Abstract
Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease (TMEV-IDD) is a relevant mouse model of multiple sclerosis. Infection of susceptible SJL/J mice leads to life-long CNS virus persistence and development of a chronic T cell-mediated autoimmune demyelinating disease triggered via epitope spreading to endogenous myelin epitopes. Potent CNS-infiltrating CD8+ T cell responses to TMEV epitopes have previously been shown to be induced in both disease-susceptible SJL/J and resistant C57BL/6 mice, in which the virus is rapidly cleared. Specific tolerization of SJL CD8+ T cells specific for the immunodominant TMEV VP3159–166 epitope has no effect on viral load or development of clinical TMEV-IDD, but adoptive transfer of activated CD8+ VP3159–166-specific T cell blasts shortly after TMEV infection to boost the early anti-viral response leads to clearance of CNS virus and protection from subsequent TMEV-IDD. These studies have important implications for vaccine strategies and treatment of chronic infections in humans.
Introduction
Theiler’s murine encephalomyelitis virus (TMEV) infection leads to a chronic-progressive, paralytic demyelinating disease (TMEV-induced demyelinating disease, TMEV-IDD) in susceptible mice that is used as a model for multiple sclerosis (MS). The clinical progression of TMEV-IDD is similar to the primary progressive form of MS, which occurs in about 15% of patients (Sospedra and Martin, 2005). Focal areas of demyelination and leukocyte infiltration are commonly found in CNS tissue from both TMEV-IDD mice and MS patients. (Bruck, 2005; Traugott et al., 1983). This infiltrate includes microglia/macrophages and clonally expanded T cells (Lipton and Jelachich, 1997).
TMEV infection leads to TMEV-IDD only in certain strains of mice, SJL mice being the prototypic susceptible strain. Following intracerebral inoculation, virus initially infects gray matter before spreading into CNS white matter tissue (Lipton and Jelachich, 1997). TMEV persists indefinitely at varying levels in various cell types including monocytes, microglia, oligodendrocytes, and astrocytes (Clatch et al., 1990; Jelachich et al., 1995; Qi and Dal, 1996; Stroop et al., 1981). The onset of clinical signs of disease occurs around day 30–35 post infection (p.i.) and demyelination is initiated by TMEV-specific pro-inflammatory CD4+ T cells which are activated by virus epitopes presented by CNS-resident macrophages, microglia and dendritic cells (DCs). Disease presents first as a gait disturbance that steadily increases in severity and progresses to incontinence and paralysis. Encephalitogenic CD4+ T cells specific for the immunodominant epitope on myelin proteolipid protein (PLP139–151) arise via epitope spreading in mice 2–4 weeks after the onset of demyelination, and immune responses to additional encephalitogenic myelin epitopes arise in an ordered fashion as disease progresses. These myelin-specific autoreactive T cells are activated directly in the inflamed CNS (McMahon et al.) and play a major role in mediating chronic pathology of TMEV-IDD.
Other strains of mice, such as C57BL/6 (B6) mice, are resistant to this demyelinating disease and efficiently clear the virus from the CNS within 2 weeks of intracranial inoculation. Since disease resistance in part maps to the H-2D MHC class I locus, the CD8+ T cell response to TMEV has been extensively studied (Bureau et al., 1998; Rodriguez and David, 1985; Rodriguez et al., 1986). In some studies, B6 mice that are genetically deficient in or have been depleted of CD8+ cells variably develop mild signs of clinical disease (Palma et al., 2001), though most mice become susceptible to demyelination but not overt disease (Fiette et al., 1993; Pullen et al., 1993; Rivera-Quinones et al., 1998; Rodriguez et al., 1993). In SJL mice, some studies have yielded similarly contradictory results, though most tend to indicate that a lack of CD8+ cells results in an earlier onset/more severe disease (Begolka et al., 2001; Borrow et al., 1992; Murray et al., 1998). The tentative association between the presence of functional CD8+ T cells and resistance to TMEV-IDD has led to direct comparison of CD8+ T cell responses to TMEV in SJL and B6 mice. It is interesting to note that though these responses have some qualitative differences, they are quantitatively quite similar (Lyman et al., 2004). Thus, the reasons for the differential ability of the two strains to clear virus is not clear. For both strains of mice, the CD8+ T cell response to TMEV consists of one immunodominant epitope and two subdominant epitopes. In SJL mice, the hierarchy of immunodominance is as follows: VP3159–166 > VP3173–181 > VP111–20 (Kang et al., 2002).
Chronic infections in humans, such as HIV, Hepatitis viruses, and human Herpes viruses, pose major public health risks. Vaccines and treatments for humans, including those designed to utilize the host’s natural CD8+ T cell response against the virus, have been investigated but have so far been largely disappointing (Letvin, 2006; Michel and Mancini-Bourgine, 2005; Riddell et al., 1996). In the current report, we used TMEV infection of SJL mice as a model of chronic virus infection leading to organ-specific autoimmunity and determined that the CD8 T cell response to the immunodominant TMEV VP3159–166 epitope, which is normally unable to clear the virus, is in fact completely dispensable during the normal immune response to the virus. However, adoptive transfer of activated VP3159–166-specific CD8+ T cells leads to complete clearance of CNS virus and subsequent protection from clinical TMEV-IDD.
Results
Tolerance to the immunodominant CD8 T cell epitope does not accelerate onset of clinical TMEV-IDD in SJL mice
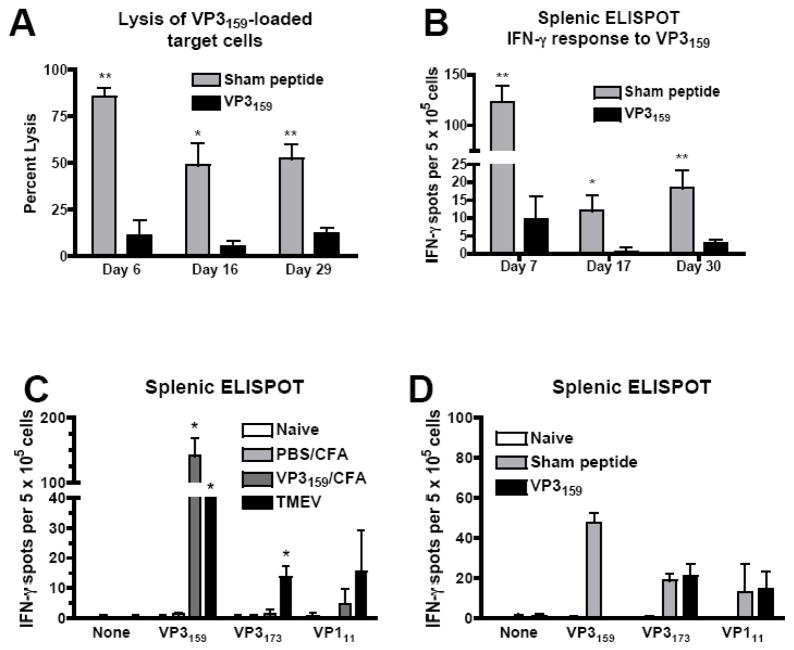
Past studies from our laboratory and others have indicated that in SJL mice lacking CD8+ cells, TMEV infection can lead to a disease of earlier onset and/or enhanced severity (Begolka et al., 2001; Borrow et al., 1992; Murray et al., 1998). However, no study to date has explored the CD8+ T cell response to this virus precisely enough to determine the extent of the role of responses to the immunodominant CD8+ T cell epitope. Our initial goal was to determine whether a lack of only the immunodominant TMEV CD8+ T cell epitope (VP3159–166) at the time of infection would lead to an enhancement of disease and viral persistence similar to that seen in studies in which the entire CD8+ cell population was absent. To approach this question, we needed to first establish a model by which we could specifically suppress this response without affecting other responses or causing undesirable side effects. To determine if the CD8+ T cell response to VP3159–166 could be prevented using the injection of soluble peptide i.v., we treated naïve SJL mice with 100 μg of VP3159–166 in PBS seven days prior to TMEV infection. On day 6–7 p.i., we performed in vivo lysis assays and splenic IFN-γ ELISPOTs to determine if this procedure prevented CD8+ T cell responses. Both the lytic response to VP3159–166 (Fig. 1A) and IFN-γ production in response to VP3159–166 (Fig 1B) were completely inhibited by this treatment. Since virus persists in SJL mice indefinitely, and therefore there is a constant supply of antigen available for T cell activation, we were also interested in whether tolerance of the CD8+ T cell response by this method would persist for several weeks. We performed IFN-γ ELISPOTs and in vivo lysis assays at approximately 2 and 4 weeks p.i. in mice that had received soluble VP3159–166 or sham peptide. While the total VP3159–166-specific CD8+ T cell response in sham peptide-treated mice waned considerably over 4 weeks p.i., the VP3159–166-specific responses in peptide-tolerized remained at background levels (Fig. 1A,B).
