Abstract
The dietary bioavailability of the isoflavone genistein is decreased in older rats compared to young adults. Since flavonoids are metabolized extensively by the UDP-glucuronosyltransferases (UGTs), we hypothesized that UGT flavonoid conjugating activity changes with age. The effect of age on flavonoid glucuronidation was determined using hepatic microsomes from male F344 rats. Kinetic models of UGT activity toward the flavonol quercetin and the isoflavone genistein were established using pooled hepatic microsomal fractions of rats at different ages, and glucuronidation rates determined using individual samples. Intrinsic clearance (Vmax/Km) values in 4, 18, and 28 mo old rats were 0.100, 0.078, and 0.087 mL/min/mg for quercetin-7-O-glucuronide, 0.138, 0.133, and 0.088 for quercetin-3′-O-glucuronide, and 0.075, 0.077, and 0.057 for quercetin-4′-O-glucuronide, respectively. While there were no differences in formation rates of total quercetin glucuronides in individual samples, the production of the primary metabolite, quercetin-7-O-glucuronide, at 30 μM quercetin concentration was increased from 3.4 and 3.1 nmol/min/mg at 4 and 18 mo to 3.8 nmol/min/mg at 28 mo, while quercetin-3′-O-glucuronide formation at 28 mo declined by a similar degree (P ≤0.05). At 30 and 300 μM quercetin concentration, the rate of quercetin-4′-O-glucuronide formation peaked at 18 mo at 0.9 nmol/min/mg. Intrinsic clearance values of genistein 7-O-glucuronide increased with age, in contrast to quercetin glucuronidation. Thus, the capacity for flavonoid glucuronidation by rat liver microsomes is dependent on age, UGT isoenzymes, and flavonoid structure.
Keywords: flavonoid, quercetin, genistein, rat, UDP-glucuronosyltransferase, acetaminophen
Introduction
Flavonoids are dietary polyphenols found ubiquitously in plant foods. Prospective cohort studies have associated generous flavonoid intake with reduced risk of cardiovascular disease, stroke, and some forms of cancer [1–3]. Flavonoids may confer protection against chronic diseases through an array of putative actions, including antioxidation, detoxification, anti-inflammation, and anti-proliferation. Any health benefits derived from dietary flavonoid intake must depend upon their bioavailability, metabolism, and distribution [4], parameters which may be affected by age-related changes in physiology. Limited flavonoid bioavailability and extensive metabolism, particularly when combined with a low intake of these phytochemicals [5], may decrease their putative contribution to health promotion and disease prevention in older adults.
Upon consumption, flavonoids are extensively transformed via Phase 2 enzymes through conjugation with glucuronide, sulfate, and/or methyl moieties, particularly in small intestine and liver [6]. This pre-systemic biotransformation of flavonoids within liver and small intestine affect their biological half-life and may decrease their efficacy. For example, glucuronidation of flavonoids limit their putative actions to protect cells against oxidative injury and inhibit lipoxygenase and xanthine oxidase activity [7, 8]. Due to the diversity of the Phase 2 enzymes, metabolites of flavonoids produced through this pathway also depend on tissue type. For example, in rats fed quercetin, the liver contained a majority of sulfated monoglucuronide derivatives, whereas intestinal tissue had the greatest proportion of monoglucuronides [6].
Physiological and environmental factors such as age, genetics, and dietary behavior affect the bioavailability of nutrients as well as drugs. However, information on the extent to which flavonoid metabolism is altered by these parameters is scarce. The Phase 2 detoxification pathway is essential to flavonoid metabolism and is partly influenced by aging [9]. van Bezooijen [10] has described how age-related changes in Phase 2 metabolism is dependent upon factors such as enzyme isoform, substrate, gender, and diet. However, the effect of age-related changes in flavonoid metabolism mediated by Phase 2 enzymes responsible for glucuronidation, sulfation, and methylation remains to be established. Characterizing this relationship is important to elucidating the impact of dietary flavonoids on the health of older adults.
Previously, we observed that fasted senescent rats fed genistein for 4 wk had a lower steady state concentration of this isoflavone in liver and plasma than adult rats [11]. The mechanisms underlying this difference remain to be determined. We hypothesized that UGT flavonoid activity changes with age. Therefore, the aim of this study was to determine whether changes in hepatic glucuronidation of selected flavonoids account for the previously observed age-related decline in their bioavailability in rats. Our objective was to determine the variation in glucuronidation kinetics of isoflavone genistein and the flavonol quercetin using pooled hepatic microsomes collected from 4, 18, and 28 mo-old rats. Acetaminophen glucuronidation was also determined in the same samples to validate enzyme kinetic modeling and as a negative control for age-related changes in glucuronidation, as hepatic microsomal rates of acetaminophen glucuronidation are reportedly unchanged with advanced age in rats and mice [12, 13].
