Abstract
We provide the first detailed description of the inner ear of a notoungulate, an extinct group of endemic South American placental mammals, based on a three-dimensional reconstruction extracted from CT imagery of a skull of Notostylops murinus. This description provides new anatomical data that should prove to be phylogenetically informative, an especially significant aspect of this research given that both the interrelationships of notoungulates and the position of Notoungulata within Placentalia are still unresolved. We also assess the locomotor agility of Notostylops based on measurements of the semicircular canals. This is the best available data on the locomotion of a notostylopid because significant postcranial remains for this group have not been described. The cochlea of Notostylops has 2.25 turns, and the stapedial ratio is 1.6. The stapedial ratio is one of the lowest recorded for a eutherian, which typically have ratios greater than 1.8. The fenestra cochleae is located posterior to the fenestra vestibuli, a condition previously only reported for some stem primates. The separation of the saccule and utricule of the vestibule is visible on the digital endocast of the bony labyrinth. The posterior arm of the LSC and the inferior arm of the PSC are confluent, but these do not form a secondary crus commune, and the phylogenetic or functional significance of this confluence is unclear at this time. Locomotor agility scores for Notostylops suggest a medium or ‘average’ degree of agility of motion compared to extant mammals. In terms of its locomotion, we tentatively predict that Notostylops was a generalized terrestrial mammal, with cursorial tendencies, based on its agility scores and the range of locomotor patterns inferred from postcranial analyses of other notoungulates.
Keywords: bony labyrinth, cochlea, computed tomography (CT), Eocene, Notostylops murinus, Notoungulata, petrosal, South America
Introduction
Notoungulata was a taxonomically diverse group of extinct, hoofed placental mammals endemic to South America throughout nearly the entire Cenozoic (Simpson, 1948, 1980; Patterson & Pascual, 1968; Rose, 2006). Notoungulates are characterized by a number of dental and auditory characters including a distinctive ‘crochet’ formed by a loph on the occlusal surfaces of upper molars and an epitympanic sinus in the squamosal (Cifelli, 1993). Members of this group include such disparate forms as rhino-sized toxodontids, rat-like basal interatheres, rabbit-like hegetotheriids, horse-like notohippids, and wombat-like mesotheriids. Notoungulates not only were remarkably diverse morphologically, they also varied widely in their locomotor specializations and paleoecology.
The relationship of Notoungulata to other placental mammals is unresolved, but a phylogenetic analysis based solely on postcranial osteology suggests that they are most closely related to Litopterna, another extinct South American ungulate-like group (Horovitz, 2004). The interrelationships among notoungulates likewise are poorly understood. The only published phylogeny of higher-level relationships within Notoungulata was that of Cifelli (1993), which was based primarily on dental characters. Other, more recent, studies examined relationships within various subgroups of notoungulates (see Cifelli, 1993; Madden, 1997; Shockey, 1997; Cerdeño & Bond, 1998; Nasif et al. 2000; Croft et al. 2004; Flynn et al. 2005; Croft & Anaya, 2006; Hitz et al. 2006; Billet et al. 2009).
Although some phylogenetic analyses have incorporated external cranial characters, the internal cranial osteology of notoungulates has been virtually unsampled. In fact, other than some studies of the auditory bulla, there are few descriptions of the internal osteology of this group. One noteworthy exception is a description of the braincase and auditory region of a specimen of Oldfieldthomasia sp. (Typotheria) based on serial sections (Simpson, 1936). Endocranial cavities and associated cranial endocasts have been described for other taxa in the context of brain evolution, but not from a phylogenetic perspective (see Simpson, 1932, 1933a,b; Patterson, 1937; Stirton, 1953; Dechaseaux, 1962; Radinsky, 1981; Dozo, 1997).
The internal anatomy of the auditory region of notoungulates in general, and the characteristic large epitympanic cavity and distinctive morphology of the auditory bullae in particular, have received considerable attention in the literature (e.g. Patterson, 1932, 1934, 1936; Gabbert, 2004). Patterson’s (1936) comparative description of the internal osteology of the auditory bullae, portions of the petrosals, and the middle ear ossicles of notoungulates, along with Simpson’s (1936) study of serial sections of the braincase and bullae, are two of the more significant contributions in this regard. Despite these and other important studies, the inner ears (i.e. bony labyrinths) of notoungulates remain entirely undescribed.
The bony labyrinth, housed in the petrosal, includes chambers for the cochlea, saccule, utricle, and semicircular ducts. The cochlea is the principal organ of hearing, whereas the other structures are associated with spatial orientation and balance. The semicircular ducts detect accelerational movements and the saccule and utricle of the vestibule help coordinate posture and body movements during locomotion by sensing position (Spoor et al. 2007). The semicircular duct system is particularly important for aiding in stabilization of vision while the body is moving so as to avoid blurred vision during locomotion (summarized by Spoor, 2003; Spoor et al. 2007).
The bony labyrinths of some groups of mammals are a significant source of evidence for inferring locomotion. For instance, measures of semicircular canal size (e.g. canal arc size, mean canal radius) relative to body mass appear to be correlated with locomotor agility in extant primates (see Spoor et al. 2007). Thus, the semicircular canals potentially can provide data on locomotion that are independent of the postcranial skeleton.
Bony labyrinths also are a source of phylogenetically informative data in at least some groups of mammals. A comparative morphological study of extant diprotodontian marsupials, for example, generated 17 quantitative and qualitative characters from this region (Schmelzle et al. 2007).
The goals of our paper are twofold. First, we provide the first description of the bony labyrinth of a notoungulate, based on a three-dimensional reconstruction from computed tomography imagery of a skull of Notostylops murinus (Notostylopidae). The tympanic bullae and external features surrounding the ear of Notostylops were discussed by others (Patterson, 1932; Simpson, 1948), but internal ear features remain undescribed. Notostylopids have been considered to be basal among notoungulates (Cifelli, 1993), or at least outside the clade uniting Typotheria and Toxodontia (Billet, 2010), the two most speciose groups of notoungulates. Therefore, Notostylops is an especially appropriate taxon for which to provide the first description of an inner ear of this group. We also make comparisons with inner ear descriptions of other therian mammals, as these may be significant in studies of the relationships and locomotor specializations of notoungulates. Secondly, we compare measurements from the bony labyrinth with published measurements from extant mammals to estimate relative locomotor agility in Notostylops. Significant portions of the postcranial skeleton of notostylopids are unknown or undescribed; therefore, inner ear morphology is currently the best available evidence for reconstructing locomotion in these extinct placental mammals.