Figure 1.
The immunodominant response to TMEV in SJL mice is specifically inhibited by tolerance induced by i.v. injection of soluble peptide prior to virus infection. (A, B, and D) SJL mice were injected i.v. with 200 μl of PBS containing 100 μg of VP3159–166 or sham peptide (VP2121–130) 7 days prior to i.c. infection with TMEV. (A) On days 6, 16, and 29 p.i., in vivo lysis assays were carried out to address the capacity of CD8 T cells to lyse VP3159–166-loaded target cells. ELISPOTs were performed to determine the frequency of splenic IFN-γ responses to VP3159–166 on days 7, 17, and 30 p.i. (B). For panel (C), naïve mice, mice primed with PBS/CFA, mice primed with VP3159–166/CFA, and mice infected with TMEV were assessed for their IFN-γ responses to VP3159–166 and the subdominant VP3173–181 and VP111–20 epitopes by splenic ELISPOT on day 7 p.i. Specificity of tolerance was tested by assessing responses to the three SJL TMEV epitopes at day 7 p.i. in VP3159–166 tolerized mice (D). All data are representative of at least 3 separate experiments containing 3–5 mice per group. Percent lysis or IFN-γ production is significantly greater than appropriate control: *p<0.01; **p<0.001.
To ensure that tolerance with soluble peptide was VP3159–166-specific, we examined peptide-specific CTL responses in response to both infection with TMEV and priming with an emulsion containing VP3159–166 and CFA. Strong VP3159–166-specific IFN-γ ELISPOTS responses were elicited in both groups, while only TMEV infection elicited a significant responses to the sub-dominant CD8+ T cell epitopes, VP3173–181 and VP111–20 (Fig. 1C). Importantly, tolerance induced by i.v. injection of soluble VP3159–166 was shown to be peptide specific, as ELISPOT responses to only the cognate peptide were suppressed in mice infected with TMEV (Fig. 1D).
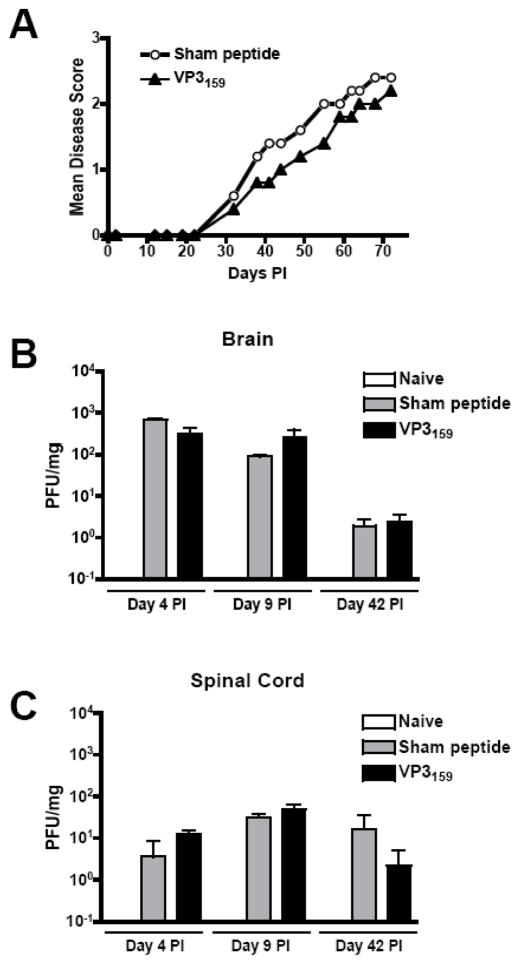
Our prediction was that in SJL mice, the lack of this immunodominant virus-specific CD8+ T cell response would impair viral clearance and accelerate or exacerbate TMEV-IDD. We monitored disease progression in SJL mice that had received 100 μg of VP3159–166 or sham peptide 7 days prior to TMEV infection. Surprisingly, there were no significant differences in the time of onset or magnitude of clinical disease score between these two groups (Fig. 2A). Viral clearance in the CNS was also unaltered by the lack of VP3159-specific cells, as mice that received soluble VP3159–166 6 days before TMEV infection had similar CNS viral titers as those that received sham peptide. This was true both at early time-points p.i. during the acute phase of CD8+ T cell activation, and at later time-points during ongoing disease (Fig. 2B,C). Therefore, the VP3159-specific response elicited by TMEV infection appeared to not play a significant role in the control of the viral infection in CNS tissue or in protection from TMEV-IDD.
Figure 2.
Abrogation of the immunodominant CTL response to TMEV in SJL mice does not influence the initiation or progression of clinical disease or the CNS viral titer. SJL mice were injected i.v. with 100 μg VP3159–166 or sham peptide in PBS 7 days prior to TMEV infection. (A) Mice were monitored for disease progression as outlined in the materials and methods for at least 75 days. Data are representative of 4 separate experiments containing at least 5 mice per group per experiment. At early (days 4 and 9 p.i.) and late (day 42 p.i.) time-points, brain (B) and spinal cord tissues (C) were harvested from 3 mice per group and assessed for the presence of replicating virus by plaque assay. Data are representative of 3 separate experiments at similar time-points. No significant difference between sham peptide and VP3159–166 recipient mice was observed at any time point in terms of disease score or CNS viral titer.
TMEV-specific CTL responses arise earlier in TMEV-resistant C57BL/6 mice than in TMEV-susceptible SJL mice
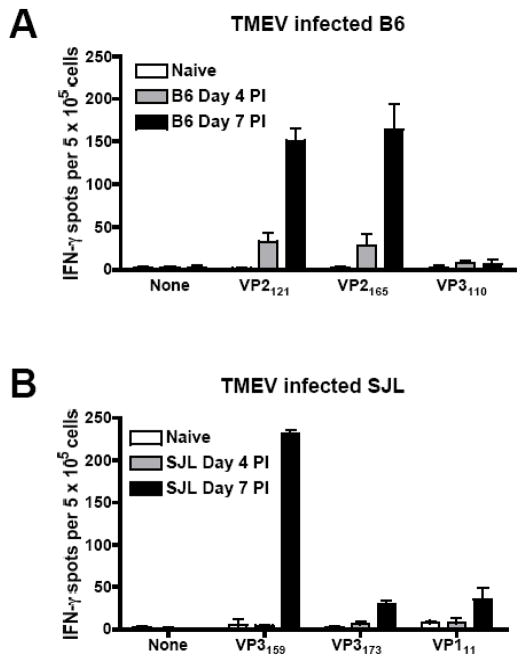
Since a lack of the CD8+ CTL response to the immunodominant T cell epitope had no effect on the progression of clinical disease or viral clearance, we considered the possibility that VP3159-specific cells are completely devoid of viral clearing capability and unable to provide any protection from disease. This idea was counter-intuitive, however, considering the fact that this CD8+ T cell response is elicited quite strongly by TMEV infection. In fact, it has been reported that the CD8+ T cell response to TMEV in SJL mice is similar in magnitude and CNS homing capability to that in TMEV-resistant B6 mice (Kang et al., 2002; Lyman et al., 2004). The TMEV-specific B6 response has been documented to be a particularly strong anti-viral response and, based on our previous studies, does influence the level of viral titer in the B6 CNS and the speed with which the virus is cleared (Getts and Miller, 2007). It has previously been shown that at day 5 p.i., virus-specific CD8+ T cells are present at a higher percentage in B6 mice than in SJL mice (Lyman et al., 2004). We thought it possible that the TMEV-specific response occurs earlier in B6 mice than it does in SJL mice, and that this plays an important role in the susceptibility of SJL mice to TMEV-IDD. To test this possibility, we compared B6 and SJL peripheral CD8+ T cell responses to TMEV infection on days 4 and 7 p.i.. We found that the B6 response to TMEV is in fact detectable at day 4 p.i., a time-point at which it is not yet detectable in SJL mice (Fig. 3).
