Abstract
Prematurely born children are at increased risk for language deficits at school age and beyond, but the neurobiological basis of these findings remains poorly understood. Thirty-one PT adolescents (600 – 1250 g birth weight) and 36 T controls were evaluated using an fMRI passive language task and neurodevelopmental assessments including: the Wechsler Intelligence Scale for Children-III (WISC-III), the Peabody Picture Vocabulary Test-Revised (PPVT-R), the Comprehensive Test of Phonological Processing (CTOPP) and the Test of Word Reading Efficiency (TOWRE) at 16 years of age. Neural activity was assessed for language processing and the data were evaluated for connectivity and correlations to cognitive outcomes. PT subjects scored significantly lower on all components of the WISC-III (p < 0.05) compared to term subjects, but there was no significant difference in PPVT-R scores between the groups. Functional connectivity (fcMRI) between Wernicke’s area (left BA 22) and the right supramarginal gyrus (BA 40) was increased in preterm subjects relative to term controls (p = 0.03), and the strength of this connection was inversely related to performance on both the PPVT-R (R2 = 0.553, p = 0.002), and the verbal comprehension index (R2 = 0.439, p = 0.019). Preterm adolescents engage a dorsal right hemisphere region for language at age 16 years. Those with the greatest cognitive deficits demonstrate increasing reliance on this alternate pathway.
Keywords: connectivity, functional magnetic resonance imaging, premature, birth weight, and outcomes
INTRODUCTION
Preterm birth represents a major pediatric public health problem in the United States and Europe today.(Behrman and Stith Butler, 2007) The neurocognitive sequelae of preterm birth have been well described,(Allin et al., 2008; Hack et al., 2009; Hille et al., 2007; Saigal and Doyle, 2008; Verrips et al., 2008) and the development of sophisticated magnetic resonance imaging (MRI) strategies has provided significant information about the impact of preterm birth on the developing brain. Preterm subjects have widespread structural and microstructural changes when compared to term control subjects in studies ranging from the newborn period to adolescence and young adulthood.(Anjari et al., 2009; Dubois et al., 2008; Gimenez et al., 2008; Nagy and Jonsson, 2009; Skranes et al., 2009) In contrast to these presumptive markers of adverse outcome, functional MRI (fMRI) strategies from several investigators demonstrate the recruitment of alternative neural systems for language in the prematurely-born,(Ment et al., 2006b; Narberhaus et al., 2009; Nosarti et al., 2009; Rushe et al., 2004) and several recent reports suggest that cognitive outcomes for this vulnerable population may depend not only on the gestational age at birth but also on the maternal level of education.(Hack et al., 2009; Weisglas-Kuperus et al., 2009) Functional connectivity (fcMRI), which assesses the functional organization of the developing brain, has been less well studied than other imaging strategies in the preterm population. By identifying those regions that activate together either during a particular task or at resting state, fcMRI may provide important information about the differential engagement of alternative neural systems in the developing preterm brain.
Numerous cohort studies have revealed inferior educational outcomes in preterm subjects. At school entry, minor developmental impairment is diagnosed in 30 – 40% and major disabilities are found in almost 20% of preterm children.(Allen, 2008; Larroque et al., 2008; Neubauer et al., 2008; Saigal and Doyle, 2008; Voss et al., 2007) Over half require special assistance in the classroom, 20% are in special education and 15% have repeated at least one grade in school.(Aylward, 2005; Bhutta et al., 2002) Furthermore, although a majority of prematurely-born neonates have recently been shown to become independent adults, the intellectual deficits of preterm subjects may persist through adolescence and young adulthood.(Saigal et al., 2006)
Imaging plays an important role in understanding the neurobiology underlying the cognitive deficits of preterm children. When compared to term control subjects, preterm subjects have global and regional decreases in cortical gray and deep gray matter, less myelinated white matter, smaller corpus callosal areas and significant ventriculomegaly.(Allin et al., 2004; Boardman et al., 2007; Huppi et al., 1998; Inder et al., 1999; Inder et al., 2005; Mewes et al., 2006; Peterson et al., 2003) Diffusion tensor imaging (DTI) provides information about the microstructure of the developing brain through measures such as fractional anisotropy (FA). FA increases with increasing myelination, axonal diameter and fiber bundle packing. Widespread decreases in FA have been reported in PT children compared to term controls in studies ranging from infancy through young adulthood(Anjari et al., 2009; Anjari et al., 2007; Skranes et al., 2007) and DTI parameters have been shown to significantly correlate with cognitive and motor outcomes for the prematurely-born.(Constable et al., 2008; Counsell et al., 2008; Skranes et al., 2009)
More recently, studies using fMRI have identified differences in the brain areas activated during language and executive tasks in preterm children when compared to term control subjects, and preliminary studies investigating fcMRI in preterm subjects during the newborn period and at school age suggest that, when compared to term control subjects, preterm subjects exhibit different patterns of connectivity between brain areas both at rest and during a functional task. (Doria et al., 2009; Doria et al., 2008; Fransson et al., 2007; Gozzo et al., 2009; Smyser et al., 2009) Resting state literature suggests that functional connectivity is at least partially anatomically determined, but the role of these alternative pathways remains poorly understood.
