermatids and step 13 spermatids, respectively, in controls, PTU- and T3-treated mice (Fig. 3). At 100 days of age, all mice investigated exhibited full, apparently normal, spermatogenesis.
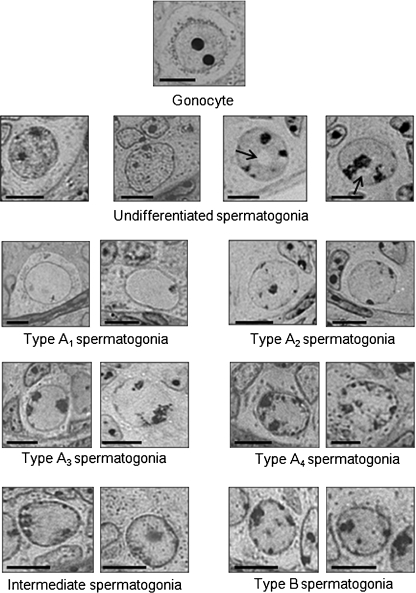
Fig. 2.
The gonocyte and spermatogonia morphology in young animals followed criteria similar to those described by Vergouwen et al. (1991) and Chiarini-Garcia & Russell (2001). Briefly, gonocytes have the largest volume with one to three nucleoli. The undifferentiated spermatogonial population shows a nucleus with mottled chromatin and nuclear vacuoles are often present in the plane of the section (arrows). Differentiated type A1 spermatogonia have a nucleus clearly demarcated from the surrounding nucleoplasm; differentiated type A2 spermatogonia have small flakes (< 10%) of heterochromatin rimming the nuclear envelope; the differentiated type A3 spermatogonia nucleus is slightly increased compared with A2 and the nucleoli are generally reticulated and not well defined but extend deeply into the nucleoplasm; and differentiated type A4 spermatogonia have 30–70% of the nuclear envelope encrusted with heterochromatin. Only small differences are noted when these cells are compared with A3. Intermediate spermatogonia present shallow heterochromatin lining 70–100% of the nucleus, making the nuclear/cytoplasmic boundary well delineated. Type B spermatogonia (B) have small, densely stained spots of heterochromatin attached to the nuclear envelope. Bar = 7 μm.
Fig. 3.
Seminiferous cord/tubule sections of controls (A, B, E, H, K, N and Q), propylthiouracil PTU-(C, F, I, L, O and R) and T3-treated mice (D, G, J, M, P and S) killed between 1 and 100 days of age. Bar = 20 μm (A–J) and 40 μm (K–S). sc, seminiferous cords; st, seminiferous tubules; asterisk, lumen; arrows, Sertoli cells. The insert indicates the most advanced cell type: G, gonocyte; A3, type A3 spermatogonia; Pl, pre-leptotene spermatocyte; P, pachytene spermatocyte; Ar 1, step 1 round spermatid; Al 7/8, step 7/8 elongated spermatid; Al 13, step 13 elongated spermatid.
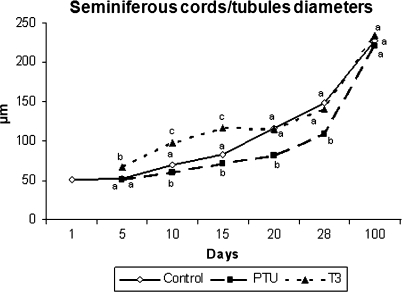
In comparison to the controls and T3-treated mice, the diameter of the seminiferous cords/tubules was always lower (P < 0.05) in PTU-treated animals from 10 to 28 days (Figs 3 and 4). Conversely, the data obtained for these parameters were higher (P < 0.05) in T3-treated animals from 5 to 15 days of age. However, there was no significant difference in tubule diameter in the three experimental groups investigated at 100 days of age.
Fig. 4.
Diameter of seminiferous cords/tubules in controls, propylthiouracil PTU- and T3-treated animals killed at different ages after birth (mean ± SEM). Different letters for the same age denote significant differences (P < 0.05).