Materials and Methods
Chemicals and Supplies
HPLC grade acetonitrile and methanol were obtained from Fisher Scientific (Thermo Fisher Scientific, Rockford, IL). Genistein was purchased from Cayman Chemical (Ann Arbor, MI) and rutin from Extrasynthese (Genay France). Quercetin, UDP-glucuronic acid, alamethicin, and all other chemicals and reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Tissues and preparation
Whole rat liver of tumor-free male Fischer F344 rats aged 4, 18, and 28 mo (n = 5/group) were obtained from the Aging Rodent Tissue Bank of the NIA/NIH (Bethesda, MD). Male rats were chosen to limit any influence of hormone fluctuation on detoxification pathways. Body weights (mean ± standard deviation) were 290 ± 12 (4 mo), 441 ± 14 (18 mo), and 406 ± 19 g (28 mo). Liver weights increased with advancing age at 12 ± 1, 17 ± 1, and 17 ± 1 g for 4, 18, and 28 mo rats, respectively.
Hepatic microsomal fractions were prepared as previously described [14] with slight modifications. Whole liver was pulverized under liquid nitrogen and stored at −80°C until homogenization. Liver aliquots (~0.5 g) were homogenized in 10 vol sucrose buffer (50 mM Tris-HCl, 0.25 M sucrose, pH 7.5) in an ice bath, using a Tekmar Tissumizer, SDT-1810 (Spokane, WA) at 85% power for 2 min. Homogenate was centrifuged at 1,000 × g for 15 min at 4°C. Supernatant kept at 4°C was successively centrifuged at 10,000 × g for 20 min to pellet nuclei and mitochondria, and the resulting supernatant was spun at 100,000 × g for 1 h to produce the microsomal pellet. After decanting the cytosol fraction, the microsomal pellet was rinsed with 400 μL suspension buffer (0.1 M potassium phosphate buffer, pH 7.5, containing 20% glycerol) and then resuspended in the same volume. Microsomal protein concentration was determined by a Pierce BCA kit (Thermo Fisher Scientific), and adjusted to 5 mg/mL with phosphate buffer (0.1 M, pH 7.5). Aliquots of the microsomal fraction were stored at −80°C until use.
Kinetics and individual rate of glucuronidation
Kinetics of quercetin, genistein, and acetaminophen glucuronidation by rat hepatic microsomes were determined as previously described [15, 16]. Acetaminophen glucuronidation was used as a positive control [12]. Briefly, microsomal protein (0–1 mg/mL, final concentration) was pre-incubated with alamethicin (0.25 μg/mL) at 37°C for 5 min in microcentrifuge tubes containing previously dried substrate. A cofactor solution of UDP-glucuronic acid (5 mM, final concentration), magnesium chloride (5 mM, final concentration), and potassium phosphate buffer, pH 7.5 (0.05 mM, final concentration) was added to initiate the reaction in a final assay volume of 0.1 mL. After incubation for selected durations at 37°C, the reaction was terminated with 0.1 mL ice-cold methanol containing either 33 μM rutin or daidzein or 0.24 mM 2-acetamidophenol as internal standards. After centrifugation at 14,000 × g for 5 min, 180 μL of supernatant was dried under a stream of purified nitrogen gas at room temperature and stored at −20°C until HPLC analysis.
The enzyme kinetics of the glucuronide formation for each age was determined in duplicate using pooled microsomes. With 0.2 mg/mL microsomal protein and 30-min incubation, formation of quercetin and genistein metabolites were linear with concentrations of 9–600 μM. Similarly, with 0.5 mg/mL protein and 30-min incubation, production of acetaminophen glucuronide was linear between 0.9–30 mM. At concentrations of 30 and 300 μM, linearity of quercetin and genistein glucuronidation was established with 0.05–5 mg/mL microsomal protein and incubation for 30 min, as well as with 0.2 mg/mL protein and incubation for up to 60 min. For acetaminophen at 0.5 and 30 mM, the glucuronidation rate was linear with 0.1–1.0 mg/mL protein for 30 min and with 0.5 mg/mL protein for up to 60 min.
The glucuronidation rate of quercetin, genistein, and acetaminophen was determined in triplicate using individual microsomal samples. Concentrations of quercetin and genistein at 30 and 300 μM, which are near the Km value and 10-fold higher, respectively, were employed. Acetaminophen glucuronidation was assayed at 0.5 and 10 mM substrate concentration, representing values 20-fold below and near its Km, respectively.
HPLC analyses of glucuronide metabolites
The products of the glucuronidation reaction were reconstituted in 90 μL of 50% methanol in water and analyzed using HPLC according to previously established methods. A Thermo-Finnigan Surveyor HPLC (Thermo Fisher Scientific, San Jose, CA) equipped with an autosampler, UV detector, and Phenomenex Synergi 10 μm Hydro-RP80 250 × 4.6 mm column (Torrance, CA), with mobile phase A consisting of 20 mM phosphate buffer, pH 2.0 and mobile phase B consisting of acetonitrile, was employed. After normalization with the respective internal standard, glucuronide metabolites were quantified using a standard curve established with the absence of UDPGA. The method of Boersma et al. [15] was used to analyze quercetin and genistein and their metabolites. Following a 20 μL injection, at 1 mL/min flow rate, mobile phase B was held at 17% for 2 min, and then increased to 25% in 5 min, to 35% in 8 min, and to 50% in 5 min. After 3 min at 50%, mobile phase B was decreased to 17% in 4 min and held for 5 min to equilibrate the column for subsequent injections.