In order to test the hypothesis that the alternative neural connections found in prematurely born subjects at adolescence may be associated with cognitive measures including language skills, the connectivity between language regions during an fMRI language task was investigated in preterm and term subjects by correlating the strength of the differential connections with language measures. Wernicke’s area, in the left superior temporal gyrus (left Brodmann’s area 22), was chosen as a reference region for functional connectivity analysis based on findings from a previous study showing significant differences in functional connectivity levels between eight year old preterms and terms. Functional connectivity to Wernicke’s area was evaluated in other primary language regions in the left hemisphere and their right-sided homologues.
Because intraventricular hemorrhage, periventricular leukomalacia and low-pressure ventriculomegaly have been associated with adverse cognitive outcomes in the prematurely born(Ment et al., 1996), our a priori hypothesis was tested in preterm subjects who were free of these lesions in both neonatal cranial ultrasound studies and magnetic resonance images at age 16 years.
METHODS
This study was performed at Yale University School of Medicine, New Haven, CT, and Warren Alpert Brown Medical School, Providence RI. The protocols were reviewed and approved by institutional review boards at each location. All scans were obtained and analyzed at Yale University.
Subjects
The preterm cohort consisted of children who were enrolled in the follow-up MRI component of the Multicenter Randomized Indomethacin IVH Prevention Trial at Yale University School of Medicine and Brown University.(Ment et al., 1994) Only those preterm children who lived within 200 miles of the Yale MRI Research Facility were eligible for this protocol. From a potential pool of 191 preterm subjects, 43 preterm children provided written assent, parental written consent, and participated in a scanning session. Of these 43, data from 12 were removed from analysis because of the presence of intraventricular hemorrhage, ventriculomegaly or periventricular leukomalacia (11) or excess motion artifact on MRI (1). Term control children were recruited from the local communities of the study children. They were group-matched to the PT children for age, sex, and minority status. Forty-two term children provided written assent, parental written consent and participated in a scanning session. Of these 42, data from 6 were removed from analysis because of excess motion artifact (4) or because of poor performance on the in magnet assessment designed to evaluate the subjects’ understanding of the in magnet task (2). Thus, of the 85 total children who participated in the scanning session, 67 were eligible for analysis, with a total of 5 subjects removed from analysis due to excess motion artifact. There was no difference in motion artifact between the groups (p = 0.38). Mothers were interviewed at the time of enrollment as to their level of educational attainment.
Neurodevelopmental Assessment
Blinded assessment was performed at 16 years of age in a separate session from the fMRI scan using the Wechsler Intelligence Scale for Children-III (WISC-III)(Wechsler, 1991), the Peabody Picture Vocabulary Test-Revised (PPVT-R)(Peabody, 1981), the Comprehensive Test of Phonological Processing (CTOPP)(Wagner et al., 1999) and the Test of Word Reading Efficiency (TOWRE)(Torgesen et al., 1999). The WISC-III is an intelligence test with scores assessing full scale intelligence quotient (FSIQ), performance IQ (PIQ), verbal IQ (VIQ) and the verbal comprehension index (VCI). The VCI score is derived from four subscales: vocabulary, similarities, information and comprehension. The PPVT-R is designed to assess receptive vocabulary, while the CTOPP provides measures of phonologic awareness, phonological memory, and rapid naming.
Task Paradigm During fMRI Scanning
We employed an event-related cue-target identity task that required a match/mismatch judgment between pictures and words that were presented acoustically and/or in printed form on each trial. Responses were made via a button press. Between 8 to 10 runs were completed per subject. This task is described in detail in Frost et al.(Frost et al., 2009)
fMRI Data Analysis
Preprocessing
All data were converted from Digital Imaging and Communication in Medicine (DICOM) format to Analyze format using XMedCon (http://xmedcon.sourceforge.net/). During the conversion process, the first four images at the beginning of each of the ten functional series were discarded to enable the signal to achieve steady-state, leaving 109 measurements for analysis. Images were first slice time corrected using sinc interpolation and then motion corrected using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). Runs with linear motion in excess of 1.5 mm or rotation greater than 2 degrees were discarded. All voxels with signal less than 5% of the maximum were set to zero, drift removal (up to 3rd order) and temporal Gaussian smoothing (standard deviation = 1) were then performed on the time-course of each voxel. Finally, the global time-course was regressed out.
Registration to a common reference space
To take individual subject data into a common reference space, three registrations were calculated within the Yale BioImage Suite software package (http://www.bioimagesuite.org).(Duncan et al., 2004) The first was a linear registration between the individual subject’s raw functional data and that subject's T1 anatomical image collected at the same slice locations. The T1 anatomical image was then linearly registered to the individual's 1 mm isotropic MP-Rage anatomical image. Finally, a non-linear registration was computed between the individual’s MP-Rage anatomical image and the Colin27 Brain (Holmes et al., 1998) in order to transform data into the standardized space defined by the Montreal Neurological Institute (MNI). The inverse transformation from the MNI space to the individual functional space was also computed.
Functional connectivity maps
A reference region in Wernicke’s area was defined anatomically on the MNI reference brain as the posterior portion of left BA 22 with center of mass Talairach coordinates of −54,−36,10 [consistent with Gozzo et al, 2009(Gozzo et al., 2009)], and transformed back (via the inverse transform obtained as described above) into individual subject space. The time-course of the reference region in a given subject was then computed as the average time-course across all pixels in the reference region. This time-course was correlated (using Pearson correlation) with the time-courses of all other voxels in the brain to create a map of r-values. These r-values were transformed to z-values using Fisher’s transform, and averaged across scans in each subject to yield one map for each subject representing the strength of correlation to Wernicke’s area in terms of Gaussian variables, which was then spatially smoothed (6mm diameter Gaussian).
