Abstract
Background
The endoscopic endonasal transsphenoidal approach (EETA) to the pituitary is performed by ear, nose, and throat (ENT) surgeons in collaboration with neurosurgeons but also by neurosurgeons alone even though neurosurgeons have not been trained in rhinological surgery.
Purpose
To register the frequency of endonasal anatomical variations and to evaluate whether these variations hinder the progress of EETA and require extra rhinological surgical skills.
Methods
A prospective cohort study of 185 consecutive patients receiving an EETA through a binostril approach was performed. All anatomical endonasal variations were noted and the relevance for the progress of surgery evaluated.
Results
In 48% of patients, anatomical variations were recognized, the majority of which were spinae septi and septum deviations. In 5% of patients, the planned binostril approach had to be converted into a mononostril approach; whereas in 18% of patients with an anatomical variation, a correction had to be performed. There was no difference between the ENT surgeon and the neurosurgeon performing the approach. Complications related to the endonasal phase of the surgery occurred in 3.8%. Fluoroscopy or electromagnetic navigation has been used during 6.5% of the surgeries.
Conclusion
Although endonasal anatomical variations are frequent, they do not pose a relevant obstacle for EETA.
Keywords: Endoscopy, Endonasal, Transsphenoidal, Pituitary surgery, Anatomy
Introduction
The endoscopic endonasal transsphenoidal approach (EETA) to the pituitary gland is becoming more and more popular and increasingly replacing the microsurgical transseptal and sublabial transsphenoidal approaches. Its protagonists claim better results through improved visualization and illumination of the operative field and less approach-related morbidity [1–5]. However, these claims are yet to be substantiated and comparative randomized studies have not been performed.
Neurosurgeons performing endoscopic pituitary surgery may be confronted with anatomic variations of the nose, which might be the reason for many to operate in collaboration with an ear, nose, and throat (ENT) surgeon. In order to evaluate, whether these anatomic variations pose serious problems in gaining adequate access to the sella, this study was performed.
Materials and methods
This is a prospective cohort study of a single center—a single neurosurgeon (EvL) consecutive series of patients with a pituitary lesion treated by an EETA between September 1999 and April 2008. The cohort comprises half of the patients operated via EETA in our institution. All patients underwent an EETA for various lesions. The data were collected during the surgical procedure.
Our standard surgical approach is a binostril approach in which the endoscope is handheld. For endonasal pituitary surgery, we use a 0º endoscope with an optic diameter of 4 mm and a separate shaft that allows easy and comfortable holding, while offering a suction–irrigation system for cleaning of the lens (Karl Storz, Tuttlingen, Germany). The maximal outer diameter of the oval-shaped shaft is 6.3 mm. A 30º optic is available for use in specific situations.
The camera used is the Endovision TRICAM® SLII three-chip camera (Karl Storz, Tuttlingen, Germany) connected to an EndoSite 3Di Digital (Viking Systems, Westborough, MA, USA), and projected onto a head-mounted display (HMD) worn by the surgeon during all phases of the surgery. The endoscopic picture is projected onto LCD screens from the HMD with a resolution of 800 × 600 pixels [6]. The instruments used are principally the same as with the microsurgical technique.
Fluoroscopy was only used when indicated, which usually meant that uncertainties existed about the anatomical situation and orientation during the procedure. This occurred only at the beginning of our learning curve. Neuronavigation has been part of the armamentarium from 1999 onwards, but only with the advent of electromagnetic (EM) navigation was it used on a regular basis. EM navigation is used in the case of large tumors that destroy the sellar floor, invade the cavernous sinus, and fill up the sphenoid sinus, because the usual anatomical landmarks are absent or poorly demarcated. In case of sphenoethmoidal cells (or Onodi cells) or a clival sella, EM navigation is also considered.
The judgment of an anatomical variation is very subjective. The incidence of anatomical variations differs significantly whether assessed by endoscopic inspection, anatomical dissection, or thin-sliced computed tomography (CT) scanning. There are also differences in definition [7]. Therefore, we practically defined an anatomical variation as any anatomical detail of the nose that obscured the direct endoscopic view of the anterior sphenoid wall or that obstructed the introduction of any instrument or second instrument through the same nostril along the endoscope (usually the aspirator). Cases in which additional surgical measures had to be taken to change the anatomy in order to achieve adequate approaches to the sphenoid sinus were noted separately (examples in Fig. 1).
