Abstract
Background and objective
Fibronectin (FN) is an important cell adhesion molecule that is used widely to characterize cell behavior. Preparations of FN purified from human plasma by gelatin-Sepharose affinity chromatography typically contain also gelatin-binding gelatinases that may cleave FN, reduce its stability, and alter its biological activities. Available methods for separating gelatinases from FN are resource demanding. Therefore, our objective was to devise a time- and cost-efficient protocol for purification of gelatinase-free FN.
Materials and Methods
Experiments tested the elution profiles for FN and gelatinases from gelatin-Sepharose using a concentration range (1-7%) of dimethyl sulfoxide (DMSO) and 4 M urea as eluants. Subsequently, we explored the sequential application of those eluants for differential elution of gelatinases and FN using a single affinity column. Finally, experiments characterized the stability of purified FN with or without contaminating gelatinases, as well as the effects of FN degradation on cell attachment and migration.
Results
Assay optimization demonstrated that pre-elution with 3% DMSO efficiently eliminated gelatinases but not FN from gelatin-Sepharose whereas subsequent elution with 4 M urea released FN. Sequential elutions with DMSO and urea produced gelatinase-free FN which was more stable than FN eluted by urea only. FN degradation did not affect human gingival fibroblast attachment, but increased cell migration significantly.
Conclusion
The present experiments devised a time- and cost-efficient protocol for eliminating gelatinases during purification of human plasma FN. Gelatinase-free FN preparations had greater stability, which may be essential for experiments because FN fragments have altered biological activities compared to intact FN.
Keywords: Fibronectin purification, gelatinase, human gingival fibroblast, dimethyl sulfoxide, cell attachment, cell migration
Fibronectin (FN) is a dimeric glycoprotein (~440 kDa) present in plasma and other body fluids, in extracellular matrix fibrils, and on cell membranes. Native FN and alternative splice forms of FN contribute to biological functions, such as cell-cell and cell-matrix interactions in wound healing, metastasis, and tissue remodeling (1, 2) and FN is widely used for biological experiments.
FN was initially identified as a serum factor which supported cell adhesion and bound collagen (3). Therefore, FN is commonly purified from human plasma, which contains ~300 μg/ml FN, by gelatin (denatured type I collagen) affinity chromatography and elution with urea (4). However, FN purified by this approach contains gelatinolytic enzymes including matrix metalloproteinases (MMPs) -2 and -9 (5). Additional steps like DEAE ion exchange (6), gel filtration (5), metal affinity (7), heparin-cellufine (8), or arginine affinity chromatography (9) can separate FN from gelatinolytic enzymes, but significantly increase labor, cost, sample handling, and reduce final yields.
To prevent FN degradation by co-purified enzymes, investigators have added protease inhibitors including ethylenediaminetetraacetic acid (EDTA), phenylmethylsulfonyl fluoride (PMSF) and ε-amino caproic acid (10). However, such inhibitors may adversely affect cells in culture. Therefore, developing efficient methods to purify gelatinase-free biologically active FN remains essential to investigate the biological functions of FN and to prevent confounding effects from proteolytically-generated FN fragments. Evidence for FN degradation in periodontal disease and diabetic wounds (11-13), as well as altered cell behavior on isolated FN fragments (12, 14) have emphasized the importance of this issue.
MMP-2 and -9 have been purified by gelatin-affinity chromatography using dimethyl sulfoxide (DMSO) elution (15) and our laboratory demonstrated that 2% DMSO disrupts MMP-2 interactions with gelatin(16, 17). Therefore, it was our working hypothesis that DMSO in this concentration range could differentially elute gelatinolytic activities but not FN bound to gelatinase-Sepharose.
To test purification conditions, we used analytical mini affinity columns (Vt 50 μL) packed with gelatin-Sepharose™ 4B (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) as detailed previously (17). Plasma was diluted 4-fold with chromatography buffer (50 mM Tris-HCl, 150 mM NaCl, pH 7.4) and centrifuged at 2,500 × g for 20 min to sediment particulate matter. Equal aliquots (500 μL) of plasma supernatant were then loaded on the analytical columns. Unbound and non-specifically bound plasma proteins were removed by extensive rinses with loading buffer (10 × Vt) and 1 M NaCl in chromatography buffer (10 × Vt). We tested elution with a concentration range of DMSO (1–7% (v/v)) in chromatography buffer (10 × Vt), 4 M urea in chromatography buffer (3 × Vt), and citrate buffer (pH 5.5) (18).
