Abstract
Verbal working memory is the ability to temporarily store and manipulate verbal information. This study tested the predictions of a neuroanatomical model of how the cerebellum contributes to verbal working memory (Desmond et al., 1997). In this model, a large bilateral region in the superior cerebellum is associated with articulatory rehearsal and a right-lateralized region in the inferior cerebellum is associated with the correction of errors within the working memory system. The Desmond et al. (1997) model was based on neuroimaging findings using item recognition tasks and comparisons between working memory and covert rehearsal tasks, whereas in this fMRI study we used a delayed serial recall (DSR) task because it relies more heavily on articulatory rehearsal, and our comparison tasks included both overt and covert speech tasks. Our results provide some support for the Desmond et al. (1997) model. In particular, we found multiple activation foci within the superior and inferior sectors of the cerebellum and evidence that these regions show different patterns of activation across working memory and speech tasks. However, the specific patterns of activation were not fully consistent with those reported by Desmond et al. (1997). Namely, our results indicate that activation in the superior sector should be functionally subdivided into a medial focus involved in speech processing and a lateral focus more specific to verbal working memory; the results also indicate that activation in the inferior sector is not uniquely right-lateralized. These complex findings speak to the need for future studies to consider the speech-motor aspects of tasks, to investigate the functional significance of adjacent peaks of activation within large regions of cerebellar activation, and to use analysis procedures that support regional distinctions through direct statistical tests. Such studies would help to refine our understanding of how the cerebellum contributes to speech and verbal working memory.
Keywords: Verbal working memory, cerebellum, speech production
1. Introduction
Working memory is the ability to actively maintain a limited amount of information in a readily available state, even in the absence of continued sensory input. This capacity for internal representation is central to complex cognition and thus it is not surprising that working memory has been an important interdisciplinary topic of research. One of the most prominent theoretical models has proposed the use of a ‘phonological’ component that is composed of two sub-systems: a short-term store that contains phonologically-based representations of the to-be-remembered items, and a rehearsal loop that acts to prevent the decay of verbal information in the phonological store (Baddeley, 1986; Salamé and Baddeley, 1982). The model developed by Baddeley and colleagues was initially intended to account for patterns of behavioral performance in the normal population and in participants with brain damage. A number of neuroimaging researchers adopted this model in an attempt to explain patterns of brain activation observed during the performance of working memory tasks. For instance, Smith et al. (1998) proposed that the left inferior parietal cortex is the site of phonological storage for verbal material and a set of regions associated with speech production are involved in articulatory rehearsal.
One of the speech-related regions associated with articulatory rehearsal is the left inferior frontal gyrus (i.e., Broca’s area)(Chein et al., 2002); such a mapping is consistent with neuropsychological findings that have found rehearsal-related deficits in verbal working memory in individuals with Broca’s aphasia (Benson, 1979; Damasio and Damasio, 2000; Dronkers et al., 2004). Motor-related speech areas have also been associated with articulatory rehearsal, including regions within the medial and lateral portions of premotor cortex and the cerebellum (Cabeza and Nyberg, 2000; Fiez, 1996; Fiez et al., 1996; Henson et al., 2000). The involvement of these areas raises interesting questions about the nature of the coding that is used in articulatory rehearsal: specifically, whether the coding is restricted to the language system, or whether it also involves the use of motor representations in brain regions that are outside of the language system per se. The neuroimaging results favor the latter interpretation, since activation during verbal working memory tasks is found in both language and motor-related brain regions. However, prior neuropsychological results favor the former interpretation. For instance, Bishop and colleagues (Bishop and Robson, 1989) examined the verbal working memory performance of individuals with cerebral palsy. These investigators found that verbal working memory performance was not different for those individuals whose motor symptoms included disturbances of speech versus those whose speech was normal. The authors concluded that the inner speech processes that are involved in working memory are at a relatively abstract level of representation since they appeared to be unaffected by difficulties in motoric aspects of speech production.
The apparent discrepancy between neuroimaging and neuropsychological findings may reflect the fact that activation in motor areas during verbal working memory tasks is incidental: that is, motor activation could be an irrelevant by-product of speech processing in the inferior frontal gyrus. This possibility has yet to be ruled out for cortical motor regions (e.g., medial and lateral premotor cortex). However, recent neuropsychological findings suggest that this account does not hold in the cerebellum. Across a relatively large number of neuropsychological studies, it has been found that individuals with damage to the cerebellum exhibit mild-to-moderate working memory deficits (Timmann and Daum, 2007), especially for verbal material (Ravizza et al., 2006).
More recent work in neuroanatomy, neuronal tracing, neuropsychology, and neuroimaging lend further support to the idea that the cerebellum is highly influential not only for motor processing, but also for cognitive processes. The cerebellum and the cerebrum are reciprocally connected through multi-synaptic closed-loop circuits (Strick et al., 2009) and the cerebellum appears to exhibit a functional topography that is consistent with specific patterns of cerebral connectivity (Stoodley and Schmahmann, 2009). The recent use of functional connectivity MRI (fcMRI) has provided a new tool for the delineation of non-motor cerebro-cerebellar circuitry. For instance, Krienen and Buckner (2009) found a set of four closed cerebro-cerebellar loops linking both motor-based and cognition-based regions in the frontal cortex with distinct loci in the cerebellum. This account of frontal-cerebellar connectivity is corroborated in another fcMRI study by Allen et al. (2005), who also found parietal-cerebellar connectivity.
Neuroimaging studies have also examined the role of the cerebellum in working memory, with the work of Desmond and colleagues best reflecting a particular focus on the cerebellum. In a series of four studies (Chen and Desmond, 2005a, 2005b; Desmond et al., 1997; Kirschen et al., 2005), they used an item recognition procedure to examine cerebellar activation during verbal working memory. The size of the memory set used in these tasks varied (1 letter or 6 letters) so that load-dependent changes in activation could be detected. The authors used a relatively brief delay (5 sec) between item presentation and the recall probe, to which participants responded by squeezing a pneumatic response bulb if the probe matched an item in the memory set. As one comparison condition, activation was monitored during a rehearsal condition, in which participants silently and repeatedly read either a low (1) or high (6) number of the letters in the display image. As a second comparison condition, activation in one study was monitored during a simple finger-tapping task, in which subjects sequentially touched their right thumb to each finger in a sequence. The findings from these studies collectively support a neuroanatomical model of the cerebellum and working memory introduced by Desmond et al (1997).
