Abstract
The bone morphogenetic protein (BMP) family of growth factors plays critical roles in bone formation. BMPs are regulated at multiple levels by various BMP antagonists. This study investigated how BMP antagonists are integrated into the cascade of events of bone formation during fracture healing. Forty mice underwent a controlled femur fracture; tissue samples at the fracture site were harvested at days 1, 3, 7, 14 and 21 after fracture, for quantification of the expression of BMPs and BMP antagonists. During fracture healing, BMP-2, -4 and -7 were up-regulated, but BMPR-1A and BMPR-2 showed reduced expression after day 14. Among BMP antagonists, the expressions of PRDC, SOST, Smad7, GREM1 and CERBERUS were generally down-regulated during fracture healing. In contrast, Noggin was significantly up-regulated in the first week after fracture; 7 days after fracture, other BMP antagonists, including DAN, CHRD, Smad6 and BAMBI, also showed significantly increased expression. In conclusion, this study indicates that BMP antagonists can be divided into two functional groups in relation to fracture healing: (1) those whose suppression may be essential for the initiation of osteogenesis; (2) those that are upregulated and may function in the remodeling of newly formed bone.
Keywords: antagonists, bone, bone morphogenetic proteins, fracture, rodent
Introduction
Bone morphogenetic proteins (BMPs), a subset of the transforming growth factor-β (TGF-β) superfamily of growth and differentiation factors (Rosen, 2006), are critical for the normal growth and development of a myriad of tissues within a wide spectrum of vertebrate species (Kingsley, 1994). Since their discovery in the 1960s, BMPs have been investigated as a potential biological tool to promote fracture healing and bone regeneration, and they are now clinically available for treating fracture non-union and augmenting bone loss in fractures (Vaibhav et al. 2007). However, the value of BMPs in enhancing fracture healing may be equivocal overall because their efficiency is no better than an autologous graft and they are more costly (Jones et al. 2006; Garrison et al. 2007). To improve the effectiveness of BMPs, a better understanding of the BMP signaling network is essential.
The secreted BMPs, of which 20 family members have been discovered (Xiao et al. 2007), bind to their specific receptors on the cell membrane and induce the dimerization of their cognate transmembrane receptors. Receptor dimerization leads to the phosphorylation of Smad 1, 2, 3, 5, and 8 in the cytoplasm. The five Smad proteins form a complex with Smad 4, which is capable of translocating the Smad complex into the nucleus. In the nucleus, Smads interact with transcription factor Runx2 and bind to DNA. Runx2, a key regulator of osteoblast differentiation, is the ultimate effector (Kloen et al. 2003). The BMP signaling cascade is sensitive to antagonists, a group of proteins and transcription factors, at almost every level of the pathway. Thus, BMP signaling can be ‘fine-tuned’ by any number of antagonists. Although they are largely overlooked compared to the research efforts given to BMPs, BMP antagonists are equally as important as BMPs in the regulation of bone formation because of their ability to interfere with and adjust BMP signaling.
The most studied BMP antagonists include noggin, chordin, sclerostin, gremlin, BAMBI (BMP and activin membrane bound inhibitor), Smad 6, and 7, DAN (differential screening-selected gene aberrant in neuroblastoma), Cerberus, and PRDC (protein related to DAN and Cerberus) (Gazzerro & Canalis, 2006). They modulate a diverse array of BMP activities, including early embryo development (Mine et al. 2008), neural development (McMahon et al. 1998), prostate development (Cook et al. 2007), inflammation of cardiac tissues (Chang et al. 2007), and bone development (Gazzerro & Minetti, 2007). Most importantly, when noggin expression was inhibited, osteoblastic differentiation and bone regeneration were enhanced both in vitro and in vivo (Wan et al. 2007). Clearly, BMP antagonists play an important role in bone regeneration and fracture healing. The functionality of BMP antagonists in the natural course of bone regeneration, however, remains unclear. For effective prompting of bone regeneration, this study was designed to clarify the role of BMP antagonists during fracture healing. In this study, the gene and protein expressions of a group of BMPs, BMP receptors, and BMP antagonists were investigated in a mouse femoral fracture model, using quantitative polymerase chain reaction (qPCR) and immunohistochemistry. The correlations of the expression of BMP antagonists with the expression of BMPs and BMP receptors as well as the natural history of fracture healing were analyzed.
