Abstract
Although 2-deoxy-2-[18F]fluoro-D-glucose (FDG) positron emission tomography (PET) has been used for the assessment of skeletal muscle activities, its application to the shoulder muscles is only sparse. The purpose of this study was to investigate the activities of the shoulder muscles during arm elevation using PET. Six healthy volunteers performed an arm elevation exercise before and after FDG injection. The exercise consisted of 200 repetitions of arm elevation in the scapular plane with a 0.25-kg weight fixed to the wrist on both arms. PET examination was performed 50 min after FDG injection. For control data, PET scan was repeated for each subject on a separate day without any exercise. The volume of interest was established for each shoulder muscle. The subscapularis was divided into three portions (superior, middle, and inferior). The standardized uptake value (SUV) was calculated in each muscle to quantify its activity. The SUVs increased significantly after exercise in the deltoid, supraspinatus, and the superior portion of subscapularis. Among three divided portions of the subscapularis, the SUV of the superior one-third was significantly greater than the rest of the muscle after exercise. Our current study clearly indicated that there were two functionally different portions in the subscapularis muscle and the superior one-third played an important role during arm elevation in the scapular plane.
Keywords: 2-deoxy-2-[18F]fluoro-D-glucose (FDG), arm elevation, muscle activity, positron emission tomography (PET), shoulder biomechanics, shoulder muscles, subscapularis muscle
Introduction
Electromyography (EMG) is a standard method to assess the in vivo function of muscles. However, there are several disadvantages when applying EMG to shoulder muscles. First, EMG using needle electrodes is the only method available for the assessment of deep muscles, but it is an invasive method which requires the insertion of the dual fine wire electrodes intramuscularly. This is technically demanding, especially for the subscapularis, which is located between the thorax and the scapula. Secondly, the insertion of the electrodes may cause serious complications such as pneumothorax. Thirdly, migration of the electrodes sometimes occurs during an exercise (Morris et al. 1998), as the shoulder joint has a wide range of motion. Fourthly, a fine needle electrode reflects only the muscle activities of its small portion. The EMG electrodes monitor a very limited area of detection, corresponding to a small number of individual muscle fibers (Malanga et al. 1996). Although EMG data could be normalized as % maximum manual muscle test, it is impossible to assess the function of the entire muscle with this method, especially in muscles with several functional units. This might explain the relatively poor reproducibility of EMG (Komi & Buskirk, 1970; Morris et al. 1998). Because of these problems, the application of EMG to shoulder muscles has been limited.
Positron emission tomography (PET) is a nuclear medicine tool for quantification of regional blood flow and tissue glucose metabolism in vivo. 2-deoxy-2-[18F]fluoro-D-glucose (FDG), as a glucose analog, is absorbed by tissues after intravenous injection, which is converted into FDG-6-phosphate (FDG-6-P) by hexokinase. FDG-6-P then escapes from further metabolism and is trapped in the cells. Radioactive 18F is subjected to beta-decay, resulting in annihilation photon emissions, which are detected by a PET scanner.
In the PET examination, the FDG accumulation in the muscle is used as a parameter of glucose uptake by the muscle, and accordingly, the muscle activity level. It is widely known that active muscle cells exhibit increased glucose uptake. Romijn et al. (1993) showed that plasma glucose uptake into muscle increased with increasing intensity of exercise. Additionally, as FDG is injected intravenously before or after exercise and FDG accumulation is assessed after exercise, the activities of any muscles with any types of exercise could be assessed. Therefore, FDG PET has been used for the assessment of skeletal muscle activities (Fujimoto et al. 1996; Tashiro et al. 1999; Ohnuma et al. 2006), which is particularly useful for measurement of the activity of deep muscles (Shimada et al. 2007). However, the application of FDG PET to the shoulder muscles is only sparse (Shinozaki et al. 2003).
Based on these backgrounds, we attempted to investigate the activities of the shoulder muscles with special interest in the rotator cuff muscles during arm elevation using FDG PET.
Materials and methods
The experimental protocol of the present study was approved by the institutional ethics committee, and a signed consent form was obtained from each subject prior to the FDG PET examination.