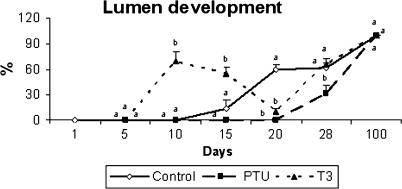
In all groups investigated, no evidence of lumen formation was observed until 5 days of age (Figs 3 and 5). At 10 days, only T3-treated mice exhibited seminiferous tubule lumens, which were present in varying degrees in approximately 70% of the cords/tubules evaluated. The PTU-treated animals remained with no apparent lumens until 20 days, whereas about 15 and 60% of the tubules in the controls had a lumen at 15 and 20 days, respectively. Surprisingly, a prominent reduction in tubules with a lumen was observed in hyperthyroidic mice from 10 to 20 days and this trend was reversed at 28 days, which is the age that precedes the full establishment of spermatogenesis, occurring at approximately 5 weeks of age in mice, when all tubules have a patent lumen (Vergouwen et al. 1993; França L.R., unpublished data). Also, at 28 days, lumen formation was first observed in PTU-treated animals although still at a lower percentage (Figs 3 and 5). At 100 days of age, all seminiferous tubules exhibited a patent lumen in the three groups investigated.
Development of lumen in the seminiferous cords/tubules in controls, propylthiouracil PTU- and T3-treated animals killed at different ages after birth (mean + SEM). Different letters for the same age denote significant differences (P < 0.05).
Spermatogonial cell size
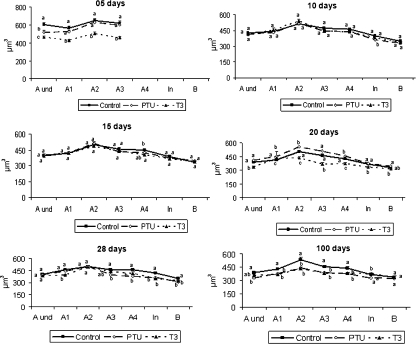
Figure 6 shows that the individual volume of undifferentiated spermatogonia (Aund) was different (P < 0.05) at 5 days of age in the three groups investigated, whereas the volumes for the other germ cells (types A1–A3 differentiated spermatogonia) at this age were significantly lower in T3-treated mice. At 10 days of age, pre-leptotene spermatocytes were present in all groups and the volumes of all different spermatogonial types could be evaluated. Therefore, it was noted that the spermatogonial volume increased 25% from undifferentiated to A2 spermatogonia (∼ 400 to ∼ 500 μm3) and then decreased gradually by ∼ 30% until type B spermatogonia (∼ 500 to ∼ 330 μm3). A later decline of a similar magnitude occurred from type B spermatogonia to primary spermatocytes in pre-leptotene (∼ 330 to ∼ 230 μm3). The same tendency was observed for the other ages investigated (Fig. 6). Moreover, a generally similar trend was found for the values related to nuclear and cytoplasm volumes. Regarding the different experimental groups investigated at different ages, germ cell volumes exhibited the same previously described trend and, amongst all of the spermatogonial types investigated, A2 spermatogonia were always the largest cells. Particularly at 10 and 15 days of age, practically no differences were found in cell volumes among the groups. However, from 20 to 100 days, cell volumes were generally greater in the control group and lower in T3-treated mice, whereas in PTU-treated mice these volumes were variable and no clear trend was observed. It should be emphasized that, for all spermatogonial types, the nuclear volume was proportionately higher and occupied approximately three-quarters of the total cell volume (data not shown). For pre-leptotene spermatocytes, however, this proportion was smaller at around two-thirds of the cell volume (data not shown).
Fig. 6.
Individual germ cell volume in control, propylthiouracil PTU- and T3-treated mice killed between 5 and 100 days of age. Different letters for the same age denote significant differences (P < 0.05) between groups. Undifferentiated type A (Aund), differentiated type A (A1–4), intermediate (In) and type B (B) spermatogonia (mean ± SEM). Different letters for the same age germ cell type indicate significant differences (P < 0.05).
Total number of spermatogonia per testis
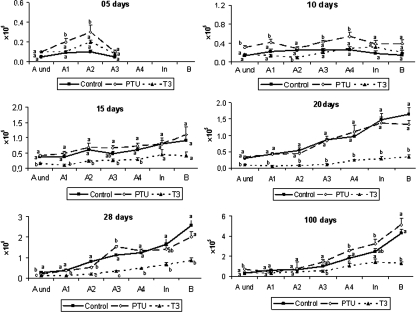
The data for the total number of all spermatogonial types investigated are displayed in Fig. 7. At 5 days, except for the Aund and A3 spermatogonia, the number of the other germ cell types present at this age (A1 and A2) was higher (P < 0.05) in hypothyroidic mice when compared with the controls. In comparison to the mice investigated at 5 days of age, the total number of gonocytes per testis obtained at birth was approximately twice the value observed for undifferentiated spermatogonia (0.5 × 105 vs. 0.24 × 105) and similar to the value found for the total number of type A spermatogonia (0.5 × 105 vs. 0.6 × 105). Therefore, some gonocytes had differentiated into spermatogonia at 5 days, whereas others probably suffered apoptosis.