Materials and methods
Specimen
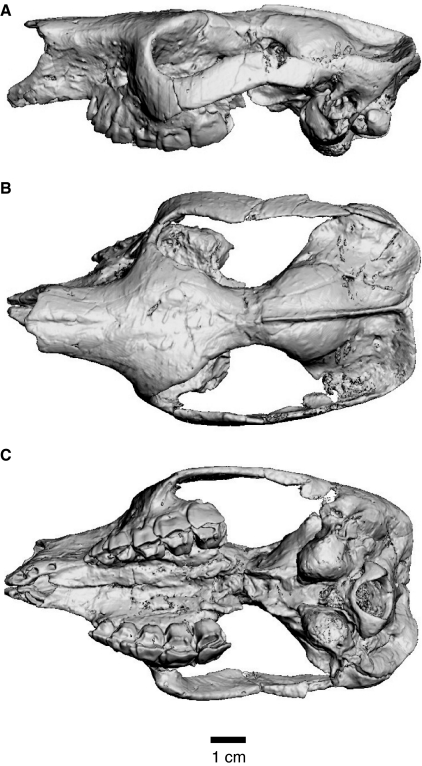
We examined a skull of N. murinus [Field Museum of Natural History (FMNH), specimen number P13319; Fig. 1], representing an advanced juvenile with deciduous teeth in place, M1 slightly worn, and M2 erupted but unworn (Riggs & Patterson, 1935; Simpson, 1948). The external morphology of the skull was described and illustrated by Riggs & Patterson (1935; plates I and II) and Simpson (1948; figure 68). Both petrosals are preserved, but only the left was sampled in detail in the CT analyses. However, ad hoc observations were also made using the right petrosal when necessary.
Fig. 1.
Digital rendering of the skull of Notostylops murinus (FMNH P13319) generated from CT imagery shown in left lateral (A), dorsal (B), and ventral (C) views.
This specimen was collected at the Gran Barranca, south of Lago Colhué-Huapí, central Chubut, Argentina (Simpson, 1948), and is derived from strata that correspond to the Barrancan subdivision of the Casamayoran South American Land Mammal ‘Age’ (SALMA; Cifelli, 1985). The age estimate for the upper boundary of the Barrancan subage from this locality is 38.5 Ma and the minimum age for the base is 41.5 Ma (Madden et al. 2005), constraining the age of this fossil as late middle Eocene (Gradstein & Ogg, 2004).
CT scanning and digital endocast extraction
The skull (Fig. 1) was imaged in its entirety in the coronal plane at the Center for Quantitative X-ray Imaging at Penn State University (http://www.cqi.psu.edu) in University Park, PA, using the HD-600 high resolution industrial X-ray system. Scan data were reconstructed as 1599 individual, 16-bit Tiff slices (i.e. images), each with pixel dimensions of 1024 × 1024, a pixel size of 0.062 mm in the X and Y planes, and an interslice spacing (Z) of 0.0684 mm. The digital endocast was extracted using the segmentation tools of avizo 5.0 (2008, Visualization Sciences Group, <http://www.vsg3d.com>).
In this description, references are made to specific CT slices through the skull of Notostylops (archived to ensure the ability to associate particular observations with individual, numbered CT slices). The prefix ‘C’ is used to designate an image from the coronal plane (i.e. transverse plane of some authors) through the skull. Groups of consecutive slices are denoted by a dash (e.g. C1000–1015).
Measurements
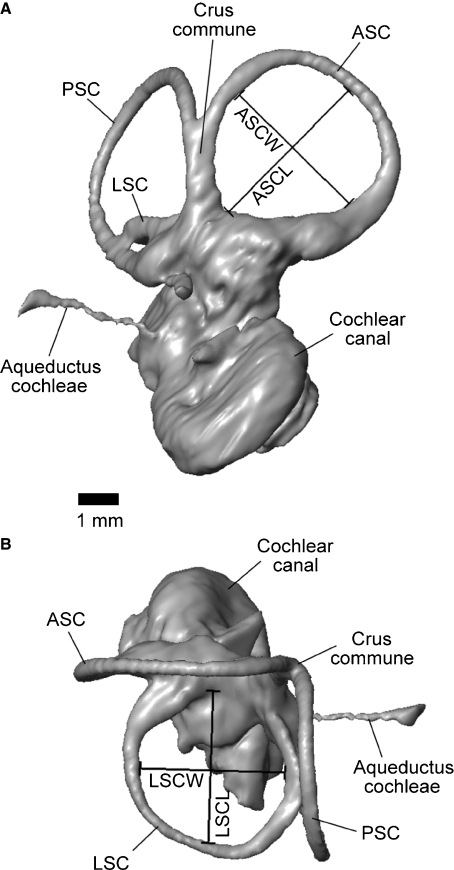
Linear measurements typically examined for inner ears (e.g. Spoor & Zonneveld, 1995) were taken from the inner ear model using the 3-D measure tool of avizo following Schmelzle et al. (2007). The inner ear model was oriented with the lateral semicircular canal (LSC) in the horizontal plane when taking measurements of the anterior and posterior semicircular canals (ASC and PSC, respectively); the ASC was oriented horizontally when measuring the LSC. Semicircular canal length was measured from the midpoint of each canal arc, which represents the point of the canal farthest away from the vestibule (Fig. 2). Semicircular canal width was measured perpendicular to semicircular canal length, at the widest expanse of the canal arc (Fig. 2). Each measurement was taken on the inner side of the bony semicircular canal, following the conventions of Schmelzle et al. (2007). All measurements were taken three times, and the mean for each was calculated and reported here (Table 1). For comparison, the same measurements were also taken from the center of each canal following the protocols of Spoor et al. (2007). However, these values are not reported here because they did not differ significantly from the other measurements and consequently did not affect any of the results, and would be repeating those given in Table 1. Radius of curvature (R) of each canal was calculated using the following equation: 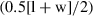 where l = length and w = width of canal (Spoor & Zonneveld, 1998).
where l = length and w = width of canal (Spoor & Zonneveld, 1998).
Fig. 2.
Representative linear semicircular canal measurements taken on the 3-D reconstruction of the left inner ear of Notostylops murinus. Measurements on anterior semicircular canal shown in (A) and measurements on lateral semicircular canal shown in (B). ASC, anterior semicircular canal; ASCL, anterior semicircular canal length; ASCW, anterior semicircular canal width; LSC, lateral semicircular canal; LSCL, lateral semicircular canal length; LSCW, lateral semicircular canal width; PSC, posterior semicircular canal.
Table 1.
Measurements and locomotor agility scores for the 3-D reconstruction of the left inner ear of Notostylops murinus (FMNH P13319).
| Body mass estimate | 3119 |
| Skull length | 99.6 |
| 1Cochlea volume | 28.0 |
| Vestibule volume | 8.8 |
| 2SC volume | 5.4 |
| ASCL | 4.28 |
| ASCW | 3.91 |
| ASCR | 2.05 |
| LSCL | 3.69 |
| LSCW | 3.51 |
| LSCR | 1.80 |
| PSCL | 4.00 |
| PSCW | 4.02 |
| PSCR | 2.01 |
| SCR | 1.95 |
| ASCR agility score | 3.4 |
| PSCR agility score | 3.6 |
| LSCR agility score | 3.9 |
| SCR agility score | 3.7 |
Body mass estimate from Croft (2000), based on this specimen. Skull length was measured from the anterior tip of the bony portion of external nares to the back of the occipital condyles. Locomotor agility scale characterizes the degree of agility of motion and is based on qualitative field observations of extant mammals (Spoor et al., 2007). Agility scores range from 1 to 6, with 1 being extremely slow and 6 being fast. 1Cochlea volume includes aqueductus cochleae, fenestra cochleae, and fenestra vestibuli; 2SC volume includes crus commune and ampullae of semicircular canals. ASC, anterior semicircular canal; LSC, lateral semicircular canal; PSC, posterior semicircular canal; SC, semicircular canal; SCR, average semicircular canal radius; L, length (following a semicircular canal name); W, width (following a semicircular canal name); R, radius (following a semicircular canal name). Mass in g; linear measurements in mm; volumes in mm3.