Fig. 1.
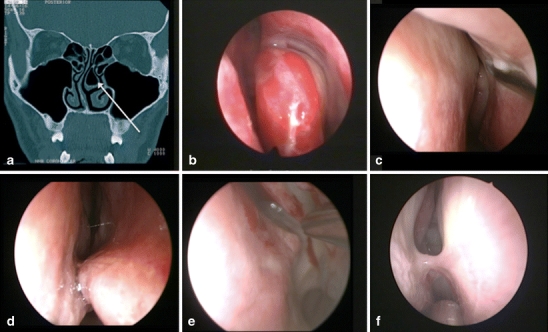
Examples of endonasal anatomical variations that required surgical correction. a Coronal CT-scan with a left bullous middle turbinate, b left endonasal bullous middle turbinate, c left septal deviation obscuring the ostium, d right spina septi, e left spina septi, and f right synechia
Complications reported in this study are those that occurred either during the surgical approach, i.e., the endonasal and transsphenoidal phases of the procedure, or postoperatively, but were clearly the result of the surgical approach. Complications attributable to the sellar phase and tumor removal are excluded from this study.
The series consists of 185 patients of whom 90 were male and 95 female. The average age is 45.6 ± 15.7 years (range 12–81 years).
Surgery was performed for various sellar lesions of which the majority was a tumor (Table 1). In 16 cases, EETA was performed because of recurrent tumor and of these ten had previously undergone endoscopic endonasal surgery and six had undergone transseptal microsurgery. Altogether, 62 of these procedures were performed in collaboration with an ENT surgeon.
Table 1.
Indications for EETA
| Lesion | No. |
|---|---|
| Pituitary adenoma | 165 |
| Craniopharyngeoma | 7 |
| Rathke’s pouch cyst | 4 |
| Xanthogranuloma | 2 |
| Carcinoid | 1 |
| CSF leak | 1 |
| Meningeoma | 1 |
| Neuroendocrine carcinoma | 1 |
| Sheehan syndrome | 1 |
| Epidermoid | 1 |
| Lymphocytic hypophysitis | 1 |
Results
Variations of the endonasal anatomy were observed in 89 cases (48.1%). The majority of these were cristae and spinae septi and septum deviations (Table 2). The incidence of observed anatomical variations was almost identical whether or not an ENT surgeon was present (45.2% with an ENT surgeon present and 49.6% with the neurosurgeon performing the surgical approach).
Table 2.
Endonasal anatomical variations
| Anatomical variation | No. | % of patients |
|---|---|---|
| Septal deviation | 56 | 30.3 |
| Spina septi | 37 | 20 |
| Concha bullosa | 5 | 2.7 |
| Synechia | 5 | 2.7 |
| Extremely narrow | 2 | 1.1 |
| Absence chondroid septum | 2 | 1.1 |
| Pansinusitis | 1 | 0.5 |
| Hypercongestive mucosa | 1 | 0.5 |
| Polyps | 1 | 0.5 |
| Patients with variation | 89 | 48.1 |
Although the binostril approach is our routine approach, in eight cases (5%) we had to perform a mononostril approach because of severe septum deviation. In all these eight cases, this adaptation of the surgical technique caused no problems.
In 16 patients (8.6% of all cases and 18% of patients with anatomical variations), some form of correction of the anatomical variation had to be performed in order to allow a sufficiently good entrance to the sphenoid sinus. Five times a septum correction was performed by the ENT surgeon, but never by the neurosurgeon. Twice a middle turbinate was completely removed (once because of a concha bullosa and once because of an extremely narrow approach), and twice a partial removal of a bullous middle turbinate (lateral part) was performed. An obstructing spina septi was drilled off with a diamond high-speed drill without opening the mucosa separately (in all cases by the neurosurgeon). In one patient, the ENT surgeon removed a nasal polyp. Overall, the ENT surgeon performed some form of correction in 9.7% of cases, while the neurosurgeon did so in 8.1% of cases. The difference is not significant. Abortion of the procedure before reaching the sella turcica did not occur.