Fig. 7.
Total number of germ cells per testis in control, propylthiouracil PTU- and T3-treated mice killed between 5 and 100 days of age. Different letters for the same age denote significant differences (P < 0.05) between groups. Undifferentiated type A (Aund), differentiated type A (A1–4), intermediate (In) and type B (B) spermatogonia (mean + SEM). Different letters for the same age germ cell type indicate significant differences (P < 0.05).
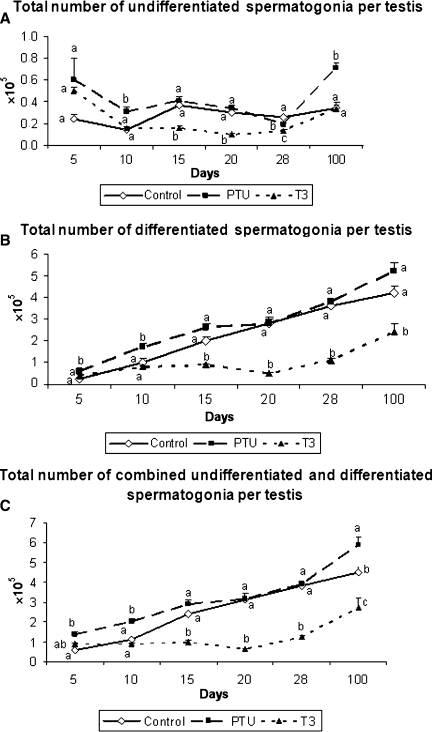
In comparison to the other two groups, the hypothyroidic mice had an overall greater (P < 0.05) number of germ cells at 10 days of age. It is important to note that the total number of type A spermatogonia, including undifferentiated spermatogonia, at this age was significantly higher in PTU-treated mice (Figs. 7 and 8). At the other ages investigated, the PTU-treated animals generally exhibited a similar number of germ cells when compared with the controls. In contrast, T3-treated mice usually had a lower (P < 0.05) number of these cells. Figure 8 summarizes the total number of undifferentiated and differentiated spermatogonia as well as the total number of spermatogonia in the three groups investigated from 5 to 100 days of age. As expected and according to what was previously described for the pool of undifferentiated and differentiated spermatogonia, a similar trend was found.
Fig. 8.
Total number of undifferentiated (A) and differentiated (B) spermatogonia, and total number of combined undifferentiated and differentiated spermatogonia (C) per testis in control, propylthiouracil PTU- and T3-treated mice killed between 5 and 100 days of age. Different letters for the same age indicate significant differences (P < 0.05) between groups (mean + SEM).
Sertoli cell stereology
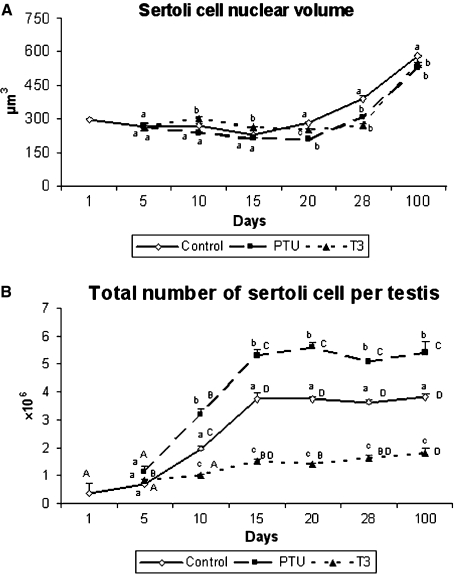
Data on the nuclear volume of Sertoli cells are displayed in Fig. 9A. In comparison with the two other experimental groups, hyperthyroidic mice exhibited a greater (P < 0.05) nuclear volume at 10 and 15 days of age. However, a significant reduction was observed for this parameter in T3-treated mice at 20 days, whereas the value found for PTU- and T3-treated mice was lower (P < 0.05) from 28 to 100 days when compared with the controls.
Fig. 9.
Sertoli cell nuclear volume (A) and total number of Sertoli cells per testis (B) in controls, propylthiouracil PTU- and T3-treated mice killed at different ages. Different lowercase letters for the same age indicate significant differences (P < 0.05) between treatments and different uppercase letters indicate significant differences between the ages investigated (P < 0.05) (mean + SEM).