Predicting locomotor agility
Spoor et al. (2007) assigned locomotor agility scores for a sample of 210 extant mammals using a scale of 1–6, with 1 being extremely slow and 6 being fast. These agility scores characterize the degree of agility of motion and are based on qualitative field observations (Spoor et al. 2007). Silcox et al. (2009) analyzed these data and determined equations to predict locomotor agility in extinct mammals. We used these equations (see below) to predict the locomotor agility for Notostylops (abbreviations: AGIL = agility; ASCR = anterior semicircular canal radius; BM = body mass in grams; LSCR = lateral semicircular canal radius; PSCR = posterior semicircular canal radius; SCR = average semicircular canal radius):
The body mass estimate for this specimen was taken from the literature (Croft, 2000). A range of locomotor agility predictions for Notostylops based on these equations is reported in Table 1.
Description
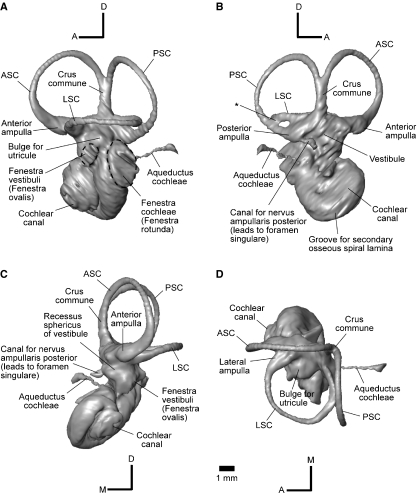
The endocast of the inner ear of Notostylops is a three-dimensional representation of the bony labyrinth of the left petrosal, and includes the cochlear canal, vestibule, and semicircular canals. As in other mammals, the anteriormost of these divisions is the cochlear canal, followed by the vestibule and semicircular canals (Fig. 3).
Fig. 3.
Three-dimensional reconstruction of the left inner ear of Notostylops murinus (FMNH P13319) shown in left lateral (A), medial (B), rostral (C), and dorsal (D) views. A, anterior; ASC, anterior semicircular canal; D, dorsal; LSC, lateral semicircular canal; M, medial; PSC, posterior semicircular canal; * marks confluence between LSC and PSC. The fenestra vestibuli and fenestra cochleae are outlined with dashed lines. Scale bar is same for all images.
Cochlear canal
The cochlear canal, which housed the membranous cochlea, is located within the pars cochlearis of the petrosal. The cochlear canal comprises the largest percentage of the bony labyrinth by volume (Table 1). The cochlea has about 2.25 turns (rotation of 810°), counted following the protocol of West (1985).
The fenestra vestibuli (fenestra ovalis) is a slightly elliptical opening located ventral and a little posterior to the lateral ampulla, and is oriented obliquely (anteroventral to posterodorsal) relative to the plane of the LSC (Fig. 3A). This opening faces ventrolaterally and holds the footplate of the stapes in mammals, but the stapes itself is not preserved on either side of this skull of Notostylops. The stapedial ratio, calculated by proxy as length divided by width of the fenestra vestibuli, is approximately 1.6.
The fenestra cochleae (fenestra rotunda) is a large, elliptical opening that is visible posterior to the much smaller fenestra vestibuli (Fig. 3A). This opening faces posterolaterally and is covered by a secondary tympanic membrane in living mammals.
The aqueductus cochleae (cochlear aqueduct) begins at the posteroventral edge of the fenestra cochleae and extends posteromedially and slightly dorsally. It is very long, extending well caudal of the PSC in superior (dorsal) view (Fig. 3D). The aqueductus cochleae is also much thinner in diameter than the semicircular canals, in contrast to the condition in some marsupials (Schmelzle et al. 2007). This canal houses the perilymphatic duct, which connects the perilymphatic space of the inner ear with the subarachnoid space of the endocranial cavity (Meng & Fox, 1995).
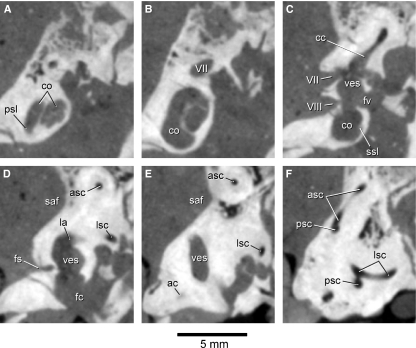
The primary and secondary osseous spiral laminae are visible on the CT images through the petrosals of Notostylops. For example, the secondary osseous spiral lamina can be seen projecting ventromedially from the radial (outer) wall of the cochlear canal (e.g. C1290; Fig. 4C). Anteriorly, this lamina is continuous with the radial wall of the cochlear canal (Meng & Fox, 1995). The secondary osseous lamina extends from the fenestra cochleae until about the first half of the basal turn (180°) of the cochlear canal (Fig. 3B). The primary osseous spiral lamina projects from the meatal (inner) wall of the cochlear canal (e.g. C1245–1248; Fig. 4A). The osseous spiral laminae mark the division between the scala tympani of the cochlear canal, typically present on the cerebellar side of the petrosal, and the scala vestibuli, which is typically located on the tympanic side of the petrosal (Meng & Fox, 1995).
Fig. 4.
Coronal CT images through the skull of Notostylops murinus (FMNH P13319) cropped to show only internal anatomy of the left petrosal. (A) is the most anterior image of the set, whereas (F) is the posteriormost. (A) corresponds to C1245, (B) C1260, (C) C1290, (D) C1305, (E) C1320, and (F) C1340. ac, aqueductus cochleae; asc, anterior semicircular canal; cc, crus commune; co, cochlear canal; fc, fenestra cochleae; fs, foramen singulare; fv, fenestra vestibuli; la, lateral ampulla aqueductus; lsc, lateral semicircular canal; psc, posterior semicircular canal; psl, primary osseous spiral lamina; saf, subarcuate fossa; ssl, secondary osseous spiral lamina; ves, vestibule; VII, opening or canal for facial nerve (Cranial Nerve VII); VIII, opening for vestibulocochlear nerve (Cranial Nerve VIII). The secondary osseous spiral lamina is visible in C1245 as a faint structure projecting from the meatal wall of the cochlear canal into the middle of the canal. This structure is more clearly visible when toggling among images C1245–1248.
Vestibule
The vestibule is housed in the pars canalicularis of the petrosal and communicates with the cochlear canal, semicircular canals, and endocranial cavity via the internal auditory meatus. The saccule and utricule of the membranous labyrinth were clearly separated within the bony labyrinth of the vestibule of Notostylops.