Three principal quercetin glucuronide metabolites were identified as previously described [15], with retention time (Rt) of 7-O-glucuronide at 11.5 min, 4′-O-glucuronide at 14.3 min, and 3′-O-glucuronide a 14.8 min. Quercetin and its metabolites and rutin (Rt 10.8 min) were monitored at 370 nm. Consistent with Doerge, et al. [17], microsomal glucuronidation incubations with genistein yielded primarily the 7-O-glucuronide of genistein (Rt 15.2 min), although with longer incubation times the 4′-O-glucuronide was observed. Genistein and its metabolites and daidzein (Rt 19.3 min) were monitored at 255 nm.
Acetaminophen and its glucuronide metabolite were analyzed according to the method of Court and Greenblatt [18]. Briefly, a 20 μL injection was eluted at 1.4 mL/min in an isocratic mode with 3% mobile phase B. Acetaminophen glucuronide (Rt 5.0 min) was the sole glucuronide metabolite formed by microsomal incubations of acetaminophen. The acetaminophen glucuronide metabolite and the internal standard 2-acetamidophenol (Rt 13.7 min) were monitored at 254 nm.
Data Analysis
Visual inspection of Michaelis-Menton, Lineweaver-Burke, Eadie-Hofstee plots and comparison of sum of squared residuals using GraphPad Prism v3.00 (GraphPad Software, San Diego, CA) were employed to select the best-fit kinetic models, as previously described by Court et al. [17, 18]. This includes the Michaelis-Menten model (eq.1) and the uncompetitive substrate inhibition model (eq. 2):
| (1) |
| (2) |
where V is the velocity, S is substrate concentration, Vmax is the maximum velocity, Km is the substrate concentration at 50% Vmax, and Ks is the substrate inhibition constant. Kinetic parameters are expressed as the estimate and 95% confidence interval.
The effect of age on glucuronidation rates of individual hepatic microsomes was assessed using one-way ANOVA. When P values were ≤0.05, post-hoc analysis was performed using Tukey’s honestly significant difference test. Statistical analyses were performed using JMP IN (SAS Inc., Cary, NC). GraphPad Prism 5.01 (GraphPad Software) was used to determine Pearson’s correlation values and significance (P ≤0.05) for microsomal glucuronidation and age.
Results
Acetaminophen Glucuronidation
The enzyme kinetics of acetaminophen glucuronidation were best fit using the Michaelis-Menten model. Although Vmax values of pooled microsomes increased with advanced age, Km and intrinsic clearance (Vmax/Km) did not follow this trend (Table 1). Age did not affect individual rates of acetaminophen glucuronidation measured at substrate concentrations at 0.5 and 10 mM (Table 2).
Table 1.
Enzyme kinetic parameters for glucuronidation of acetaminophen AAP), genistein (G), and quercetin (Q) by hepatic microsomes of different aged male F344 rat.
| Metabolite | Age mo | Vmax nmol/min/mg | Km μM | Ks μM | CLinta mL/min/mg | Trend CLint |
|---|---|---|---|---|---|---|
| AAP-4-O- gluc | 4 | 4.6 ± 0.6 | 9600 ± 3800 | - | 0.000479 | R = 0.02 |
| 18 | 4.8 ± 0.2 | 1800 ± 200 | - | 0.000261 | P =0.99 | |
| 28 | 5.3 ± 0.2 | 1300 ± 100 | - | 0.000510 | ||
| G-7-O-gluc | 4 | 3.0 ± 0.3 | 23 ± 6 | - | 0.130 | R = 0.99 |
| 18 | 3.0 ± 0.3 | 21 ± 4 | - | 0.138 | P = 0.10 | |
| 28 | 4.4 ± 0.8 | 30 ± 5 | - | 0.148 | ||
| Q-7-O-gluc | 4 | 9.7 ± 2.4 | 97 ± 38 | 469 ± 237 | 0.100 | R = −0.66 |
| 18 | 10 ± 3 | 133 ± 55 | 440 ± 233 | 0.078 | P = 0.54 | |
| 28 | 19 ± 7 | 219 ± 114 | 370 ± 238 | 0.087 | ||
| Q-4′-O-gluc | 4 | 0.63 ± 0.08 | 4.6 ± 2.6 | 456 ± 177 | 0.138 | R = −0.86 |
| 18 | 0.96 ± 0.09 | 7.2 ± 2.5 | 633 ± 202 | 0.133 | P = 0.34 | |
| 28 | 0.72 ± 0.07 | 8.2 ± 2.8 | 473 ± 145 | 0.088 | ||
| Q-3′-O-gluc | 4 | 4.9 ± 0.8 | 66 ± 22 | 695 ± 322 | 0.075 | R = −0.76 |
| 18 | 6.3 ± 1.0 | 81 ± 23 | 672 ± 268 | 0.077 | P = 0.45 | |
| 28 | 4.3 ± 0.6 | 75 ± 21 | 1017 ± 472 | 0.057 | ||
AAP and G glucuronidation were modeled by Michaelis-Menten kinetics, whereas Q glucuronidation was modeled by uncompetitive substrate inhibition kinetics. Data is the average of duplicate determinations. Data are model estimate with standard error in the parentheses.