At 5 days of age, the total number of Sertoli cells per testis demonstrated no significant differences in the three groups (Fig. 9B). However, the total number of these cells from 10 to 100 days of age always remained higher (P < 0.05) in PTU-treated mice and lower (P < 0.05) in those that received T3 in comparison to the controls. From 15 to 100 days of age, there was a strong trend toward stabilization in the number of Sertoli cells per testis in all three groups.
Sertoli cell and spermatogenic efficiencies
These evaluations were only made for adult mice. Both the meiotic index and Sertoli cell efficiency were similar (P > 0.05) in the three experimental groups. These indices demonstrated that approximately 10 spermatids were supported per Sertoli cell in mice at 100 days of age and that 25–30% of germ cell loss (apoptosis) occurred during meiosis. However, in comparison to the control mice (4.2 ± 0.5 × 106), the total daily sperm production per testis was significantly higher (5.6 ± 0.5 × 106) and lower (2.4 ± 0.2 × 106) in PTU- and T3-treated mice, respectively.
Blood and lymphatic vessel stereology
The results related to the volume and volume density of the different testis vascular components are displayed in Table 1. For most ages investigated (particularly at 10–15 days), hypothyroidic mice had higher (P < 0.05) values for the percentage and absolute volume of the vascular components investigated, especially capillaries.
Table 1.
Volume (μL) and volume density (% in parenthesis) of the different vascular components at different ages in C57BL/6J mice (mean ± SEM).
| Age | Control | PTU | T3 |
|---|---|---|---|
| 5 days | |||
| Capillary | 0.02 ± 0.003a (1.2 ± 0.2a) | 0.03 ± 0.006b (1.5 ± 0.2a) | 0.01 ± 0.002a (0.8 ± 0.2a) |
| Lymphatic vessel | 0.006 ± 0.001a (0.5 ± 0.06a) | 0.005 ± 0.001a (0.3 ± 0.03a) | 0.004 ± 0a (0.3 ± 0.1b) |
| Total vessels | 0.02 ± 0.003a (1.8 ± 0.2a) | 0.03 ± 0.01a (1.6 ± 0.4a) | 0.02 ± 0.003a (1.3 ± 0.2a) |
| 10 days | |||
| Capillary | 0.04 ± 0.01a (1.2 ± 0.3a) | 0.17 ± 0.04b (3.2 ± 0.4b) | 0.05 ± 0.007a (1.6 ± 0.2a) |
| Lymphatic vessel | 0.02 ± 0.004a (0.6 ± 0.1a) | 0.05 ± 0.008b (1 ± 0.1a) | 0.02 ± 0.003a (0.5 ± 0.1a) |
| Total vessels | 0.07 ± 0.02a (2.2 ± 0.5a) | 0.25 ± 0.04b (4.8 ± 0.7b) | 0.07 ± 0.007a (2.3 ± 0.2a) |
| 15 days | |||
| Capillary | 0.1 ± 0.02a (1.6 ± 0.3a) | 0.2 ± 0.03b (2.6 ± 0.3b) | 0.1 ± 0.01a (2.1 ± 0.1ab) |
| Lymphatic vessel | 0.05 ± 0.01a (0.8 ± 0.2a) | 0.1 ± 0.02b (1.4 ± 0.2b) | 0.01 ± 0.01a (0.2 ± 0.1a) |
| Total vessels | 0.2 ± 0.05a (2.5 ± 0.6ab) | 0.3 ± 0.06b (4.2 ± 0.5a) | 0.1 ± 0.01a (2.7 ± 0.1b) |
| 20 days | |||
| Capillary | 0.2 ± 0.04a (1.5 ± 0.3a) | 0.3 ± 0.04a (2.6 ± 0.3b) | 0.07 ± 0.02b (1.5 ± 0.4ab) |
| Lymphatic vessel | 0.03 ± 0.02a (0.2 ± 0.1a) | 0.1 ± 0.02a (1 ± 0.2b) | 0.01 ± 0.005b (0.3 ± 0.1a) |
| Total vessels | 0.3 ± 0.05a (2 ± 0.3a) | 0.4 ± 0.05a (3.7 ± 0.3b) | 0.1 ± 0.02b (1.9 ± 0.4a) |
| 28 days | |||
| Capillary | 0.4 ± 0.02a (1.3 ± 0.06a) | 0.3 ± 0.06a (1.7 ± 0.3a) | 0.2 ± 0.03b (1.5 ± 0.2a) |
| Lymphatic vessel | 0.1 ± 0.01a (0.3 ± 0.05a) | 0.15 ± 0.04a (0.8 ± 0.2a) | 0.07 ± 0.01a (0.7 ± 0.08a) |
| Total vessels | 0.4 ± 0.1a (1.8 ± 0.1b) | 0.5 ± 0.1a (2.8 ± 0.4a) | 0.3 ± 0.04a (2.5 ± 0.2ab) |
| 100 days | |||
| Capillary | 1.1 ± 0.2a (1.2 ± 0.2a) | 1.5 ± 0.2a (1.4 ± 0.2a) | 0.4 ± 0.1b (1 ± 0.3a) |
| Lymphatic vessel | 0.3 ± 0.1a (0.3 ± 0.1a) | 0.4 ± 0.1a (0.4 ± 0.07a) | 0.08 ± 0.02a (0.2 ± 0.07a) |
| Total vessels | 1.5 ± 0.3a (1.6 ± 0.3ab) | 2.6 ± 0.2b (2.3 ± 0.2a) | 0.5 ± 0.1c (1.2 ± 0.3b) |
Different letters in same line indicate statistical difference (P < 0.05).