Figure 3.
TMEV-specific CD8+ T cell response are elicited earlier in TMEV-resistant B6 mice compared to TMEV-susceptible SJL mice. B6 (A) and SJL (B) mice were infected i.c. with TMEV. On days 4 and 7 p.i., mice were sacrificed and splenic IFN-γ ELISPOTs were performed to determine the frequency of CTL responses to the relevant immunodominant and subdominant B6 or SJL TMEV CD8+ T cell epitopes (A and B, respectively). Data are representative of 3 mice per time-point in 3 separate experiments.
These data led us to hypothesize that the SJL CD8+ T cell response to TMEV may be potent enough to mediate viral clearance and protection from TMEV-IDD, but may not be elicited early enough after infection to control the viral load before it reaches a critical point beyond which the virus cannot be cleared.
Transfer of activated VP3159–166-specific T cells protects SJL mice from development of clinical and histologic signs of TMEV-IDD by facilitating CNS virus clearance
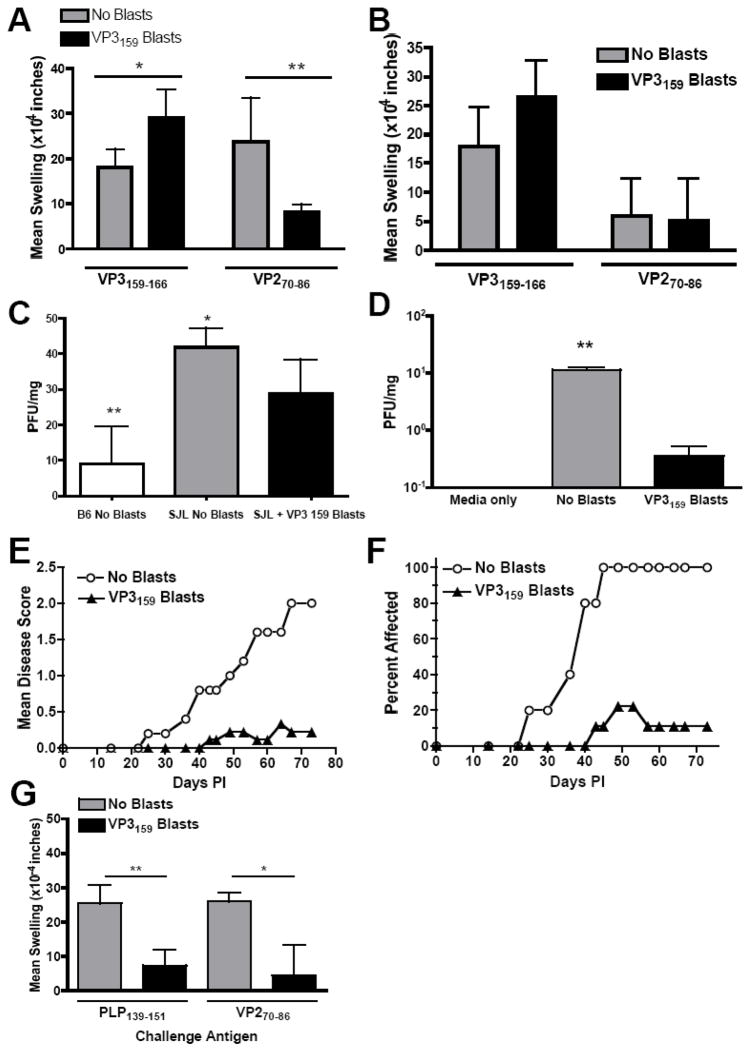
To address the hypothesis that an anti-viral CD8+ T cell response that is present earlier in infection may allow better control of TMEV infection in SJL animals, we asked if adoptive transfer of activated VP3159-specific T cell blasts could lead to virus clearance and protection from TMEV-IDD. Since priming SJL mice with the VP3159–166/CFA emulsion elicits higher numbers of peripheral CD8+ T cell responders to VP3159–166 than can be achieved with TMEV infection (Fig. 1C), and since tetramers are not available for the SJL strain of mouse, we chose to prime rather than infect mice to generate VP3159–specific cells. Six days after VP3159–166/CFA priming, lymph node cells were cultured with 50 μM VP3159–166 and IL-2. After 4 days in culture, 2×106 blasts were injected i.v. into SJL mice that had been infected with TMEV 2 days prior. To determine what effect the transfer of VP3159 blasts early in infection has on virus-specific CD4+ and CD8+ T cell responses during the acute phase of viral clearance, DTH responses against both the CD8+ immunodominant epitope (VP3159=166) and the CD4+ immunodominant epitope (VP270–86) were measured at days 7 and 14 p.i. Mice that received VP3159 blasts at day 2 p.i. had an increased CD8+ T cell response at both time points (Fig. 4A & B). The response to the viral CD4+ epitope VP270–86 was reduced in VP3159 blast recipient mice at day 7 p.i., (Fig. 4A), and was equivalent to that of mice that did not receive blasts at day 14 p.i., (Fig. 4B).
Figure 4.
Transfer of VP3159–166-specific T cell blasts increases early anti-viral CD8+ T cell responses, reduces CNS virus persistence, and protects SJL mice from the development of TMEV-IDD. Mice were administered 2×106 VP3159–166 specific blasts in PBS on day 2 post TMEV infection. DTH responses to the dominant viral CD4+ (VP270–86, left ear) and CD8+ (VP3159–166, right ear) epitopes were measured at days 7 (A) and 14 (B) p.i. Data are represented as mean ear swelling ± SEM and are representative of 4 mice per group; *, p<0.03, **p<0.02. (C) Mice were sacrificed on day 14 p.i. and assessed for viral titer in brain tissue by plaque assay. Data are represented as mean pfu/mg of tissue ± SEM and are representative of 3 mice per group. *, p<0.07 between blast recipient SJL and control SJL mice, between B6 animals and blast recipient SJL mice **, p<0.02. (D) On day 75 p.i., 3 mice per group were sacrificed and assessed for viral titer in brain tissue by plaque assay. CNS virus was essentially undetectable (fewer than 1 PFU/mg tissue) in the recipients of VP3159–166-specific blasts as compared PBS treated controls: *, p<0.001. Clinical disease was monitored by mean clinical disease score (E) and percentage of mice displaying clinical symptoms (F) for at least 75 days p.i. Data are representative of 5 experiments of 5–10 mice per group. Blast recipient scores and percent affected over the course of disease were significantly lower than control group, p<0.001. DTH in response to the dominant CD4 myelin autoepitope PLP139–151 (left ear) or the dominant TMEV CD4 epitope VP270–86 (right ear) was performed on day 75 p.i. (G). Data are presented as mean ear swelling ± SD and are representative of 2 separate experiments of 5 mice per group. Responses to both the dominant myelin and TMEV CD4 epitopes were significantly lower in VP3159–166 blast recipients than PBS treated controls: *, p=0.01; **, p=0.002.
We next sought to determine what effect the increased antiviral CD8+ T cell response had on virus persistence and the development and progression of TMEV-IDD. B6 mice, which clear TMEV from the CNS approximately 2 weeks after infection (Lipton and Jelachich, 1997), were used as a reference to determine whether the addition of VP3159 blasts early in infection would lead to similar clearance rates of TMEV in SJL mice. CNS tissue taken at day 14 p.i. from TMEV infected B6 mice, TMEV infected SJL mice, and TMEV infected SJL mice that had received VP3159 specific blasts at day 2 p.i. showed that as expected, B6 mice had significantly lower viral load compared to SJL mice that had not received VP3159 blasts, as two out of three B6 mice had cleared the virus completely from the brain and the third mouse had a four fold lower titer compared to the TMEV infected SJL group. (Fig. 4C). At this time point, SJL mice that had received blasts exhibited lower viral loads compared to mice that had not received blasts, but no mice in this group had undetectable levels of virus. Therefore, the transfer of VP3159 blasts results in an increased rate of viral clearance, but not to the same rate as that of the resistant B6 strain. However, transfer of VP3159–161 blasts led to essentially total clearance of CNS virus measured at 75 days p.i., in stark contrast to control TMEV infected SJL mice that had not received blasts, which exhibited a significant amount of CNS virus at this time point (Fig. 4D).