CLint is defined as Vmax/Km.
Table 2.
Rates of acetaminophen (AAP) and genistein (G) glucuronidation by hepatic microsomes of different aged male F344 rat. Changes in rates are not significantly correlated with age (P < 0.05).
| Metabolite | Age (mo) | Rate (nmol/min/mg) | |
|---|---|---|---|
| 0.05 mM AAPa | 10 mM AAP | ||
| AAP-4-O-glucuronide | 4 | 0.34 ± 0.08 | 2.4 ± 0.6 |
| 18 | 0.24 ± 0.18 | 2.1 ± 1.4 | |
| 28 | 0.39 ± 0.10 | 3.0 ± 0.8 | |
| 30 μM Gb | 300 μM G | ||
| G-7-O-glucuronide | 4 | 1.7 ± 0.2 | 3.6 ± 0.3 |
| 18 | 1.8 ± 0.4 | 3.8 ± 1.0 | |
| 28 | 2.2 ± 0.5 | 4.9 ± 1.4 | |
|
|
|||
Concentrations of acetaminophen employed in microsomal incubations.
Concentrations of genistein employed in microsomal incubations.
Values are expressed as mean ± SD, n=5.
Genistein Glucuronidation
Genistein 7-O-glucuronidation kinetics were best modeled by the Michaelis-Menten equation (Fig. 1). Age did not affect the Vmax or Km of genistein, with their values ranging from 3.0–4.4 nmol/min/mg and from 21–30 μM, respectively (Table 1). However, the intrinsic clearance (Vmax/Km) of genistein was increased modestly by age (R = 0.99, P = 0.10), with a 14% larger clearance at 28 mo than at 4 mo (Table 1). However, these increments were not confirmed in individual samples, as the rate of 7-O-glucuronide-genistein formation was unaltered overall by age (Table 2). Nevertheless, glucuronidation rate of the 28 mo livers were 29% and 34% higher than the 4 mo livers at genistein concentrations of 30 μM and 300 μM, respectively.
Fig. 1.
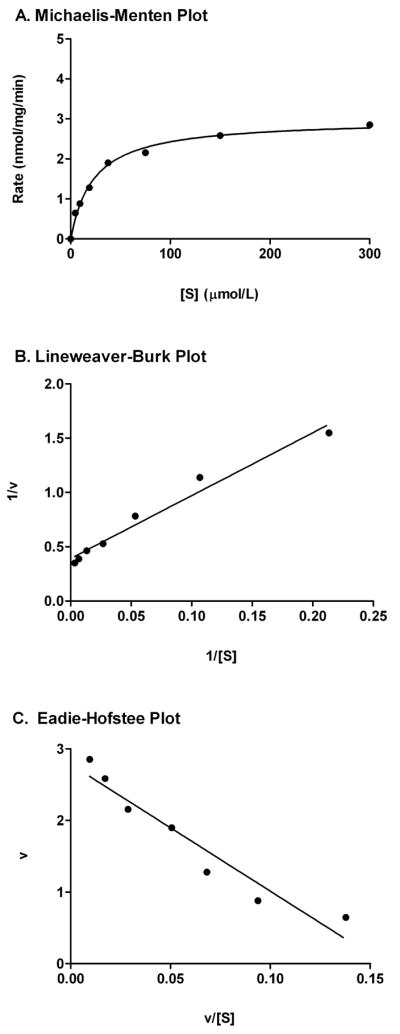
Kinetic plots of genistein glucuronidation using pooled hepatic microsomes from five 4 mo male F344 rats. A. Michaelis-Menton plot, with estimate best modeled by Michaelis-Menten kinetics. B. Lineweaver-Burk plot. C. Eadie-Hofstee plot. The kinetic parameters are summarized in the Table 1.
Quercetin Glucuronidation
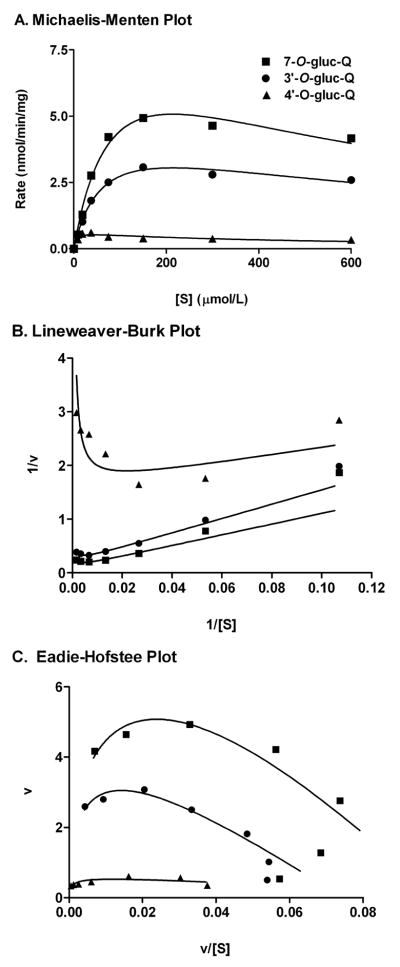
In contrast to genistein and acetaminophen glucuronidation, the enzyme kinetics of quercetin glucuronidation was described best by the uncompetitive substrate inhibition model (Fig. 2). Intrinsic clearance values declined from 4 to 28 mo by 13, 36, and 24% for the 7-O-, 3′-O-, and 4′-O- glucuronides, respectively (Table 1). This age-related decline of intrinsic clearance appear principally due to the disproportionate increases in Vmax and Km. For example, for the most abundant quercetin metabolite, quercetin-7-O-glucuronide, the Vmax and Km increased by 125% and 96%, respectively from 4 to 28 mo.