Desmond et al. (1997) identified a set of bilateral regions in the superior cerebellum (in lobule VI and the superior portion of lobule VIIA) that were more active for a high- versus the low-load working memory contrast, and also for a high- versus low-load rehearsal contrast. This observation, coupled with evidence that this portion of the cerebellum is likely to be connected with the inferior frontal gyrus, led the authors to propose that the identified superior cerebellar regions participate in an articulatory rehearsal loop. In contrast, activation in the inferior cerebellum (in lobule VIIB) was only found in the working memory contrast, and only in the right cerebellar hemisphere. This observation, coupled with evidence that this portion of the cerebellum is likely to be interconnected with the temporoparietal cortex (a potential locus of the phonological store (Paulesu et al., 1993)) led the authors to propose a different function for this cerebellar region. In particular, they posited that the right inferior cerebellum helps to correct for errors in the articulatory rehearsal loop by comparing information in the loop with the contents of the phonological store.
The findings reported by Desmond and colleagues are compelling, and they dovetail with recent behavioral and neuropsychological research which also point toward an error-correction role for the cerebellum in verbal working memory (Guediche et al., 2006). At the same time, however, a number of important issues remain. The goal of the present paper is to build upon the work of Desmond and colleagues by addressing three issues. One issue is whether the pattern of activation observed by in the Desmond et al. (1997) study can be replicated using a serial recall task, rather than an item recognition procedure. While this might seem like a trivial distinction, behavioral evidence suggests that inner speech rehearsal mechanisms are more important for serial recall tasks, and thus it is possible that activity in brain regions associated with inner speech might be affected by a manipulation of the working memory task (Henson, 2001; Henson et al., 2003). To address this question, we designed our working memory paradigm to include a serial recall task with an overt retrieval epoch.
A second question is whether Desmond and colleagues’ findings concerning the relationship between working memory and covert rehearsal will also be found for a comparison of working memory and overt speech – both for overt speech performed in the context of working memory (i.e., during spoken recall of the presented items) and for overt speech performed in the context of a simple repetition task. While simple motor accounts of the cerebellum and working memory might predict few differences between overt and covert speech conditions, several studies have found this to be an important distinction in cortical patterns of brain activity (Barch et al., 1999; Huang et al., 2001; Palmer et al., 2001; Shuster and Lemieux, 2005). To address this question, we examine subjects’ brain activity during simple overt and covert speech tasks that we directly compare to the pattern of brain activity observed during a delayed serial recall task.
Finally, the analysis approaches used by Desmond et al. (1997) relied heavily upon statistical thresholding approaches that yielded voxel-wise maps of activation in the cerebellum. Such approaches are powerful, but they can obscure important sub-threshold levels of activation in other task conditions and they do not generally lend themselves to direct tests of whether activation patterns differ significantly across regions. To address this issue, we complemented voxel-wise analysis procedures with region of interest (ROI) approaches that allowed us to conduct inferential statistical tests designed to directly compare activation patterns across tasks and regions of the cerebellum.
2. Methods
2.1 Subjects
A total of 19 (7 female, 12 male) subjects were recruited from the University of Pittsburgh and surrounding metropolitan area for inclusion in this research study. Subjects were right handed (determined by questionnaire), between 19-33 yrs of age (mean age of 23 yrs), and all subjects were native English speakers with no reported history of illiteracy, learning disability, illicit drug use, psychiatric illness, or traumatic brain injury. To ensure safety in the MR environment, subjects were also screened for MRI contra-indications, claustrophobia, and females of childbearing potential were asked to complete a urine pregnancy test prior to participation. Each subject provided informed consent on a University of Pittsburgh Institutional Review Board approved document prior to participation, and they received monetary compensation for their time and participation.
2.2 Experimental Tasks
Subjects performed a delayed serial recall task (DSR). For each trial of this task, subjects were visually presented with a sequential series of six letters that were randomly selected from a set of eight letters (F, H, K, L, M, Q, R, S), and they were asked to remember the letters in the order in which they were presented. All subjects were explicitly instructed not to utilize any mnemonic devices such as chunking or name designation. During the encoding interval, each of the letters appeared in succession at the center of a computer screen for 1 sec each, to give a total encoding duration of 6 secs. During the subsequent maintenance interval, subjects were instructed to silently (covertly) repeat the letters using subvocal speech. They were then prompted to retrieve the items by the appearance of six red question marks at the center of the screen, which remained present during a 6 sec retrieval epoch. During this retrieval epoch subjects attempted to recall the letters aloud in the order in which they had been presented. If they did not remember a letter in the series, they said, “skip” and continued with the sequence as they could remember it. Following the retrieval period, there was a 14 sec baseline during which subjects fixated on a small cross at the center of the computer screen; this epoch allowed the hemodynamic response to return to a baseline, non-task level. Subjects performed 50 trials of the DSR task over five runs. The entire experiment lasted 26 mins and 40 secs.
Subjects also performed four simple motor tasks: covert speech (silently repeat the word “the”), overt speech (repeat the word “the” aloud), covert tapping (imagine tapping their right index finger), and overt tapping (depress a response key using their right index finger). The start of each task was cued by a brief, 2 sec instruction at the center of the screen that provided the details for the type of trial to follow. After the instruction, subjects carried out the instructed movement tasks at the rate of approximately three times per second for the duration of a 6 sec production epoch. All subjects went through a series of practice trials with a metronome to become accustomed to the rate of speech and tapping prior to beginning the experiment. Similar to the DSR task, these simple motor tasks were followed by a 14 sec baseline fixation. This portion of the experiment was also split into five runs, with each task-type occurring randomly four times per run for a total of 20 trials of each. The total duration of the simple motor task portion of the experiment was 29 mins, 20 secs.