Complications strictly related to the approach and the endonasal phase of the surgery occurred in seven patients (3.8%). In three cases (1.6%), a severe epistaxis had to be treated. One of these patients even went into shock and had to be operated on. A bilateral bleeding from a branch of the sphenopalatine artery had to be coagulated after which a speedy recovery ensued. Two other patients required a Bellocq tamponade for 3 days. Two patients were noted as having a sinusitis that required antibiotic therapy.
Twice, an anatomical disorientation with loss of route occurred, which resulted in an unwanted opening of the frontobasis with undesired cerebrospinal fluid (CSF) flow (1% of patients). With the use of fluoroscopy, the anatomical orientation was easily restored and the surgery completed uneventfully. The CSF leak was treated with a fat graft and external lumbar drainage for 3 days. In both cases, the anatomical disorientation was considered to be caused by a large Onodi cell (sphenoethmoidal cell), which was mistakenly held for the sphenoid sinus after entering the space. In 93.5% of our patients, we used no form of assistance in orientation. Fluoroscopy was used in six patients (twice after getting anatomically lost) and six times EM neuronavigation was used.
Discussion
The introduction of endoscopes into a special field of surgery seems to be held equivalent with minimal invasiveness, less traumatization and better results, even when no evidence exists. The EETA is increasingly popularized, replacing the golden standard of the microsurgical transsphenoidal approach for pituitary lesions more and more. The claims of the protagonists of EETA of better results, less complications and decreased morbidity have not yet been substantiated. However, there are indications that endonasal approaches have less approach-related morbidity and a lower approach-related complication rate than transseptal and sublabial transsphenoidal approaches [8–13]. Whether a microsurgical endonasal approach or an EETA differ in their results is not yet clear [9]. In a series of patients that had previously undergone sublabial transsphenoidal surgery and later microsurgical endonasal surgery for recurrent tumor about 80% of patients reported easier recovery, less pain and better nasal airflow after the second surgery than after the first sublabial surgery [9].
Although endoscopic endonasal surgery is relatively new to the neurosurgeon, ENT surgeons are already well-acquainted with endoscopic sinus surgery. Therefore, it is relatively easy for the ENT surgeon to switch from a standard microsurgical technique to an endoscopic technique.
In low and moderate caseload pituitary centers, pituitary surgery is most often performed by a neurosurgeon in collaboration with an ENT surgeon, whereas we observed that in high-volume centers, the neurosurgeon quite often completes the procedure from the start. For these neurosurgeons, the switch to an endoscopic approach might be more difficult, even more so when confronted with anatomical variations.
For the transition from a microsurgical approach to an endoscopic approach, it is helpful to already have some experience with endoscopy, but also to have performed anatomical studies and training on human cadavers [14, 15]. One should also study the different techniques of EETA in use. Some surgeons prefer a mononostril approach, whereas others advocate a binostril approach. The protagonists of the first approach claim less traumatization of the nose when using one nostril compared with the binostril approach. However, the introduction of three instruments (endoscope, aspirator, and any other instrument) through a single nostril might harm the nasal mucosa more than in the binostril technique, whereas the size of the opening of the anterior sphenoid wall is the same for both techniques, as claimed by surgeons favoring the binostril technique. The mononostril technique, therefore, also more often requires the removal of the middle turbinate to allow a successful procedure and sufficient space for the instruments. Since we do not consider this “minimally invasive”, we prefer the binostril technique.
Our study shows that EETA can be well-performed by an experienced neurosurgeon without the collaboration with an ENT surgeon and that approach-related problems are hardly encountered. The advantage is that surgery can be easier planned without logistic problems and requires less manpower, which also decreases the cost of surgery. However, this only accounts for straightforward sellar pathology. In case of more extensive lesions and/or extended EETA, we still seek and advise the collaboration with the ENT surgeon.