Fig. 2.
Kinetic plots of quercetin glucuronidation using pooled hepatic microsomes from five 4 mo male F344 rats. A. Michaelis-Menton plot, with estimate best modeled by uncompetitive substrate inhibition kinetics. B. Lineweaver-Burk plot. C. Eadie-Hofstee plot. The kinetic parameters are summarized in the Table 1.
While there were no differences in the rate of total quercetin glucuronidation from individual hepatic microsomes, the production of quercetin-7-O-glucuronide at 30 μM quercetin concentration increased 12% from 4 mo to 28 mo, while 3′-glucuronide formation at 28 mo declined by a similar degree (P ≤0.05) (Fig. 3). The rate of quercetin-4′-glucuronide formation peaked at 18 mo. The magnitude and pattern of age-related changes in glucuronidation rates were similar between quercetin concentration at 30 and 300 μM. However, at 300 μM, changes in 7-O-glucuronide rates were insignificant (Fig. 3).
Fig. 3.
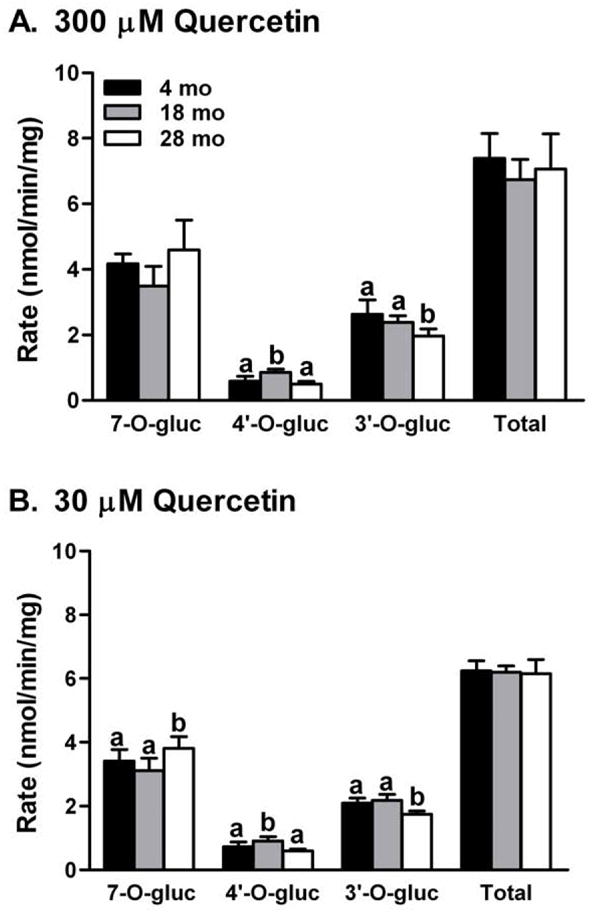
Age-related changes in glucuronidation activities toward quercetin catalyzed by hepatic microsomal UGT of male F344 rats. Quercetin concentration at 30 μM was in range of the Km value for total glucuronide metabolite formation. Data are expressed as mean ± SD (n = 5/group). P values of one-way ANOVA for 30 μM quercetin were 0.0381 for 7-O-gluc, 0.0034 for 4′-O-gluc, 0.00089 for 3′-O-gluc, and 0.93 for total glucuronides, respectively. P values of one-way ANOVA for 300 μM were 0.058 for 7-O-gluc, 0.0009 for 4′-O-gluc, 0.016 for 3′-O-gluc, and 0.50 for total glucuronides, respectively. abcMeans within the same metabolite without the same letter differ, Tukey’s HSD multiple comparison test, P ≤0.05.
Discussion
A wide variation in flavonoid bioavailability has been observed in clinical trials [19], which can be attributed to environmental, physiological, dietary, and genetic factors. Age may be a contributing factor to this variation of flavonoid bioavailability, since we have previously observed older rats had lower steady state of genistein disposition than younger rats [11]. Although the mechanisms responsible for this difference have not been explored, physiological changes associated with advancing age may complicate or distort patterns of metabolism that could affect the bioavailability and bioactivity of dietary polyphenols. Such age-related changes could include decreased liver size and volume of distribution, and increased intestinal transit time [20]. Furthermore, age-related changes in metabolic enzymatic activity may alter the absorption and biotransformation of both nutrients and drugs [21]. Thus, in this study of hepatic microsomes in vitro, we examined the impact of age on the activity of UDP-glucuronosyltransferase activity toward genistein and quercetin.