The saccule (sacculus) lies in the recessus sphericus, an anterior chamber of the vestibule (Meng & Fox, 1995). The recessus sphericus is visible on the endocast as a bulge lying dorsal and medial to the fenestra vestibuli (Fig. 3C).
The utricule (utriculus) of Notostylops is much larger than the saccule, and would have been positioned in the vestibule posterodorsal to the fenestra vestibuli. This space is indicated on the endocast by a large bulge between and just below the ends of the LSC (Fig. 3A,D).
The internal auditory meatus (internal acoustic meatus) is located on the medial side of the petrosal and provides communication between the endocranial volume and the inner ear. Cranial Nerve VII (facial nerve) passes through the dorsal opening of the internal auditory meatus (IAM) but does not actually enter the bony labyrinth. The ventral portion of the IAM is pierced by various branches of Cranial Nerve VIII (vestibulocochlear nerve) in mammals, and some of these openings are discernible on the CT images of Notostylops. For example, a well-developed canal (Fig. 3B,C) extends ventrally from the posterior ampulla to an opening in the internal auditory meatus that was termed the foramen vestibuli by Meng & Fox (1995). This canal likely transmitted the nervus ampullaris posterior, a branch of the vestibular nerve (Meng & Fox, 1995). The tractus spiralis foraminosus, the spiral distribution of the foramina of the cochlear nerve (Luo & Marsh, 1996), is not visible on either petrosal of this specimen.
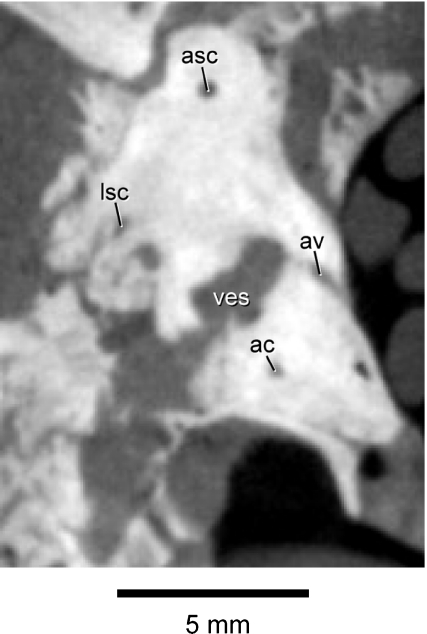
The aqueductus vestibuli (vestibular aqueduct), which housed the endolymphatic duct in life, is not visible on the left petrosal, but it is present on the right petrosal (Fig. 5). This canal extends ventromedially from the portion of the vestibule just anterior to the crus commune.
Fig. 5.
Coronal CT image (C1319) through the skull of Notostylops murinus (FMNH P13319) cropped to show only the right petrosal. Abbreviations are as in Fig. 4, with the addition of av, aqueductus vestibuli.
Semicircular canals
The three semicircular canals are located in the pars canalicularis of the petrosal. The ASC has the largest radius of the three semicircular canals, followed by the PSC and then the LSC (Table 1). The ASC is the most oblong in overall form, whereas the PSC is nearly circular (Table 1). All of the semicircular canals are relatively straight, that is, they lack any major undulations along their entire length in the respective plane of each canal (Fig. 3), unlike the condition in some marsupials (Schmelzle et al. 2007). The petrosal of Notostylops has a very shallow subarcuate fossa (Fig. 4D,E) (Simpson, 1933a).
Each semicircular canal swells to form an ampulla at one end, where it connects with the vestibule. The anterior and lateral ampullae attach to the vestibule in the same horizontal plane (Fig. 3A). However, the posterior ampulla is located significantly below (ventral to) the crus commune (Fig. 3B), similar to the condition in Macropus (Schmelzle et al. 2007) but unlike that of some other mammals (see Meng & Fox, 1995; Sánchez-Villagra & Schmelzle, 2007; Schmelzle et al. 2007).
The posterior arm of the ASC and the anterior arm of the PSC form the crus commune (Figs 3 and 4), a standard condition in mammals (see Luo & Ketten, 1991; Meng & Fox, 1995; Sánchez-Villagra & Schmelzle, 2007; Schmelzle et al. 2007). The osseous canals of the posterior arm of the LSC and the inferior arm of the PSC are confluent at the posteriormost extension of the LSC (Figs 3B and 4F). This same confluence also is visible in the right inner ear of Notostylops. However, the posterior arm of the LSC enters the vestibule slightly dorsal to the entrance of the inferior arm of the PSC such that there is no secondary crus commune in Notostylops (Fig. 3), contra the condition in some other mammals (see Meng & Fox, 1995; Sánchez-Villagra & Schmelzle, 2007; Horovitz et al. 2008; Ladevèze et al. 2008). Besides the crus commune, the posterior arm of the LSC forms the only other connection between a semicircular canal and the vestibule that is not made via an ampulla.
The maximum lateral extent of the LSC is even with the lateralmost extension of the PSC (Fig. 3D). The posterior arm of the LSC attaches anterodorsally to the position of the posterior ampulla (Fig. 3A).
Discussion
Comparative anatomy and character states
Below we further discuss the anatomy of the inner ear of Notostylops in comparison with other notoungulates and mammals. We also review relevant character states of previously published characters for Notostylops, other notoungulates, and other mammals based on data available from the literature.
The cochlear canal of Notostylops constitutes more than 66% of the bony labyrinth space, compared with around 21% for the vestibule (Table 1). Relative to vestibule size, the cochlea is much larger in Notostylops than in non-therian mammals such as multituberculates when skull length is taken into consideration (Luo & Ketten, 1991). Instead, the size of the vestibule of Notostylops relative to skull length more closely resembles the opossum Didelphis (Luo & Ketten, 1991).
Notostylops has both primary and secondary osseous laminae in its cochlear canal. The primary bony lamina occurs in all extant therian mammals and some of their closest extinct relatives, whereas the secondary bony lamina is variably present or absent in stem and crown therians (Meng & Fox, 1995; Ruf et al. 2009). The evolutionary acquisition of this latter structure in various mammalian groups is unclear at this time (Ruf et al. 2009).
The secondary osseous lamina of Notostylops extends for the first half turn of the basal cochlear canal. In contrast, the secondary osseous lamina extends beyond the half turn in at least some cetaceans (Fleischer, 1976; Luo & Eastman, 1995; Geisler & Luo, 1996; Luo & Marsh, 1996). This lamina extends for less than half of the basal turn of the cochlear canal in the stem therian mammal Henkelotherium (Ruf et al. 2009).
The cochlea of Notostylops has 2.25 turns, whereas the cochlea of the notoungulate Hegetotherium reportedly has 2.5 turns, based on a broken specimen (Simpson, 1936). The cochleae of therian mammals have at least one complete turn, but there is a considerable range of variation in the number of turns among extant species (see Hyrtl, 1845; Gray, 1907, 1908; Fleischer, 1973; West, 1985). At this point, the ancestral condition for the number of cochlear turns is unclear for specific extant and extinct placental groups.