To understand to which extent DMSO and urea differentially eluted gelatinases and FN from the analytical gelatin-Sepharose columns, fractions were analyzed by the highly sensitive gelatin substrate enzymography as detailed previously (17). Briefly, samples were separated by SDS-PAGE under non-reducing conditions using 10% minislab gels co-polymerized with 150 μg/ml heat-denatured type I collagen prepared from rat tails. Gels were then washed in 5% Triton X-100, equilibrated with collagenase assay buffer (CAB) (50 mM Tris, 200 mM NaCl, 5 mM CaCl2, 0.05% Brij 35, 0.02% NaN3, pH 7.4), and incubated at 37 °C for 16 h. Counterstaining with Coomassie Brilliant Blue R-250 revealed enzyme activities as cleared bands against a blue background. Relative amounts of FN in fractions were quantified from digitized gel images using the ChemiDoc XRS imaging system and Quantity One® software version 4.5 (Bio-Rad Laboratories, Hercules, CA).
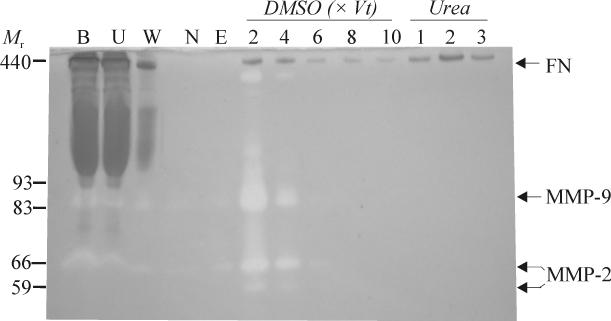
Fractions eluted with urea only contained both FN and high levels of gelatinolytic activities corresponding primarily to MMP-2 and -9 (not shown). However, the contaminating gelatinolytic activities could be eluted with DMSO, thereby eliminating the enzymes from the final urea fractions (Fig. 1). Increasing concentrations of DMSO more efficiently eluted the gelatinases, but decreased the recovery of FN in the final urea fractions from 63 to 41% over the concentration range of 1-7% DMSO. Among a series of concentrations and elution volumes, 6-10 bed volumes of 3% DMSO was optimal for eliminating detectable gelatinase activities from the gelatin-Sepharose columns while providing the highest recovery of gelatinase-free FN. It is plausible, but not demonstrated here, that additional proteins and proteases interacting with gelatin and FN were also eluted by DMSO.
Fig. 1.
Purification of gelatinase-free human plasma fibronectin (FN) by gelatin affinity chromatography and sequential DMSO and urea elution. Diluted human plasma was centrifuged to eliminate aggregates and 500 μL sample was loaded on an analytical gelatin-Sepharose mini column (Vt 50 μL). Fractions representing loaded plasma (B), unbound protein (U), washes with chromatography buffer alone (W) or containing 1 M NaCl (N), equilibration with chromatography buffer (E), pre-elution with 3% DMSO (DMSO, 2-10 x Vt), and final elution with 4 M urea (Urea; 1-3) were analyzed by gelatin enzymography. This purification strategy produced gelatinase-free FN. Masses (Mr) and positions corresponding to FN and MMPs-2 and -9 are indicated
The sequential DMSO-Urea purification protocol was applied to a larger scale gelatin-Sepharose column (Vt 15 mL). A total of 50 mL human plasma diluted to 200 mL in chromatography buffer and sedimented as described above was loaded on the column and washed with 10 × Vt each of loading buffer alone or containing 1 M NaCl. After pre-elution with 10 × Vt of 3% DMSO, final elution consisted of 3 × Vt of 4 M urea in chromatography buffer. Analyses by enzymography revealed a pattern of gelatinase and FN elution corresponding to that observed with analytical mini affinity columns. Final elution with 4 M urea yielded in excess of 5 mg gelatinase-free purified FN (not shown).