The maximum plasma concentrations following ingestion of flavonoid-containing foods or supplements in humans ranges from 0.1 to 8 μM for flavonol metabolites and 0.3 to 25 μM for isoflavone metabolites [19]. We utilized a range of flavonoid concentrations up to 600 μM for kinetic determinations of glucuronidation, and tested individual microsomal preparations from different ages at 30 and 300 μM on the basis of kinetic determinations, approximating Km values and 10-times the Km value. In a previous study, rats fed 4.45 g/kg quercetin for 6 weeks resulted in metabolite concentrations of 14 μM in plasma and 2.6 nmol/g in liver tissue [6]. Thus, while 300 μM flavonoid may be achieved in the stomach or gastrointestinal lumen after supplementation, concentrations of ≤30 μM flavonoid are commonly reported in plasma and tissue and plasma.
Acetaminophen has often been used as a model compound to evaluate glucuronidation activity. We did not find age-related changes in glucuronidation activity of hepatic microsomes toward this compound. This result is consistent with studies of hepatic microsomal acetaminophen glucuronidation in male and female Brown Norwegian rats at 4 and 34 mo [12] and male C57BL/6NNia mice at 3–5, 11–13, 18–20, and 24–26 mo [13]. However, Sweeny and Weiner [13] did find that acetaminophen glucuronidation by cultured hepatocytes was increased in old compared to young and adult rats. Therefore, the effect of advanced age on UGT activity toward acetaminophen may be influenced by the species and strain of the animal model.
Phase 2 conjugation reactions facilitate elimination of flavonoids from the body. Thus, increased microsomal rates of genistein glucuronidation may have contributed to the reported decrease in the steady-state concentration of genistein in older rats [11]. We found the kinetic parameters of microsomal genistein glucuronidation were similar to those reported by Chen et al. [22] in adult male Sprague-Dawley rats. However, we did not observe an age-related change in glucuronidation activity toward genistein in vitro, although there was a tendency toward increased intrinsic clearance with age. Since the variability between individual rats increased with age, a larger sample size per age group or more time points may be necessary to better reveal an age-dependent effect on genistein glucuronidation. These in vitro results do not support our hypothesis that hepatic microsomal rates of glucuronidation significantly change with age. However, the effects of age on intestinal and renal glucuronidation or other Phase 2 conjugation enzymes warrant further investigation.
Although it was previously reported that age affects the steady-state concentration of genistein, evidence for age-related changes in isoflavone metabolism in humans is scarce and inconsistent. Cassidy et al. [23] found that bioavailability of daidzein was 30% higher in postmenopausal women than in pre-menopausal women, while Faughnan et al. [24] reported that age did not affect urinary isoflavone excretion in women. Thus, the impact of advanced age on the bioavailability of flavonoids requires further study with careful consideration to hormonal status, sample size, dosage, and magnitude of age difference.
Quercetin glucuronide metabolites have been characterized extensively while enzyme kinetic modeling of quercetin glucuronidation in rat hepatic microsomes has been lacking. We found UGT-catalyzed quercetin glucuronidation exhibited uncompetitive substrate inhibition kinetics, consistent with a previous study using recombinant human UGT 1A3 and 1A9 [25]. Enzyme kinetic modeling suggested hepatic quercetin glucuronidation decreased with age. The Km and Vmax values for 7-, 4′-, and 3′-glucuronidation of quercetin in older rats was increased compared to those at 4 mo of age, but was associated with a decrease in CLint values. However, quercetin glucuronidation rates of individual microsome preparations declined for the 3′- and 4′-metabolite and were constant when considering total glucuronidation activity (sum of the metabolites). The preservation of total glucuronidation activity toward quercetin was due to differential age-related changes in the activity of different UGT isoenzymes. At 28 mo, the increase in production of quercetin 7-O-glucuronide was offset by decreased production of the 3′-O-glucuronide. Since the bioactivity of metabolized flavonoids is dependent upon the location of hydroxyl moiety conjugation [8], quercetin glucuronides may have different actions at different ages.
While age affected the hepatic glucuronidation of quercetin, both genistein and acetaminophen were not subject to the same influence. Previous animal studies have shown age-related changes in glucuronidation capacity are substrate dependent. Substrates absent an influence of aging include acetaminophen, bilirubin, p-nitrophenol, and estrone, while glucuronidation activity toward morphine, 1-naphthol, and testosterone appear to be modified by advancing age [12, 26, 27]. The lack of a consistent age-related effect in flavonoid glucuronidation activity toward different substrates may arise from the diversity of UGT isoenzymes. Using human recombinant UGT (rUGT), Boersma et al. [15] demonstrated several UGT isoenzymes metabolized quercetin with different conversion rates, of which UGT1A9, 1A1, 1A3, 1A8, and 2B7 were the most efficient. Consistent with our results, tissue obtained from animals of different ages and with similar net glucuronidation rates but divergent UGT regioselectivity produced different metabolite profiles. For example, Boersma et al. [15] reported that half of the quercetin glucuronides formed by UGT1A3 were the 3′-O- form, while UGT1A9 produced the 7-O- form to the same extent. Liu et al. [28] found that UGT1A1 was primarily responsible for genistein glucuronidation, while the work of Bock et al. [29] indicated that UGT1A6 principally glucuronidated acetaminophen. This diversity of UGTs might also explain the observation by Brett et al. [30] that urinary excretion of hesperitin, but not naringenin, declined with advanced age in humans after drinking orange juice. Therefore, age-related effects on UGT activity toward flavonoid are likely dependent on isoenzyme specific changes.