Regions of Interest Analyses
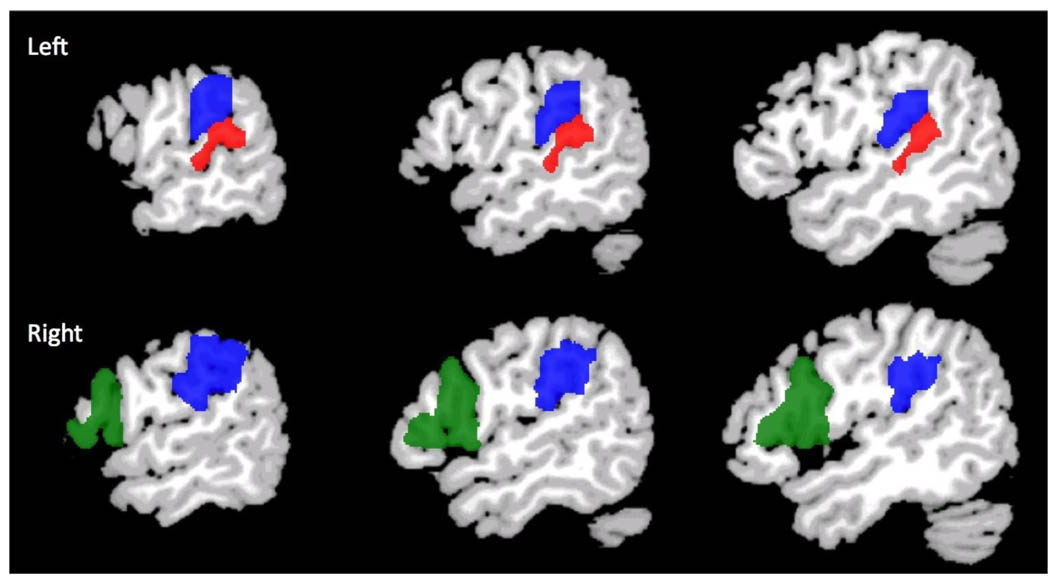
Three a-priori regions of interest (ROIs), shown in Figure 1, were defined as those areas that showed significant differences in activation levels between 8 year old preterms and terms in a previous study.(Gozzo et al., 2009) These were anatomically delineated regions defined to include the inferior portion of the left supramarginal gyrus (left BA 40), the inferior portion of the right supramarginal gyrus (right BA 40) and the homologue of Broca’s area (right BA 44/45). Talairach coordinates of center of mass and volumes (mm3) for these regions are given in Table 1. For all three regions, the inverse transformation from the MNI space to the individual functional space was also computed.
Figure 1.
Anatomic ROIs where connectivity to left Wernicke’s area (red) was assessed. Left and right BA 40 are shown in blue, and the ROI in right BA 44/45 is green.
Table 1.
Talairach coordinates for connectivity from Wernicke's area (−54,−36,10)
| Brodmann's Areas (BA) | Talairach coordinates | Volume (mm3) |
|---|---|---|
| Left BA 40 | 40, −53,−33 | 8122 |
| Right BA 40 | 40, 56, −30 | 12443 |
| Right BA 44–45 | 46, 15, 14 | 20874 |
Of note, the children in the 8 year old study from which the ROIs were taken performed a different in-magnet task than that employed for the 16 year old subjects.
Statistical Analyses
Demographic and cognitive data were analyzed using standard chi-squared statistics for categorical data, and nonparametric Wilcoxon rank sum tests for continuous-valued data. Individual general linear models were run with connectivity data for each of the three designated ROI as the dependent variable to determine the contribution of group (PT vs. T) to regional connectivity. The independent variables included group, age at scan and gender as well as the group-by-gender interaction term.
Correlations between Functional Connectivity and Language Measures
For the region of interest that showed significant differences in connectivity to Wernicke’s in PT compared to T, R BA 40, general linear models were run with language measures including standard scores for PPVT-R, VCI, the WISC-III vocabulary subscore, the CTOPP and the TOWRE as the dependent variables. The independent variables were strength of connection, age at scan, gender, group, maternal education (4 level) and the R BA 40 connectivity-by-group interaction term. In addition to main effects, interactions between the strength of connection and gender and group were examined. Where significant strength of connection by group effects were observed, secondary analyses were run for each group separately with language measure as the dependent variable and strength of connection, age at scan, gender and maternal education as independent variables.
Scatter plots showing the relationship between cognitive variables and connectivity were made. To assess the effects of leverage in these plots, robust regressions were performed for each analysis.
Correlations between connectivity and perinatal variables
The relationship between connectivity data (described below) and neonatal variables was assessed in the preterm group using a general linear model. Perinatal variables included gender, birth weight, randomization to indomethacin vs. placebo, bronchopulmonary dysplasia(Tooley, 1979) and maternal education in four levels (< high school, high school graduate, some college, and college graduate). GLM also were used to investigate the contribution of maternal education to connectivity from L Wernicke’s area to R BA 40 for all study subjects.
All statistical analyses were performed using SAS 9.1.3, and all p-values are two-sided.
RESULTS
Subjects and Neurodevelopmental Assessment
Thirty-one preterm children and 36 term controls participated in this study. Perinatal data for preterm children are described in Table 2. As shown in Table 3, there were no significant differences between the preterm and term groups in the number of males, minority status, special services received or handedness. There were differences between the groups in maternal education (p = 0.03) and participant age at the time of scan (p = 0.04). Although preterm adolescents scored significantly lower than term controls on FSIQ, VIQ, VCI, PIQ and TOWRE, there were no significant differences in the PPVT-R, CTOPP or WISC-III vocabulary scores for the groups.