To determine whether the increase in viral clearance translated to a protective effect against TMEV-IDD, mice were monitored for clinical signs of disease for 75 days. Transfer of VP3159–166 blasts afforded nearly complete protection from the development of TMEV-IDD (Fig. 4E). In each experiment, approximately 20% of VP3159–166 blast recipients broke through and exhibited clinical signs of TMEV-IDD; however, disease in these mice was of later onset and much less severe compared to control TMEV infected animals, and was in some cases transient (Fig. 4F). Concomitant with protection from clinical disease, responses to the immunodominant TMEV CD4+ T cell viral epitope (VP270–86) and the immunodominant CD4+ T cell myelin epitope (PLP139–151) were also significantly reduced in recipients of VP3159 blasts compared to control TMEV infected mice on day 77 p.i., indicating that the activity of VP3159–166 blasts early in infection resulted in the resolution of the viral infection and therefore a decline in the virus-specific T cell response and the prevention of spreading to the myelin-specific autoimmune response (Fig. 4G).
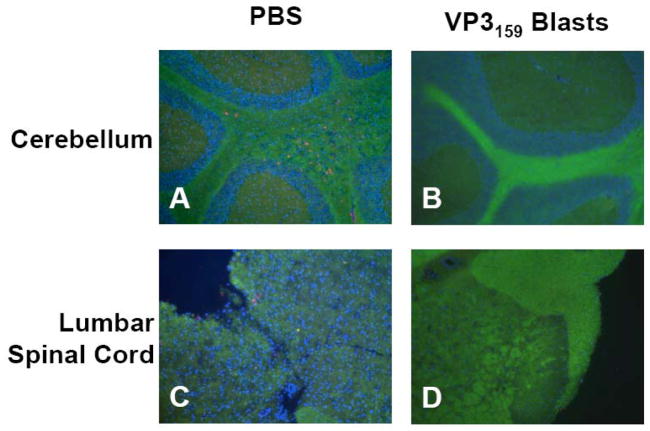
Lack of CNS inflammation and demyelination correlated with lowered clinical disease, as control mice had distinct demyelination in brain and spinal cord tissue as well as considerable infiltration of CD4+ T cells at day 75 p.i. (Fig. 5A,C). At this time-point, VP3159–166 blast recipients at a score of 0 did not exhibit any T cell infiltration or evidence of demyelination in the brain or spinal cord (Fig. 5B,D). Those few VP3159–166 blast recipients that did break through and develop low disease scores had correspondingly low levels of CD4+ T cell infiltration and demyelination in CNS tissues (data not shown).
Figure 5.
Transfer of VP3159–166 specific T cell blasts protects SJL mice from CNS demyelination and CD4+ T cell infiltration. SJL mice were infected with TMEV on day 0, then mice were injected i.v. with PBS (A, C) or 2×106 VP3159–166-specific T cell blasts (B, D) on day 2 p.i. On day 75 p.i., 5 mice per group were sacrificed and brain and spinal cord tissues were harvested and frozen in liquid nitrogen. Sections were cut from frozen tissues and stained with a fluoromyelin stain (green) and anti-CD4 (red), then counterstained with DAPI (blue). Representative cerebellum (A, B) and lumbar spinal cord (C, D) tissue sections are shown. The PBS treated mouse shown was at a score of 3 at the time of sacrifice. Both CD4+ cells and a lack of myelin staining are evident in both cerebellum and spinal cord tissue (A, C). The VP3159–166 T cell blast recipient pictured was at a score of 0 and shows intact myelin and a lack of CD4+ T cell infiltration (B,D).
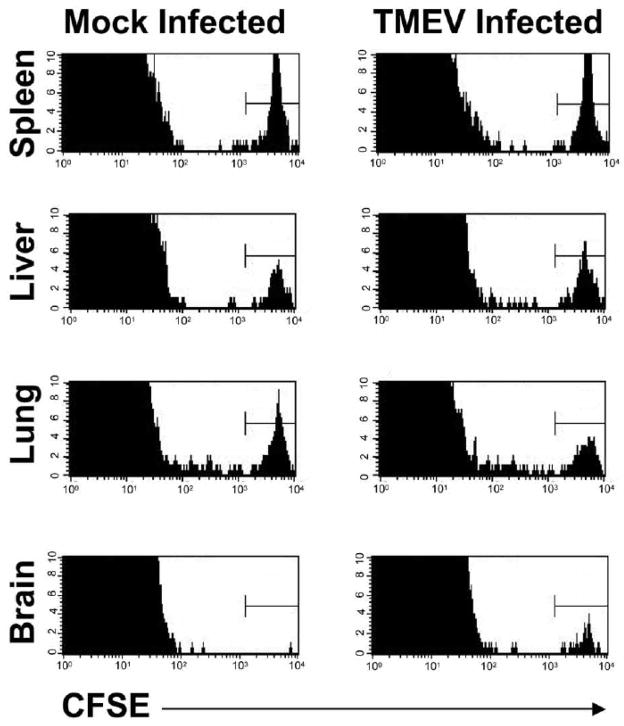
Since the VP3159–166 CD8+ T cell blasts were transferred into SJL mice two days after i.c. TMEV infection, we hypothesized that these transferred CTLs would have to enter the CNS to explain the fact that mice that are administered VP3159 blasts are able to clear persistent CNS virus when the endogenous VP3159 response is not able to do so. Indeed, two days post i.v. transfer, CFSE-labeled VP3159–166-specific T cell blasts were demonstrable in the spleen, liver, and lung tissue of both TMEV-infected and mock-infected recipients, but were present in the brains of only virus-infected, and not mock-infected, mice (Fig. 6).
Figure 6.
VP3159–166-specific T cell blasts are retained in the CNS of TMEV-infected, but not mock-infected, SJL mice. VP3159 blasts were labeled with 2μM CFSE prior to transfer into SJL mice that were mock-infected or infected with TMEV 2 days previously. 2 days post-transfer (day 4 p.i.), mice were sacrificed and spleen, liver, lung, and brain tissues were harvested, and infiltrating cells analyzed by flow cytometry for CFSE+ cells. For each histogram, organs from 3 mice per group were pooled. Data are representative of 3 separate experiments.
Taken together, these data indicated that the absence of the VP3159-specific CD8+ T cell response does not lead to an increase in viral titer or disease, but that the transfer of VP3159 specific blasts into SJL mice at day 2 p.i. results in increased CD8+ DTH responses, increased viral clearance, and protection from TMEV-IDD. The fact that B6 animals have a detectable response to TMEV at least 2 days earlier than SJL mice suggested to us that the timing of the generation and arrival in the CNS of the anti-TMEV CD8+ T cell response may play an important role, and may help us to reconcile these apparently contradictory results. We hypothesize that the immunodominant CD8+ T cell response in SJL mice is capable of contributing to viral clearance, but is not generated early enough after infection to control the virus, and therefore disease development.
