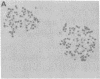
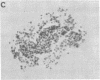
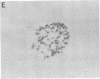
Abstract
The mechanism of determination of early embryonic cells has been investigated using sea urchin embryos. An efficacious method of isolating blastomere pairs from the animal or vegetal half of sea urchin embryos was developed. The overt differentiation of separated animal and vegetal blastomere pairs resembles that of separated animal and vegetal hemispheres isolated by manual dissection. Treatment of animal blastomeres with LiCl caused them to display a morphology resembling that of isolated vegetal blastomeres. The effects of separation of animal and vegetal blastomeres and of treatment of animal blastomeres with LiCl were examined at the molecular level using gut alkaline phosphatase and a spicule matrix protein RNA as markers of differentiation. Histochemical staining and in situ hybridization studies showed that these markers are normally only expressed in vegetal blastomeres but that their expression can be evoked in animal blastomeres by treatment with LiCl.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar S., Schreiber G., Danon A., Belmaker R. H. Lithium inhibits adrenergic and cholinergic increases in GTP binding in rat cortex. Nature. 1988 Feb 4;331(6155):440–442. doi: 10.1038/331440a0. [DOI] [PubMed] [Google Scholar]
- Benson S., Sucov H., Stephens L., Davidson E., Wilt F. A lineage-specific gene encoding a major matrix protein of the sea urchin embryo spicule. I. Authentication of the cloned gene and its developmental expression. Dev Biol. 1987 Apr;120(2):499–506. doi: 10.1016/0012-1606(87)90253-3. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Hough-Evans B. R., Britten R. J., Davidson E. H. Lineage and fate of each blastomere of the eight-cell sea urchin embryo. Genes Dev. 1987 Mar;1(1):75–85. doi: 10.1101/gad.1.1.75. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Lineage-specific gene expression and the regulative capacities of the sea urchin embryo: a proposed mechanism. Development. 1989 Mar;105(3):421–445. doi: 10.1242/dev.105.3.421. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988 Jul 1;54(1):95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Hall H. G. Hardening of the sea urchin fertilization envelope by peroxidase-catalyzed phenolic coupling of tyrosines. Cell. 1978 Oct;15(2):343–355. doi: 10.1016/0092-8674(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Hough-Evans B. R., Britten R. J., Davidson E. H. Mosaic incorporation and regulated expression of an exogenous gene in the sea urchin embryo. Dev Biol. 1988 Sep;129(1):198–208. doi: 10.1016/0012-1606(88)90174-1. [DOI] [PubMed] [Google Scholar]
- Kao K. R., Elinson R. P. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988 May;127(1):64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- LALLIER R. BIOCHEMICAL ASPECTS OF ANIMALIZATION AND VEGETALIZATION IN THE SEA URCHIN EMBRYO. Adv Morphog. 1964;4:147–196. doi: 10.1016/b978-1-4831-9950-4.50007-2. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Deckmyn H., Ross T. S., Bross T. E., Ishii H., Bansal V. S., Wilson D. B. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986 Dec 19;234(4783):1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfohl R. J. Alkaline phosphatase of sea urchin embryos: Chromatographic and electrophoretic characterization. Dev Biol. 1975 Jun;44(2):333–345. doi: 10.1016/0012-1606(75)90404-2. [DOI] [PubMed] [Google Scholar]
- Pfohl R. J., Giudice G. The role of cell interaction in the control of enzyme activity during embryogenesis. Biochim Biophys Acta. 1967 Jun 20;142(1):263–266. doi: 10.1016/0005-2787(67)90534-5. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Benson S., Robinson J. J., Britten R. J., Wilt F., Davidson E. H. A lineage-specific gene encoding a major matrix protein of the sea urchin embryo spicule. II. Structure of the gene and derived sequence of the protein. Dev Biol. 1987 Apr;120(2):507–519. doi: 10.1016/0012-1606(87)90254-5. [DOI] [PubMed] [Google Scholar]
- Van Belle H. Kinetics and inhibition of alkaline phosphatases from canine tissues. Biochim Biophys Acta. 1972 Nov 10;289(1):158–168. doi: 10.1016/0005-2744(72)90118-0. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. Determination and morphogenesis in the sea urchin embryo. Development. 1987 Aug;100(4):559–576. doi: 10.1242/dev.100.4.559. [DOI] [PubMed] [Google Scholar]