The stapedial ratio of 1.6 for Notostylops is significantly lower than ratios reported for most placental mammals, which are typically greater than 1.8 (Segall, 1970). Besides Notostylops, Prokennalestes (1.7; Wible et al. 2001) and ‘zhelestids’ (average 1.6; Ekdale et al. 2004) are the only other eutherians reported to have stapedial ratios below 1.8. Metatherians generally have stapedial ratios below 1.8, with a few exceptions (Segall, 1970; Rougier et al. 1998; Horovitz et al. 2008). The fenestra vestibuli also is oval in the notoungulate Hegetotherium, suggesting a low stapedial ratio, but precise measurements were not reported (Patterson, 1936).
The fenestra cochleae is directed posterolaterally (character state 106.0 of Bloch et al. 2007) in Notostylops, Hegetotherium (Patterson, 1936), and Scarrittia (Gabbert, 2004), the primitive condition in eutherians (Novacek, 1986; Novacek & Wyss, 1986). However, the fenestra cochleae faces directly posteriorly (character state 106.1 of Bloch et al. 2007) in the notoungulates Interatherium and Nesodon (Patterson, 1936). This derived posteriorly facing condition also was reported for lagomorphs, chiropterans, dermopterans, and sirenians (Novacek, 1986).
The fenestra cochleae is located posteromedial to the fenestra vestibuli (character state 303.0 of Wible et al. 2007) in Scarrittia (Gabbert, 2004). However, the fenestra cochleae is situated directly posterior to the fenestra vestibuli (character state 303.1 of Wible et al. 2007) in Notostylops, Oldfieldthomasia (Simpson, 1936), and Nesodon (Patterson, 1936). The derived state (posteriorly positioned) for this character is also reported for some stem primates (Wible et al. 2007).
The fenestra cochleae is larger in diameter than the fenestra vestibuli in Notostylops and Oldfieldthomasia (Simpson, 1936) as well as the marsupials Caluromys, Macropus, Bettongia, Phascolarctos, and Vombatus (Sánchez-Villagra & Schmelzle, 2007; Schmelzle et al. 2007). In contrast, these fenestrae are subequal in size in Kulbeckia, a zalambdalestid eutherian (Ekdale et al. 2004), and the marsupial Dendrolagus (Schmelzle et al. 2007). The fenestra vestibuli is larger than the fenestra cochleae in humans (Hyrtl, 1845).
The maximum lateral extent of the LSC is the same as the lateralmost extension of the PSC in Notostylops (character state 4.0 of Schmelzle et al. 2007), similar to the condition in the marsupials Petauroides, Pseudocheirus, and Phascolarctos. The LSC of Notostylops is straight in its transition to the lateral ampulla (character state 6.0 of Schmelzle et al. 2007) and also in its transition to the vestibule (character state 7.0 of Schmelzle et al. 2007). The posterior arm of the LSC attaches anterior to the level of the PSC in Notostylops (character state 8.0 from Schmelzle et al. 2007), as in the marsupials Phascolarctos, Macropus, Dorcopsis, and Vombatus.
The overall shape of the PSC of Notostylops is straight when viewed in the plane of the canal (character state 9.0 from Schmelzle et al. 2007), similar to the condition in the marsupials Pseudocheirus, Dendrolagus, and Phascolarctos. The overall shape of the PSC of Notostylops is round when viewed perpendicular to the plane of the canal (character state 10.0 from Schmelzle et al. 2007), as in the marsupials Petauroides, Pseudocheirus, Dendrolagus, Bettongia, Macropus, and Dorcopsis.
The phylogenetic or functional significance of the confluence between the posterior arm of the LSC and the inferior arm of the PSC of Notostylops is unclear at this time. The only reported confluence between these two semicircular canals in other mammals is associated with the formation of a secondary crus commune (Hyrtl, 1845; Meng & Fox, 1995; Sánchez-Villagra & Schmelzle, 2007; Horovitz et al. 2008). However, the confluence present in Notostylops does not result in a secondary crus commune. Furthermore, this condition is unlikely to be an incipient stage in the development of a crus, based on a study of the ontogeny of the bony labyrinth in the marsupial Caluromys that reports a secondary crus commune for all individuals in a growth series (Sánchez-Villagra & Schmelzle, 2007). It is possible that, because the specimen of Notostylops is a subadult, the space between the canals had not completely ossified. It is also possible that the bone separating the two canals was broken postmortem, due to being very thin or fragile, although this is unlikely given that other delicate structures, such as the osseous spiral laminae, are preserved in this specimen. Regardless, it is unlikely that the membranous portions of these two canals were actually confluent in Notostylops, as extant taxa possessing a second bony crus commune do not exhibit any confluence between the participating membranous canals (Sánchez-Villagra & Schmelzle, 2007).
The inner ear of Notostylops differs from those of other eutherians in several regards. First, the saccule and utricule are separated in the bony labyrinth, a condition otherwise reported only in Henkelotherium (Ruf et al. 2009). Secondly, the above-mentioned confluence between the LSC and the PSC, which does not form a secondary crus commune, is unique to Notostylops (assuming this is not an artifact of ontogeny or preservation). Thirdly, to our knowledge, the extension of the aqueductus cochleae posterior to the plane of the PSC has not been reported for other eutherians. Fourthly, the bony canal containing the aqueductus cochleae of Notostylops also is thin compared to the thickness of the semicircular canals, a condition present in the membranous labyrinths of a variety of extant placentals (Gray, 1907, 1908) including some primates, the European mole (Talpa europaea), Indian gazelle (Gazella bennetti), capybara (Hydrochoerus hydrochaeris), Eurasian red squirrel (Sciurus vulgaris), and the three-toed sloth (Bradypus tridactylus). Fifthly, the fenestra cochleae is situated posterior to the fenestra vestibuli in some, but not all, notoungulates, a condition previously reported only for some stem primates (Wible et al. 2007). Sixthly, the fenestra cochleae is larger than the fenestra vestibuli in Notostylops and Oldfeldthomasia (Simpson, 1936), in contrast to humans (in which the opposite is true; Hyrtl, 1845), and some zalambdalestid eutherians which have fenestrae of subequal size (Ekdale et al. 2004). Finally, the secondary osseous lamina of Notostylops extends for the first half turn of the basal cochlear canal, as opposed to some cetaceans in which the lamina extends beyond the half turn (Fleischer, 1976; Luo & Eastman, 1995; Geisler & Luo, 1996; Luo & Marsh, 1996).
The characters pertaining to the semicircular canals that were described by Schmelzle et al. (2007) for marsupials have yet to be evaluated in eutherians other than Notostylops. These differences are potentially phylogenetically informative, but need to be systematically evaluated within a larger sample of eutherian mammals.
Locomotor agility
The postcranial skeleton of notostylopids is not well known due to the paucity of skeletal specimens for this group. Consequently, we know virtually nothing about the locomotor adaptations or behaviors of this group. The data presented in this paper on the semicircular canals provide the only information to date on the locomotion of any notostylopid.