Protection from TMEV-IDD in susceptible SJL mice requires transfer of CD8+ T cells specific for the immunodominant VP3159–166 epitope early after infection
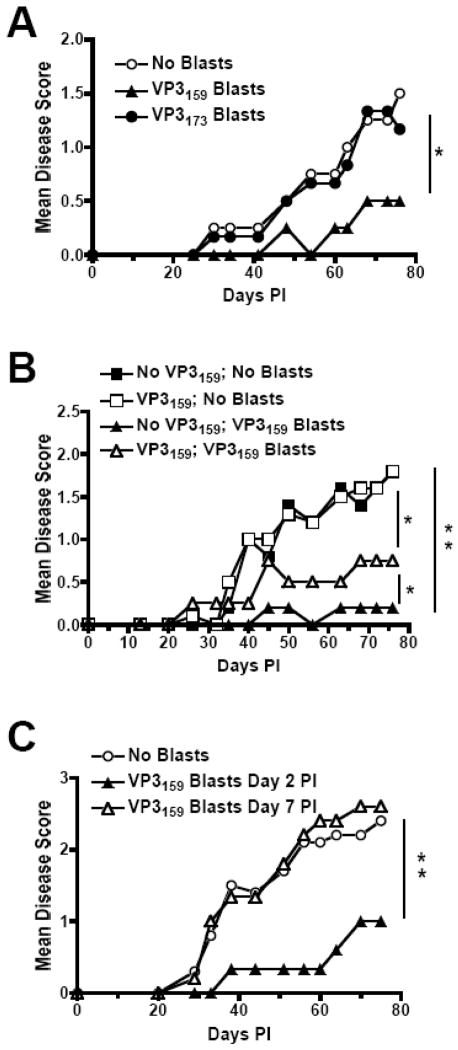
Of the two sub-dominant SJL TMEV CD8+ T cell epitopes, one (VP111–20) is of extremely low strength, while the other (VP3173–181) induces a strong IFN-γ response as measured by IFN-γ ELISPOT (Fig. 1D) and a relatively strong lytic response as measured by in vivo lysis (data not shown). We asked whether activated CD8+ T cells specific for the subdominant VP3173–181 epitope could similarly protect SJL mice from TMEV-IDD. Unlike VP3159–166-specific CD8+ T cells, transfer of 2×106 VP173–181-specific T cells provided no protection from development of clinical disease in TMEV-infected SJL mice (Fig. 7A).
Figure 7.
CD8+ T cell blast-mediated protection from TMEV-IDD requires specificity for the immunodominant CD8 epitope, the endogenous TMEV-specific response, and early administration following TMEV infection. For disease courses, SJL mice were infected with TMEV on day 0 and monitored for clinical signs of TMEV-IDD for 75 days p.i. (A) Mice were administered 2×106 VP3159–166 or VP3173–180 blasts in PBS or PBS alone on day 2 p.i. (B) Seven days prior to TMEV infection, SJL mice were administered 100 μg of soluble VP3159–166 i.v. to deplete the endogenous VP3159-specific response, or PBS as a control. 2 days p.i., the two groups of mice (depleted and non-depleted) were divided into 4 equal groups and mice were administered either VP3159 blasts or PBS. (C) SJL mice were administered VP3159–166 blasts on either day 2 or day 7 p.i. For each graph, data are representative of 3 experiments of at least 5 mice per group. Blast recipient groups disease course significantly less than appropriate No Blast controls or indicated group: *, p<0.05; **, p<0.005.
To determine whether endogenous VP3159–166-specific CTLs were required for clearance of CNS-persisting virus, 2×106 activated VP3159–166-specific T cells were transferred into TMEV-infected SJL mice whose endogenous response had been tolerized to the VP3159–166 peptide two weeks previously. We found that VP3159–166 blasts were still protective in mice without functional endogenous VP3159-specific cells, but protection was not as complete as when the endogenous population was intact, suggesting that the number of antigen-specific cells present, or the later arrival of these endogenous cells, is an important factor in VP3159–166-dependent protection (Fig. 7B). These data also show that the endogenous population of VP3159-specific cells is functional, and not dispensable in this adoptive transfer scenario, unlike under the normal circumstances of TMEV infection in SJL mice.
Finally, we investigated when during the course of infection that adoptively transferred VP3159–166-specific CTLs were required to mediate protection from TMEV-IDD. VP3159–166-specific blasts were protective when transferred at day 2 p.i., but provided no protection when injected on day 7 p.i. (Fig. 7C), the time point at which the endogenous TMEV-specific response reaches its peak in SJL mice. This observation indicated that the blasts must be provided early after infection, and prior to the peak of the naturally occurring response, to offer any protection from the onset of TMEV-IDD.
Discussion
The CD8+ cytolytic T cell response to TMEV in SJL mice has been shown to be relatively robust as indicated by measurements of IFN-γ production and lytic capacity, yet the response is incapable of clearing virus, allowing establishment of a life-long persistent infection and resulting in the induction of a chronic CNS demyelinating disease via epitope spreading (Ercolini and Miller, 2009; Munz et al., 2009). We sought to define the anti-viral capacity of the CD8+ T cell response in TMEV-susceptible SJL mice to the immunodominant VP3159–166 epitope. Surprisingly, specific inactivation of the response to the immunodominant VP3 epitope in peptide tolerized mice had no effect on disease course or viral load (Figs. 1 and 2). This result was interesting in light of the fact that past experiments have shown that SJL mice lacking the entire CD8+ cell population (which would include CD8+ cells of any type including CD8α+ dendritic cells), or more specifically lacking functional CD8+ cytolytic T cells, are susceptible to an earlier onset and/or more severe form of TMEV-IDD (Begolka et al., 2001; Borrow et al., 1992; Murray et al., 1998). These results appear to indicate that the VP3159–166-specific CD8+ T cell response does not contribute significantly to the protection conferred by the CD8+ T cell response as a whole. Therefore, we reasoned that the CD8+ T cell-mediated protection evidenced by past studies must be mediated by one or several other CD8+ T cell populations directed against one or both of the two known sub-dominant epitopes. However, this possibility seems unlikely due to the minimal nature of the VP111–20-specific response and the fact that VP3173–181-specific blasts were not protective upon adoptive transfer to SJL mice two days p.i. (Fig. 7). Alternatively, or perhaps additionally, it could be the activity or lack thereof of some other CD8+ T cell population, such as a population of regulatory cells, that leads to enhanced disease in CD8-deficient SJL mice. As precedent for this possibility, BALB/cByJ mice, which are resistant to TMEV, have been shown to possess a population of CD8+ T cells that mediates protection not by enhancing viral clearance, but by directly suppressing auto-reactive CD4+ T cell responses (Haynes et al., 2000; Nicholson et al., 1999). Whether a similar CD8+ regulatory population exists in SJL mice is currently not known.
It was especially surprising that specific tolerization of the VP3159–166-specific response had no effect on TMEV titers in infected SJL mice. It was once thought that the TMEV-specific CD8+ T cell response in SJL mice was far inferior to that in B6 mice. However, a direct comparison of the CD8+ T cell responses in these strains of mice revealed that, in fact, the responses in SJL and B6 mice were quantitatively comparable, and both are strongly activated in the CNS (Lyman et al., 2004). Therefore, we had expected that the absence of this virus-specific CD8+ T cell response, which is high frequency, functionally effective in lysing virus-infected target cells, and capable of migrating to the site of infection, would have obvious effects on the viral load of the CNS, if not in the development of TMEV-IDD. Our observation that the CD8+ T cell response to TMEV is mounted earlier in resistant B6 mice as compared to susceptible SJL mice provided a possible explanation for this discrepancy in virus clearance and ultimate disease susceptibility between the two strains (Fig. 3).
Surprisingly, we found that although the absence of the VP3159-specific CD8+ T cell response had no effect on viral clearance or TMEV-IDD outcome, these parameters could be drastically affected by boosting the same CD8+ T cell response through supplying VP3159-specific CD8+ T cell blasts early after infection. The temporal aspect of the CD8+ T cell response may explain these apparently contradictory findings. In B6 mice, the CD8+ T cell response is elicited early after infection and is capable of eradicating the virus before it becomes overwhelming. Our findings suggest that the TMEV-specific CD8+ T cell response in SJL mice is less able to clear TMEV compared to B6 mice because the response in SJL mice is not elicited early enough to control the infection, allowing eventual development of disease. Therefore, absence of the VP3159 response may have little effect due to its inability to arrive in the CNS in time to control viral replication, but the early boost of this response may have a significant effect on early control of viral replication, resulting in better viral clearance and protection from the development of TMEV-IDD. Studies to further test this hypothesis are ongoing.
The endogenous VP3159–166-specific population appears to be required for optimal protection. We however did not address the possibility that the requirement for the presence of the endogenous CTLs could be overcome by the injection of higher numbers of blasts on day +2 p.i. It is also possible that the endogenous VP3159–166-specific population comes into the CNS as a second wave attack on the virus after the adoptively supplied T cell blasts have performed their function and died.
