Abstract
Contributions of cerebro-cerebellar function to executive verbal working memory were examined using event-related functional magnetic resonance imaging (fMRI) while 16 subjects completed two versions of the Sternberg task. In both versions subjects were presented with two or six target letters during the encoding phase, which were held in memory during the maintenance phase. A single probe letter was presented during the retrieval phase. In the “match condition”, subjects decided whether the probe matched the target letters. In the “executive condition”, subjects created a new probe by counting two alphabetical letters forward (e.g., f → h) and decided whether the new probe matched the target letters. Neural activity during the match and executive conditions was compared during each phase of the task. There were four main findings. First, cerebro-cerebellar activity increased as a function of executive load. Second, the dorsal cerebellar dentate co-activated with the supplementary motor area (SMA) during encoding. This likely represented the formation of an articulatory (motor) trajectory. Third, the ventral cerebellar dentate co-activated with anterior prefrontal regions BA 9/46 and the pre-SMA during retrieval. This likely represented the manipulation of information and formation of a response. A functional dissociation between the dorsal “motor” dentate and “cognitive” ventral dentate agrees with neuroanatomical tract tracing studies that have demonstrated separate neural pathways involving each region of the dentate: the dorsal dentate projects to frontal motor areas (including the SMA), and the ventral dentate projects to frontal cognitive areas (including BA 9/46 and the pre-SMA). Finally, activity during the maintenance phase in BA 9, anterior insula, pre-SMA and ventral dentate predicted subsequent accuracy of response to the probe during the retrieval phase. This finding underscored the significant contribution of the pre-SMA/ventral dentate pathway – observed several seconds prior to any motor response to the probe -- to executive verbal working memory.
Keywords: cerebellum, fMRI, Sternberg, verbal working memory, executive function
1. Introduction
Over the past 20 years, neuroimaging has consistently revealed cerebellar activity during cognitive tasks, such as language, working memory, attention, and executive function (for reviews see Ben-Yehudah, Guediche et al., 2007; Desmond and Fiez, 1998; Desmond and Marvel, 2009). Researchers often did not fully understand these activations given that the cerebellum was traditionally thought to be primarily responsible for motor coordination and balance. As a result, the cerebellum was sometimes used as a control region to compare against activations in other brain regions. Other times, the lower half of the cerebellum was not even included in the scanner’s field of view (FOV). Therefore, the contributions of the cerebellum to cognition, for the most part, went undetected.
Clinical data support the notion that cerebellar function extends beyond the motor domain into the cognitive domain. Studies of clinical populations in which the cerebellum is implicated have reported cognitive deficits consistent with reports from the neuroimaging literature (i.e., deficits in language, working memory, and executive function) (Desmond, Chen et al., 2003; Desmond and Marvel, 2009; Fiez, Petersen et al., 1992; Leggio, Silveri et al., 2000; Nicolson and Fawcett, 2005; Pascual-Leone, Grafman et al., 1993; Valera, Faraone et al., 2005). Notably, Schmahmann and Sherman (1997) proposed a cerebellar cognitive affective syndrome based on observations of cognitive and emotional impairments in patients with diseases confined to the cerebellum. They found that these impairments were most clinically prominent in patients with lesions involving the inferior cerebellum (the region of the cerebellum that was often truncated from the FOV during neuroimaging). Children with cerebellar tumor resection have shown verbal working memory deficits that, interestingly, corresponded to the location of the lesion: damage to the left inferior cerebellum was associated with impaired performance when stimuli were delivered in the auditory modality, whereas damage to the right inferior cerebellum was associated with impaired performance when stimuli were delivered in the visual modality (Kirschen, Davis-Ratner et al., 2008). Several other disorders that are said to involve the cerebellum have also been associated with language deficits, such as dyslexia (Nicolson and Fawcett, 2005), attention deficit-hyperactivity disorder (Valera, Faraone et al., 2005), alcoholism (Desmond, Chen et al., 2003), fetal alcohol exposure(O'Hare, Kan et al., 2005), and schizophrenia (Marvel, Schwartz et al., 2004).
Neuroanatomical evidence supports a pathway of communication between the cerebellum and the frontal lobe. Retrograde tracers have been used in non-human primates to map regions that project from the cerebellum (Akkal, Dum et al., 2007; Dum and Strick, 2003; Hoover and Strick, 1999; Middleton and Strick, 2000, 2001). In such studies, labeled neurons in prefrontal Brodmanns Area (BA) 9 and 46 and the pre-supplementary motor area (pre-SMA), which are considered to be cognitive, traced back to the dentate (Bates and Goldman-Rakic, 1993; Hoshi and Tanji, 2004). Similarly, labeled neurons in the primary motor cortex (M1) and the supplementary motor area (SMA), which are considered to be motor, also traced back to the dentate. Importantly, neurons in BA regions 9/46 and the pre-SMA traced back to ventral portions of the dentate, whereas neurons in the M1 and the SMA traced back to dorsal portions of the dentate. This topographical organization suggests that there are two cerebro-cerebellar pathways— one that supports cognition, and one that supports motor function.