Surgical orientation
The endoscopic endonasal approach to the sphenoid sinus is straightforward, relatively easy, and can be performed in 10–15 min (binostril approach). In the ideal situation, the ostium to the sphenoid sinus is unobscured, allowing straightforward anatomical orientation and entrance to the sphenoid sinus. However, anatomical variations do occur frequently and it is in these situations that surgery might become more difficult and require surgical experience. Septum deviations (present in 63% of patients with sinonasal symptoms [16] and 54% in a series of patients operated by a sublabial transseptal approach [17]), spina septi, concha bullosa present in 22–53% on thin-sliced CT scanning and often associated with the deviation of the nasal septum to the contralateral side [18] and a narrow lumen of the nose all might hinder the unobscured vision and unhampered introduction of the endoscope and/or various instruments. Also, one can become anatomically disoriented, which can cause serious morbidity, e.g., CSF fistula, internal carotid artery injury, and optic nerve injury.
The risk of loss of orientation can be reduced by carefully studying the preoperative MRI to which a preoperative CT scan of the nose and sinuses may be added. The surgeon should be especially aware of Onodi cells (which can be found in up to 8.4–9% of cases) [19, 20], enlarged posterior ethmoid cells and the exact localization of septae in the sphenoid sinus. During the learning curve after starting EETA, the use of routine CT scanning of the sinuses should be advised until sufficient expertise has been acquired. In case of anticipated anatomical difficulties, including a clival sella or sphenoid sinus that is totally filled by tumor, one should be prepared to take extra measures for orientation. This may be simple fluoroscopy, but intraoperative neuronavigation (and especially EM neuronavigation) is preferable. In our series, loss of orientation has occurred only twice, leading in both cases to inadvertent CSF leakage, whereas in 93.5% of patients no additional means for orientation were used.
Anatomical variations
Our series has shown that anatomical variations are frequent (seen in almost half of patients), but the number might be lower than in radiological studies. Bolger et al. even found bony paranasal sinus anatomical variations in 65% of patients both with and without sinus complaints, whereas mucosal abnormalities were seen in 41.7% of patients without sinus complaints in a coronal CT study of the sinuses [7]. Whereas we decided to distinguish spinae septi and septum deviations as separate entities for practical reasons, Mladina has classified these as different forms of septum deformities distinguishing seven different types [21]. In a multicenter, multinational study on 2,589 patients in different geographic areas of the world, Mladina et al. [22] even found almost 90% of septum deformities in anterior rhinoscopy.
Nevertheless, in the majority of the cases, these anatomical variations do not hamper the progress of surgery. In less than 20% of our patients with a variation, special measures had to be taken to allow access of the sphenoid ostium; while in eight patients, a planned binostril approach was converted into a mononostril approach. In most cases, however, these modifications were relatively small and easy to perform, while there was no case in which surgery had to be aborted because of approach-related surgical problems. Whether an ENT surgeon or a neurosurgeon was performing the approach did not affect these results; although the ENT surgeon tends to correct a septum deviation to allow a binostril approach, while the neurosurgeon converts to a mononostril approach to deal with this problem. The advantage of a direct reconstruction of the septal alignment may be a subjective improvement in nasal function [17]. Therefore, in patients with nasal airway obstruction symptoms before surgery because of septal deviation, septal correction should be reconsidered.
The majority of these anatomical variations can be seen on preoperative CT scanning of the nose and sinuses and, thus, the surgeon can be prepared. However, after a sufficient learning curve this is no longer strictly needed because the surgeon knows how to deal with any obstacles encountered. It is important to prepare the surgery well with adequate decongestion of the nasal mucosa. This can be achieved with patties drenched in a mixture of cocaine hydrochloride 5%/epinephrine 1 mg/ml placed medially to the middle and inferior turbinates.
Approach-related complications
Postoperative complications related to the endonasal transsphenoidal approach are rare and occurred in only 3.8% of patients. Besides a loss of orientation, which resulted in CSF leakage (0.9%), three cases showed a severe epistaxis (1.6%). Two of these three cases could be treated conservatively with a Bellocq tamponade, while one patient, who was in hemodynamic shock, had to return to the OR. In the latter case, branches of the sphenopalatine arteries had to be coagulated at both inferolateral borders of the middle turbinate. In a large national survey by Ciric et al. in 1997 in the USA, the reported rate of epistaxis in transsphenoidal surgery was 3.4% and, thus, twice as high [23]. However, they found that the rate of epistaxis is dependant on caseload. Surgeons having performed more than 500 cases had an epistaxis rate of 0.4%, surgeons with a caseload of 200–500 had an epistaxis rate of 1.7%, while the less experienced surgeons (<200 cases) had postoperative epistaxis rate of 4.3% [23]. Our result is, therefore, in line with the epistaxis rate after a microsurgical transsphenoidal approach of moderate experienced pituitary surgeons. White et al. [13] showed in 2004 that their transition from a sublabial to an endoscopic approach significantly reduced the rate of postoperative epistaxis from 16% to 2%. This reduction was also seen by Koren et al. [11].