2.3 Collection of Data from Overt Conditions
Subjects’ overt verbal responses during the retrieval portion of the DSR task and the overt speech task were recorded with a microphone that is integrated in the Brain Imaging Research Center’s MR compatible headphones (Avotec, Inc., Stuart, FL). The data were audio-recorded with Sony Sound Forge 8 software (Sony Corporation of America) on a PC in the control room. Because the microphone also collected scanner sounds and disturbances, the digitized data were subsequently processed using a noise cancellation algorithm to eliminate the majority of these extraneous MR sounds so that what remained were the subjects’ overtly spoken words (Jung et al., 2005). Extraneous sounds that were not eliminated by the noise cancellation algorithm were random across the conditions. Subjects’ overt finger tapping responses were obtained from a MR compatible button glove placed on their right hand and output was recorded in E-prime format text files (Psychology Software Tools, Inc., Pittsburgh, PA).
2.4 fMRI Data Acquisition
All MRI data were collected on a 3.0 T Siemens Magnetom Allegra (Siemens AG, USA) head-only research scanner with CP transmit/receive head coil and an integrated mirror at the Brain Imaging Research Center, a joint University of Pittsburgh and Carnegie Mellon University facility. Subjects’ heads were padded in order to help minimize movement during scanning.
Structural scans were obtained prior to functional scans. In particular, 39, T2-weighted in-plane and high-resolution structural scans were obtained using a standard EPI pulse sequence. During functional scanning, 39, 3.5 mm thick, oblique slices parallel to the plane of the anterior - posterior commissure were obtained (TR = 2000 ms, TE = 25 ms, Flip Angle= 79, FOV=205) for 13.65 cm of whole-brain coverage on most subjects. All subjects were placed in the scanner so that we received full cerebellar coverage on all subjects. Although we scanned the entire brain, the focus of the current research is solely on the cerebellum. Activations in other regions in cortex were obtained, but they are not reported in the current paper.
2.5 fMRI Data Analysis
Data were reconstructed and preprocessed using Neuroimaging Software Package, NIS 3.6 (University of Pittsburgh, Princeton University) and an integrative software package, Functional Imaging Software Widgets, Fiswidgets (Fissell et al., 2003). After reconstruction and quality checks, the data were corrected for motion with Automated Image Registration, AIR 3.08 (Woods et al., 1993). Subject movement beyond 4 degrees or 4 mm was considered unacceptable and data with this amount of movement were excluded from the analysis. After motion correction was completed, the images were corrected for linear trends to adjust for possible scanner drift. A reference brain was then chosen from among the structural images of the subjects, and the skull was removed from the structural images of the subjects’ brains. These stripped structural scans were transformed into the reference brain space. To get the functional data into the same space, the images were scaled to a global mean and a three dimensional Gaussian filter (8 mm FWHM) was used to smooth the data to account for between subject anatomical differences. Finally, the reference brain and functional maps were converted into Talairach space (Talairach and Tournoux, 1988) so that statistical analyses could be performed.
2.6 fMRI Voxel-wise Contrasts
Further data analysis was performed using voxel-wise repeated measures ANOVA on the data for each task. Because we had an a priori interest in both working memory and speech we performed two contrasts within the DSR task. In the first, we examined the encoding interval versus baseline. More specifically, the blood-oxygen-level dependent (BOLD) signal during the last 2 secs of encoding and first 4 secs of maintenance was contrasted with the BOLD signal during the last 4 secs of the baseline fixation interval. In the second contrast, we looked at the activation in the cerebellum during the retrieval epoch, and contrasted the BOLD signal during the last 2 secs of retrieval and first 4 secs of baseline with the BOLD signal during the last 4 secs of the baseline fixation interval. For these contrasts we used a threshold criterion of p=.00001 and a contiguity of three voxels. The analysis of the different epochs in the DSR task help to elucidate the regions in the cerebellum associated with the encoding/maintenance and retrieval of information in verbal working memory. We focused our analysis primarily on the encoding interval because preliminary data had shown that it was during this task epoch that we have the greatest BOLD signal, and previous research in our laboratory indicated that the BOLD signal decreases in some regions over an extended maintenance interval (Chein and Fiez, 2001). Specific cerebellar lobules were designated using a cerebellar atlas (Schmahmann et al., 2000).
To further identify the role of specific regions in verbal working memory and their potential relationship to speech processing and domain general motor execution, we used the same significance threshold and voxel contiguity to perform voxel-wise ANOVA in the simple speech and non-speech tasks.
2.7 fMRI Conjunction Analyses
As a complement to the voxel-wise ANOVAs, we utilized the statistical maps from these contrasts and AFNI neuroimaging software (Cox, 1996) to perform conjunction analyses to more closely identify the areas of overlap between all of the tasks. To perform the overlap analysis we collapsed across both the encoding and retrieval epochs of the DSR task, and identified voxels that were common to the DSR task and simple speech tasks regions. These analyses are designed to help to further elucidate and establish interpretations of functional specificity in the cerebellum.
2.8 fMRI Timecourse Analysis
The conjunction analyses do not provide a complete picture of the differences between the tasks. However, with the threshold procedure utilized in the voxel-wise ANOVA we cannot be certain that the separations between regions are not the result of a region being less ‘active’ but still enlisted for the different tasks: i.e., the thresholding procedure for the ANOVA does not establish a significant difference between our tasks, nor does it permit a direct comparison between regions. Thus, in order to further clarify the role that overt and covert speech play in verbal working memory, we utilized the seven regions identified from the encoding and retrieval contrasts to extract timeseries data from each of the regions found in our DSR task (we did not separately examine the regions from our simple speech tasks, because our overlap analysis indicates that these regions are subsets of those that are active during the DSR task).
We then used the timeseries data to compute the average percent signal change in each region for the encoding epoch versus baseline, the retrieval epoch versus baseline, covert speech versus baseline, and overt speech versus baseline (Figure 1c). These percent signal change data were then subject to further inferential statistical tests to probe for differences between task conditions and regions of activation within the cerebellum. Specifically, we examined the differences in percent signal change among four factors: 1) the sector location of the region (superio-medial; superio-lateral; inferior), 2) the hemisphere location of the region (left, right), 3) the type of speech condition posited to be involved (covert/encoding, overt/retrieval), and 4) the task type (DSR, simple speech). We performed a 3x2x2x2 repeated-measures ANOVA. The assumption of sphericity was not met and a Greenhouse Geiser correction was utilized, with an alpha of p =.01. Simple main effects analyses were used to further explore any significant interactions.