Table 2.
Perinatal Data for Preterm Subjects
| N (%) | |
|---|---|
| Number | 31 |
| Male | 19 (61) |
| Gestational Age (weeks) | 27.7 ± 2.0 |
| Birth Weight (grams) | 933.1 ± 189.3 |
| Randomized to Indomethacin | 17 (55) |
| Antenatal Steroids | 11 (35) |
| Bronchopulmonary Dysplasia | 9 (29) |
| Necrotizing Enterocolitis | 4 (13) |
Table 3.
Demographic and Cognitive Data for Study Children
| Preterm | Term | p value | |
|---|---|---|---|
| Number | 31 | 36 | |
| Male | 19 (61%) | 15 (42%) | 0.14 |
| Right-handed | 26 (84%) | 35 (97%) | 0.08 |
| Minority | 13 (42%) | 13 (36%) | 0.89 |
| Full Scale IQ | 92 ± 17 | 108 ± 17 | 0.003 |
| Verbal IQ | 95 ± 18 | 107 ± 16 | 0.02 |
| Verbal Comprehension Index | 96 ± 17 | 107 ± 16 | 0.02 |
| WISC Subtest: Vocabulary | 8.9 ± 4.0 | 10.6 ± 2.8 | 0.17 |
| Performance IQ | 90 ± 17 | 107 ± 18 | 0.001 |
| PPVT-R | 98 ± 22 | 107 ± 23 | 0.16 |
| TOWRE: Standard Score | 89 ± 11 | 97 ± 14 | 0.03 |
| CTOPP: Rapid Naming Composite | 97 ± 16 | 101 ± 14 | 0.36 |
| Special Services | 8 (25%) | 3 (8%) | 0.10 |
| Age at Scan (years) | 16 ± 0.3 | 16 ± 0.4 | 0.04 |
| Maternal Education | |||
| < High School Graduate | 4 (13%) | 1 (3%) | 0.03 |
| High School Graduate | 13 (42%) | 13 (36%) | |
| Some College | 9 (29%) | 7 (19%) | |
| College Graduate | 5 (16%) | 15 (42%) |
ROI Analyses
As shown in Table 4, the right supramarginal gyrus, BA 40, was significantly more functionally connected to Wernicke’s area in preterms than in terms (p = 0.025). There was a trend (p = 0.058) in the difference between groups in functional connectivity from left supramarginal gyrus to Wernicke’s area. In contrast, there was no difference between groups in connectivity from Wernicke’s area to the right homologue of Broca’s area, BA 44 – 45(p = 0.84).
Table 4.
Connectivity to Left Wernicke's Region (Least Square Means)
| BA Regions | Preterm | Term | p value |
|---|---|---|---|
| Left BA 40 | 0.148 ± 0.01 | 0.126 ± 0.01 | 0.058 |
| Right BA 40 | 0.099 ± 0.01 | 0.077 ± 0.01 | 0.025 |
| Right BA 44–45 | 0.016 ± 0.01 | 0.018 ± 0.01 | 0.838 |
data covaried for age at scan, gender and group-by-gender interaction
In addition, since 25 subjects (13 PT and 12 term controls) took part in both the 8 year old connectivity study from which the ROIs were taken, the connectivity analysis for L Wernicke’s area to R BA 40 was repeated with the 42 cross-sectional non-longitudinal subjects; a trend significance was noted for greater connectivity in the PT group (p = 0.06).
Connectivity-Cognition Relationships
There was a significant effect of the strength of the connection between Wernicke’s area and right BA 40 on PPVT-R scores (R2 = 0.357, p = 0.02), a significant effect of group on PPVT-R scores (p = 0.024) and a significant interaction between strength of connection and group (p = 0.009). A significant effect of maternal education (p = 0.002) also was found, indicating the important and independent effect of several factors. To explore the interaction between strength of connection and group in predicting PPVT-R scores, separate general linear model analysis was performed for each group. As shown in Table 5, the strength of the connection to right BA 40 had a significant effect on PPVT-R scores in the preterm group (R2 = 0.553, p < 0.002), but in not the term controls (Table 6). For the preterm group, there was an effect of maternal education (p = 0.02) but no main effects of age at scan or gender. These results were confirmed using a robust regression analysis evaluating the impact of connectivity to the PPVT-R score (p < 0.001 for both adjusted and unadjusted analyses).
Table 5.
GLM for Connectivity to R BA 40 and Cognitive Measures for Preterm Subjects
| Cognitive Measures | R2 | p value |
|---|---|---|
| PPVT-R | 0.553 | 0.002 |
| VCI | 0.439 | 0.019 |
| WISC Subtest: Vocabulary | 0.502 | 0.004 |
data covaried for age at scan, gender and maternal education
Table 6.