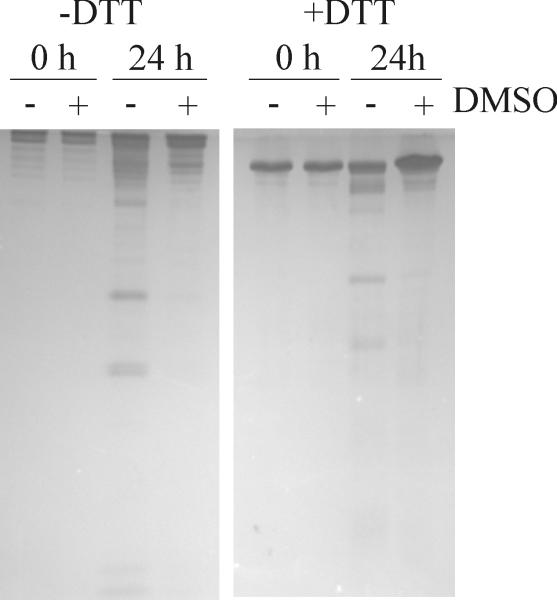
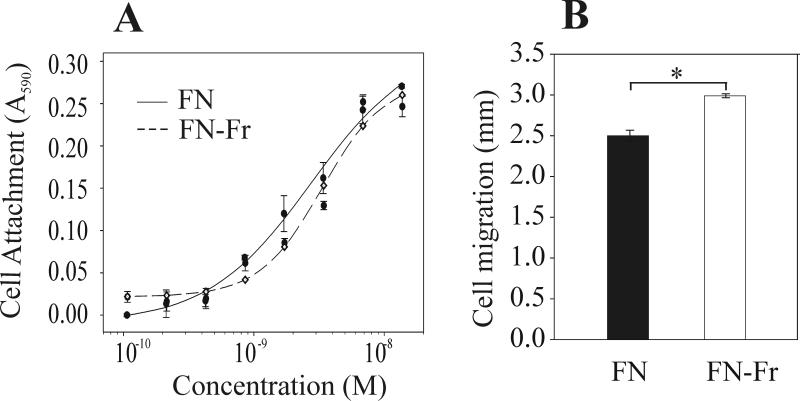
The stability of FN purified with and without DMSO pre-elution of gelatinases was tested by incubation at 0, 4, 22, or 37 °C for 1-7 days. FN fragmentation was substantial in preparations obtained by urea elution only, but significantly less after sequential DMSO-urea elution, suggesting that eliminating gelatinases from FN by pre-elution with DMSO enhanced the storage stability (Fig 2; 24 h at 22 °C is shown). Subsequent analysis of the biological activities of the two preparations in cell attachment and migration assays on protein coated surfaces as detailed previously (17). In brief, 40,000 human gingival fibroblasts (hGF) were added per well in 96 microwell plates coated with FN preparations obtained using the urea only or sequential DMSO-urea elution. Cell attached after 90 min were fixed, stained, and quantified by optical density after dissolution of the cell-bound stain. Cell migration assays measured the expansion on similarly coated surfaces of identical numbers of cells allowed to attach in standard sized wells over 3 days in 0.25% serum-containing medium. Expansion was measured from digitized images of fixed and stained cell areas (12). Cell attachment assays revealed that ~3 nM FN purified by either of the two strategies was required to support half-maximal attachment of hGF cells (Fig. 3A). In comparison, cells demonstrated significantly less migration (P<0.05) on surfaces that were coated with sequentially DMSO-urea eluted FN (Fig. 3B) compared with urea only eluted FN that also displayed more FN fragmentation (see Fig. 2). Overall, these cell experiments point to significant impact of the mode of FN purification on biological activities.
Fig. 2.
Stability of gelatinase-free FN. Human plasma FN purified by gelatin affinity chromatography and sequential DMSO-urea elution (+DMSO) or urea elution only (−DMSO) were incubated at 22 °C for 24 h and analyzed for protein stability by 7.5% SDS-PAGE under non-reducing (−DTT) and reducing (+DTT) conditions. Eliminating the gelatinolytic activities enhanced the stability of FN.
Fig. 3.
Biological activities of gelatinase-free human plasma FN. Fragmented FN (FN-Fr) from urea elution and intact gelatinase-free FN from sequential DMSO-urea elution (FN) (See also Fig. 2) were compared in cell based assays. FN fragmentation did not alter cell attachment (Panel A) but significantly increased cell migration (Panel B). Asterisk (*) denotes statistically significant difference (P<0.05).