Fig 1.
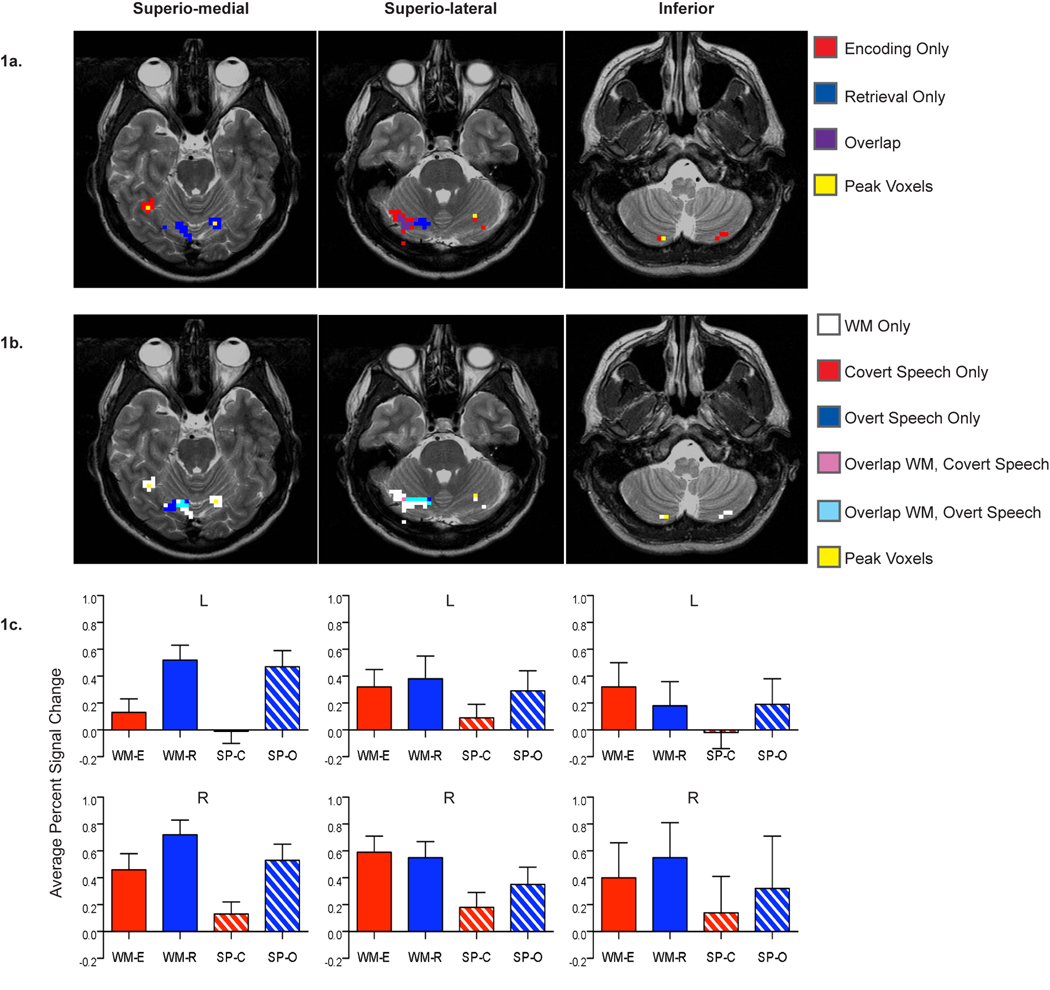
Functional activations during the verbal working memory and simple speech tasks across the superior and inferior sectors of the cerebellum (superior split into: superio-medial, superio-lateral). (a) Overlap of the seven regions identified from the encoding and the retrieval epochs of the DSR task. (b) Overlap of the regions identified from the DSR task and simple covert and overt speech tasks. (c) Average percent signal change across the DSR and speech tasks in six of the bilateral regions identified from the encoding and retrieval contrasts. Note that data is shown for only one of the two left inferior regions listed in Table 1. The excluded left inferior cerebellar region showed a similar pattern of activation.
2.9 fMRI Simple Finger-tapping Analyses
As a final step in our analyses, we examined data from our overt and covert tapping tasks. We ran a voxel-wise ANOVA on the simple tapping data at the same significance threshold and voxel contiguity as the working memory and simple speech tasks. We expected that there would be no overlap between verbal working memory and tapping in areas that were specific to working memory. Once the set of regions active for overt tapping were identified, we performed a conjunction analysis between the overt tapping conditions and DSR task as was done with the simple speech tasks.
3. Results
3.1 fMRI Voxel-wise Contrast Results
From our voxel-wise repeated measures ANOVAs of encoding versus baseline and retrieval versus baseline, we identified a number of cortical activations; as expected these included regions in the left inferior frontal cortex, bilateral premotor cortex, left motor cortex, and left inferior parietal cortex. Within the cerebellum we identified a set of seven active regions (Table 1). All seven of the regions identified in this initial voxel-wise ANOVA came from either the encoding contrast or the retrieval contrast, but not both. They show some peripheral overlap in their extent of activation, but in general the voxel clusters are distinct from one another and they lie in different sectors in the cerebellum. In particular, the regions identified from the retrieval task are located in the superior sector of the cerebellum and they have a relatively medial extent (superio-medial); those from the encoding contrast are in both the superior and inferior sectors of the cerebellum, with those in the superior sector having a more lateral extent (superio-lateral) than those in the superior sector identified in the retrieval contrast. The locations of these separate, non-overlapping foci for the encoding and retrieval contrasts can be seen in Figure 1a and Table 1. Across both the superior and inferior cerebellar sectors, the regions generally show a bilateral pattern of activation for the encoding and retrieval epochs of our verbal working memory task.
Table 1.