Materials and methods
Fracture model
Forty 10-week-old male C57BL/6 mice (Harlan, Indianapolis, IN, USA) were used in this study (approved by the Saint Louis University Animal Care and Use Committee). The mice were anesthetized by injection of a ketamine/xylazine cocktail. On the randomly selected hind limb, the lateral aspect of the thigh was shaved and sterilized with betadine. The skin was incised and soft tissues were bluntly dissected to expose the mid-shaft femur. A transverse fracture was created with a pair of scissors. Skin closure was accomplished with a single horizontal mattress stitch using a 4–0 Monocryl suture (Ethicon, Piscataway, NJ, USA). Animals were allowed to use the fractured extremity ad lib. Post-surgery analgesia was given for the first 3 days and afterwards when necessary. Radiographs were taken weekly after surgery using a Porta-Ray MISIII X-ray machine (Pro-Rad, Deer Park, NY, USA). Animals, eight in each group, were sacrificed by cervical dislocation at 1, 3, 7, 14, and 21 days after fracture. The fractured femur was harvested, and the standardized tissue blocks centered at the fracture line were created. These tissue blocks included the fracture site as well as surrounding hematoma, soft callus, and/or hard callus. Mid-shaft femoral bone segments from the contralateral unfractured side were harvested for experimental controls. From each time-point group, seven tissue blocks were placed in TriZol RNA extraction solution (Sigma-Adrich, St. Louis, MO, USA) and frozen at −80 °C until used for RNA extraction. One tissue sample at each time-point was fixed in 4% paraformaldehyde at 4 °C overnight and decalcified in 10% EDTA for histology.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
The tissue blocks were homogenized in a liquid nitrogen-cooled mortar. Tissue powders were then processed for RNA extraction using the TriZol method. Using the SuperScript™ first-strand synthesis system (Invitrogen, Carlsbad, CA, USA), 2.5 μg of total RNA was reverse-transcribed and the products of reverse-transcription were treated with RNase H before storage at −20 °C. Real-time PCR was performed on a MyiQ real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). Using Sybr® green PCR Master Mix reagents (Bio-Rad Laboratories), each reaction mixture consisted of 12.5 μL SYBR green PCR reagent, 2.5 μL of 1 : 50 diluted reverse-transcription product, optimized volume of 5 mm primers and diethylpyrocarbonate (DEPC)-treated water, for a total volume of 25 μL. No-template and no-reverse-transcription reactions were included in each PCR plate as negative controls. 18S was used as an internal standard in each PCR plate. After 10 min at 95 °C, the PCR amplification was performed for 40 cycles; each cycle consisted of amplification at 95 °C for 50 s and 65 °C for 30 s. Primers supplied by Integrated DNA Technologies, Coralville, IA, USA, are detailed in Table 1. Amplification efficiency of > 90% was required for further processing of the data. Five replicates of each reaction were performed. The cycle at which the fluorescent level was statistically above the background was defined as the threshold cycle (Ct). The Ct values of the gene under investigation were first normalized by subtraction of the Ct value of 18S, and the relative expression of this gene at fracture site (ΔΔCt) is a subtraction of the normalized Ct (ΔCt) of this gene at fracture site from the one in normal bone.
Table 1.
A list of primers for real-time RT-PCR.