By virtue of its high efficiency, 4 M urea has been the most commonly used eluting agent for purification of FN by gelatin-Sepharose affinity chromatography (8, 10). In our experiments, DMSO up to a concentration of 7% eluted a proportion, but not all FN bound on the columns. The choice of elution strategy should take into consideration the intended use of the purified FN. If preserving an intact and functional conformation is important, mild eluting agents like low pH citric acid buffer (pH range 5.5-6.0), arginine, KBr, 1 M NaBr (1), or DMSO may be advantageous. Strong chaotropic agents like urea, guanidine hydrochloride, and lithium di-iodosalicylic acid efficiently elute FN from affinity media, but may affect the structure and function of FN. For example, lithium di-iodosalicylic acid is 20-fold more efficient for FN elution than even 8 M urea in 0.1 M citric acid, pH 4.7, but the eluted FN has significantly reduced activity in cell attachment assays (19). Furthermore, urea can induce conformational changes and expose protease-sensitive sites in FN (20), which may be subject to cleavage by gelatinases such as MMP-2 and -9 that have proteolytic activity on FN (21).
Since it had not been established whether these eluants separate gelatinases from FN, we tested and demonstrated that the commonly used low-pH eluant citric acid, pH 5.5, released gelatinases and FN from gelatin columns in the same fractions (not shown) apparently resulting from the closeness in isoelectric points for these molecules (5.39, 5.70, and 5.31, respectively). In comparison, the utility of DMSO for differential elution is consistent with reports that gelatin binding by these molecules is primarily hydrophobic in nature (22). DMSO below 10% concentrations exerts little or no denaturing effect on lysozyme (23) and creatine kinase (24).
An intriguing question raised, but not resolved by the present series of experiments, is whether different eluants release different FN isoforms. For example, different site-specific glycopeptides of FN have been identified in plasma and cellular FN by hydrophilic affinity approaches that take advantage of the hydrogen bonding between carbohydrates of the glycopeptides in carbohydrate-based gels such as cellulose or related substrates (25). Our experiments point to heterogeneity in gelatin binding of what could represent different forms of FN resulting from alternative splicing or differences in glycosylation.
In summary, our experiments have demonstrated that co-purified gelatinases may alter both the stability and biological functions of FN preparation. To address this problem, we here present a time- and cost-efficient procedure for purification of gelatinase-free FN that may be applied in most laboratories to purify FN for use in biological and biochemical experiments.
Acknowledgements
We gratefully acknowledge experimental advice by Dr. Robert J. Klebe, University of Texas Health Science Center at San Antonio, Texas. Supported by grants DE017139, DE 016312, and DE018135 from the National Institutes of Health.
References
- 1.Hynes RO. Fibronectins. Springer-Verlag; New York: 1990. [Google Scholar]
- 2.Kaspar M, Zardi L, Neri D. Fibronectin as target for tumor therapy. Int J Cancer. 2006;118:1331–1339. doi: 10.1002/ijc.21677. [DOI] [PubMed] [Google Scholar]
- 3.Klebe RJ. Isolation of a collagen-dependent cell attachment factor. Nature. 1974;250:248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- 4.Engvall E, Roushlahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int.J.Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 5.Johansson S, Smedsrod B. Identification of a plasma gelatinase in preparations of fibronectin. J.Biol.Chem. 1986;261:4363–4366. [PubMed] [Google Scholar]
- 6.Aggeler J, Engvall E, Werb Z. An irreversible tissue inhibitor of collagenase in human amniotic fluid: characterization and separation from fibronectin. Biochem Biophys Res Commun. 1981;100:1195–1201. doi: 10.1016/0006-291x(81)91950-1. [DOI] [PubMed] [Google Scholar]
- 7.Smilenov L, Forsberg E, Zeligman I, Sparrman M, Johansson S. Separation of Fibronectin from A Plasma Gelatinase Using Immobilized Metal Affinity-Chromatography. FEBS Lett. 1992;302:227–230. doi: 10.1016/0014-5793(92)80447-o. [DOI] [PubMed] [Google Scholar]