Regions active for working memory task epoch vs. fixation baseline, simple speech vs. baseline, and simple overt tapping vs. baseline at p=.00001, voxel contiguity=3.
| Contrast | Hemisphere | Sector | Talairach Coordinate |
Cerebellar Region |
|---|---|---|---|---|
| WM: | ||||
| Retrieve | L | Superior,Medial | −16, −61, −22 | VI |
| R | Superior,Medial | 25, −64, −26 | VI | |
| WM: | ||||
| Encode | R | Superior,Lateral | 36, −48, −21 | VI |
| L | Superior,Lateral | −25, −54, −29 | VI | |
| L | Inferior | −15, −68, −51 | VIIB / VIIIA | |
| L | Inferior | −31, −61, −58 | VIIIA | |
| R | Inferior | 13, −68, −55 | VIIIA | |
| Speech: | ||||
| Overt Speech | R | Superior | 7, −61, −25 | VI / V |
| Speech: | ||||
| Covert Speech | R | Superior | 25, −58, −26 | VI |
| Tapping: | ||||
| Overt Tapping | R | Middle | 33, −51, −30 | VI |
| L | Superior | −32, −58, −26 | VI | |
| R | Inferior | 16, −61, −55 | VIIIB | |
Next, we examined the data from our covert and overt simple speech tasks, which resulted in the identification of two right lateralized regions from our speech tasks’ data, one for overt speech and one for covert speech (Table 1). The peaks of the regions found in our speech contrasts did not exactly match those found in the DSR task, but they were close in proximity (Table 1).
3.2 fMRI Conjunction Analysis Results
We looked at the overlap of the seven regions identified in DSR task and the two regions identified in our simple speech tasks. We found that the set of two right lateralized speech regions appear to be a subset of the regions found in the DSR task (Figure 1b).
3.3 fMRI Timecourse Analysis Results
Results of the timecourse analysis show that the most inferior regions of the cerebellum tend to be specific to working memory, whereas the superior regions show activity across all four of the tasks. The more superio-medial regions seem to demonstrate a more general speech motor processing role, since the activity in this sector is highest during periods of overt articulation.
The pattern of differences on percent signal change among the four-way condition did not meet our relatively stringent threshold for significance, though it was close, F(2,26) = 7.117, p = .012, partial η2 = .354. None of the three-way interactions reached our strict significance criterion either, p ≥ .021. However, two of our two-way interactions were highly significant. First, the pattern of activation among the sectors was significantly influenced by whether the task contrast involved covert versus overt speech, F(2,26) = 12.192, p = .001, partial η2 = .484. Second, the degree to which the activation was lateralized to the right hemisphere was significantly influenced by the task type (DSR, simple speech), F(1,13) = 17.266, p = .001, partial η2 = .570. No other two-way interactions reached our significance threshold, p ≥ .037.
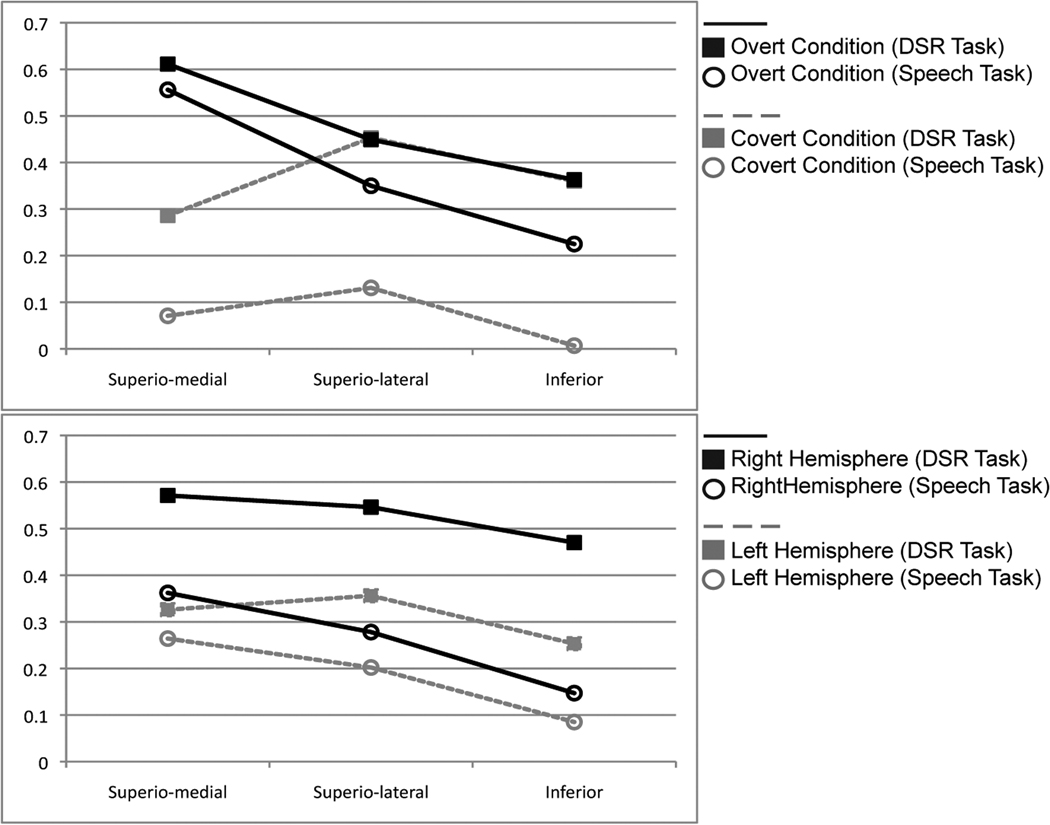
In order to further probe the percent signal change differences in our significant two-way interaction between sector and speech condition, we performed simple main effects analyses. In the first, we looked at the activation in each of the three sectors (superio-medial; superio-lateral; inferior) as a function of speech condition (covert versus overt speech), collapsing across task type (DSR, speech) and hemisphere (left, right). We found that covert speech had a significantly lower percent signal change in the superio-medial sector (M = .178, SE = .017) than overt speech (M = .584, SE = .044), F(1,16) = 50.948, p<.001, partial η2 = .761. In contrast, there was no significant difference between the covert and overt speech in the superio-lateral sector (M = .292, SE = .023; M = .399, SE = .065 respectively), F(1,16) = 2.939, p = .106, partial η2 = .155, or the inferior sector (M = .183, SE = .040; M = .294, SE = .074 respectively), F(1,16) = 2.621, p = .125, partial η2 = .141 (Figure 2a).
Fig 2.
Graphical representation of the two-way interactions from the ANOVAs. (a) Interaction between condition and sector showing the significant difference between overt and covert speech in the superio-medial sector. (b) Interaction between task and hemisphere showing that the DSR task had significantly higher percent signal change in the right hemisphere than the simple speech task.