| Gene Name | GenBank No. | Forward primer | Reverse primer |
|---|---|---|---|
| BMP2 | NM007553 | CAGGAAGCTTTGGGAAACAG | GTCGAAGCTCTCCCACTGAC |
| BMP4 | NM007554 | GCCATTGTGCAGACCCTAGT | ACCCCTCTACCACCATCTCC |
| BMP7 | NM007557 | GGGCTTACAGCTCTCTGTGG | CCGGATACTACGGAGATGGA |
| BMPR1A | NM009758 | CGTGCGAATCAGACAATGAC | CTGGCTTCTTCTGGTCCAAG |
| BMPR2 | NM007561 | TATGCAGAATGAACGCAACC | CTGGACATCGAATGCTCAGA |
| NOGGIN | NM008711 | TGTGGTCACAGACCTTCTGC | GTGAGGTGCACAGACTTGGA |
| CHRD | NM009893 | CACAGGCAACATCCTGTTTG | CCTGAAGGGTGAGTGGATGT |
| SOST | NM024449 | GTGTGATGTTGGGCTACGTG | CCACCACAATCTCTCCCCTA |
| PRDC | NM011825 | GGATGTTCTGGAAGCTCTCG | GATCTGGTGATGCCACCTCT |
| BAMBI | NM026505 | CTGTGATAGCGGTTCCCATT | TGGTGTCCGTGAAAGCTGTA |
| SMAD7 | NM001042660 | GAGTCTCCCCCTCCTCCTTA | CAGGCTCCAGAAGAAGTTGG |
| SMAD6 | NM008542 | CGGGTTACTCCATCAAGGTG | GGCAGGAGGTGATGAACTGT |
| CERBERUS | NM009887 | ACAGGAGGAAGCCAAGAGGT | AGTCTTCATGGGCAATGGTC |
| GREM1 | NM011824 | AAGCGAGATTGGTGCAAAAC | TGAAAGGACCCTTCCTCCTT |
| DAN | NM008675 | CCCTTCAATGGAGTGGCTTA | CCTAAAGGGTCCAGAACACG |
| 18S | NR003278 | CTCAACACGGGAAACCTCAC | ATGCCAGAGTCTCGTTCGTT |
Histology and immunohistochemistry
Tissue blocks were embedded in O.C.T. (optimal cutting temperature) compound and sectioned longitudinally with a cryostat at a thickness of 10 μm. Selected tissue sections from each time-point were stained with hematoxylin and eosin (H & E) for histologic observation.
The primary antibodies for immunohistochemistry were goat anti-mouse DAN (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Sections were autoclaved in Tris-EDTA buffer (pH 9.0) for antigen retrieval. Following blocking incubation, primary antibody (1 : 50 in 1% bovine serum albumin) was added to the sections and incubated overnight at 4 °C. Texas Red conjugated bovine anti-goat secondary antibody was incubated for 1 h at room temperature. Sections were counterstained with DAPI (4′,6-diamidino-2-phenylindole) for nuclear staining. Primary antibody was replaced with 1% bovine serum album in phosphate-balanced saline for the negative staining controls. Tissue staining was examined under a fluorescent microscope (DMI4000; Leica). Images were taken using a cooled digital camera (Leica DFC340) and processed with Leica Application Suite 2.8 (Leica).
Statistical analysis
Data are presented as mean ± standard deviation. One-way anova was performed for multiple comparison of gene expression (ΔΔCt) among different time-points, followed by post-hoc t-test. Gene expression (ΔΔCt) in the non-fractured normal bone is defined as 1.0 and a significant difference is defined as P < 0.05, and the average ΔΔCt of five replicates as < 0.5 for downregulation or > 1.5 for upregulation.
Results
Radiography demonstrated that callus formation appeared as early as day 7 after fracture, despite displacement between the distal and proximal fracture ends and unrestricted use of the limbs. Complete healing – diminished fracture line and bony callus reuniting the two fracture ends – was observed at day 21 in all the animals of this study group.
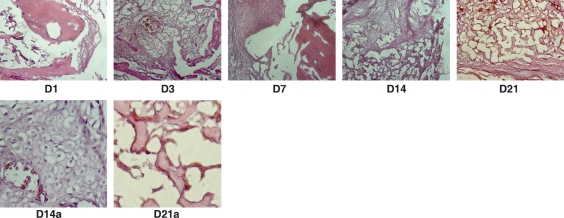
Histology of fracture healing (Fig. 1) was in line with the radiography. At day 1, blood clot was formed at the fracture ends. At day 3, there were inflammatory reactions around the fracture site: a significantly increased number of leukocytes in the blood clot and cell proliferation under the periosteum. At day 7, cells condensed and formed cartilaginous tissue around the fracture ends. At week 2, callus tissue, a mixture of condensed fibrous tissue, cartilage and osteoids, formed between the fractured ends. By week 3, a large amount of bony callus developed around the fracture site. Cartilage was still seen but had significantly reduced in the callus compared with week 2. Bone marrow was observed in the new bone, indicating maturation.
Fig. 1.