Subjects
Six healthy volunteers without any history of shoulder pain or trauma were examined using FDG PET in the present study. There were four males and two females with an average age of 42 years (range; 28–65). Magnetic resonance imaging (MRI) was performed in all subjects to confirm that there were no pathologic conditions around the shoulder, including rotator cuff tears. All the subjects refrained from eating and drinking for at least 3 h before the examination.
Experimental protocol
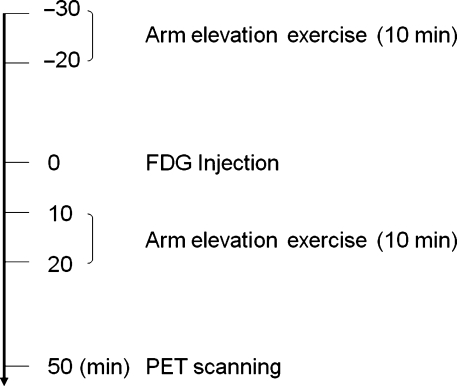
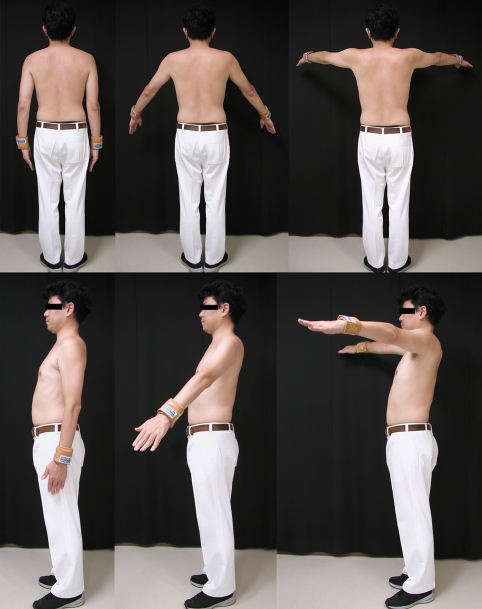
The FDG, radioactive glucose analog, was injected intravenously via the cubital vein after elevation exercise of bilateral arms (200 repetitions in 10 min) in the scapular plane with a 0.25-kg weight (Steel Band; Tiger Medical Instruments, Osaka, Japan) fixed to both wrists. The mean dose and standard deviation (SD) of injected FDG was 86.0 ± 7.1 MBq for the exercise condition. After FDG injection, the subjects were asked to repeat the same exercise of 200 repetitions (Fig. 1). Each subject took a 20-s rest after every 40-s exercise (20 repetitions of arm elevation exercise) to avoid excessive muscle fatigue. The exercise of arm elevation was performed from 0 to 90º elevation in the scapular plane, which inclines 30º anteriorly from the coronal plane. In each time of elevation exercise, the subjects were asked to elevate their arm to 90º in 1 s. In the current study, great care was taken that both shoulders of each subject were kept in the neutral rotation during the entire exercise protocol. As Fig. 2 shows, each subject was requested to elevate the arm, palm down, until it reached 90º to keep the arm in neutral rotation.
Fig. 1.
Time schedule of examination. The same arm elevation exercises in the scapular plane are performed for 10 min before and after FDG injection. PET scanning is initiated 50 min after FDG injection.
Fig. 2.
Arm elevation exercise in the scapular plane. We define the scapular plane as a plane which inclines 30º anteriorly from the coronal plane.
PET scanning was initiated 50 min after injection of FDG. For control data, PET scan was repeated for each subject on a separate day without any exercise. The mean dose of injected FDG for the control condition was 81.8 ± 22.7 MBq, giving no statistically significant difference of radiological doses in the two conditions.
PET examination
A set of emission scans in three-dimensional data acquisition mode was performed 50 min after injection of FDG from the base of the neck to the middle of the upper arm, using a PET scanner (SET-2400W; Shimadzu Inc., Kyoto, Japan) with an intrinsic spatial resolution of 3.9 mm full width at half maximum. The axial field of view of this scanner is 200 mm and images were obtained by performing two incremental scans, which took 8 min each. Transmission scans, which lasted 5 min each, using 68Ge/68Ga external rotating line source, were obtained after the emission scans (post-injection transmission) to correct for tissue attenuation.