Consistent with a crucial link between early temporal appearance of TMEV-specific CTL activity and effective clearance of CNS-persistent virus, we showed that peripheral adoptive transfer of 2×106 activated VP3159–166-specific T cells on day +2, but not on day +7 p.i., resulted in drastically reduced viral titers and highly significant protection from both the clinical and histopathologic signs of TMEV-IDD (Figs. 4, 5 & 7). Complete protection from TMEV-IDD occurred in 80% of virus-infected SJL mice receiving activated VP3159–166-specific T cell blasts, and mice that did not develop signs of clinical disease were also free of T cell infiltration and demyelinating lesions in the CNS. In the remaining 20% of mice, a later onset, significantly less severe form of TMEV-IDD occurred, with lesser CNS inflammation and demyelination correlating with reduced clinical disease. Additionally SJL mice receiving VP3159–166-specific T cell blasts cleared virus from the brain earlier than untreated animals (Fig. 4). Early temporal appearance of TMEV-specific CTL activity also allowed for an increase in the DTH response to the CD8+ immunodominant epitope VP3159–166 early after infection. Interestingly, the CD4+ T cell anti-viral response was decreased early after infection (Fig. 4). Since the VP3159–166 CTLs were provided on day 2 p.i., we predicted that they would likely be recruited to and retained in the inflamed, virus-infected CNS. Indeed, CFSE-labeled VP3159 blasts were detectable in the brain 2 days post-transfer in mice infected with TMEV, but could not be detected in brains of mock-infected mice (Fig. 5). Therefore, these cells were retained in the CNS by the presence of the viral infection and their presence in the site of virus persistence led to essentially total clearance of the infection (Fig. 4).
In addition, long after the initial infection (on day 77 p.i.), SJL recipients of VP3159–166 T cell blasts displayed significantly lower DTH responses to the immunodominant TMEV CD4 T cell VP270–86 epitope, which was anticipated based on the decreased viral load at this time-point and therefore lack of antigen for maintaining virus-specific T cell responses. More important to chronic CNS demyelinating pathology, VP3159–166 T cell blast recipients had a significantly diminished DTH response to PLP139–151 than control mice. This endogenous myelin peptide is the immunodominant CD4+ T cell myelin epitope in SJL mice, and is the first and most important of the autoreactive responses triggered by epitope spreading that characterize the chronic autoimmune phase of TMEV-IDD (McMahon et al., 2005; Miller et al., 1997; Munz et al., 2009; Neville et al., 2002).
To our knowledge, this is the initial observation that the immunodominant CD8+ T cell response to TMEV in SJL mice is by itself capable of protecting SJL mice from TMEV-IDD by mediating CNS virus clearance. Collectively, our data strongly suggest that when VP3159–166 blasts are provided to SJL mice early after i.c. infection, these cells are able to clear the viral infection, vastly reducing the amount of inflammation in the CNS. This rapid resolution of the virus infection prevents early myelin damage and the later activation of myelin-specific T cells via epitope spreading, reducing or preventing autoimmune-mediated demyelination and protecting mice from the chronic stages of clinical TMEV-IDD. Interestingly, our results indicate that a virus-specific CD8+ T cell response that does not normally significantly contribute to the control of viral load or protection from virus-induced disease can be enhanced to provide complete protection. This has interesting implications related to the use of amplified specific CD8+ T cells in humans, as therapies against acute and chronic viral infections as well as tumors. Tumor-specific CD8+ T cells are often rendered tolerant within hosts (Frey and Monu, 2006). In several studies, investigators have attempted to reverse tolerance or overcome the immunosuppressive local environment of a tumor by supplying tumor-specific CD8+ T cells (Dudley and Rosenberg, 2003). Studies comparing injection of CD8+ T cells at varying stages of differentiation revealed that the less differentiated the cells, the more protection is provided against tumors in mice (Gattinoni et al., 2005). The cells used in our studies may correspond to the least differentiated activated CD8+ T cells in this study, as they were subjected to only one round of in vitro stimulation, suggesting that the requirement for minimal differentiation extends to CD8+ T cell responses to viral infection.
Chronic viral infections in humans, such as HIV, hepatitis viruses, and human herpes viruses, induce CD8+ T cell responses, but these are insufficient for viral clearance (Goulder et al., 2001; McMichael, 1998; Webster and Bertoletti, 2001). In contrast to tumor studies, few studies have been published in which antigen-specific CD8+ T cells transfers are used for antiviral responses, though in one study the reactivation of CMV following bone marrow transplant could be prevented by the administration of CMV-specific CD8+ T cell clones isolated from the bone marrow donor (Walter et al., 1995). Many studies have attempted to utilize other methods of amplifying host anti-HIV CD8+ T cell responses, including the use of various methods of targeting antigen to DCs, which in turn prime endogenous CD8+ T cells in infected hosts [reviewed in (Walsh et al., 2003)]. Our approach, which is a more direct way of using the host’s endogenous CD8+ T cell response, may provide a new method to accomplish adoptive immunotherapy. Immunodominant viral epitopes from an individual could be determined and that individual’s virus-specific CD8+ T cells could be amplified ex vivo as we have described, and re-administered to the individual to achieve effective pathogen-specific immunotherapy. A caveat to this approach was illustrated in our system by the necessity that the activated VP3159–166-specific T cells be transferred early after infection to be effective against viral and autoimmune pathogenesis. Thus, this approach may have limitations and may not be suitable for long-established chronic infections in humans. Regardless, our approach may be well suited to supplement vaccine therapies, and/or anti-tumor therapies, although further research will be required to confirm and extend these initial observations.
Materials and methods
Mice
SJL mice were purchased from Harlan Laboratories, Bethesda, MD. C57BL/6 mice were purchased from Jackson Laboratories, Bar Harbor, ME. All mice were housed in the Center for Comparative Medicine, Northwestern University, Chicago IL, under the guidelines of the Animal Care and Use Committee (ACUC).
Peptides
All synthetic peptides were obtained from Genemed Synthesis, San Francisco, CA. These included TMEV peptides VP2121–130 (FHAGSLLVFM), VP2165–173 (TGYRYDSRT), VP3159–166 (FNFTAPFI), VP3173–181 (QTSYTSPTI), VP111–20 (SNDDASVDFV), VP3110–120 (NFLFVFTGAAM), and VP270–86 (WTSQEAFSHIRIPLP), as well as PLP139–151 (HSLGKWLGHPDKF). All peptides were purified by HPLC and were certified to be ≥95% pure.,
TMEV inoculation and evaluation of clinical disease
For all TMEV infections, strain BeAn 8386 was used. Mice were infected by i.c. injection with 4.0 – 10 × 106 PFU of BeAn in a volume of 30μL serum-free Dulbecco’s Modified Eagle’s Medium (DMEM). For long-term experiments, mice were monitored every 3 to 4 days and scored for clinical disease severity. For TMEV infected SJL mice, the grading scale was as follows: 0, asymptomatic; 1, mild to moderate waddling gait; 2, moderate to severe waddling gait with extensor spasms; 3, extreme difficulty walking, impaired righting reflex; 4, paralysis of 1 or more limbs, inability to walk; 5, moribund. All mice were monitored daily and afforded easier access to food and water once their symptoms became severe.
TMEV plaque assay
Mice were anesthetized with 50 mg/kg Nembutal and perfused with 30–50 ml of PBS through the left ventricle. Spinal cords and brains were collected from perfused mice, weighed, and homogenized on ice using a Polytron System PT1200C tissue homogenizer (Kinematica AG, Switzerland). Homogenates were serially diluted and added to tissue culture treated plates (Nunc, Roskilde, Denmark) of confluent BHK-21 cells for a 1 h incubation at room temperature, with periodic gentle rocking. Cells were then covered with a media/agar solution containing 1% Noble Agar (BD, Sparks, MD) and 2X DMEM. Following a 5 day incubation at 34°C, formalin (Fisher Scientific, Fair Lawn, NJ) was added and incubated at room temperature for 1–2 hours to fix the BHK monolayer. The agar was removed and plaques were visualized by staining with crystal violet. To determine PFU/ml homogenate, the number of plaques on each plate was multiplied by the dilution factor of the homogenate and divided by the amount of homogenate added per plate. The PFU/ml was divided by the weight of the tissue to calculate PFU/mg tissue.