Histology of bone healing. Day 1: blood clot formed at the fracture end. Day 3: cell density increased in the clot as results of cell proliferation and local inflammation. Day 7: fibroblasts condensed at the fracture end. Day 14: callus formed at the fracture site with mixed tissues of fibrous, cartilaginous and bone. Day 14a: enlarged area of the cartilaginous tissue in the callus. Day 21: callus matured with mostly bone and bone marrow formed in the bone (Day 21a). Note: hematoxylin and eosin staining.
The gene expression during fracture healing was compared with normal, non-fractured control bone taken from the contralateral limbs, functionally grouped as follows:
BMPs and BMP receptors
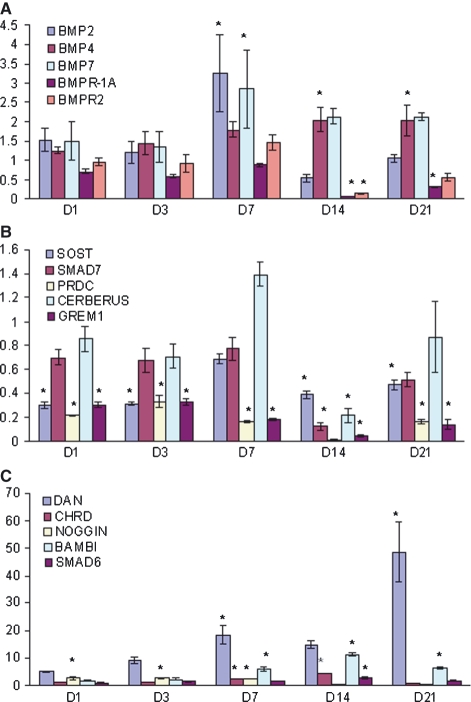
The expression of BMP-2 and -7 gradually increased after the fracture and became statistically significant at day 7 (3.27- and 2.83-fold, respectively) (Fig. 2A). Whereas the upregulation of BMP-2 was brief, the decrease of BMP-7 expression was slower than expression of BMP-2. BMP-7 expression was still more than twofold that found in the normal bone at week 3. BMP-4 was expressed in a different pattern from BMP-2 and -7. BMP-4 was slightly increased over the first week after fracture and reached a twofold increase at weeks 2 and 3, which was statistically significant. The expression of BMPR-1A was statistically unchanged in the first week of fracture compared to the non-fractured bone, but downregulated in weeks 2 and 3. For BMPR-2, its expression in the fractured tissue blocks was comparable to the normal or non-fractured bone, except for downregulation at week 2. The increase of BMPR-2 expression (1.5-fold) at day 7 corresponded well with the expression of BMPs. However, the increase was not statistically significant.
Fig. 2.
Quantitative RT-PCR. Gene expression in the controls, i.e. normal non-fractured bone, is defined as 1. Y-axis shows the fold number of the expression of genes investigated relative to the controls ((ΔΔCt). (A) There is a significant upregulation of BMP-2 and -7 at day 7, and the upregulation of BMP-7 continued into week 3. The expression of BMP-4 is above the control throughout the fracture-healing period, but reaches significant levels only after week 2. The expression of BMPR-1A and BMPR-2 in the callus is insignificantly different from the controls, except at day 14. (B) Among the BMP antagonists, PRDC and GREM1 in callus are expressed less than half of that in normal non-fractured bone. CERBERUS and Smad7 are expressed at a lower than normal level at all time-points, but this is only significant at day 14. The expression of SOST is downregulated during fracture healing, except at day 7. (C) DAN, CHRD, noggin, BAMBI, and Smad6 are upregulated during fracture healing. Most of these show a trend of a gradual increase of expression, but noggin is upregulated only during the first week of fracture. The greatest upregulation is nearly 50-fold of DAN at day 21. *Indicates where P < 0.05 when the gene expression is compared between the fracture tissue and normal bone.
BMP antagonists that downregulated during fracture healing
PRDC, SOST, Smad7, GREM1, and CERBERUS were a group of BMP antagonists that downregulated during the 3-week course of fracture healing (Fig. 2B). Among them, GREM1 and PRDC had the most significantly reduced expression, less than half of the control, during 3 weeks of fracture healing. Smad7 and SOST were expressed at a steady level, except for a relatively greater decrease of Smad7 (< 20% of the control) at week 2. The expression of CERBERUS was greater than the control at day 7 (1.4-fold), but it was statistically insignificant.