PTU, propylthiouracil; T3, triidothryronine.
Discussion
This is the first study to investigate the effects of transient neonatal hypo- and hyperthyroidism, induced, respectively, by PTU and T3, on Sertoli cell proliferation/differentiation and the correlation of these important events with the evolution of spermatogenesis, spermatogonial cell size, total number of spermatogonia per testis and total volume and volume density of vascular components in the testis. Based on the most advanced germ cell type found at 28 days of age, spermatogenesis was only slightly accelerated and delayed in T3- and PTU-treated mice, respectively, and unpublished data from our laboratory demonstrate that full spermatogenesis is present around 5 weeks of age in control, T3- and PTU-treated mice.
Unlike rats (Van Haaster et al. 1992; Hess et al. 1993; França et al. 1995) and other mouse strains (Swiss mice; Joyce et al. 1993), our results suggest that the period of Sertoli cell proliferation was apparently not affected by the treatments, at least under the conditions of the present study, in which mice were killed at 5-day intervals from birth to 20 days of age. However, the total number of Sertoli cells per testis was significantly increased in hypothyroidic mice in comparison to the controls beginning at 10 days of age, whereas the opposite occurred in the T3-treated mice. As the mice used in the present study were C57BL/6J and those investigated by Joyce et al. (1993) were Swiss-Webster, the unexpected finding that the treatments did not change the Sertoli cell proliferation period could be explained by strain specificity, at least in part. Although we have not evaluated the Sertoli cell proliferation rate, based on the Sertoli cell numbers found we assume that this mitotic index was increased and decreased, respectively, due to the hypo- and hyperthyroidic conditions of the mice. In accordance with the literature (Ross et al. 2001; Russell et al. 2001), it is worth mentioning that, in the present study, Sertoli cell apoptosis was not observed during this cell proliferation period. In particular, mature Sertoli cells only suffer apoptosis when they lack Bclw (Ross et al. 2001; Russell et al. 2001).
The total number of undifferentiated spermatogonia was increased in PTU-treated mice, particularly at 10 days of age, probably due to the higher number of Sertoli cells, and this finding was associated with significantly more vessels. Although functional experiments should be performed on this particular aspect, our data suggest that this particular age may represent a critical window in which potentially more undifferentiated cells and consequently more spermatogonial niches would be available.
We did not measure thyroid hormone levels in the present investigation. However, several critical parameters that were evaluated, such as gonadosomatic index, tubular lumen formation and the total number of Sertoli cells per testis, strongly suggest that the treatments and doses employed were effective in inducing the hyper- and hypothyroidism conditions. Nevertheless, it should be emphasized that, as observed by Joyce et al. (1993), the magnitude of the increase in testicular weight induced by PTU in our study is much less pronounced than that observed in rats (Hess et al. 1993; França et al. 1995; Cooke et al. 2005).
The diameter of seminiferous cords/tubules is an excellent parameter for evaluating the evolution of spermatogenesis during post-natal testis development and the degree of Sertoli cell maturation and fluid secretion that result in lumen formation. Therefore, there was a parallelism between the results obtained for the lumen of the seminiferous tubules and those found for the nuclear volume of the Sertoli cells.