Correlations for Connectivity to R BA 40 and Cognitive Measures for Term Subjects
| Cognitive Measures | R2 | p value |
|---|---|---|
| PPVT-R | 0.167 | 0.746 |
| VCI | 0.213 | 0.411 |
| WISC Subtest: Vocabulary | 0.229 | 0.370 |
data covaried for age at scan, gender and maternal education
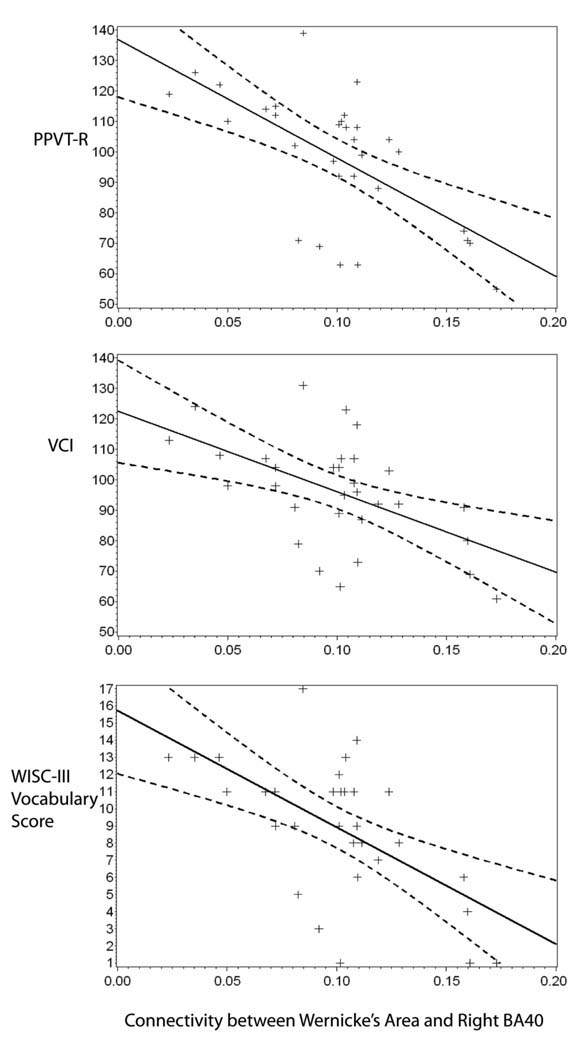
A scatter plot showing the relationship between the strength of this connection in the preterm subjects and their PPVT-R scores is provided in Figure 2, top panel. This figure shows that a stronger connectivity between Wernicke’s area and right BA40 in preterm subjects was associated with decreasing performance on the PPVT-R.
Figure 2.
Scatterplots depict connectivity-cognitive relationships in the preterm subjects. The strength of connection between Wernicke’s area and the ROI in right BA40 was inversely related to scores on the PPVT-R (top plot, y = 137–388*x), the VCI (middle plot, y = 122–264*x) and the vocabulary score of the WISC-III (bottom plot, y = 16–68*x).
In addition, there was a significant group-by-region effect for the strength of the connection between Wernicke’s area and right BA 40 on VCI scores (R2 = 0.391, p = 0.01), trend effects for both group and ROI on VCI scores (p = 0.07 and p = 0.096, respectively), and a main effect of maternal education (p < 0.001). Group-specific general linear models revealed that the strength of the connection to right BA 40 had a significant effect on VCI scores in the preterm group (R2 = 0.439, p = 0.019, Table 5), but in not the term controls (Table 6). Neither age at scan nor gender were significant predictors in the preterm subjects, while maternal education was statistically significant (p = 0.031). As shown in the middle panel of Figure 2, greater connectivity between these regions was associated with decreasing VCI scores for the preterm group.
In contrast, there were no significant effects of the strength of the connection between Wernicke’s area and right BA 40 on either the CTOPP or the TOWRE scores (p ≥ 0.8 for both).
Because the vocabulary subtest of the WISC-III assesses a similar language function as the PPVT-R, a post-hoc analysis was performed to test the hypothesis that this language measure would also correlate with connectivity between Wernicke’s area and right BA 40. A significant effect of the strength of this connection on vocabulary scores was detected (R2 = 0.425, p = 0.02), along with an effect of group on vocabulary scores (p = 0.005), a significant interaction between strength of connection and group (p = 0.001), and a main effect of maternal education (p < 0.001). Group-specific general linear models were performed and revealed that the strength of the connection to right BA 40 had a significant effect on vocabulary scores in the preterm group (R2 = 0.502, p = 0.004, Table 5), but not in the term controls (R2 = 0.23, p = 37, Table 6). Neither age at scan nor gender were significant, but maternal education was important in the preterm group (p = 0.035) and the term group (p = 0.009). As shown in the bottom panel of Figure 2, greater connectivity between these regions was associated with decreasing vocabulary scores for the preterm group (R2 = 0.502, p = 0.004, Table 5).
Exploratory analyses of perinatal risk factors
Exploratory analyses were performed to examine those factors that have been associated with microstructural changes in the preterm brain. These included gender, age at scan, birth weight, randomization to early indomethacin, bronchopulmonary dysplasia and levels of maternal education. Regression analysis suggested that, in the presence of gender, age at scan, birth weight and randomization to indomethacin, both bronchopulmonary dysplasia and level of maternal education (model R2 = 0.408, p = 0.04 and 0.03, respectively) contributed to connectivity between Wernicke’s and right BA 40. In this analysis, increasing years of maternal education was inversely related to connectivity between these two regions.
Although perinatal data are not available for the term control group, a second GLM investigating the impact of maternal education on connectivity between Wernicke’s and right BA 40 for the term controls demonstrated that, in the presence of age at scan and gender, there was a trend for maternal education (p = 0.07).
DISCUSSION
Preterm birth and, by extension, the longterm neurocognitive sequelae of the prematurely born represent major public health problems in the United States today. Thus, understanding the influence of birth at an early gestational age on brain development becomes a priority for neonatologists and neuroscientists alike. The development of sophisticated neuroimaging strategies has facilitated this goal, and we report alterations in neural connectivity for language in preterm adolescents when compared to term controls.