All data were corrected for dead time, decay, and measured photon attenuation, and reconstructed into a 128 × 128 × 63 matrix for a set of three-dimensional volume images, using Fourier rebinning (Defrise et al. 1997) and the ordered subset Expectation Maximization (Hudson & Larkin, 1994) algorithm, with the aid of a supercomputer SX-7 at the Information Synergy Center, Tohoku University.
MRI examination
To quantify the muscle activities in each muscle, we needed to determine the exact location of each muscle on the PET image. For that purpose, MR images were taken on both shoulders of all subjects using the FSE-XL Sequence (Signa Horizon LX 1.5T Ver.9.1; GE Healthcare, Milwaukee, Wisconsin, USA). The measurement conditions were as follows: Repetition time/Echo time was 3000/85 ms, number of excitations was one, the field of view was 46 cm, number of matrices was 512 × 512, slice thickness was 3 mm, and a slice gap was 1.5 mm.
Image analyses
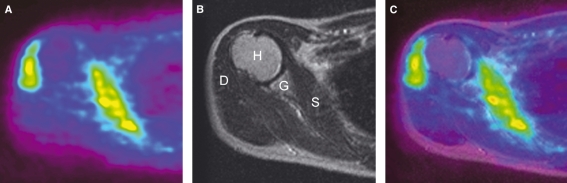
We analyzed the dominant (right side in all subjects) shoulder in each subject. It was difficult to identify the anatomical location of each muscle only with the PET images. Thus, the PET image was fused to the MR image at the same level using a software Dr. View/LINUX (AJS Inc., Tokyo, Japan), which enabled us to delineate the contour of each muscle (Fig. 3). The subject’s own MR image was used as a reference for that purpose.
Fig. 3.
(A) PET image of axial view of the right shoulder at the level of humeral head; (B) MR image of same subject as A; (C) the fused image of A to B. H, humeral head; G, glenoid; D, deltoid muscle; S, subscapularis muscle. High FDG uptakes are observed in the deltoid and subscapularis muscles.
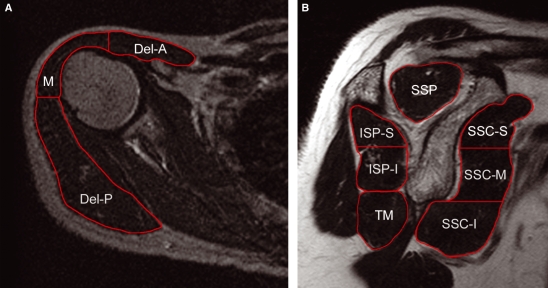
Subsequently, the volume of interest (VOI) was placed on the MR image for each shoulder muscle, including the deltoid, supraspinatus, subscapularis, infraspinatus, and teres minor. The deltoid and subscapularis muscles were divided into three portions (anterior, middle, and posterior portions for the deltoid, and superior, middle, and inferior portions for the subscapularis, respectively), and the infraspinatus muscle was divided into two portions (superior and inferior). These muscles with broad origins were thought to have several independent portions which act differently. Each portion of the deltoid muscle was divided by the lines drawn from the centre of humerus to 12 o’clock and 9 o’clock directions. On the other hand, the subscapularis and the infraspinatus muscles were equally divided along the craniocaudal axis (Fig. 4A,B).
Fig. 4.
The volume of interest (VOI) (red-edged area) of the shoulder muscles. (A) MRI of axial view of right shoulder at the level of humeral head; and (B) MRI of oblique sagittal Y-shaped view of right shoulder. Del-A, anterior portion of deltoid; M, middle portion of deltoid; Del-P, posterior portion of deltoid; SSP, supraspinatus; SSC-S, superior portion of subscapularis; SSC-M, middle portion of subscapularis; SSC-I, inferior portion of subscapularis; ISP-S, superior portion of infraspinatus; ISP-I, inferior portion of infraspinatus; and TM, teres minor. The deltoid muscle is divided into three portions (anterior, middle and posterior). B is a schema of how the infraspinatus and the subscapularis muscles are divided into two and three portions, respectively.