Although this epistaxis occurs only rarely, it can be a severe complication and should be prevented. Since the origin of the bleeding is usually a branch of the sphenopalatine artery at the inferolateral border of the middle turbinate, this site has to be inspected carefully after performing the anterior sphenoidectomy and again at the end of surgery. Simple bipolar coagulation deals with the problem in case of an arterial bleeding. The use of epinephrine at the ostium to the sphenoid sinus causes arterial vasoconstriction and, therefore, may obscure arterial damage. However, 1–2 h later, the vasoconstrictive effect runs out and bleeding may start from the artery causing epistaxis, which usually occurs in the recovery room. Therefore, we advise not to use epinephrine at the ostium before performing an anterior sphenoidectomy.
The low number of postoperative sinusitis is most likely biased by the fact that some cases may have been treated by antibiotic therapy by their general practitioners before having established a diagnosis of sinusitis. Also sphenoid sinusitis may be asymptomatic [8]. In the large survey by Ciric et al., the rate of postoperative sinusitis of transseptal or sublabial transsphenoidal surgery was 8.5% and, thus, much higher [23]. The difference between the endoscopic or microsurgical endonasal approach (1% in this series and 0.8–2% reported in other larger series [8, 9]) in the rate of postoperative sinusitis may be attributed to the absence of nasal packing after the endonasal approach.
Nasal septum perforation, which is quite a common complication of the transseptal and sublabial transsphenoidal approaches (being 6.7% in the survey of Ciric et al. [23]), did not occur in our series and this represents the decreased trauma to the septum by the endonasal approach, as was also confirmed by Koren et al. [11].
Conclusion
Although endonasal anatomic variations occur frequently, they only rarely cause trouble in gaining access to the sellar region and, therefore, can be dealt with by the experienced pituitary surgeon in practically all cases.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Cappabianca P, Alfieri A, de Divitiis E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS) Minim Invasive Neurosurg. 1998;41:66–73. doi: 10.1055/s-2008-1052019. [DOI] [PubMed] [Google Scholar]
- 2.Cappabianca P, de Divitiis E. Endoscopy and transsphenoidal surgery. Neurosurgery. 2004;54:1043–1048. doi: 10.1227/01.NEU.0000119325.14116.9C. [DOI] [PubMed] [Google Scholar]
- 3.de Divitiis E, Cappabianca P. Endoscopic endonasal transsphenoidal surgery. Adv Tech Stand Neurosurg. 2002;27:137–177. doi: 10.1007/978-3-7091-6174-6_4. [DOI] [PubMed] [Google Scholar]
- 4.Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44–51. doi: 10.3171/jns.1997.87.1.0044. [DOI] [PubMed] [Google Scholar]
- 5.Netea-Maier RT, van Lindert EJ, den Heijer M, van der Eerden A, Pieters GF, Sweep CG, Grotenhuis JA, Hermus AR. Transsphenoidal pituitary surgery via the endoscopic technique: results in 35 consecutive patients with Cushing's disease. Eur J Endocrinol. 2006;154:675–684. doi: 10.1530/eje.1.02133. [DOI] [PubMed] [Google Scholar]
- 6.van Lindert EJ, Grotenhuis JA, Beems T. The use of a head-mounted display for visualization in neuroendoscopy. Comput Aided Surg. 2004;9:251–256. doi: 10.1080/10929080500165476. [DOI] [PubMed] [Google Scholar]
- 7.Bolger WE, Butzin CA, Parsons DS. Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope. 1991;101:56–64. doi: 10.1288/00005537-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97:293–298. doi: 10.3171/jns.2002.97.2.0293. [DOI] [PubMed] [Google Scholar]