Preterm subjects exhibited increased connectivity from Wernicke’s region to a dorsal right hemisphere language region: right BA 40. The strength of this connection was inversely related to performance for the preterm adolescents on three well-standardized semantic tasks: the PPVT-R, the VCI and the WISC-III vocabulary subtest. Microstructural findings have been reported in preterm neonates with respiratory difficulties(Anjari et al., 2009), and our analyses suggest that adolescents with neonatal BPD exhibit greater connectivity from Wernicke’s area to right BA 40. Further, maternal education is a major predictor of cognitive outcome in preterm subjects at young adulthood(Weisglas-Kuperus et al., 2009), and those subjects whose mothers had the lowest levels of education have the greatest connectivity between Wernicke’s and R BA 40 in our analyses. This supports our finding that this pathway is used most by those with the lowest testing scores.
Adolescence is a time of significant cortical maturation, and the present studies expand and extend our previous work investigating the impact of preterm birth on the developing brain. Earlier fMRI studies from this cohort have shown that preterm children recruit right hemisphere regions in response to a passive listening task at age 12 years,(Ment et al., 2006a) and our recent fcMRI analyses suggest increased connectivity to right hemisphere language regions at age 8 years(Gozzo et al., 2009). At 8 years, however, there were no significant cognitive correlates in this cohort, while at 16 years we identified a similar increased right sided connectivity, now correlating significantly to poor cognitive task performance. Recent descriptions of language systems in typically developing adults and children suggest the engagement of a ventral pathway for semantic processing and a dorsal system for phonologic needs.(Hickok and Poeppel, 2007; Saur et al., 2008) This study reports the engagement of the dorsal right hemisphere language center for semantic tasks and increased use of this putative phonologic connection for semantic processing by those subjects with the greatest semantic deficits.
Recent studies of functional connectivity in typically developing children reveal age-dependent patterns of cortical maturation. Resting state networks are present in infancy (Fransson et al., 2009), and the maturation of neural networks continues throughout adolescence and young adulthood.(Fair et al., 2008; Fair et al., 2007) As maturation progresses, the developing brain increases the strength of connections that exist in spatially remote regions in an anterior-posterior direction to create highly cohesive functional circuits, while simultaneously reducing the strength of connections between adjacent regions and contralateral homologues.(Fair et al., 2009; Stevens et al., 2009; Supekar et al., 2009) Further, although preliminary data from adolescents with dyslexia suggest that abnormalities in functional connectivity may correlate with cognitive measures, the neurocognitive correlates of maturing cortical connections are as yet unknown.(Wolf et al., 2009)
The limitations of this study include the small sample size, incomplete voxel-based morphometric and diffusion tensor data for the cohort and the lack of serial functional connectivity studies over time. The subjects we report are part of a well-studied cohort with neuroimaging available from the earliest postnatal hours. Although MRI investigations were not available in the newborn period for these subjects, sequential evaluation with a variety of imaging modalities has documented their progress in terms of cognitive performance, volumetric differences, cortical activation and microstructural alterations in normal cortical development over time. Although recent data in adult subjects suggest a correlation between structure and function, we had neither complete volumetric data nor diffusion tensor imaging in this cohort of adolescents. Further, although a subset of subjects in the current report participated in our previous functional connectivity report of 8 year old preterm subjects and term controls, two factors prevent the report of a longitudinal analysis: the selection of a different language task for the 16 year old cohort, and the unavailability of study subjects secondary to both orthodontic appliances and geographic availability. The use of fMRI to evaluate functional connectivity in adolescents is an emerging technology with little known yet about those complex factors such as maternal education which contribute to the findings we report.
Preterm birth results in alterations in connectivity in the developing brain. The engagement of an auxiliary right hemisphere language region in those preterm children with the greatest cognitive need suggests that longitudinal studies must be done. The connectivity to right BA 40 may represent either a delay in cortical maturation in the prematurely born, or the recruitment of alternate circuits for language in the developing preterm brain.
Acknowledgements
This work was supported in part by NS 27116, MO1-RR06022, MO1-RR00125, and T32 HD 07094
We thank Drs. Deborah Hirtz and Walter Allan for their scientific expertise; Marjorene Ainley for follow-up coordination; Jill Maller-Kesselman, Susan Delancy and Victoria Watson for neurodevelopmental testing; and Hedy Sarofin and Terry Hickey for their technical assistance.