Soluble peptide tolerance
Mice were injected intravenously via the lateral tail vein with 100 μg of the indicated peptide in 200 μl PBS, 6 or 7 days prior to TMEV infection.
In vivo cytolysis assay
Splenocytes were collected from naive animals, treated with NH4Cl to remove red blood cells, and divided into two populations. Each population was pulsed with either cognate or irrelevant peptide, then the two populations were labeled with differential concentrations of 5-carboxyfluorescein diacetate succinimidyl ester (CFSE). These two populations were mixed together and injected in equal numbers into immune (infected) or naïve animals, at 6 to 10×106 total cells per mouse. After 18 to 24 h, spleens were collected from recipients and analyzed by flow cytometry on a BD LSRII for the presence and relative numbers of cells in each CFSE peak. Two equations were used to determine the percent lysis. The adjustment factor (A) was calculated by dividing the percent of cells loaded with irrelevant peptide by the percent of cells loaded with cognate peptide in naïve controls. For the percent lysis equation, the average of the A from 2 to 3 naïve mice was calculated, and the following equation was used:
Enzyme-linked immunoSPOT (ELISPOT)
ELISPOT assays were carried out as previously described (Tompkins et al., 2002), with the following modifications. Unless otherwise noted, 5×105 bulk splenocytes were plated per well with 0 or 50 μg of the indicated antigen. For some experiments, CD4+ and/or CD8+ cells were positively or negatively selected prior to plating using Auto-Macs bead separation (Miltenyi Biotech, Auburn, CA) according to manufacturers’ instructions. All ELISPOT data are presented as mean number of spots per well ± SD.
Adoptive transfer of CD8+ T cell blasts
Donor SJL mice were primed with an emulsion containing 1 mg/ml peptide and incomplete Freund’s adjuvant (IFA) mixed with 2mg/mL Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) to make Complete Freund’s adjuvant. 100 μl of emulsion per mouse was injected subcutaneously in 3 spots on the flank, approximately 33.3 μl per spot. Seven days after priming, the axial, brachial, and inguinal lymph nodes were collected. Single cell suspensions were placed in culture with 50 μM antigen and 200 U/ml rIL-2 (Roche, Nutley, NJ). For some experiments, splenocytes were also collected from donor mice, and depleted of CD4+ cells using Auto-Macs bead separation (Miltenyi Biotech, Auburn, CA) prior to placing them in culture with antigen and rIL-2. After 4 days in culture, cells were washed and blasts counted. Blasts were identified by their large size under trypan blue examination, and 2×106 blasts in PBS were injected i.v. per mouse via the lateral tail vein. Among experiments, blast percentage ranged from 12% to 26%, so the total number of cells injected varied; however the blast numbers remained constant.
Histology and immunohistochemistry
Brains, spinal cords, and spleens from perfused mice were harvested and immunohistochemistry for the detection of CD4+ cells was performed as previously described (Tompkins et al., 2002). Myelin visualization was carried out using a fluoromyelin stain (Molecular Probes, Eugene, OR), following manufacturers instructions. Slides were coverslipped in Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA) and analyzed using a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI) and Metamorph imaging software (Universal Imaging, Downingtown, PA).
Isolation of lymphocytes from CNS, lung, and liver
Mice were anesthetized with 50 mg/kg Nembutal, then perfused with 30–50 ml of PBS through the left ventricle. Brains, lungs, and livers were harvested, minced with scissors and treated with Liberase R1 (Roche, Indianapolis, IN) and DNase I (Invitrogen, Carlsbad, CA) at 37°C with periodic gentle mixing for 45 minutes (for brain tissue) or 1 h (for lung and liver tissue). Samples were pushed through 40 μm Nytex (Sefar America, Kansas City, MO) to yield a single cell suspension. Mononuclear cells were isolated using a 30%/70% Percoll (Amersham, Piscataway, NJ) gradient centrifuged at 2500 × g for 20 minutes at room temperature.
Delayed type hypersensitivity (DTH)
For each mouse, the thickness of both ears was measured using a Mitutoyo model 7326 micrometer (Schlesinger’s Tools, Brooklyn, NY) to provide baseline ear thickness. Ears were then injected s.c. with 10 μg of peptide in 10 μl PBS, using a 100 μl Hamilton syringe and a 30 gauge needle. For each mouse, PLP139–151 or VP3159–166 was injected into in one ear, VP270–86 into the other. 24 h after injection of peptide, ear thickness was again measured. Mean ear swelling was determined by subtracting the baseline ear width from the second measurement.
Statistical analysis
For all in vitro and ex vivo assays, comparisons between groups were analyzed using unpaired Student’s t test. For all disease courses, comparisons between groups were made using ANOVA. p values < 0.05 were considered significant.
Acknowledgments
This work was supported in part by United States Public Health Service, NIH Grant NS-023349 and National Multiple Sclerosis Society Grant RG4294A9/1. M.T.G. was supported by an NIH Individual Predoctoral Research Fellowship (F31 AI-060338) and M.C.R. by NIH Training Grant T32 AI-0007476.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Begolka WS, Haynes LM, Olson JK, Padilla J, Neville KL, Canto MD, Palma J, Kim BS, Miller SD. CD8-deficient SJL mice display enhanced susceptibility to Theiler’s virus infection and increased demyelinating pathology. J Neurovirol. 2001;7:409–420. doi: 10.1080/135502801753170264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begolka WS, Vanderlugt CL, Rahbe SM, Miller SD. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J Immunol. 1998;161:4437–4446. [PubMed] [Google Scholar]
- Borrow P, Tonks P, Welsh CJR, Nash AA. The role of CD8 + T cells in the acute and chronic phases of Theiler’s murine encephalomyelitis virus-induced disease in mice. J Gen Virol. 1992;73:1861–1865. doi: 10.1099/0022-1317-73-7-1861. [DOI] [PubMed] [Google Scholar]
- Bruck W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252(Suppl 5):v3–9. doi: 10.1007/s00415-005-5002-7. [DOI] [PubMed] [Google Scholar]
- Bureau JF, Drescher KM, Pease LR, Vikoren T, Delcroix M, Zoecklein L, Brahic M, Rodriguez M. Chromosome 14 contains determinants that regulate susceptibility to Theiler’s virus-induced demyelination in the mouse. Genetics. 1998;148:1941–1949. doi: 10.1093/genetics/148.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatch RJ, Melvold RW, Miller SD, Lipton HL. Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TMEV-specific delayed-type hypersensitivity. J Immunol. 1985;135:1408–1414. [PubMed] [Google Scholar]
- Clatch RJ, Miller SD, Metzner R, Dal Canto MC, Lipton HL. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler’s murine encephalomyelitis virus (TMEV) Virology. 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- Dal Canto MC, Calenoff MA, Miller SD, Vanderlugt CL. Lymphocytes from mice chronically infected with Theiler’s murine encephalomyelitis virus produce demyelination of organotypic cultures after stimulation with the major encephalitogenic epitope of myelin proteolipid protein. Epitope spreading in TMEV infection has functional activity. J Neuroimmunol. 2000;104:79–84. doi: 10.1016/s0165-5728(99)00230-1. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nature reviews. 2003;3:666–75. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. 2009;155:1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiette L, Aubert C, Brahic M, Pena Rossi C. Theiler’s virus infection of beta2-microglobulin-deficient mice. J Virol. 1993;67:589–592. doi: 10.1128/jvi.67.1.589-592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey AB, Monu N. Effector-phase tolerance: another mechanism of how cancer escapes antitumor immune response. J Leukoc Biol. 2006;79:652–62. doi: 10.1189/jlb.1105628. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety SJ, Rundell MK, Dal Canto MC, Miller SD. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. VI Potentiation of demyelination with and characterization of an immunopathologic CD4 + T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994;152:919–929. [PubMed] [Google Scholar]