Then, the VOIs of each portion of muscle defined on MR images were copied onto fused PET images using Dr. View/LINUX for evaluation of radioactivity in each muscle. The standardized uptake value (SUV) was calculated to quantify their activities.
SUV was calculated with the following equation;
The SUV expresses the ratio of the amount of FDG accumulated in a certain VOI compared to the situation where the FDG is distributed equally over the entire body.
Statistical analyses
All experimental data were expressed as mean value with SD. Statistical analyses of the data were performed using jmp 6.0 software (SAS Institute Inc., Cary, NC, USA). The two-way factorial analysis of variance (anova) followed by Tukey’s Honestly Significant Difference (HSD) test was employed for multiple comparison of the SUVs of each portion of shoulder muscle. A P-value < 0.05 was considered statistically significant.
Results
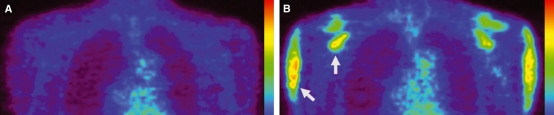
There were no abnormal artifactual accumulations in the PET images at rest (Fig. 5A). High FDG uptakes were observed in all the shoulder girdle muscles after exercise (Fig. 5B). Interestingly, one of the areas with the highest uptake was located in the superior one-third of the subscapularis (Fig. 6).
Fig. 5.
PET images of bilateral shoulder girdles in a representative subject, coronal view at the site of the humeral head. (A) at rest; (B) after exercise. A and B are the images of identical site of same subject. High FDG uptakes are observed in the shoulder girdle muscles (shown with white arrows). The color scales indicates the level of FDG uptake. Red expresses the maximum uptake of FDG.
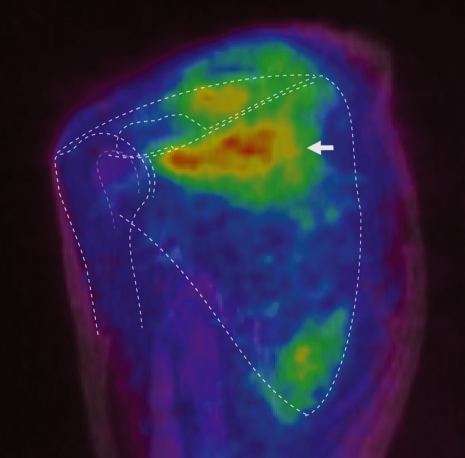
Fig. 6.
The oblique coronal fused image of PET and MRI. High FDG uptake is observed in the superior one-third of the subscapularis muscle (shown with arrow).
Assessment of the activities in whole muscle
After the arm elevation exercise in the scapular plane, the SUVs increased significantly in the deltoid, supraspinatus, and subscapularis muscles. Although the SUVs of the infraspinatus and the teres minor increased slightly after exercise, the differences between at rest and after exercise conditions were not statistically significant (Table 1).
Table 1.
The standardized uptake value (SUV) for each shoulder girdle muscle.
| Muscle | Rest | Exercise |
|---|---|---|
| Deltoid | 0.68 ± 0.07 | 1.31 ± 0.24* |
| Anterior | 0.65 ± 0.05 | 0.85 ± 0.17 |
| Middle | 0.61 ± 0.07 | 1.67 ± 0.36* |
| Posterior | 0.73 ± 0.10 | 1.28 ± 0.30* |
| Supraspinatus | 1.03 ± 0.10 | 2.05 ± 0.31* |
| Subscapularis | 1.17 ± 0.15 | 1.71 ± 0.30* |
| Superior | 1.24 ± 0.18 | 2.29 ± 0.57* |
| Middle | 1.15 ± 0.13 | 1.48 ± 0.24 |
| Inferior | 1.12 ± 0.13 | 1.21 ± 0.15 |
| Infraspinatus | 0.88 ± 0.12 | 1.11 ± 0.24 |
| Superior | 0.94 ± 0.11 | 1.22 ± 0.25 |
| Inferior | 0.76 ± 0.16 | 0.90 ± 0.23 |
| Teres minor | 1.10 ± 0.11 | 1.19 ± 0.26 |
*Statistically significant increase compared to at rest (P < 0.05).