To explore the differences in percent signal change in the other significant two-way interaction between the tasks (DSR and speech) and the hemisphere (left and right) we again performed simple main effects analyses. In this analysis we executed the main effects analyses on the task conditions at each level of hemisphere (left and right), collapsing across sectors (superio-medial; superio-lateral; inferior) and conditions (covert, overt). The DSR task (M = .529, SE = .035) had significantly higher percent signal change in the right hemisphere than the simple speech task (M = .262, SE = .044), F(1,13) = 20.576, p = .001, partial η2 = .613. There was no significant difference between the DSR task (M = .312, SE = .031) and the simple speech task (M = .184, SE = .049) in the left hemisphere, F(1,13) = 4.234, p = .060, partial η2 = .246 (Figure 2b).
3.4 fMRI Simple Finger-tapping Results
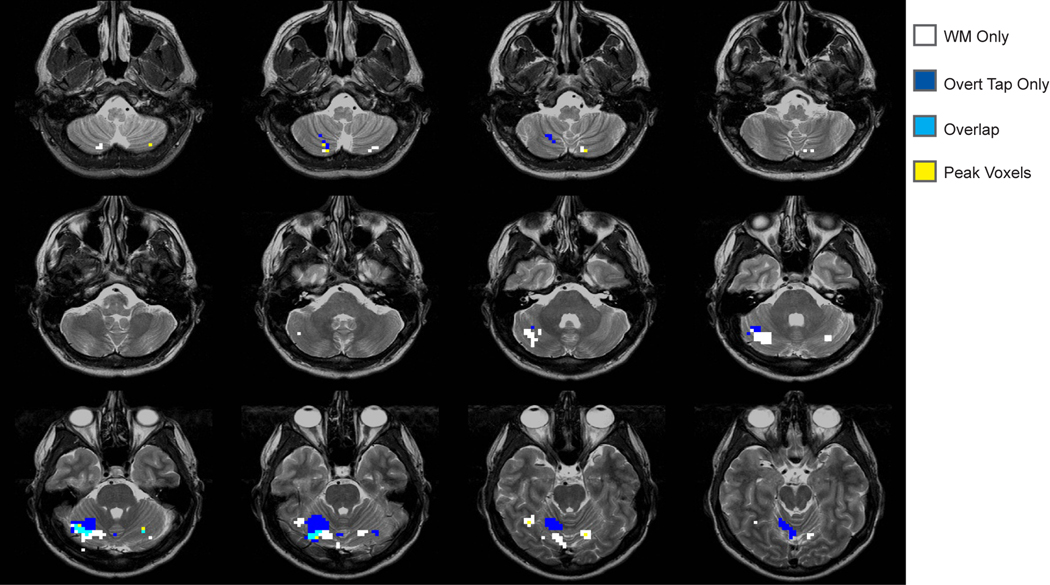
In our voxel-wise analysis of the simple tapping tasks, we found three regions that were active for overt tapping, and no voxel clusters that reached our threshold for covert tapping (Table 1). In the subsequent conjunction analysis, the overlap map (Figure 3) indicates a large superior area of activation for tapping that is generally anterior and lateral to the working memory regions and that extends through several lobules. The regions identified for overt tapping only show marginal overlap with the DSR and speech tasks.
Fig 3.
Overlap of the regions identified from the DSR task and simple overt tapping tasks.
4. Discussion
Desmond et al. (Chen and Desmond, 2005a, 2005b; Desmond et al., 1997; Kirschen et al., 2005) used results from a series of studies to propose a neuroanatomical model of phonological processing in verbal working memory (Chen and Desmond, 2005a, 2005b; Desmond et al., 1997; Kirschen et al., 2005). One key idea in this model is a distinction between the roles of the bilateral superior (lobule VI and superior lobule VIIA) and right inferior (lobule VIIB) sectors of the cerebellum. Specifically, the model proposes an error correction role for the right inferior cerebellum, in which a region that is recruited during verbal working memory tasks helps to align items that are active in an articulatory rehearsal loop (which involves bilateral regions in the superior cerebellum) with those in the short-term store located in the temporoparietal cortex. Thus, regions in the superior cerebellum act to set up and maintain the memory trace, and a region in the right inferior cerebellum works like a foreman, in a position of oversight, to correct and reconcile the items in the articulatory trace with those in the store.
The results of this study generally substantiated our expectations; specifically, both speech tasks and working memory tasks activate similar areas within the cerebellum. Further, the data demonstrate an overlap between speech processing and working memory, but not simple tapping and working memory. Thus, our data provide support for some aspects of the Desmond et al. (1997) model. In agreement with Desmond and collaborators, we find that that there are multiple sectors of the cerebellum that are engaged during a verbal working memory task, including regions located in both the superior and inferior lobules. This alone is important, because the inferior portions of the cerebellum are often not fully imaged and thus past research has tended to generate an incomplete perspective on the regions of the cerebellum that contribute to working memory performance. Our results also support the idea that the cerebellum should not be treated as a single entity, and indicate the need for more precision in specifying and referring to loci of activation within the cerebellum. Finally, we note that this more complex picture of the cerebellum and working memory might explain why neuropsychological research has yet to reveal a clear mapping between specific patterns of cognitive deficits and specific loci of activation within the cerebellum (Strick et al., 2009). In other words, in the domain of working memory it is possible that many sites of damage within the cerebellum could give rise to impaired performance on general measures of working memory (e.g., reductions in verbal span), though potentially more fine-grained assessments of behavior might reveal subtle differences in performance patterns associated with damage to particular subregions within the cerebellum.