The agility scores calculated for Notostylops range from 3.4 to 3.9 (Table 1) suggesting that this taxon had a medium or ‘average’ degree of agility of motion compared to extant mammals (Spoor et al. 2007). Of the 210 species sampled by Spoor et al. (2007), 96 had an agility score of 3 or 4. This corresponds to extant mammals exhibiting general terrestrial, cursorial, scansorial, arboreal, and semiaquatic locomotor styles (e.g. platypus), but not gliding, flying, saltatorial, or fully aquatic locomotion.
Analyses of postcranial morphology of various notoungulates (other than notostylopids) suggest that some taxa, such as interatheriids, were generalized terrestrial locomotors with cursorial or fossorial tendencies (Croft & Anderson, 2008; Shockey & Anaya, 2008). Pachyrukhos (Hegetotheriidae) may have been saltatory (Reguero et al. 2007), whereas mesotheriids were likely scratch-diggers (Shockey et al. 2007). Among extant mammals sampled, diggers have agility scores of 2–3, whereas saltatorial locomotors have scores of 5–6 (Spoor et al. 2007). Neither of these locomotor types fits well with locomotor inferences for Notostylops based on its agility scores, which are closer to 4. Consequently, based on the information from its auditory region alone, we might tentatively predict that Notostylops, poorly characterized postcranially, was a generalized terrestrial mammal with cursorial tendencies based on the range of locomotor types known for notoungulates and agility scores. The terrestrial artiodactyls sampled by Spoor et al. (2007) and some carnivorans (such as the domestic dog and cat), all have agility scores of 3–4. However, we caution that this locomotor prediction for Notostylops should be tested with postcranial data when and if such data become available; several other extant mammals with different locomotor styles have similar agility scores (e.g. arboreal tree shrews), and there is a broad overlap of agility scores among taxa with quite different locomotor styles or agility (e.g. those with ‘average’ values; see above). Furthermore, two main sources of error for locomotor agility scores, discussed below, may affect the scores calculated for Notostylops.
One source of potentially significant error in calculating agility scores for extinct taxa such as notoungulates is uncertainty in body mass estimation. Different methods for estimating body mass for extinct mammals, whether based on skeletal or dental proxies, can result in a broad range of mass values for an individual (see Damuth & MacFadden, 1990, for a review of techniques). Such uncertainty would result in a range of potential agility scores and inferred locomotor styles for that same individual. Using multiple lines of evidence from different parts of the skeleton can help minimize such issues, but such data often are unavailable for extinct species, and are not currently available for any notostylopid.
A second source of error, assigning locomotor agility scores for extant mammals based on qualitative field observations (e.g. Spoor et al. 2007), involves some degree of subjectivity – on the part of both the observer and of the scorer. One way to further test the correlation between agility scores and attributes of the semicircular canal system in extant mammals, as well as to increase objectivity in coding agility scores in extant species, would be to use an osteological proxy of locomotor performance. The metatarsal-to-femur ratio (‘cursorial ratio’; see Janis & Wilhlem, 1993) represents one readily measurable proxy that could be used in specimens preserving both of these elements, though other possibilities no doubt exist. Such an analysis is beyond the scope of this paper, but would be an intriguing avenue of future investigation into notoungulate locomotor styles.
Conclusions
We provide the first description of the inner ear of a notoungulate using a three-dimensional reconstruction based on CT imagery of a skull of Notostylops murinus. A principal goal of this study was to provide new, potentially phylogenetically informative inner ear endocast data for future analyses of interrelationships among notoungulates, as well as the placement of Notoungulata within Placentalia. Secondly, we provide the first published inferences of the locomotor agility of Notostylops, based on measurements from the semicircular canals. This is noteworthy because significant portions of the postcranial skeleton of notostylopids have never been described, precluding locomotor reconstructions directly from their skeletal morphology.
We observe that the cochlea of Notostylops has 2.25 turns and the stapedial ratio is 1.6, one of the lowest recorded for any eutherian. The following observations are potentially phylogenetically informative. First, the fenestra cochleae is located posterior to the fenestra vestibuli, a condition previously only reported for some stem primates (Wible et al. 2007). Secondly, the posterior arm of the LSC and the inferior arm of the PSC are confluent without forming a secondary crus commune, but the significance of this confluence is unclear at this time. Thirdly, the separation of the saccule and utricule in the bony labyrinth, as reflected in the digital endocast, is only known in Notostylops and the stem therian Henkelotherium (Ruf et al. 2009). Fourthly, the posterior extension of the aqueductus cochleae beyond the PSC is not known in other eutherians. Fifthly, the bony canal containing the aqueductus cochleae is thin compared to the thickness of the semicircular canals, a condition also present in a variety of extant placentals (Gray, 1907, 1908). Sixthly, the fenestra cochleae is larger than the fenestra vestibuli in Notostylops and Oldfeldthomasia (Simpson, 1936), in contrast to other eutherians for which this character has been examined (Hyrtl, 1845; Ekdale et al. 2004). Finally, the secondary osseous lamina extends only to the first half turn of the basal cochlear canal as opposed to some cetaceans in which the lamina extends beyond this turn (Fleischer, 1976; Luo & Eastman, 1995; Geisler & Luo, 1996; Luo & Marsh, 1996).
The locomotor agility scores calculated for Notostylops range from 3.4 to 3.9, suggesting a medium or ‘average’ degree of agility of motion compared to extant mammals. This range of scores is comparable with members of a variety of groups of extant mammals and locomotor patterns. We tentatively predict, based on these scores and the range of locomotor styles inferred from postcranial skeletal morphology analyses of other notoungulates, that Notostylops was a generalized terrestrial locomotor with cursorial tendencies. However, this prediction will need to be tested with postcranial material when it becomes available.
Our study identifies a number of potentially phylogenetically informative characters that differ from those observed in other mammals, emphasizing the need for additional descriptions of other notoungulate inner ears for comparison, and also for more comparative work on mammalian bony labyrinths in general. Published phylogenetic characters for mammalian inner ears only have been examined in a limited taxonomic sample. With additional comparisons, more characters pertaining to this anatomical region are likely to be described.
Acknowledgments
We thank Tim Ryan (Department of Anthropology, Center for Quantitative X-ray Imaging at Penn State University) for scanning the specimen. Bruce Shockey provided helpful advice about notoungulate literature and postcrania. Funding for this research was provided by NSF DEB-0513476 to J.J.F. and a Frick Postdoctoral Fellowship from the Department of Vertebrate Paleontology at the A.M.N.H. to T.E.M. This work also represents a contribution to the AToL-Mammal Morphology studies supported by BIO EF- 0629811 (to J.J.F. and colleagues). We thank Zhe-Xi Luo, editor Julia Clarke, and an anonymous reviewer for helpful constructive comments on this paper.