BMP antagonists that upregulated in the course of fracture healing
Among the upregulated BMP antagonists, noggin was expressed with a unique pattern that was upregulated earlier, from day 1 through day 7, and substantially downregulated at weeks 2 and 3. DAN, CHRD, BAMBI, and Smad6 shared a common pattern of a gradual increase in expression, which reached a peak from day 7 to week 3, followed with less upregulation (Fig. 2C). The most significant increase of expression was DAN, which was at a greater level at any time-point than the other antagonists. A steady increase of DAN expression lasted to the final time-point of the study, up to a 50-fold increase compared to the control at week 3. CHRD and BAMBI had increased expression only after day 7 and CHRD was downregulated at week 3.
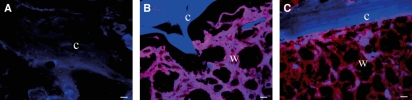
Immunohistochemistry (Fig. 3). Because DAN had the greatest increase of gene expression among the BMP antagonists, the localization of DAN was verified by immunohistochemistry. DAN was not detected in bone in early fracture healing (Fig. 3A). In the callus formed between week 1 and week 2, DAN was positively stained along the surface of newly formed woven bone, but not in the mature cortical bone (Fig. 3B,C).
Fig. 3.
Immunohistochemistry for DAN. At week 1, the fractured bone is not stained with DAN (A). In weeks 2 (B) and 3 (C), DAN is strongly stained in the newly formed woven bone, but not in the mature lamellar cortical bone. DAN is stained with Texas Red, red. Nuclear is counterstained with DAPI, blue. C: cortical bone; W: woven bone; bar = 100μm.
Discussion
BMP antagonists, aside from association with BMP signaling pathways, have diverse roles in development (Abe, 2006; Stabile et al. 2007). We hypothesized that, although BMP and BMP antagonists are in concert in molecular regulation, individual BMP antagonists may have specialized functionality in the event of fracture healing. This fracture model was produced with regular fracture ends to aid sample collection. Leaving the fracture unfixed was intended to allow natural (non-interfered with) fracture healing. Although C57BL/6 mice are probably the most widely used laboratory animals and have been used in standardized fracture models (Hiltunen et al. 1993; Marturano et al. 2008), it is noteworthy that this strain of mice has unique immunological macrophage reactions (Mills et al. 2000), which may potentially influence the outcome of fracture healing.
The effects of BMP-2 and -7 in fracture healing have been well studied (Tsuji et al. 2006; Tsiridis et al. 2007). The upregulation of BMP-2 and -7 in this mouse model was consistent with other animal models (Khanal et al. 2008). BMP-4 was steadily expressed, but its role in fracture healing is undetermined, as fractures heal normally in BMP-4 knockout animals (Wutzl et al. 2006; Tsuji et al. 2008). BMP receptors are expressed by multiple cell types, including fibroblasts, endothelial cells, chondrocytes, and osteoblasts (Kloen et al. 2003). The reduced expression of BMPR-1A and BMPR-2 at days 14 and 21 may relate to the changes of callus composition and reduced cell density, as the histology demonstrated abundant extracellular matrix had been deposited in the cartilaginous and bony callus in the late stage of fracture healing. The quantified expression of BMPs and BMP receptors in this animal model serves as references of the molecular biology of fracture healing in analyzing the functions of BMP antagonists.
The biological effects of BMPs in bone formation are the sum of BMP expression and the activities of BMP antagonists. Although antagonizing BMP signals through various mechanisms (Gazzerro & Canalis, 2006), PRDC, SOST, Smad7, GREM1, and CERBERUS were expressed significantly less in the bone callus than in the non-fractured bone, suggesting that they may have contributed to bone formation by reducing the levels of antagonizing BMP signaling. It could be proven that the reduction of BMP antagonists during fracture healing is as critical as the increase of BMPs is to bone formation.