We found several differences between our data and the results we expected based on the Desmond et al. (1997) model and the prior findings from this research group (Chen and Desmond, 2005a, 2005b; Desmond et al., 1997; Kirschen et al., 2005). These differences suggest that the proposed distinction between the roles of the superior and inferior cerebellum in verbal working memory may be incomplete. In their study, Desmond et al. (1997) proposed a bilateral superior focus as the locus for the articulatory rehearsal loop and a right-lateralized focus as the locus for a more specific working memory region involved in error correction. Their localization of activation in the superior sector consisted of a large cluster that covered both our superio-medial and superio-lateral sectors. As a whole, Desmond et al. (1997) consider this large region to perform a unitary function, though as noted in the tables that report their research findings, they consistently find distinct superior-medial and superio-lateral peaks within their clusters of voxels that exceeded their statistical thresholds. Two aspects of our findings indicate the importance of distinguishing between subregions (superio-medial and superio-lateral) within the superior cerebellum. First, the robustness of the activation in the superio-medial regions for overt speech and the retrieval epoch of the DSR task suggest that the superio-medial cerebellum is generally more motoric in nature (Figure 1). Second, we found that the superio-lateral and the inferior regions both show greater engagement in the working memory task than the speech conditions (overt/covert). It seems the individual peaks within the large superior sector in the Desmond model are in fact separate regions that perform distinct functions.
Another point of difference between our findings and the Desmond et al. (1997) model concerns the pattern of lateralization across superior and inferior sectors of the cerebellum. The Desmond et al. (1997) model posits that the region in the inferior sector of the cerebellum that is associated with error correction is right lateralized, whereas the superior regions that are associated with articulatory rehearsal are engaged bilaterally. Our ANOVA showed significantly higher right hemisphere activation for the verbal working memory task, but there was no evidence for sector differences. However, regions in the inferior sector of the cerebellum showed an overall lower magnitude of activation; thus, with voxel-wise methods activation is more likely to surpass threshold levels for significance in the superior sector as compared to the inferior sector of the cerebellum, especially in the right hemisphere. Our region-of-interest approach suggests that differences in activation foci revealed with such voxel-wise methods may be misleading: the overall pattern of activity in inferior regions appears to be right-dominant, but no more so than the pattern of lateralization found in regions located in the superio-medial and superio-lateral sectors of the cerebellum.
Finally, according to the Baddeley et al. model of working memory, a covert inner speech process is thought to support the articulatory rehearsal loop (Baddeley, 1986; Salamé and Baddeley, 1982), an idea that led Desmond et al. (1997) to expect that covert speech production and the inner speech process associated with articulatory rehearsal may activate similar regions within the cerebellum. Based on the same general logic that both tasks involve a common inner speech component, we had expected to extract similar regions for the covert speech task and the encoding portion of our working memory task. Consistent with other studies using covert speech and speech perception tasks, we found one region in the right superior cerebellum that was minimally active (Ackermann et al., 1998; Chen and Desmond, 2005a; Mathiak et al., 2002; Papathanassiou et al., 2000; Riecker et al., 2000; Strelnikov et al., 2006). Nevertheless, we were surprised to find very little activation for covert speech across the cerebellum, even when the threshold was lowered in exploratory analyses of our data. The lack of activation could be used as evidence that none of the regions within the cerebellum play a key role in the articulatory rehearsal process. However, it is possible that the nature of our simple speech tasks hindered our ability to see covert speech related activation in the cerebellum. Research has shown increased bilateral activity in the superior cerebellum with more complex tasks (Bohland and Guenther, 2006; Riecker et al, 2006; Riecker et al., 2008; Soros et al., 2006). For example, Bohland and Guenther (2006) found increased bilateral activation of superior cerebellum in Lobule VI and Crus I / II, as well as, inferior cerebellum in Lobule VIII when subjects spoke complex sequences (with three unique syllables) versus simpler sequences. We also note that our findings are consistent with other research that has compared covert and overt speech tasks and generally found that response magnitudes are blunted in covert task contexts (Barch et al., 1999; Huang et al., 2001; Palmer et al., 2001; Shuster and Lemieux, 2005). This blunted activation may have inhibited our ability to detect differences between the working memory task and covert speech task as well as our ability to find areas particularly important for covert speech. Overall, our findings and previous work suggest that overt speech and covert speech are not the same (Barch et al., 1999; Huang et al., 2001; Palmer et al., 2001; Shuster and Lemieux, 2005), with overt speech recruiting motor-processing regions and inner speech recruiting regions in which coding occurs at more abstract levels.
5. Conclusion
We provide some support for the Desmond et al. (1997) model of working memory in the cerebellum. Specifically, we find that the cerebellum shows functional specificity across the superior and inferior sectors. However, we also find some important points of divergence. First, the superior cerebellum should be functionally subdivided into at least two regions, a superio-medial region involved in more motor-speech based processes and a superio-lateral region that is more specific to working memory processing. Second, in our serial recall task we found that the inferior sector of the cerebellum is right-dominant, but the activation is not exclusive to the right hemisphere nor does the pattern of lateralization differ from that observed in the other sectors of the cerebellum. Finally, it may be important to reconsider the role of covert speech in working memory and how it may be mediated and detected in the brain. Finally, though our findings do not support some of the evidential basis for the model proposed by Desmond et al. (1997), it would be premature to conclude that key conceptual components of the model are necessarily wrong. In fact, in other lines of work we have found the model provides a useful framework for understanding how cerebellar brain damage and stimulus manipulations may affect verbal working memory performance. Instead, we believe that the current results underscore the need for further research on the role of the cerebellum in working memory, especially work that examines the specific contributions of distinct subregions of the cerebellum and that seeks to delineate the interrelationship between cortical and cerebellar processing.
Acknowledgements
This work was supported by a grant from the National Institutes of Health, MH59256.