References
- Billet G. New observations on the skull of Pyrotherium (Pyrotheria, Mammalia) and new phylogenetic hypotheses on South American ungulates. J Mammal Evol. 2010;17:21–59. [Google Scholar]
- Billet G, Patterson B, Muizon C. Craniodental anatomy of late Oligocene archaeohyracids (Notoungulata, Mammalia) from Bolivia and Argentina and new phylogenetic hypotheses. Zool J Linn Soc. 2009;155:458–509. [Google Scholar]
- Bloch JI, Silcox MT, Boyer DM, et al. New Paleocene skeletons and the relationship of plesiadapiforms to crown-clade primates. Proc Natl Acad Sci USA. 2007;104:1159–1164. doi: 10.1073/pnas.0610579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdeño E, Bond M. Taxonomic revision and phylogeny of Paedotherium and Tremacyllus (Pachyrukhinae, Hegetotheriidae, Notoungulata) from the late Miocene to Pleistocene of Argentina. J Vertebr Paleontol. 1998;18:799–811. [Google Scholar]
- Cifelli RL. Biostratigraphy of the Casamayoran, early Eocene, of Patagonia. Am Mus Novit. 1985;2820:1–26. [Google Scholar]
- Cifelli RL. The phylogeny of the native South American ungulates. In: Szalay FS, Novacek MJ, McKenna MC, editors. Mammal Phylogeny. Volume 2: Placentals. New York: Springer-Verlag; 1993. pp. 195–216. [Google Scholar]
- Croft DA. Archaeohyracidae (Mammalia: Notoungulata) from the Tinguiririca Fauna, Central Chile, and the Evolution and Paleoecology of South American Mammalian Herbivores. University of Chicago; 2000. PhD dissertation. [Google Scholar]
- Croft DA, Anaya F. A new middle Miocene hegetotheriid (Notoungulata: Typotheria) and a phylogeny of the Hegetotheriidae. J Vertebr Paleontol. 2006;26:387–399. [Google Scholar]
- Croft DA, Anderson LC. Locomotion in the extinct notoungulate Protypotherium. Palaeontol Electronica. 2008;11:1–20. [Google Scholar]
- Croft DA, Flynn JJ, Wyss AR. Notoungulata and Litopterna of the early Miocene Chucal Fauna, northern Chile. Fieldiana Geol New Ser. 2004;No. 50:1–52. [Google Scholar]
- Damuth J, MacFadden BJ, editors. Body Size in Mammalian Paleobiology: Estimation and Biological Implications. New York: Cambridge University Press; 1990. [Google Scholar]
- Dechaseaux C. Encéfalos de notoungulados y de desdentados xenartros fósiles. Ameghiniana. 1962;2:193–209. [Google Scholar]
- Dozo MT. Paleoneurología de Dolicavia minuscula (Rodentia, Caviidae) y Paedotherium insigne (Notoungulata, Hegetotheriidae) del Plioceno de Buenos Aires, Argentina. Ameghiniana. 1997;34:427–435. [Google Scholar]
- Ekdale EG, Archibald JD, Averianov AO. Petrosal bones of placental mammals from the Late Cretaceous of Uzbekistan. Acta Palaeontol Pol. 2004;49:161–176. [Google Scholar]
- Fleischer G. Studien am Skelett des Gehörorgans der Säugetiere, einschließlich des Menschen. Säugetierkd Mitt. 1973;21:131–239. [Google Scholar]
- Fleischer G. Hearing in extinct cetaceans as determined by cochlear structure. J Paleontol. 1976;50:133–152. [Google Scholar]
- Flynn JJ, Croft DA, Charrier R, et al. New Mesotheriidae (Mammalia, Notoungulata, Typotheria), geochronology and tectonics of the Caragua area, northernmost Chile. J South Am Earth Sci. 2005;19:55–74. [Google Scholar]
- Gabbert SL. The basicranium and posterior cranial anatomy of the families of the Toxodontia. Bull Am Mus Nat Hist. 2004;285:177–190. [Google Scholar]
- Geisler JH, Luo ZX. The petrosal and inner ear of Herpetocetus sp. (Mammalia: Cetacea) and their implications for the phylogeny and hearing of archaic mysticetes. J Paleontol. 1996;70:1045–1066. [Google Scholar]
- Gradstein FM, Ogg JG. Geologic time scale 2004 – why, how, and where next! Lethaia. 2004;37:175–181. [Google Scholar]
- Gray AA. The Labyrinth of Animals: Including Mammals, Birds, Reptiles and Amphibians. London: J & A Churchill; 1907. Vol. 1. [Google Scholar]
- Gray AA. The Labyrinth of Animals: Including Mammals, Birds, Reptiles and Amphibians. London: J & A Churchill; 1908. Vol. 2. [Google Scholar]
- Hitz R, Flynn JJ, Wyss AR. New basal Interatheriidae (Typotheria, Notoungulata, Mammalia) from the Paleogene of central Chile. Am Mus Novit. 2006;3520:1–32. [Google Scholar]
- Horovitz I. Eutherian mammal systematics and the origins of South American ungulates as based on postcranial osteology. Bull Carnegie Mus Nat Hist. 2004;36:63–79. [Google Scholar]
- Horovitz I, Ladevèze S, Argot C, et al. The anatomy of Herpetotherium cf. fugax Cope, 1873, a metatherian from the Oligocene of North America. Palaeontographica (A) 2008;284:109–141. [Google Scholar]
- Hyrtl J. Vergleichend-anatomische Untersuchungen über das innere Gehörorgan des Menschen und der Säugethiere. Prague: Friedrich Ehrlich; 1845. [Google Scholar]
- Janis CM, Wilhlem PB. Were there mammalian pursuit predators in the Tertiary? Dances with wolf avatars. J Mammal Evol. 1993;1:103–125. [Google Scholar]
- Ladevèze S, Asher RJ, Sánchez-Villagra MR. Petrosal anatomy in the fossil mammal Necrolestes: evidence for metatherian affinities and comparisons with the extant marsupial mole. J Anat. 2008;213:686–697. doi: 10.1111/j.1469-7580.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZX, Eastman ER. Petrosal and inner ear of a squalodontoid whale: implications for evolution of hearing in odontocetes. J Vertebr Paleontol. 1995;15:431–442. [Google Scholar]
- Luo ZX, Ketten DR. CT scanning and computerized reconstructions of the inner ear of multituberculate mammals. J Vertebr Paleontol. 1991;11:220–228. [Google Scholar]
- Luo ZX, Marsh K. Petrosal (periotic) and inner ear of a Pliocene kogiine whale (Kogiinae, Odontoceti): implications on relationships and hearing evolution of toothed whales. J Vertebr Paleontol. 1996;16:328–348. [Google Scholar]
- Madden RH. A new toxodontid notoungulate. In: Kay RF, Madden RH, Cifelli RL, Flynn JJ, editors. Vertebrate Paleontology in the Neotropics: The Miocene Fauna of La Venta, Colombia. Washington, DC: Smithsonian Institution Press; 1997. pp. 335–354. [Google Scholar]
- Madden RH, Bellosi E, Carlini AA, et al. Geochronology of the Sarmiento Formation at Gran Barranca and elsewhere in Patagonia: calibrating middle Cenozoic mammal evolution in South America. Actas 16˚ Cong. Geol. Arg., La Plata. 2005;4:411–412. [Google Scholar]