- 9.Dusick JR, Esposito F, Mattozo CA, Chaloner C, McArthur DL, Kelly DF. Endonasal transsphenoidal surgery: the patient’s perspective—survey results from 259 patients. Surg Neurol. 2006;65:332–341. doi: 10.1016/j.surneu.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Kawamata T, Iseki H, Ishizaki R, Hori T. Minimally invasive endoscope-assisted endonasal trans-sphenoidal microsurgery for pituitary tumors: experience with 215 cases comparing with sublabial trans-sphenoidal approach. Neurol Res. 2002;24:259–265. doi: 10.1179/016164102101199882. [DOI] [PubMed] [Google Scholar]
- 11.Koren I, Hadar T, Rappaport ZH, Yaniv E. Endoscopic transnasal transsphenoidal microsurgery versus the sublabial approach for the treatment of pituitary tumors: endonasal complications. Laryngoscope. 1999;109:1838–1840. doi: 10.1097/00005537-199911000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Roux FX, Page P, Nataf F, Devaux B, Djian MC, Joly LM. The endonasal approach to pituitary adenomas: experience in 105 procedures. Ann Endocrinol (Paris) 2002;63:187–192. [PubMed] [Google Scholar]
- 13.White DR, Sonnenburg RE, Ewend MG, Senior BA. Safety of minimally invasive pituitary surgery (MIPS) compared with a traditional approach. Laryngoscope. 2004;114:1945–1948. doi: 10.1097/01.mlg.0000147925.04605.cc. [DOI] [PubMed] [Google Scholar]
- 14.Cavallo LM, Dal Fabbro M, Jalalod'din H, Messina A, Esposito I, Esposito F, de Divitiis E, Cappabianca P. Endoscopic endonasal transsphenoidal surgery. Before scrubbing in: tips and tricks. Surg Neurol. 2007;67:342–347. doi: 10.1016/j.surneu.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 15.Snyderman C, Kassam A, Carrau R, Mintz A, Gardner P, Prevedello DM. Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope. 2007;117:699–705. doi: 10.1097/MLG.0b013e318031c817. [DOI] [PubMed] [Google Scholar]
- 16.Sazgar AA, Massah J, Sadeghi M, Bagheri A, Rasool E. The incidence of concha bullosa and the correlation with nasal septal deviation. B-ENT. 2008;4:87–91. [PubMed] [Google Scholar]
- 17.Urquhart AC, Bersalona FB, Ejercito VS, Holt JJ. Nasal septum after sublabial transseptal transsphenoidal pituitary surgery. Otolaryngol Head Neck Surg. 1996;115:64–69. doi: 10.1016/S0194-5998(96)70138-9. [DOI] [PubMed] [Google Scholar]
- 18.Stallman JS, Lobo JN, Som PM. The incidence of concha bullosa and its relationship to nasal septal deviation and paranasal sinus disease. AJNR Am J Neuroradiol. 2004;25:1613–1618. [PMC free article] [PubMed] [Google Scholar]
- 19.Leunig A, Betz CS, Sommer B, Sommer F. Anatomic variations of the sinuses; multiplanar CT analysis in 641 patients. Laryngorhinootologie. 2008;87:482–489. doi: 10.1055/s-2007-995572. [DOI] [PubMed] [Google Scholar]
- 20.Mazza D, Bontempi E, Guerrisi A, Del Monte S, Cipolla G, Perrone A, Lo Mele L, Marini M. Paranasal sinuses anatomic variants: 64-slice CT evaluation. Minerva Stomatol. 2007;56:311–318. [PubMed] [Google Scholar]
- 21.Mladina R. The role of maxillar morphology in the development of pathological septal deformities. Rhinology. 1987;25:199–205. [PubMed] [Google Scholar]
- 22.Mladina R, Cujic E, Subaric M, Vukovic K. Nasal septal deformities in ear, nose, and throat patients: an international study. Am J Otolaryngol. 2008;29:75–82. doi: 10.1016/j.amjoto.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–236. doi: 10.1097/00006123-199702000-00001. [DOI] [PubMed] [Google Scholar]