Assessment of divided portions of each muscle
Among the divided portions in each muscle, the SUVs increased significantly after exercise in the middle and posterior portions of the deltoid muscle and the superior portion of subscapularis muscle. Interestingly, the average SUV in the superior portion of the subscapularis muscle was 2.29, which was the greatest value among all portions of muscles measured in the present study. On the other hand, the differences of SUVs between at rest and after exercise conditions were again not statistically significant in either the superior or the inferior portions of the infraspinatus muscle (Table 1).
Comparison among the three divided portions in the subscapularis muscle
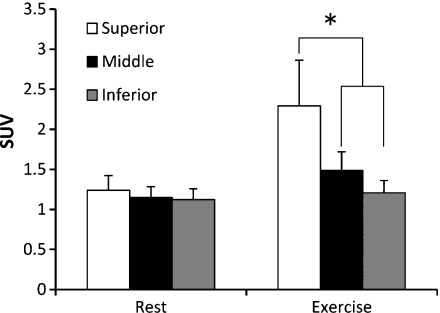
There were no significant differences among three divided portions of the subscapularis muscle at rest. After the exercise, the SUV of the superior one-third of subscapularis muscle was significantly greater than that of the rest of the muscle (Fig. 7).
Fig. 7.
Average and standard deviation of SUVs among each divided portion of the subscapularis muscle both at rest and after exercise conditions. There are no significant differences among three divided portions of the subscapularis muscle at rest. After the exercise, the SUV of the superior one-third of subscapularis muscle is significantly greater than that for the rest of the muscle (*P < 0.05).
Discussion
Pappas et al. (2001) found a positive correlation between the normalized FDG uptake in the biceps brachii and the number of repetitions of elbow flexion. They concluded that FDG PET was capable of measuring task-specific muscle activity within exercising skeletal muscle. Based on their report, FDG PET imaging seems to be highly reproducible in biomechanical and physiological analyses of muscle activities.
Glucose transporter, GLUT4, is known to undergo translocation from intercellular sites to the plasma membrane in response to muscle contraction (Hirshman et al. 1988; Douen et al. 1990). However, it is also known that a small number of muscle contractions do not cause enough glucose uptakes in the skeletal muscles. Thus, a certain number of repetitions are necessary to measure the muscle activity using PET.
In the previous PET studies focusing on the shoulder muscles, Shinozaki et al. (2003) applied 480 repetitions of exercise including arm elevation, internal or external rotation with Thera-Band, a rubber band for resistive exercise, which loaded the arms with more than 1-kg in each muscle contraction. In our pilot study, we realized that it was difficult even for the normal subjects to complete their exercise protocol because of muscle fatigue. Our aim was to assess the muscle function using this new technology, which can also be applied to patients with shoulder pain or limited range of motion. Based on these backgrounds, we reduced both the weight and the number of repetitions to 0.25 kg and 400 times, respectively. Each subject took a 20-s rest after every 40-s exercise (20 repetitions of arm elevation exercise) to avoid causing muscle fatigue. The elevation angle was also limited to a maximum of 90º to avoid shoulder pain during exercise. With these modifications of the exercise protocol, all of the subjects in the current series successfully completed the exercise protocol without complaining of any muscle fatigue or pain.
During arm elevation in the scapular plane, the middle portion of deltoid and the supraspinatus are believed to be the two most important elevators (McMahon et al. 1996). As expected, the activities of the deltoid and supraspinatus increased after the arm elevation exercise. On the other hand, the biomechanical roles of the subscapularis muscle during arm elevation are still controversial. The present study demonstrated that the activities of the subscapularis increased significantly after the exercise. Does this mean that the subscapularis functions as an elevator of the arm or a stabilizer of the shoulder during arm elevation?
As a mover of the glenohumeral joint, Poppen & Walker (1976) reported that the subscapularis had less elevation function. Howell et al. (1986) also believed the elevation function of the subscapularis muscle was negligible based on the calculation of the torque during elevation in the scapular plane. Contrary to their reports, it was reported that the EMG activity of subscapularis was the highest during elevation in the scapular plane among various shoulder motions (Townsend et al. 1991). Kuechle et al. (1997) demonstrated in their kinematic moment arm study that the subscapularis muscle might potentially be a more important elevator in the scapular plane than either the supraspinatus or infraspinatus.