Another group of BMP antagonists – noggin, BAMBI, DAN, Smad6, and chordin – was upregulated at various stages of fracture healing. Noggin, which directly binds BMP extracellularly and blocks the interaction of BMPs with their receptors, has shown a unique expression pattern to other BMP antagonists during fracture healing. Reacting to the fracture, noggin was upregulated in the early stage of fracture healing in the current study. This upregulation of noggin, which is in agreement with another study (Niikura et al. 2006), seems associated with the expression of BMP-2. Indeed, noggin has a higher affinity with BMP-2 than with BMP-7 (Aspenberg et al. 2001; Gazzerro & Canalis, 2006) and injection of rhBMP-2 into the paraspinal muscles increased noggin expression in 2–4 days (Nakamura et al. 2003). In a recent study, when noggin was knocked down with RNA interference (RNAi), osteogenesis of preosteoblasts was significantly enhanced both in vitro and in vivo (Wan et al. 2007).
Chordin, Smad6, and BAMBI shared the same pattern of expression during fracture healing, peaking at week 2. BAMBI, a kinase-deficient decoy pseudo-receptor of BMPs (Higashihori et al. 2008), was expressed 10 times more in the callus than in normal bone. The significant increase of BAMBI expression is regarded as a negative feedback to BMP activities during fracture healing (Onichtchouk et al. 1999; Higashihori et al. 2008). BAMBI has been found co-expressing with BMP-4 (Grotewold et al. 2001). The similar expression patterns of BMP-4 and BAMBI found in this study may explain in part why BMP-4 is dispensable in fracture healing (Tsuji et al. 2008).
In the current study, the increase of chordin and Smad6 expression was coupled with a period of fracture healing which is active in bone formation and featured with upregulation of BMPs. The involvement of chordin in bone formation is also evident in a recent study in which osteogenesis of bone marrow stem cells was increased twofold when chordin was selectively knocked down with RNAi (Kwong et al. 2008). However, the consequence of suppression of BAMBI, Smad6, and chordin in fracture healing is uncertain.
DAN is important for growth and development across the spectrum of vertebrates (Balesman & Van Hul, 2002), although its null mutations demonstrate a subtle different phenotype from controls (Gazzerro & Canalis, 2006). In this study, DAN is one of the most upregulated BMP antagonists compared to the non-fractured bone. Like BAMBI, the expression of DAN steadily increased throughout the fracture-healing course. The extraordinary upregulation in the late stage of fracture healing links the role of DAN with bone remodeling, in which bone formation and resorption are kept in balance. This is further supported by the localization of DAN overwhelmingly in the newly formed woven bone, in which the matrix is not organized in an orderly fashion. On the other hand, DAN was not detected in the mature lamellar bone in fracture callus.
The emergence of new BMP antagonists is expanding the list of those ubiquitous factors (Haque et al. 2008). BMP antagonists are dynamically involved in the initiation and modulation of fracture healing. The increase of BMP antagonists in the late stage of fracture healing may serve to repress BMP signaling and restore homeostasis in repaired bone (Haque et al. 2008; Higashihori et al. 2008). For the purpose of amplifying BMP signaling and enhancing bone formation, however, they are the potential targets for suppression, especially in established fracture non-union which has passed the stage of active bone formation.
Acknowledgments
This project is funded in part by a grant awarded to J.T.W. and Z.Z. by the Foundation for Orthopaedic Trauma. The authors thank Mrs. Alice Overby for her editing assistance.