The authors would like to thank Kate Fissell and Kwan-Jin Jung for technical assistance and Gal Ben-Yehudah for discussion. We would also like to thank the anonymous reviewers for their suggestions and insight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H, Wildgruber D, Daum I, Grodd W. Does the cerebellum contribute to cognitive aspects of speech production? A functional magnetic resonance imaging study (fMRI) in humans. Neuroscience Letters. 1998;247:187–190. doi: 10.1016/s0304-3940(98)00328-0. [DOI] [PubMed] [Google Scholar]
- Allen G, McColl R, Barnard H, Ringe WK, Fleckenstein J, Cullum CM. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. NeuroImage. 1998;28:39–48. doi: 10.1016/j.neuroimage.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. New York, NY: Clarendon Press/Oxford University Press; 1986. [Google Scholar]
- Baddeley AD. The episodic buffer: A new component of working memory? Trends in Cognitive Sciences. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. In: The psychology of learning and motivation. GH B, editor. San Diego: Academic Press; 1974. pp. 47–90. [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DG, Cohen JD. Overt verbal responding during fmri scanning: Empirical investigations of problems and potential solutions. NeuroImage. 1999;10:642–657. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Benson DF. Aphasia, alexia, and agraphia. New York, NY: Churchill Livingstone; 1979. [Google Scholar]
- Bishop DV, Robson J. Unimpaired short-term memory and rhyme judgments in congenitally speechless individuals: Implications for the notion of "Articulatory coding". Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 1989;41:123–140. [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. NeuroImage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition ii: An empirical review of 275 pet and fmri studies. Journal of Cognitve Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fiez JA. Dissociation of verbal working memory system components using a delayed serial recall task. Cerebral Cortex. 2001;11:1003–1014. doi: 10.1093/cercor/11.11.1003. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fissell K, Jacobs S, Fiez JA. Functional heterogeneity within broca's area during verbal working memory. Physiology and Behavior. 2002;77:635–639. doi: 10.1016/s0031-9384(02)00899-5. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage. 2005a;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005b;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Cox RW. Afni: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damasio A, Damasio H. Aphasia and the neural basis of language. In: Mesulum MM, editor. Principals of behavioral and cognitive neurology. New York, NY: Oxford University Press; 2000. pp. 294–315. [Google Scholar]
- Desmond JE, Gabrieli JDE, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional mri. Journal of Neuroscience. 1997;17(24):9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers N, Wilkins D, Van Valin R, Redfern B, Jaeger J. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Cerebellar contributions to cognition. Neuron. 1996;16:13–15. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Peterson SE. A positron emission tomography study of the short-term maintenance of verbal information. Journal of Neuroscience. 1996;16(2):808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissell K, Tseytlin E, Cunningham D, Iyer K, Carter CS, Schneider W, et al. A graphical computing environment for neuroimaging analysis. Neuroinformatics. 2003;1:111–125. doi: 10.1385/ni:1:1:111. [DOI] [PubMed] [Google Scholar]
- Guediche S, Ben-Yehudah G, Fiez JA. Examining the neural basis of the phonological similarity effect in verbal working memory; Paper presented at the 14th Annual Meeting of the Cognitive Neuroscience Society; San Francisco, CA, USA. 2006. [Google Scholar]
- Henson R. Serial order in short-term memory. Psychologist. 2001;14:70–73. [Google Scholar]
- Henson R, Hartley T, Burgess NA. Selective interference with verbal short-term memory for serial order information: A new paradigm and tests of a timing-signal hypothesis. The Quarterly Journal of Experimental Psychology. 2003;56A:1307–1334. doi: 10.1080/02724980244000747. [DOI] [PubMed] [Google Scholar]
- Henson RN, Burgess N, Frith CD. Recoding, storage, rehearsal and grouping in verbal short-term memory: An fmri study. Neuropsychologia. 2000;38:426–440. doi: 10.1016/s0028-3932(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using eventrelated fmri. Human Brain Mapping. 2001;15:39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K, Prasad P, Qin Y, Anderson JR. Extraction of overt verbal response from the acoustic noise in a functional magnetic resonance imaging scan by use of segmented active noise cancellation. Magnetic Resonance in Medicine. 2005;53:739–744. doi: 10.1002/mrm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Chen SHA, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: An fmri study. NeuroImage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cerebral Cortex. doi: 10.1093/cercor/bhp135. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak K, Hertrich I, Grodd W, Ackermann H. Cerebellum and speech perception: A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2002;14:902–912. doi: 10.1162/089892902760191126. [DOI] [PubMed] [Google Scholar]
- Palmer ED, Rosen HJ, Ojemann JG, Buckner RL, Kelley WM, Petersen SE. An event-related fmri study of overt and covert word stem completion. NeuroImage. 2001;14:182–193. doi: 10.1006/nimg.2001.0779. [DOI] [PubMed] [Google Scholar]
- Papathanassiou D, Etard O, Mellet E, Zago L, Mazoyer B, Tzourio-Mazoyer N. A common language network for comprehension and production: A contribution to the definition of language epicenters with PET. NeuroImage. 2000;11:347–357. doi: 10.1006/nimg.2000.0546. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. NeuroReport. 2000;11:1997–2000. doi: 10.1097/00001756-200006260-00038. [DOI] [PubMed] [Google Scholar]
- Riecker A, Kassubeck J, Groschel K, Grodd W, Ackermann H. The cerebral control of speech tempo: Opposite relationship between speaking rate and BOLD signal changes at striatal and cerebellar structures. NeuroImage. 2006;29:46–53. doi: 10.1016/j.neuroimage.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Riecker A, Brendel B, Ziegler W, Erb M, Ackermann H. The influence of syllable onset complexity and syllable frequency on speech motor control. Brain and Language. 2008;107:102–113. doi: 10.1016/j.bandl.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Salamé P, Baddeley AD. Disruption of short-term memory by unattended speech: Implications for the structure of working memory. Journal of Verbal Learning and Verbal Behavior. 1982;21(2):150–164. [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. Mri atlas of the human cerebellum. San Diego, CA: Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
- Shuster LI, Lemieux SK. An fmri investigation of covertly and overtly produced mono- and multisyllabic words. Brain and Language. 2005;93(1):20–31. doi: 10.1016/j.bandl.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal working memory: Evidence from neuroimaging. Proceedings of the National Academy of Science. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros P, Sokoloff LG, Bose A, McIntosh AR, Graham SJ, Stuss DT. Clustered functional MRI of overt speech production. NeuroImage. 2006;32:376–387. doi: 10.1016/j.neuroimage.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strelnikov KN, Vorobyev VA, Chernigovsky TV, Medvedev SV. Prosodic cues to syntactic processing - a PET and ERP study. NeuroImage. 2006;29:1127–1134. doi: 10.1016/j.neuroimage.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and non-motor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: An approach to medical cerebral imaging. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Timmann D, Daum I. Cerebellar contributions to cognitive functions: A progress report after two decades of research. The Cerebellum. 2007;6:159–162. doi: 10.1080/14734220701496448. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. Rapid automated algorithm for aligning and reslicing pet images. Journal of Computer Assited Tomography. 1993;17:536–546. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]