ABBREVIATIONS
- BA
Brodmann’s area
- CTOPP
Comprehensive Test of Phonological Processing
- fcMRI
Functional Connectivity Magnetic Resonance Imaging
- fMRI
Functional Magnetic Resonance Imaging
- FSIQ
Full Scale Intelligence Quotient
- GLM
General linear model
- PIQ
Performance Intelligence Quotient
- PPVT-R
Peabody Picture Vocabulary Test-Revised
- PT
Preterm
- ROI
Region of Interest
- SPM
Statistical Parametric mapping
- T
Term
- TOWRE
Test of Word Reading Efficiency
- VCI
Verbal Comprehension Index
- VIQ
Verbal Intelligence Quotient
- WISC-III
Wechsler Intelligence Scale for Children-III
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21:123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- Allin M, Henderson M, Suckling J, Nosarti C, Rushe TM, Fearon P, Stewart AL, Bullmore ET, Rifkin L, Murray RM. Effects of very low birthweight on brain structure in adulthood. Dev Med Child Neurol. 2004;46:46–53. doi: 10.1017/s0012162204000088. [DOI] [PubMed] [Google Scholar]
- Allin M, Walshe M, Fern A, Nosarti C, Cuddy M, Rifkin L, Murray R, Rushe T, Wyatt J. Cognitive maturation in preterm and term born adolescents. J Neurol Neurosurg Psychiatry. 2008;79:381–386. doi: 10.1136/jnnp.2006.110858. [DOI] [PubMed] [Google Scholar]
- Anjari M, Counsell SJ, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD. The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics. 2009;124:268–276. doi: 10.1542/peds.2008-1294. [DOI] [PubMed] [Google Scholar]
- Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards DA, Counsell SJ. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Neurodevelopmental outcomes of infants born prematurely. Devel Behav Ped. 2005;26:427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- Behrman RE, Stith Butler A. Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes Board on Health Sciences Outcomes: Preterm Birth: Causes, Consequences, and Prevention. Washington, D.C.: The National Academies Press; 2007. [PubMed] [Google Scholar]
- Bhutta AD, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm. A meta-analysis. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Boardman JP, Counsell SJ, Rueckert D, Hajnal JV, Bhatia KK, Srinivasan L, Kapellou O, Aljabar P, Dyet LE, Rutherford MA, Allsop JM, Edwards AD. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol. 2007;62:185–192. doi: 10.1002/ana.21171. [DOI] [PubMed] [Google Scholar]
- Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, DeLancy S, Katz KH, Schneider C, Schafer RJ, Makuch RW, Reiss AL. Prematurely born children demonstrate white matter microstructural differences at age 12 years relative to term controls: An investigation of group and gender effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Edwards AD, Chew ATM, Anjari M, Dyet LE, Srinivasan L, Boardman JP, Allsop JM, Hajnal JV, Rutherford MA, Cowan FM. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Rees G, Counsell SJ, Merchant N, Edwards DA. Spontaneous activity in the developing preterm brain: a functional magnetic responance imaging study. 2009 PAS Abstract Book, 2525.2521. [Google Scholar]
- Doria V, Beckmann CF, Rees G, Nunes RG, Merchant N, Counsell S, Edwards D. Spontaneous brain activity in infants from 33 weeks gestational age. Neuroscience. 2008 Abstracts 2008 787.13/SS50. [Google Scholar]
- Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, Borradori-Tolsa C, Mangin JF, Huppi PS. Mapping the early cortical folding process in the preterm newborn brain. Cerebral Cortex. 2008;18:1444–1454. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH. Geometric strategies for neuroanatomic analysis from MRI. Neuroimage. 2004;23 Suppl 1:S34–S45. doi: 10.1016/j.neuroimage.2004.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. PNAS. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a "local to distributed" organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. PNAS. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Engstrom M, Hallberg B, Mosskin M, Aden U, Lagercrantz H, Blennow M. Spontaneous brain activity in the newborn brain during natural sleep - An fMRI study in infants born at full term. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e3181b1bd84. epub ahead of print 6-09. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U. Resting-state networks in the infant brain. PNAS. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SJ, Landi N, Mencl WE, Sandak R, Fulbright RK, Tejada ET, Jacobsen L, Grigorenko EL, Constable RT, Pugh KR. Phonological awareness predicts activation patterns for print and speech. Ann Dyslexia. 2009;59:78–97. doi: 10.1007/s11881-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez M, Miranda MJ, Born AP, Nagy Z, Rostrup E, Jernigan TL. Accelerated cerebral white matter development in preterm infants: A voxel-based morphometry study with diffusion tensor MR imaging. Neuroimage. 2008;41:728–734. doi: 10.1016/j.neuroimage.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, Schneider KC, Peterson BS, Rajeevan N, Makuch RW, Constable RT, Ment LR. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. J Dev Behav Pediatr. 2009;30:122–130. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hille ETM, Weisglas-Kuperus N, van Goudoever JB, Jacobusse GW, Ens-Dokkum MH, de Groot L, Wit JM, Geven WB, Kok JH, de Kleine MJK, Kollee LAA, Mulder ALM, van Straaten HLM, de Vries LS, van Meissenbruch MM, Verloove-Vanhorick SP. Functional outcomes and participation in young adulthood for very preterm and very low birth weight infants: The Dutch project on preterm and small for gestational age infants at 19 years of age. Pediatrics. 2007;120:e587–e595. doi: 10.1542/peds.2006-2407. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Warfield S, Kikinis R, Barnes P, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ. Periventricular white matter injury in the premature neonate is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield S, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Larroque B, Ancel P-Y, Marret S, Marchand L, Andre M, Arnaud C, Pierrat V, Roze J-C, Messer J, Thiriez G, Burguet A, Picaud J-C, Breart G, Kaminski M. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): A longitudinal cohort study. Lancet. 2008;371:813–820. doi: 10.1016/S0140-6736(08)60380-3. [DOI] [PubMed] [Google Scholar]
- Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, Duncan CC, Scott DT, Taylor KJ, Katz KH, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: A multicenter randomized trial. Pediatrics. 1994;93:543–550. [PubMed] [Google Scholar]
- Ment LR, Peterson BS, Meltzer JA, Vohr B, Allan WA, Katz KH, Lacadie C, Schneider KC, Duncan CC, Makuch RW, Constable RT. A functional MRI study of the long-term influences of early indomethacin exposure on language processing in the brains of prematurely born children. Pediatrics. 2006a;118:961–970. doi: 10.1542/peds.2005-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Peterson BS, Vohr B, Allan WA, Schneider C, Lacadie C, Katz KH, Maller-Kesselman J, Pugh KR, Duncan CC, Makuch RW, Constable RT. Cortical recruitment patterns in children born prematurely compared with control children druing a passive listening functional magnetic resonance imaging task. J Pediatr. 2006b;149:490–498. doi: 10.1016/j.jpeds.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Vohr B, Oh W. Neurodevelopmental outcome at 36 months C.A. of preterm infants in the Multicenter Indomethacin IVH Prevention Trial. Pediatrics. 1996;98:714–718. [PubMed] [Google Scholar]
- Mewes AUJ, Huppi PS, Als H, Rybicki FJ, Inder TE, McAnulty GB, Mulkern RV, Robertson RL, Rivkin MJ, Warfield SK. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118:23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Jonsson B. Cerebral MRI findings in a cohort of ex-preterm and control adolescents. Acta Paediatr. 2009;98:996–1001. doi: 10.1111/j.1651-2227.2009.01278.x. [DOI] [PubMed] [Google Scholar]
- Narberhaus A, Lawrence E, Allin MP, Walshe M, McGuire P, Rifkin L, Murray R, Nosarti C. Neural substrates of visual paired associates in young adults with a history of very preterm birth: Alterations in fronto-parieto-occipital networks and caudate nucleus. Neuroimage. 2009;47:1884–1893. doi: 10.1016/j.neuroimage.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Neubauer AP, Voss W, Kattner E. Outcome of extremely low birth weight survivors at school age: The influence of perinatal parameters on neurodevelopment. Eur J Pediatr. 2008;167:87–95. doi: 10.1007/s00431-007-0435-x. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Shergill SS, Allin MP, Walshe M, Rifkin L, Murray RM, McGuire PK. Neural substrates of letter fluency processing in young adults who were born very preterm: Alterations in frontal and striatal regions. Neuroimage. 2009;47:1904–1913. doi: 10.1016/j.neuroimage.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Peabody. Peabody Picture Vocabulary Test-Revised. Circle Pines, NM: American Guidance Service; 1981. [Google Scholar]
- Peterson BS, Anderson AW, Ehrenkranz RA, Staib LH, Tageldin M, Colson ER, Gore JC, Duncan CC, Makuch RW, Ment LR. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- Rushe TM, Temple CM, Rifkin L, Woodruff PWR, Bullmore ET, Stewart AL, Simmons AD, Russell TA, Murray RM. Lateralisation of language function in young adults born very preterm. Arch Dis Child Fetal Neonatal Ed. 2004;89:F112–F118. doi: 10.1136/adc.2001.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf B, Streiner D, Boyle M, Pinelli J, Paneth N, Goddeeris J. Transition of extremely low-birth-weight infants from adolescence to young adulthood: Comparison with normal birth-weight controls. JAMA. 2006;295:667–675. doi: 10.1001/jama.295.6.667. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Magnus-Sebastian Y, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes J, Lohaugen GC, Martinussen M, Indredavik MS, Dale AM, Haraldseth O, Vangberg TR, Brubakk AM. White matter abnormalities and executive function in children with very low birth weight. Neuroreport. 2009;20:263–266. doi: 10.1097/wnr.0b013e32832027fe. [DOI] [PubMed] [Google Scholar]
- Skranes JS, Vangberg TR, Kulseng S, Indredavik MS, Evensen KAI, Martinussen M, Dale AM, Haraldseth O, Brubakk A-M. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Hill JE, Degnan AJ, Snyder AZ, Shimony JS, Neil JJ. Longitudinal analysis of neural network development in preterm infants. 2009 doi: 10.1093/cercor/bhq035. PAS Abstract Book, 4765.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley WH. Epidemiology of bronchopulmonary dysplasia. J Pediatr. 1979;95:851–858. doi: 10.1016/s0022-3476(79)80451-5. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading. Austin, TX: Efficiency (TOWRE). Pro-Ed; 1999. [Google Scholar]
- Verrips E, Vogels T, Saigal S, Wolke D, Meyer R, Hoult L, Verloove-Vanhorick SP. Health-related quality of life for extremely low birth weight adolescents in Canada, Germany and the Netherlands. Pediatrics. 2008;122:556–561. doi: 10.1542/peds.2007-1043. [DOI] [PubMed] [Google Scholar]
- Voss W, Neubauer AP, Wachtendorf M, Verhey JF, Kattner E. Neurodevelopmental outcome in extremely low birth weight infants: What is the minimum age for reliable developmental prognosis? Acta Paediatr. 2007;96:342–347. doi: 10.1111/j.1651-2227.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. The Comprehensive Test of Phonological Processing. Austin, Texas: PRO-ED, Inc.; 1999. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children- Third Edition. New York: The Psychological Corporation Harcourt Brace Co; 1991. [Google Scholar]
- Weisglas-Kuperus N, Hille ET, Duivenvoorden HJ, Finken MJ, Wit JM, van Buuren S, van Goudoever JB, Verloove-Vanhorick SP. Intelligence of very preterm or very low birthweight infants in young adulthood. Arch Dis Child Fetal Neonatal Ed. 2009;94:F196–F200. doi: 10.1136/adc.2007.135095. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Lohr C, Steinbrink C, Martin C, Vasic N. Functional brain network abnormalities during verbal working memory performance in adolescents and young adults with dyslexia. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.09.020. [DOI] [PubMed] [Google Scholar]