- Meng J, Fox RC. Osseous inner ear structures and hearing in early marsupials and placentals. Zool J Linn Soc. 1995;115:47–71. [Google Scholar]
- Nasif NL, Musalem S, Cerdeño E. A new toxodont from the Late Miocene of Catamarca, Argentina, and a phylogenetic analysis of the Toxodontidae. J Vertebr Paleontol. 2000;20:591–600. [Google Scholar]
- Novacek MJ. The skull of leptictid insectivorans and the higher-level classification of eutherian mammals. Bull Am Mus Nat Hist. 1986;183:1–111. [Google Scholar]
- Novacek MJ, Wyss AR. Higher-level relationships of the recent eutherian orders: morphological evidence. Cladistics. 1986;2:257–287. doi: 10.1111/j.1096-0031.1986.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Patterson B. The auditory region of the Toxodontia. Fieldiana Geol. 1932;6:5–27. [Google Scholar]
- Patterson B. The auditory region of an upper Pliocene typotherid. Fieldiana Geol. 1934;6:83–89. [Google Scholar]
- Patterson B. The internal structure of the ear in some notoungulates. Fieldiania Geol. 1936;6:199–227. [Google Scholar]
- Patterson B. Some notoungulate braincasts. Geol Ser, Field Mus Nat Hist. 1937;6:273–301. [Google Scholar]
- Patterson B, Pascual R. The fossil mammal fauna of South America. Q Rev Biol. 1968;43:409–451. [Google Scholar]
- Radinsky L. Brain evolution in extinct South American ungulates. Brain Behav Evol. 1981;18:169–187. doi: 10.1159/000121785. [DOI] [PubMed] [Google Scholar]
- Reguero MA, Dozo MT, Cerdeño E. A poorly known rodentlike mammal (Pachyrukhinae, Hegetotheriidae, Notoungulata) from the Deseadan (Late Oligocene) of Argentina. Paleoecology, biogeography, and radiation of the rodentlike ungulates in South America. J Paleontol. 2007;81:1301–1307. [Google Scholar]
- Riggs ES, Patterson B. Description of some notoungulates from the Casamayor (Notostylops) beds of Patagonia. Proc Am Philos Soc. 1935;75:163–215. [Google Scholar]
- Rose KD. The Beginning of the Age of Mammals. Baltimore: Johns Hopkins University Press; 2006. [Google Scholar]
- Rougier GW, Wible JR, Novacek MJ. Implications of Deltatheridium specimens for early marsupial history. Nature. 1998;396:459–463. doi: 10.1038/24856. [DOI] [PubMed] [Google Scholar]
- Ruf I, Luo ZX, Wible JR, et al. Petrosal anatomy and inner ear structures of the Late Jurassic Henkelotherium (Mammalia, Cladotheria, Dryolestoidea): insight into the early evolution of the ear region in cladotherian mammals. J Anat. 2009;214:679–693. doi: 10.1111/j.1469-7580.2009.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Schmelzle T. Anatomy and development of the bony inner ear in the woolly opossum, Caluromys philander (Didelphimorphia, Marsupialia) Mastozool Neotrop. 2007;14:53–60. [Google Scholar]
- Schmelzle T, Sánchez-Villagra MR, Maier W. Vestibular labyrinth diversity in diprotodontian marsupial mammals. Mammal Study. 2007;32:83–97. [Google Scholar]
- Segall W. Morphological parallelisms of the bulla and auditory ossicles in some insectivores and marsupials. Fieldiana Zool. 1970;51:169–205. [Google Scholar]
- Shockey BJ. Two new notoungulates (Family Notohippidae) from the Salla Beds of Bolivia (Deseadan: late Oligocene): systematics and functional morphology. J Vertebr Paleontol. 1997;17:584–599. [Google Scholar]
- Shockey BJ, Anaya F. Postcranial osteology of mammals of Salla, Bolivia (late Oligocene): form, function, and phylogenetic implications. In: Sargis E, Dagosto M, editors. Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. Dordrecht: Springer; 2008. pp. 135–157. [Google Scholar]
- Shockey BJ, Croft DA, Anaya F. Analysis of function in the absence of extant functional homologues: a case study using mesotheriid notoungulates (Mammalia) Paleobiology. 2007;33:227–247. [Google Scholar]
- Silcox MT, Bloch JI, Boyer DM, et al. Semicircular canal system in early primates. J Hum Evol. 2009;56:315–327. doi: 10.1016/j.jhevol.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Simpson GG. Skulls and brains of some mammals from the Notostylops beds of Patagonia. Am Mus Novit. 1932;578:1–11. [Google Scholar]
- Simpson GG. Braincasts of Phenacodus, Notostylops, and Rhyphodon. Am Mus Novit. 1933a;622:1–19. [Google Scholar]
- Simpson GG. Braincasts of two typotheres and a litoptern. Am Mus Novit. 1933b;629:1–18. [Google Scholar]
- Simpson GG. Structure of a primitive notoungulate cranium. Am Mus Novit. 1936;824:1–32. [Google Scholar]
- Simpson GG. The beginning of the age of mammals in South America. Part 1. Bull Am Mus Nat Hist. 1948;91:1–232. [Google Scholar]
- Simpson GG. Splendid Isolation: The Curious History of South American Mammals. New Haven: Yale University Press; 1980. [Google Scholar]
- Spoor F. The semicircular canal system and locomotor behaviour, with special reference to hominin evolution. Cour Forschr-Inst Senckenberg. 2003;243:93–104. [Google Scholar]
- Spoor F, Zonneveld F. Morphometry of the primate bony labyrinth: a new method based on high-resolution computed tomography. J Anat. 1995;186:271–286. [PMC free article] [PubMed] [Google Scholar]
- Spoor F, Zonneveld F. Comparative review of the human bony labyrinth. Yearb Phys Anthropol. 1998;41:211–251. doi: 10.1002/(sici)1096-8644(1998)107:27+<211::aid-ajpa8>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- Spoor F, Garland T, Krovitz G, et al. The primate semicircular canal system and locomotion. Proc Natl Acad Sci USA. 2007;104:10808–10812. doi: 10.1073/pnas.0704250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirton RA. A new genus of interatheres from the Miocene of Colombia. Univ Calif Publ Geol Sci. 1953;29:265–348. [Google Scholar]
- West CD. The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J Acoust Soc Am. 1985;77:1091–1101. doi: 10.1121/1.392227. [DOI] [PubMed] [Google Scholar]
- Wible JR, Rougier GW, Novacek MJ, et al. Earliest eutherian ear region: a petrosal referred to Prokennalestes from the Early Cretaceous of Mongolia. Am Mus Novit. 2001;3322:1–44. [Google Scholar]
- Wible JR, Rougier GW, Novacek MJ, et al. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature. 2007;447:1003–1006. doi: 10.1038/nature05854. [DOI] [PubMed] [Google Scholar]