Although Sertoli cell maturation was accelerated in T3-treated mice, an unexpected result was the drastic decrease in the percentage of tubules with a lumen, observed in T3-treated mice killed at 20 days of age. Apparently, this treatment altered the functional activity of Sertoli cells, resulting in a smaller nuclear volume for this cell in this time period. As Sertoli cell fluid secretion is mainly regulated by androgens (De Gendt et al. 2005), the plasma levels of this male steroid could be altered before puberty in T3-treated mice. A direct effect of T3 on other testicular somatic elements such as Leydig and peritubular myoid cells could not be excluded. The results found for the lumen of seminiferous tubules and Sertoli cell efficiency in adult mice strongly suggest that Sertoli cell function was apparently normal in the treated mice, at least at 100 days of age. The changes found regarding spermatogenic efficiency at the same age were merely a reflection of the total number of Sertoli cells per testis, which resulted in smaller and larger testis size in T3- and PTU-treated mice, respectively. Although we did not evaluate any sperm parameter in the present study, in adult rats made transiently hypothyroidic during the neonatal period, sperm concentration and motility in the epididymis cauda as well as litter size have been found to be unaffected by PTU treatment (Cooke & Meisami, 1991).
To our knowledge, there are no longitudinal published data regarding the size and number of different spermatogonial types (Aund to B) in mice or any other mammalian species. Although the different types of undifferentiated and differentiated type A spermatogonia are not discriminated, the literature for mammals indicates that spermatogonial nuclear volume decreases gradually from type A to type B spermatogonia (França, 1991; Neves, 2001). In fish investigated at our laboratory, such as tilapia (Schulz et al. 2005) and zebrafish (Leal et al. 2009), this trend is very clear and dramatic decreases are observed for cell and nuclear sizes from undifferentiated or initial type A spermatogonia to the last generation of type B spermatogonia, which involves approximately 10 spermatogonial generations (Schulz et al. 2005; Leal et al. 2009). Our results demonstrating that undifferentiated type A spermatogonia have lesser nuclear and cell volumes may be useful for the identification of undifferentiated spermatogonia, employing approaches or methods such as in-vitro studies and/or confocal, scanning microscopy and image analysis programs, which could be helpful in the investigation of spermatogonial biology. Although we have no explanation for this finding, in comparison to the other type A spermatogonia evaluated in the present study, A2 spermatogonia were consistently larger in all groups and ages investigated.
Based on the finding that the total number of undifferentiated spermatogonia at 10 days of age was higher in hypothyroidic animals and on the knowledge that undifferentiated spermatogonia are preferentially located adjacent to the interstitium (Chiarini-Garcia et al. 2001) – specifically blood vessels (Yoshida et al. 2007) – we investigated the volume and volume density of the different vascular components in the present study. These unique findings demonstrate that the testis of hypothyroidic mice had a greater volume and volume density of the vascular components evaluated, particularly capillaries at 10–15 days of age. These results suggest that capillaries and blood vessels may play an important role in the spermatogonial environment (Shetty & Meistrich, 2007; Yoshida et al. 2007). Therefore, hypothyroidism may represent an interesting approach for investigating important aspects of spermatogonial biology, including aspects involving the influence of Sertoli cells on blood vessel growth and function (Golat & Cameron, 2008).
The investigations performed in the present study provide important morphological and stereological data related to testis function, particularly spermatogonial cell development and Sertoli cell proliferation/differentiation, in mice made transiently hypo- and hyperthyroidic during the neonatal period and evaluated from birth to adulthood. Interestingly, type A2 spermatogonia were always the largest spermatogonial cells in all groups and ages investigated. Our results suggest that neonatal hypothyroidism may be a useful tool for studying the biology of spermatogonial cells and a potential tool for providing more undifferentiated spermatogonial cells that could potentially be used for spermatogonial transplantation, thereby optimizing the efficiency of this technique when young mice are used as donors.
Acknowledgments
Technical help from Adriano Moreira and Mara Lívia Santos is highly appreciated. The authors also thank Dr Sergio Batlouni for help with the T3 experiments. Financial support from the Minas Gerais State Foundation (FAPEMIG) and the Brazilian National Research Council (CNPq) is gratefully acknowledged. A scholarship awarded to S.A.A. from Coordination for the Improvement of Higher Level Personnel (CAPES) is also greatly appreciated. This was supported by CNPq and FAPEMIG.