- Getts MT, Miller SD. Differential outcome of tolerance induction in naive vs. activated Theiler’s virus epitope-specific CD8+ cytotoxic. T cells J Virol. 2007;81:6584–6593. doi: 10.1128/JVI.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJ, Altfeld MA, Rosenberg ES, Nguyen T, Tang Y, Eldridge RL, Addo MM, He S, Mukherjee JS, Phillips MN, Bunce M, Kalams SA, Sekaly RP, Walker BD, Brander C. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J Exp Med. 2001;193:181–94. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LM, Vanderlugt CL, Dal Canto MC, Melvold RW, Miller SD. CD8 + T cells from Theiler’s virus-resistant BALB/cByJ mice downregulate pathogenic virus-specific CD4 + T cells. J Neuroimmunol. 2000;106:43–52. doi: 10.1016/s0165-5728(00)00212-5. [DOI] [PubMed] [Google Scholar]
- Jelachich ML, Bandyopadhyay P, Blum K, Lipton HL. Theiler’s virus growth in murine macrophage cell lines depends on the state of differentiation. Virology. 1995;209:437–444. doi: 10.1006/viro.1995.1276. [DOI] [PubMed] [Google Scholar]
- Kang BS, Lyman MA, Kim BS. The majority of infiltrating CD8+ T cells in the central nervous system of susceptible SJL/J mice infected with Theiler’s virus are virus specific and fully functional. J Virol. 2002;76:6577–6585. doi: 10.1128/JVI.76.13.6577-6585.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpus WJ, Peterson JD, Miller SD. Anergy in vivo: Down-regulation of antigen-specific CD4+ Th1 but not Th2 cytokine responses. Int Immunol. 1994;6:721–730. doi: 10.1093/intimm/6.5.721. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Pope JG, Peterson JD, Dal Canto MC, Miller SD. Inhibition of Theiler’s virus-mediated demyelination by peripheral immune tolerance induction. J Immunol. 1995;155:947–957. [PubMed] [Google Scholar]
- Katz-Levy Y, Neville KL, Padilla J, Rahbe SM, Begolka WS, Girvin AM, Olson JK, Vanderlugt CL, Miller SD. Temporal development of autoreactive Th1 responses and endogenous antigen presentation of self myelin epitopes by CNS-resident APCs in Theiler’s virus-infected mice. J Immunol. 2000;165:5304–5314. doi: 10.4049/jimmunol.165.9.5304. [DOI] [PubMed] [Google Scholar]
- Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6:930–9. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Jelachich ML. Molecular pathogenesis of Theiler’s murine encephalomyelitis virus-induced demyelinating disease in mice. Intervirology. 1997;40:143–152. doi: 10.1159/000150541. [DOI] [PubMed] [Google Scholar]
- Lyman MA, Myoung J, Mohindru M, Kim BS. Quantitative, not qualitative, differences in CD8(+) T cell responses to Theiler’s murine encephalomyelitis virus between resistant C57BL/6 and susceptible SJL/J mice. Eur J Immunol. 2004;34:2730–2739. doi: 10.1002/eji.200324811. [DOI] [PubMed] [Google Scholar]
- McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- McMichael A. T cell responses and viral escape. Cell. 1998;93:673–6. doi: 10.1016/s0092-8674(00)81428-2. [DOI] [PubMed] [Google Scholar]
- Michel ML, Mancini-Bourgine M. Therapeutic vaccination against chronic hepatitis B virus infection. J Clin Virol. 2005;34(Suppl 1):S108–14. doi: 10.1016/s1386-6532(05)80019-8. [DOI] [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9:246–58. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PD, Pavelko KD, Leibowitz J, Lin X, Rodriguez M. CD4 + and CD8 + T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J Virol. 1998;72:7320–7329. doi: 10.1128/jvi.72.9.7320-7329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville KL, Padilla J, Miller SD. Myelin-specific tolerance attenuates the progression of a virus-induced demyelinating disease: Implications for the treatment of MS. J Neuroimmunol. 2002;123:18–29. doi: 10.1016/s0165-5728(01)00479-9. [DOI] [PubMed] [Google Scholar]
- Nicholson SM, Haynes LM, Miller SD, Melvold RW. The role of protective CD8 + T cells in resistance of BALB/c mice to TMEV-induced demyelinating disease: regulatory vs. lytic. J Neuroimmunol. 1999;98:136–146. doi: 10.1016/s0165-5728(99)00090-9. [DOI] [PubMed] [Google Scholar]
- Olson JK, Croxford JL, Miller SD. Virus-induced autoimmunity: Potential role of viruses in initiation, perpetuation, and progression of T cell-mediated autoimmune diseases. Viral Immunol. 2001;14:227–250. doi: 10.1089/088282401753266756. [DOI] [PubMed] [Google Scholar]
- Palma JP, Lee HG, Mohindru M, Kim BS, Dal Canto MC, Miller SD, Kim BS. Enhanced susceptibility to Theiler’s virus-induced demyelinating disease in perforin-deficient mice. J Neuroimmunol. 2001;116:25–35. doi: 10.1016/s0165-5728(01)00293-4. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Karpus WJ, Clatch RJ, Miller SD. Split tolerance of Th1 and Th2 cells in tolerance to Theiler’s murine encephalomyelitis virus. Eur J Immunol. 1993;23:46–55. doi: 10.1002/eji.1830230109. [DOI] [PubMed] [Google Scholar]
- Pope JG, Karpus WJ, Vanderlugt CL, Miller SD. Flow cytometric and functional analyses of CNS-infiltrating cells in SJL/J mice with Theiler’s virus-induced demyelinating disease: Evidence for a CD4 + T cell-mediated pathology. J Immunol. 1996;156:4050–4058. [PubMed] [Google Scholar]
- Pullen LC, Miller SD, Dal Canto MC, Kim BS. Class I-deficient resistant mice intracerebrally inoculated with Theiler’s virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur J Immunol. 1993;23:2287–2293. doi: 10.1002/eji.1830230935. [DOI] [PubMed] [Google Scholar]
- Qi Y, Dal CMC. Effect of Theiler’s murine encephalomyelitis virus and cytokines on cultured oligodendrocytes and astrocytes. J Neurosci Res. 1996;45:364–374. doi: 10.1002/(SICI)1097-4547(19960815)45:4<364::AID-JNR5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, Lupton SD, Overell RW, Reynolds TC, Corey L, Greenberg PD. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–23. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- Rivera-Quinones C, McGavern D, Schmelzer JD, Hunter SF, Low PA, Rodriguez M. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat Med. 1998:187–193. doi: 10.1038/nm0298-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, David CS. Demyelination induced by Theiler’s virus: influence of the H-2 haplotype. J Immunol. 1985;135:2145–2148. [PubMed] [Google Scholar]
- Rodriguez M, Dunkel AJ, Thiemann RL, Leibowitz J, Zijlstra M, Jaenisch R. Abrogation of resistance to Theiler’s virus-induced demyelination in H-2b mice deficient in beta2-microglobulin. J Immunol. 1993;151:266–276. [PubMed] [Google Scholar]
- Rodriguez M, Leibowitz J, David CS. Susceptibility to Theiler’s virus-induced demyelination. Mapping of the gene within the H-2D region. J Exp Med. 1986;163:620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Stroop WG, Baringer JR, Brahic M. Detection of Theiler’s virus RNA in mouse central nervous system by in situ hybridization. Lab Invest. 1981;45:504–509. [PubMed] [Google Scholar]
- Tompkins SM, Padilla J, Dal Canto MC, Ting JP, Van Kaer L, Miller SD. De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:4173–4183. doi: 10.4049/jimmunol.168.8.4173. [DOI] [PubMed] [Google Scholar]
- Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: Distribution of T cell subsets within active chronic lesions. Science. 1983;219:308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- Walsh SR, Bhardwaj N, Gandhil RT. Dendritic cells and the promise of therapeutic vaccines for human immunodeficiency virus (HIV)-1. Current HIV research. 2003;1:205–16. doi: 10.2174/1570162033485285. [DOI] [PubMed] [Google Scholar]
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- Webster G, Bertoletti A. Quantity and quality of virus-specific CD8 cell response: relevance to the design of a therapeutic vaccine for chronic HBV infection. Mol Immunol. 2001;38:467–73. doi: 10.1016/s0161-5890(01)00082-7. [DOI] [PubMed] [Google Scholar]
- Welsh CJ, Tonks P, Nash AA, Blakemore WF. The effect of L3T4 T cell depletion on the pathogenesis of Theiler’s murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68:1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]