- 8.Poulouin L, Gallet O, Rouahi M, Imhoff JM. Plasma fibronectin: Three steps to purification and stability. Protein Expr Purif. 1999;17:146–152. doi: 10.1006/prep.1999.1103. [DOI] [PubMed] [Google Scholar]
- 9.Speziale P, Visai L, Rindi S, Di Poto A. Purification of human plasma fibronectin using immobilized gelatin and Arg affinity chromatography. Nature Protocols. 2008;3:525–533. doi: 10.1038/nprot.2008.12. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama SK. Purification of Fibronectin. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM, editors. Current Protocols in Cell Biology. John Wiley & Sons, Inc; 2001. [Google Scholar]
- 11.Talonpoika J, Heino J, Larjava H, Hakkinen L, Paunio K. Gingival crevicular fluid fibronectin degradation in periodontal health and disease. Scand J Dent Res. 1989;97:415–421. doi: 10.1111/j.1600-0722.1989.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 12.Stanley CM, Wang Y, Pal S, Klebe RJ, Harkless LB, Xu X, Chen Z, Steffensen B. Fibronectin fragmentation is a feature of periodontal disease sites and diabetic foot and leg wounds and modifies cell behavior. J Periodontol. 2008;79:861–875. doi: 10.1902/jop.2008.070492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huynh QN, Wang S, Tafolla E, Gansky SA, Kapila S, Armitage GC, Kapila YL. Specific fibronectin fragments as markers of periodontal disease status. J Periodontol. 2002;73:1101–1110. doi: 10.1902/jop.2002.73.10.1101. [DOI] [PubMed] [Google Scholar]
- 14.Clark RA, An JQ, Greiling D, Khan A, Schwarzbauer JE. Fibroblast migration on fibronectin requires three distinct functional domains. J Invest Dermatol. 2003;121:695–705. doi: 10.1046/j.1523-1747.2003.12484.x. [DOI] [PubMed] [Google Scholar]
- 15.Shimokawa K, Nagase H. Purification of MMPs and TIMPs. In: Ian M, editor. Matrix Metalloproteinase Protocols. Humana Press; 2001. pp. 275–304. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Wang Y, Lauer-Fields JL, Fields GB, Steffensen B. Contributions of the MMP-2 collagen binding domain to gelatin cleavage - Substrate binding via the collagen binding domain is required for hydrolysis of gelatin but not short peptides. Matrix Biol. 2004;23:171–181. doi: 10.1016/j.matbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Steffensen B, Xu X, Martin PA, Zardeneta G. Human fibronectin and MMP-2 collagen binding domains compete for collagen binding sites and modify cellular activation of MMP-2. Matrix Biol. 2002;21:399–414. doi: 10.1016/s0945-053x(02)00032-x. [DOI] [PubMed] [Google Scholar]
- 18.Miekka S, Ingham K, Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1989;27:1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- 19.Klebe RJ, Bentley KL, Sasser PJ, Schoen RC. Elution of fibronectin from collagen with chaotrophic agents. Exp Cell Res. 1980;130:111–117. doi: 10.1016/0014-4827(80)90047-6. [DOI] [PubMed] [Google Scholar]
- 20.Markovic Z, Lustig A, Engel J. Shape and stability of fibronectin in solutions of different pH and ionic strength. Hoppe-Seyler.’s.Z.Physiol.Chem. 1983;364:1795–1804. doi: 10.1515/bchm2.1983.364.2.1795. [DOI] [PubMed] [Google Scholar]
- 21.Steffensen B, Hakkinen L, Larjava H. Proteolytic events of wound-healing--coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev.Oral Biol.Med. 2001;12:373–398. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- 22.Pickford AR, Smith SP, Staunton D, Boyd J, Campbell ID. The hairpin structure of the (6)F1(1)F2(2)F2 fragment from human fibronectin enhances gelatin binding. EMBO J. 2001;20:1519–1529. doi: 10.1093/emboj/20.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharjya S, Balaram P. Effects of organic solvents on protein structures: observation of a structured helical core in hen egg-white lysozyme in aqueous dimethylsulfoxide. Proteins. 1997;29:492–507. doi: 10.1002/(sici)1097-0134(199712)29:4<492::aid-prot9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Ou WB, Park YD, Zhou HM. Effect of osmolytes as folding aids on creatine kinase refolding pathway. Int J Biochem Cell Biol. 2002;34:136–147. doi: 10.1016/s1357-2725(01)00113-3. [DOI] [PubMed] [Google Scholar]
- 25.Tajiri M, Yoshida S, Wada Y. Differential analysis of site-specific glycans on plasma and cellular fibronectins: application of a hydrophilic affinity method for glycopeptide enrichment. Glycobiology. 2005;15:1332–1340. doi: 10.1093/glycob/cwj019. [DOI] [PubMed] [Google Scholar]