References
- Fig. 5.Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:238–248. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Amann RP, Almquist JO. Reproductive capacity of dairy bulls. Direct and indirect measurement of testicular sperm production. J Dairy Sci. 1962;45:774–781. [Google Scholar]
- Capel B. The battle of the sexes. Mech Dev. 2000;92:89–103. doi: 10.1016/s0925-4773(99)00327-5. [DOI] [PubMed] [Google Scholar]
- Chiarini-Garcia H, Russell LD. High resolution light microscopic characterization of mouse spermatogonia. Biol Reprod. 2001;65:1170–1178. doi: 10.1095/biolreprod65.4.1170. [DOI] [PubMed] [Google Scholar]
- Chiarini-Garcia H, Russell LD. Characterization of mouse spermatogonia by transmission electron microscopy. Reproduction. 2002;123:567–577. doi: 10.1530/rep.0.1230567. [DOI] [PubMed] [Google Scholar]
- Chiarini-Garcia H, Hornick JR, Grswold MD, et al. The distribution of type A spermatogonia in the mouse is not random. Biol Reprod. 2001;65:1179–1185. doi: 10.1095/biolreprod65.4.1179. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Meisami E. Early hypothyroidism in rats causes increased adult testis and reproductive organ size but does not change testosterone levels. Endocrinology. 1991;129:237–243. doi: 10.1210/endo-129-1-237. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Hess RA, Porcelli J, et al. Increased sperm production in the adult rats after transient neonatal hypothyroidism. Endocrinology. 1991;129:244–248. doi: 10.1210/endo-129-1-244. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Holsberger DR, França LR. Thyroid hormone regulation of Sertoli cell development. In: Skinner MK, Griswold MD, editors. The Sertoli Cell Biology. USA: Elsevier; 2005. pp. 217–226. [Google Scholar]
- De Gendt K, Atanassova N, Tan KA, et al. Development and function of the adult generation of Leydig cells in mice with Sertoli cell-selective or total ablation of the androgen receptor. Endocrinology. 2005;146:4117–4126. doi: 10.1210/en.2005-0300. [DOI] [PubMed] [Google Scholar]
- De Rooij DG. Stem cells in the testis. Int J Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol. 1998;10:694–701. doi: 10.1016/s0955-0674(98)80109-9. [DOI] [PubMed] [Google Scholar]
- De Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- DiNapoli L, Capel B. SRY and the standoff in sex determination. Mol Endocrinol. 2008;22:1–9. doi: 10.1210/me.2007-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França LR. Belo Horizonte, Brazil: Federal University of Minas Gerais; 1991. Análise morfofuncional da espermatogênese de suínos adultos da raça Piau. Thesis. [Google Scholar]
- França LR. Daily sperm production in Piau boars estimated from Sertoli cell population and Sertoli cell index. In: Dieleman SJ, editor. Proceedings of the 12th International Congress on Animal Reproduction and Artificial Insemination. The Hague: The ICAR; 1992. pp. 1716–1718. [Google Scholar]
- França LR, Godinho CL. Testis morphometry, seminiferous epithelium cycle length, and daily sperm production in domestic cats (Felis catus) Biol Reprod. 2003;68:1554–1561. doi: 10.1095/biolreprod.102.010652. [DOI] [PubMed] [Google Scholar]
- França LR, Hess RA, Cooke PS, et al. Neonatal hypothyroidism causes delayed Sertoli cell maturation in rats treated with propylthiouracil: evidence that the Sertoli cell controls testis growth. Anat Rec. 1995;242:57–69. doi: 10.1002/ar.1092420108. [DOI] [PubMed] [Google Scholar]
- França LR, Avelar GF, Almeida FFL. Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology. 2005;63:300–318. doi: 10.1016/j.theriogenology.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Golat BT, Cameron DF. Sertoli cells enhance formation of capillary-like structures in vitro. Cell Transplant. 2008;17:1135–1144. doi: 10.3727/096368908787236512. [DOI] [PubMed] [Google Scholar]
- Hess RA, Cooke PS, Bunick D, et al. Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli and germ cell numbers. Endocrinology. 1993;132:2607–2613. doi: 10.1210/endo.132.6.8504761. [DOI] [PubMed] [Google Scholar]
- Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288:95–103. doi: 10.1016/j.mce.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsberger DR, Cooke PS. Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res. 2005;322:133–140. doi: 10.1007/s00441-005-1082-z. [DOI] [PubMed] [Google Scholar]