As a stabilizer of the glenohumeral joint, Inman et al. (1944) reported that the primary function of subscapularis was to depress the humeral head. Kronberg et al. (1990) found that the subscapularis worked as a stabilizer together with the infraspinatus and latissimus dorsi during abduction of the shoulder. More recently, Reddy et al. (2000) demonstrated that the inferior force vector produced by the subscapularis and infraspinatus prevented subacromial impingement. These reports suggest that the subscapularis plays an important role during arm elevation.
The increased activities of the subscapularis after the exercise observed in this study could be interpreted as a mover function or a stabilizer function or both. As these data were obtained from normal volunteers without shoulder instability, we assumed that this increased activity might reflect the function of the subscapularis as a mover.
Most of the previous investigators looked at the subscapularis as one muscle unit from the functional point of view. However, the subscapularis has a wide origin from the anterior aspect of the scapular body. As a result, the function may change according to different parts of the muscle. In 1989, Kato investigated the innervating patterns to the subscapularis muscle in 40 cadavers. He found three primary branches to the subscapularis, namely, the superior, middle, and inferior subscapular branches (Kato, 1989). On the basis of this report, Kadaba et al. considered the upper and lower subscapularis as two functionally separate muscles with independent innervation. They divided the muscle into two portions and examined their EMG activity during isometric internal rotation exercises with the arm in 0 or 90º of abduction. They found that the most active portion among the subscapularis muscle differed with the angle of the abduction (Kadaba et al. 1992). Otis et al. (1994) divided the subscapularis muscle into three potions in their moment arm study using 10 cadaver shoulders. They reported that the upper subscapularis had a significant elevator function in the scapular plane.
In the current study, we clearly demonstrated that the deltoid, supraspinatus, and subscapularis muscles worked considerably during arm elevation in the scapular plane. The activity of the superior one-third of the subscapularis muscle during the exercise was significantly greater than the inferior two-thirds. The activities of the infraspinatus and teres minor muscles were relatively low. These results suggest that the superior one-third of this muscle contributes to arm elevation from 0 to 90º in the scapular plane in neutral rotation of the arm.
Clinically, Gerber & Krushell (1991) reported 16 cases of the isolated rupture of the tendon of the subscapularis muscle. In their series, seven patients showed decreased strength at the Jobe’s supraspinatus test, which meant the decreased strength of elevation in the scapular plane. They emphasized that the most important clinical findings of isolated subscapularis tendon rupture were the increased range of external rotation and the loss of muscle power in the internal rotation with a positive lift-off test. However, they did not explain why some patients showed decreased muscle power in elevation in the scapular plane. We assumed that muscle weakness in elevation might be caused by the rupture of the tendon of the superior portion of subscapularis muscle.
Measurements of FDG PET during exercise for determining muscle activities have some advantage over intramuscular wire EMG study. One of the biggest advantages was that we could quantify the activities for any portions of any muscles non-invasively. On the other hand, there were several disadvantages in the FDG PET measurements. First, the resolution of PET images was relatively low, which made it difficult to identify the contour of each muscle. Thus, in the current study, we needed to fuse the PET image to the subject’s own MR image taken at the same level as PET. Secondly, PET scans required some exposure to radiation. To reduce the amount of radiation, we used PET scanning in three-dimensional mode in the current study, which required a smaller dose of FDG than in other studies. As a result, the injection dose of FDG in the current study was approximately 80 MBq, which corresponded to the estimated radiation exposure of 3.6 mSv. Although it did not exceed the annual permissible exposure level of radiation (5 mSv per year) in our institutional guideline, we enrolled the least required number of subjects in this study. The number of subjects in the current study is also comparable to that of the previous studies (Tashiro et al. 1999: n = 7; Fujimoto et al. 2000: n = 7; Ohnuma et al. 2006: n = 6). Thirdly, FDG uptake in PET study does not reflect a real-time contraction of each muscle, which may change at different phases of a motion. Rather, it reflects a sum of muscle activities during the motion. We were unable to detect in which phase of the motion each muscle was active with FDG uptake. Although Fujimoto et al. (2000) demonstrated that both FDG uptake and EMG data were reliable and comparable parameters in the assessment of the muscle activity, such differences should always be taken into consideration.