References
- Abe E. Function of BMPs and BMP antagonists in adult bone. Ann N Y Acad Sci. 2006;1068:41–53. doi: 10.1196/annals.1346.007. [DOI] [PubMed] [Google Scholar]
- Aspenberg P, Jeppsson C, Economides AN. The bone morphogenetic proteins antagonist noggin inhibits membranous ossification. J Bone Miner Res. 2001;16:497–500. doi: 10.1359/jbmr.2001.16.3.497. [DOI] [PubMed] [Google Scholar]
- Balesman W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Chang K, Weiss D, Suo J, et al. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- Cook C, Vezina CM, Allgeier SH, et al. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol. 2007;312:217–230. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KR, Donell S, Ryder J, et al. Clinical effectiveness and cost–effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess. 2007;11:1–150. doi: 10.3310/hta11300. iii–iv. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord. 2006;7:51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Minetti C. Potential drug targets within bone morphogenetic protein signaling pathways. Curr Opin Pharmacol. 2007;7:325–333. doi: 10.1016/j.coph.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Grotewold L, Plum M, Dildrop R, et al. Bambi is coexpressed with Bmp-4 during mouse embryogenesis. Mech Dev. 2001;100:327–330. doi: 10.1016/s0925-4773(00)00524-4. [DOI] [PubMed] [Google Scholar]
- Haque T, Hamade F, Alam N, et al. Characterizing the BMP pathway in a wild type mouse model of distraction osteogenesis. Bone. 2008;42:1144–1153. doi: 10.1016/j.bone.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Higashihori N, Song Y, Richman JM. Expression and regulation of the decoy bone morphogenetic protein receptor BAMBI in the developing avian face. Dev Dyn. 2008;237:1500–1508. doi: 10.1002/dvdy.21529. [DOI] [PubMed] [Google Scholar]
- Hiltunen A, Vuorio E, Aro HT. A standardized experimental fracture in the mouse tibia. J Orthop Res. 1993;11:305–312. doi: 10.1002/jor.1100110219. [DOI] [PubMed] [Google Scholar]
- Jones AL, Bucholz RW, Bosse MJ, et al. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1431–1441. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- Khanal A, Yoshioka I, Tominaga K, et al. The BMP signaling and its Smads in mandibular distraction osteogenesis. Oral Dis. 2008;14:347–355. doi: 10.1111/j.1601-0825.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. The TGF-beta superfamily: new members, new receptors and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kloen P, Di Paola M, Borens O, et al. BMP signaling components are expressed in human fracture callus. Bone. 2003;33:362–371. doi: 10.1016/s8756-3282(03)00191-1. [DOI] [PubMed] [Google Scholar]
- Kwong FN, Richardson SM, Evans CH. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res Ther. 2008;10:R65. doi: 10.1186/ar2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, Cleveland BC, Byrne MA, et al. An improved murine femur fracture device for bone healing studies. J Biomech. 2008;41:1222–1228. doi: 10.1016/j.jbiomech.2008.01.029. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, et al. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Mine N, Anderson RM, Klingensmith J. BMP antagonism is required in both the node and lateral plate mesoderm for mammalian left-right axis establishment. Development. 2008;135:2425–2434. doi: 10.1242/dev.018986. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Wakitani S, Nakayama J, et al. Temporal and spatial expression profiles of BMP receptors and noggin during BMP-2 induced ectopic bone formation. J Bone Miner Res. 2003;18:1854–1862. doi: 10.1359/jbmr.2003.18.10.1854. [DOI] [PubMed] [Google Scholar]
- Niikura T, Hak DJ, Reddi AH. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J Orthop Res. 2006;24:1463–1471. doi: 10.1002/jor.20182. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Chen YG, Dosch R, et al. Silencing of TGF-beta signaling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- Rosen V. BMP and BMP inhibitors in bone. Ann NY Acad Sci. 2006;1068:19–25. doi: 10.1196/annals.1346.005. [DOI] [PubMed] [Google Scholar]
- Stabile H, Mitola S, Moroni E, et al. Bone morphogenic protein antagonist Drm/gremlin is a novel proangiogenic factor. Blood. 2007;109:1834–1840. doi: 10.1182/blood-2006-06-032276. [DOI] [PubMed] [Google Scholar]
- Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(S1):S11–S25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1428. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Cox K, Bandyopadhyay A, et al. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am. 2008;90(Suppl 1):14–18. doi: 10.2106/JBJS.G.01109. [DOI] [PubMed] [Google Scholar]
- Vaibhav B, Nilesh P, Vikram S, et al. Bone morphogenic protein and its application in trauma cases: a current concept update. Injury. 2007;38:1227–1235. doi: 10.1016/j.injury.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Wan DC, Pomerantz JH, Brunet LJ, et al. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–26459. doi: 10.1074/jbc.M703282200. [DOI] [PubMed] [Google Scholar]
- Wutzl A, Brozek W, Lernbass I, et al. Bone morphogenetic proteins 5 and 6 stimulate osteoclast generation. J Biomed Mater Res (A) 2006;77:75–83. doi: 10.1002/jbm.a.30615. [DOI] [PubMed] [Google Scholar]
- Xiao YT, Xiang LX, Shao JZ. Bone morphogenetic protein. Biochem Biophys Res Commun. 2007;362:550–553. doi: 10.1016/j.bbrc.2007.08.045. [DOI] [PubMed] [Google Scholar]