- Holsberger DR, Buchold GM, Leal MC, et al. Cell-cycle inhibitors p27kip1 and p21Cip1 regulate murine Sertoli cell proliferation. Biol Reprod. 2005a;72:1429–1436. doi: 10.1095/biolreprod.105.040386. [DOI] [PubMed] [Google Scholar]
- Holsberger DR, Kiesewetter SE, Cooke PS. Regulation of neonatal Sertoli cell development by thyroid hormone receptor alpha1. Biol Reprod. 2005b;73:396–403. doi: 10.1095/biolreprod.105.041426. [DOI] [PubMed] [Google Scholar]
- Joyce KL, Porcelli J, Cooke PS. Neonatal goitogen treatment increases adult testis size and sperm production in the mouse. J Androl. 1993;14:448–455. [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;202:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Leal MC, Cardoso ER, Nóbrega RH, et al. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol Reprod. 2009;81:177–187. doi: 10.1095/biolreprod.109.076299. [DOI] [PubMed] [Google Scholar]
- Neves ES. Belo Horizonte, Brazil: Federal University of Minas Gerais; 2001. Estudo comparativo da estrutura do testículo e do processo espermatogênico em jumentos (Equus asinus) e burros (Equus mulus mulus) Thesis. [Google Scholar]
- Ogawa T, Ohmura M, Ohbo K. The niche for spermatogonial stem cells in the mammalian testis. Int J Hematol. 2005;82:381–388. doi: 10.1532/IJH97.05088. [DOI] [PubMed] [Google Scholar]
- Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Capel B. Signaling at the crossroads of gonad development. Trends Endocrinol Metab. 2005;16:19–25. doi: 10.1016/j.tem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Amy SP, Mahar PL, et al. BCLW mediates survival of postmitotic Sertoli cells by regulating BAX activity. Dev Biol. 2001;239:295–308. doi: 10.1006/dbio.2001.0445. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha-Hikim AP. Histological and Histopathological Evaluation of the Testis. Vienna, IL: Cache River Press; 1990. [Google Scholar]
- Russell LD, Warren J, Debeljuk L, et al. Spermatogenesis in BCLw-deficient mice. Biol Reprod. 2001;65:318–332. doi: 10.1095/biolreprod65.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz RW, Menting S, Bogerd J, et al. Sertoli cell proliferation in the adult testis – evidence from two fish species belonging to different orders. Biol Reprod. 2005;73:891–898. doi: 10.1095/biolreprod.105.039891. [DOI] [PubMed] [Google Scholar]
- Shetty G, Meistrich ML. The missing niche for spermatogonial stem cells: do blood vessels point the way? Cell Stem Cell. 2007;1:361–363. doi: 10.1016/j.stem.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Sprando RL. Perfusion of the testis through the heart using heparin. In: Russell LD, Ettlin RA, Sinha-Hikim AP, Clegg ED, editors. Histological and Histopathological Evaluation of the Testis. Vienna, IL: Cache River Press; 1990. pp. 277–280. [Google Scholar]
- Steinberger A, Steinberger E. Replication pattern of Sertoli cells in maturing rat testis in vivo and organ culture. Biol Reprod. 1971;4:84–87. doi: 10.1093/biolreprod/4.1.84. [DOI] [PubMed] [Google Scholar]
- Van Haaster LH, De Jong FH, Docter R, et al. The effect of hypothyroidism on Sertoli cell proliferation and differentiation and hormone levels during testicular development in the rat. Endocrinology. 1992;131:1574–1576. doi: 10.1210/endo.131.3.1505485. [DOI] [PubMed] [Google Scholar]
- Van Haaster LH, De Jong FH, Docter R, et al. High neonatal triiodothyronine levels reduce the period of Sertoli cell proliferation and accelerate tubular lumen formation in the rat testis, and increase serum inhibin levels. Endocrinology. 1993;133:755–760. doi: 10.1210/endo.133.2.8344214. [DOI] [PubMed] [Google Scholar]
- Vergouwen RPFA, Jacobs SGPM, Huiskamp R, et al. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil. 1991;93:233–243. doi: 10.1530/jrf.0.0930233. [DOI] [PubMed] [Google Scholar]
- Vergouwen RFPA, Huiskamp R, Bas RJ, et al. Postnatal development of testicular cell populations in mice. J Reprod Fertil. 1993;99:479–485. doi: 10.1530/jrf.0.0990479. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nakagawa T, et al. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukemo M, Nabeshima YI. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1696–1703. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]