As reported in previous studies, incubations of rat liver microsomes yield the 7-, 3′-, and 4′-glucuronides of quercetin. However, after quercetin consumption, glucuronides, diglucuronides, mixed sulfates, and methyl conjugates are also present in rat tissues [6]. As reviewed by Mackenzie et. al [31], humans and rats have orthologous UGT1A1, 1A3, 1A5, 1A6, 1A7, 1A8, and 1A10. Humans have functional UGT1A4 and UGT1A9, whereas rats do not [31]. The human S9 liver fraction also forms the 3-glucuronide of quercetin, whereas in rat it is produced in the intestine [32]. The equivalency of genistein or quercetin glucuronidation kinetics between recombinant human and rat UGT has not been established. Although the F344 rat is widely used as an animal model of aging, the homology of its metabolite profile and UGT enzymes to humans does limit extrapolating our data to humans.
In conclusion, age-related changes in male rat hepatic microsomal glucuronidation of flavonoids were statistically significant but modest in their magnitude. The glucuronidation of genistein decreased with age, although the trend of increasing CLint was small. Quercetin total glucuronidation capacity was constant with age, but young and old rats had a different metabolite profiles. Further studies of the impact of age-related changes on specific UGT isoenzymes toward quercetin and other flavonoids are warranted, as more than 15 isoenzymes compose the UGT super-family. In vivo studies are required to demonstrate the extent to which advancing age alters flavonoid bioavailability, metabolomics, and bioactivity. As flavonoids are also metabolized extensively by Phase 2 enzymes in the intestine and kidney, research determining the potential for age-related changes in these organs is required.
Acknowledgments
This work is funded through the USDA ARS Cooperative Agreement #58-1950-7-707. Dr. Court was supported by grant R01GM061834 from the National Institute of General Medical Sciences (NIGMS), National Institutes of Health (Bethesda, MD). Dr. Bolling was supported by NIH IRACDA Training Grant K12 GM074869.
Funding: This work is funded through the USDA ARS Cooperative Agreement #58-1950-7-707. Dr. Court was supported by grant R01GM061834 from the National Institute of General Medical Sciences (NIGMS), National Institutes of Health (Bethesda, MD). Dr. Bolling was supported by NIH IRACDA Training Grant K12 GM074869.
Footnotes
The contents of this publication do not necessarily reflect the views or policies of the NIH or USDA nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cutler G, Nettleton J, Ross J, Harnack L, Jacobs D, Jr, Scrafford C, Barraj L, Mink P, Robien K. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women’s Health Study. Int J Cancer. 2008;123:664–71. doi: 10.1002/ijc.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geleijnse J, Launer L, Hofman A, Pols H, Witteman J. Tea flavonoids may protect against atherosclerosis: the Rotterdam Study. Arch Intern Med. 1999;159:2170–4. doi: 10.1001/archinte.159.18.2170. [DOI] [PubMed] [Google Scholar]
- 3.Keli S, Hertog M, Feskens E, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–42. [PubMed] [Google Scholar]
- 4.Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19:73–82. doi: 10.1016/j.copbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Chun O, Chung S, Song W. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007;137:1244–52. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 6.Graf B, Ameho C, Dolnikowski G, Milbury P, Chen C-Y, Blumberg J. Rat gastrointestinal tissues metabolize quercetin. J Nutr. 2006;136:39–44. doi: 10.1093/jn/136.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Day A, Bao Y, Morgan M, Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic Biol Med. 2000;29:1234–43. doi: 10.1016/s0891-5849(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson D, Cooney J, Jensen D, Wibisono R, Adaim A, Skinner M, Zhang J. Comparison of enzymically glucuronidated flavonoids with flavonoid aglycones in an in vitro cellular model of oxidative stress protection. In Vitro Cell Dev Biol –Animal. 2008;44:73–80. doi: 10.1007/s11626-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 9.McLean A, Le Couteur D. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56:163–84. doi: 10.1124/pr.56.2.4. [DOI] [PubMed] [Google Scholar]
- 10.van Bezooijen C. Influence of age-related changes in rodent liver morphology and physiology on drug metabolism--a review. Mech Ageing Dev. 1984;25:1–22. doi: 10.1016/0047-6374(84)90126-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen C-Y, Bakhiet R. Age decreased steady-state concentrations of genistein in plasma, liver, and skeletal muscle in Sprague-Dawley rats. Mech Ageing Dev. 2006;127:344–8. doi: 10.1016/j.mad.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Woodhouse K, Herd B. The effect of age and gender on glucuronidation and sulphation in rat liver: a study using paracetamol as a model substrate. Arch Gerontol Geriatr. 1993;16:111–5. doi: 10.1016/0167-4943(93)90002-y. [DOI] [PubMed] [Google Scholar]