In conclusion, this study provided new information concerning the muscle contribution during arm elevation. There were two functionally different portions in the subscapularis muscle: the superior one-third played an important role during arm elevation in the scapular plane. We would suggest that FDG PET is applicable to patients with various shoulder problems to clarify the biomechanical contribution of each muscle from the aspect of glucose metabolism.
Acknowledgments
This study was partly supported by a JST grant on research and education in molecular imaging. Part of the experimental results in this research was obtained using supercomputing resources at Information Synergy Center, Tohoku University.
The authors thank Dr. Toshihiko Fujimoto, Dr. Mitsuyoshi Mineta, Dr. Hidemitsu Miyazawa, Dr. Takashi Sakata and Dr. Yoshimasa Sakoma for their technical assistance.
Author contributions
There are seven authors on our article. The contributions of each author to this work are as follows: Omi: main researcher (collection of volunteers, performing the shoulder exercise, MR examination and statistical analyses); Sano: corresponding author (obtaining approval from the institutional ethics committee, controlling the whole project and data interpretation); Ohnuma: co-author (supervising the experimental protocols both for the exercise and for the PET examination); Kishimoto: co-author (obtaining permission to use the radioactive agent (FDG) and supervising the experimental protocol for the PET examination); Watanuki: co-author (controlling PET scanner and analyzing the PET data); Tashiro: co-author (supervising the PET examination, preparing radioactive FDG for the current study and analyzing the PET data); Itoi: senior author (first planning the current study and then supervising the whole project).
References
- Defrise M, Kinahan PE, Townsend DW, et al. Exact and approximate rebinning algorithms for 3-D PET data. IEEE Trans Med Imaging. 1997;16:145–158. doi: 10.1109/42.563660. [DOI] [PubMed] [Google Scholar]
- Douen AG, Ramlal T, Rastogi S, et al. Exercise induces recruitment of the ‘insulin-responsive glucose transporter’. Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990;265:13427–13430. [PubMed] [Google Scholar]
- Fujimoto T, Itoh M, Kumano H, et al. Whole-body metabolic map with positron emission tomography of a man after running. Lancet. 1996;348:266. doi: 10.1016/s0140-6736(05)65572-9. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Itoh M, Tashiro M, et al. Glucose uptake by individual skeletal muscles during running using whole-body positron emission tomography. Eur J Appl Physiol. 2000;83:297–302. doi: 10.1007/s004210000254. [DOI] [PubMed] [Google Scholar]
- Gerber C, Krushell RJ. Isolated rupture of the tendon of the subscapularis muscle. Clinical features in 16 cases. J Bone Joint Surg Br. 1991;73:389–394. doi: 10.1302/0301-620X.73B3.1670434. [DOI] [PubMed] [Google Scholar]
- Hirshman MF, Wallberg-Henriksson H, Wardzala LJ, et al. Acute exercise increases the number of plasma membrane glucose transporters in rat skeletal muscle. FEBS Lett. 1988;238:235–239. doi: 10.1016/0014-5793(88)80486-1. [DOI] [PubMed] [Google Scholar]
- Howell SM, Imobersteg AM, Seger DH, et al. Clarification of the role of the supraspinatus muscle in shoulder function. J Bone Joint Surg Am. 1986;68:398–404. [PubMed] [Google Scholar]
- Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13:601–609. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- Inman VT, Saunders JB, Abbott LC. Observations on the function of the shoulder joint. J Bone Joint Surg Am. 1944;26:1–30. [Google Scholar]
- Kadaba MP, Cole A, Wootten ME, et al. Intramuscular wire electromyography of the subscapularis. J Orthop Res. 1992;10:394–397. doi: 10.1002/jor.1100100312. [DOI] [PubMed] [Google Scholar]