- 13.Sweeny D, Weiner M. Metabolism of acetaminophen in hepatocytes isolated from mice and rats of various ages. Drug Metab Dispos. 1985;13:377–9. [PubMed] [Google Scholar]
- 14.Court M, Duan S, Guillemette C, Journault K, Krishnaswamy S, Von Moltke L, Greenblatt D. Stereoselective conjugation of oxazepam by human UDP-glucuronosyltransferases (UGTs): S-oxazepam is glucuronidated by UGT2B15, while R-oxazepam is glucuronidated by UGT2B7 and UGT1A9. Drug Metab Dispos. 2002;30:1257–65. doi: 10.1124/dmd.30.11.1257. [DOI] [PubMed] [Google Scholar]
- 15.Boersma M, van der Woude H, Bogaards J, Boeren S, Vervoort J, Cnubben N, van Iersel M, van Bladeren P, Rietjens I. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem Res Toxicol. 2002;15:662–70. doi: 10.1021/tx0101705. [DOI] [PubMed] [Google Scholar]
- 16.Court M. Isoform-selective probe substrates for in vitro studies of human UDP-glucuronosyltransferases. Methods Enzymol. 2005;400:104–16. doi: 10.1016/S0076-6879(05)00007-8. [DOI] [PubMed] [Google Scholar]
- 17.Doerge D, Chang H, Churchwell M, Holder C. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos. 2000;28:298–307. [PubMed] [Google Scholar]
- 18.Court M, Greenblatt D. Molecular basis for deficient acetaminophen glucuronidation in cats. An interspecies comparison of enzyme kinetics in liver microsomes. Biochem Pharmacol. 1997;53:1041–7. doi: 10.1016/s0006-2952(97)00072-5. [DOI] [PubMed] [Google Scholar]
- 19.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 20.Le Couteur D, McLean A. The aging liver. Drug clearance and an oxygen diffusion barrier hypothesis. Clin Pharmacokinet. 1998;34:359–73. doi: 10.2165/00003088-199834050-00003. [DOI] [PubMed] [Google Scholar]
- 21.Patki K, von Moltke L, Harmatz J, Hesse L, Court M, Greenblatt D. Effect of age on in vitro triazolam biotransformation in male human liver microsomes. J Pharmacol Exp Ther. 2004;308:874–9. doi: 10.1124/jpet.103.059311. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Wang S, Jia X, Bajimaya S, Lin H, Tam V, Hu M. Disposition of flavonoids via recycling: comparison of intestinal versus hepatic disposition. Drug Metab Dispos. 2005;33:1777–84. doi: 10.1124/dmd.105.003673. [DOI] [PubMed] [Google Scholar]
- 23.Cassidy A, Brown J, Hawdon A, Faughnan M, King L, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell K. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;136:45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Faughnan M, Hawdon A, Ah-Singh E, Brown J, Millward D, Cassidy A. Urinary isoflavone kinetics: the effect of age, gender, food matrix and chemical composition. Br J Nutr. 2004;91:567–74. doi: 10.1079/BJN20041087. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Xie S, Chen S, Zeng S. Glucuronidation of flavonoids by recombinant UGT1A3 and UGT1A9. Biochem Pharmacol. 2008;76:416–25. doi: 10.1016/j.bcp.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Galinsky R, Manning B, Kimura R, Franklin M. Changes in conjugative enzyme activity and acetaminophen metabolism in young and senescent male F-344 rats following prolonged exposure to buthionine sulfoximine. Exp Gerontol. 1992;27:221–32. doi: 10.1016/0531-5565(92)90046-3. [DOI] [PubMed] [Google Scholar]
- 27.Leakey J, Cunny H, Bazare J, Jr, Webb P, Lipscomb J, Slikker W, Jr, Feuers R, Duffy P, Hart R. Effects of aging and caloric restriction on hepatic drug metabolizing enzymes in the Fischer 344 rat. II: Effects on conjugating enzymes. Mech Ageing Dev. 1989;48:157–66. doi: 10.1016/0047-6374(89)90047-x. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Tam V, Hu M. Disposition of flavonoids via enteric recycling: determination of the UDP-glucuronosyltransferase isoforms responsible for the metabolism of flavonoids in intact Caco-2 TC7 cells using siRNA. Mol Pharm. 2007;4:873–82. doi: 10.1021/mp0601190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bock K, Forster A, Gschaidmeier H, Bruck M, Munzel P, Schareck W, Fournel-Gigleux S, Burchell B. Paracetamol glucuronidation by recombinant rat and human phenol UDP-glucuronosyltransferases. Biochem Pharmacol. 1993;45:1809–14. doi: 10.1016/0006-2952(93)90437-2. [DOI] [PubMed] [Google Scholar]
- 30.Brett G, Hollands W, Needs P, Teucher B, Davis B, Brodbelt J, Kroon P. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br J Nutr. 2008 doi: 10.1017/S000711450803081X. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie P, Bock K, Burchell B, Guillemete C, Ikushiro S, Iyanagi T, Miners J, Owens I, Nebert D. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogen Genomics. 2005;15:677–85. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- 32.van der Woude H, Boersma M, Vervoort J, Rietjens I. Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem Res Toxicol. 2004;17:1520–30. doi: 10.1021/tx049826v. [DOI] [PubMed] [Google Scholar]