- Kato K. Innervation of the scapular muscles and its morphological significance in man. Anat Anz. 1989;168:155–168. [PubMed] [Google Scholar]
- Komi PV, Buskirk ER. Reproducibility of electromyographic measurements with inserted wire electrodes and surface electrodes. Electromyography. 1970;10:357–367. [PubMed] [Google Scholar]
- Kronberg M, Nemeth G, Brostrom LA. Muscle activity and coordination in the normal shoulder. An electromyographic study. Clin Orthop Relat Res. 1990;257:76–85. [PubMed] [Google Scholar]
- Kuechle DK, Newman SR, Itoi E, et al. Shoulder muscle moment arms during horizontal flexion and elevation. J Shoulder Elbow Surg. 1997;6:429–439. doi: 10.1016/s1058-2746(97)70049-1. [DOI] [PubMed] [Google Scholar]
- Malanga GA, Jenp YN, Growney ES, et al. EMG analysis of shoulder positioning in testing and strengthening the supraspinatus. Med Sci Sports Exerc. 1996;28:661–664. doi: 10.1097/00005768-199606000-00003. [DOI] [PubMed] [Google Scholar]
- McMahon PJ, Jobe FW, Pink MM, et al. Comparative electromyographic analysis of shoulder muscles during planar motions: anterior glenohumeral instability versus normal. J Shoulder Elbow Surg. 1996;5:118–123. doi: 10.1016/s1058-2746(96)80006-1. [DOI] [PubMed] [Google Scholar]
- Morris AD, Kemp GJ, Lees A, et al. A study of the reproducibility of three different normalisation methods in intramuscular dual fine wire electromyography of the shoulder. J Electromyogr Kinesiol. 1998;8:317–322. doi: 10.1016/s1050-6411(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Ohnuma M, Sugita T, Kokubun S, et al. Muscle activity during a dash shown by 18F-fluorodeoxyglucose positron emission tomography. J Orthop Sci. 2006;11:42–45. doi: 10.1007/s00776-005-0972-y. [DOI] [PubMed] [Google Scholar]
- Otis JC, Jiang CC, Wickiewicz TL, et al. Changes in the moment arms of the rotator cuff and deltoid muscles with abduction and rotation. J Bone Joint Surg Am. 1994;76:667–676. doi: 10.2106/00004623-199405000-00007. [DOI] [PubMed] [Google Scholar]
- Pappas GP, Olcott EW, Drace JE. Imaging of skeletal muscle function using (18)FDG PET: force production, activation, and metabolism. J Appl Physiol. 2001;90:329–337. doi: 10.1152/jappl.2001.90.1.329. [DOI] [PubMed] [Google Scholar]
- Poppen NK, Walker PS. Normal and abnormal motion of the shoulder. J Bone Joint Surg Am. 1976;58:195–201. [PubMed] [Google Scholar]
- Reddy AS, Mohr KJ, Pink MM, et al. Electromyographic analysis of the deltoid and rotator cuff muscles in persons with subacromial impingement. J Shoulder Elbow Surg. 2000;9:519–523. doi: 10.1067/mse.2000.109410. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Shimada H, Kimura Y, Suzuki T, et al. The use of positron emission tomography and [18F]fluorodeoxyglucose for functional imaging of muscular activity during exercise with a stride assistance system. IEEE Trans Neural Syst Rehabil Eng. 2007;15:442–448. doi: 10.1109/TNSRE.2007.903978. [DOI] [PubMed] [Google Scholar]
- Shinozaki T, Takagishi K, Ichikawa A, et al. Use of 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) imaging for the evaluation of muscle metabolic activity in ruptured rotator cuffs: identification of shoulder muscles by fusion imaging studies involving both FDG PET and magnetic resonance imaging. J Shoulder Elbow Surg. 2003;12:544–549. doi: 10.1016/s1058-2746(03)00208-8. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Fujimoto T, Itoh M, et al. 18F-FDG PET imaging of muscle activity in runners. J Nucl Med. 1999;40:70–76. [PubMed] [Google Scholar]
- Townsend H, Jobe FW, Pink M, et al. Electromyographic analysis of the glenohumeral muscles during a baseball rehabilitation program. Am J Sports Med. 1991;19:264–272. doi: 10.1177/036354659101900309. [DOI] [PubMed] [Google Scholar]