The role of the cerebellum in verbal working memory and executive function has often been studied using variations of the Sternberg paradigm (Sternberg, 1966). In a typical Sternberg task, a subject judges whether a stimulus (probe) is contained in a memorized set of previously presented stimuli (targets). The Sternberg paradigm, therefore, can be divided into three phases: encoding (when targets are presented), maintenance (when target information is rehearsed), and retrieval (when a probe is presented and compared to the encoded targets). Response time (RT) on the Sternberg paradigm is linearly related to verbal working memory load. For example, the greater the number of targets to hold in mind, the longer the RT. Researchers can examine behavior during each phase of the Sternberg task in order to better understand the elements of working memory.
Baddeley’s model of working memory models a process for the temporary storage and manipulation of visual and verbal information (Baddeley, 1992). It can be divided into three subcomponents: 1) the executive system, which controls and regulates other cognitive processes; 2) the visuospatial sketch pad, which stores and manipulates images; and 3) the phonological loop, which stores and manipulates language-related information. The phonological loop is assumed to be further comprised of two components, a phonological store that can hold speech-related information for 1–2 seconds, and an articulatory control, which refreshes the contents of the information via rehearsal so that it may be held longer in working memory. Articulatory control can also register visually presented material, such as letters, into the phonological store by subvocalization. According to Baddeley (Baddeley, Gathercole et al., 1998), the evolutionary function of the phonological loop is to create a reliable representation of a novel speech event (e.g., a new word). This concept has direct implications for language skills because the ability to hold unfamiliar phonemes in mind is important to learning and expanding one’s vocabulary. Understanding the neural substrates of working memory, and the phonological loop in particular, is therefore critical to understanding language and its related disorders.
Neuroimaging studies have embraced Baddeley’s model, capitalizing on the wealth of behavioral studies supporting it. These efforts have linked articulatory control processes to left inferior frontal regions, such as BA 6/44/45/47 and phonological storage to left inferior parietal regions, such as BA 40 (Fiez and Raichle, 1997; Fiez, Raife et al., 1996; Jonides, Schumacher et al., 1998; Paulesu, Frith et al., 1993; Smith and Jonides, 1998; Smith, Jonides et al., 1998). Desmond et al. (Desmond, Gabrieli et al., 1997) have proposed a model of cerebro-cerebellar function during verbal working memory that combines Baddeley’s model with the neuroimaging literature. This model proposes that separate regions of the cerebellum, working with the neocortex, differentially support the subcomponents of working memory. In a series of studies using fMRI, Desmond and colleagues observed that when participants performed the Sternberg paradigm, neural activity revealed two spatially and functionally distinct regions in the cerebellum (Chen and Desmond, 2005b; Desmond, Gabrieli et al., 1997). One region appeared bilaterally in the superior hemisphere (lobule VI/Crus I) during the encoding phase, and another region was located predominantly in the right inferior hemisphere (lobule VIIB/VIII) during the maintenance phase. The superior cerebellum was activated in association with a verbal working memory task and a subvocal rehearsal control task, presumably engaging the process of registering the visually presented letters into a phonological code as part of the articulatory control system. Activation in the left inferior frontal regions, such as BA 45/47, was observed in conjunction with the superior cerebellum in both types of tasks. In contrast, activations in the inferior cerebellum, elicited during the maintenance phase, were associated specifically with the verbal working memory task (and not with the motor control task). This activation was accompanied by activity in the left inferior parietal lobule, which has been associated with phonological storage and rehearsal (Becker, MacAndrew et al., 1999; Ravizza, Delgado et al., 2004).
Initial observations indicated that little cerebellar activation was observed during the retrieval phase (i.e., once the probe was presented and subjects made a comparison to the targets held in working memory). The retrieval phase of the Sternberg task arguably involved more manipulation of information than did the encoding or maintenance phases, and required greater allocation of limited cognitive resources (Chen and Desmond, 2005b; D'Esposito, Detre et al., 1995). Therefore, low cerebellar activity in the presence of increased executive load seemed inconsistent with the reports in the neuroimaging and neuropsychological literature. Yet, preliminary evidence obtained from 14 subjects scanned in a limited FOV that was restricted to the cerebellum suggested that the executive requirements for responding to a single probe may not have been sufficient to recruit cerebellar participation (Chen and Desmond, 2005b). When subjects were presented with two probes concurrently, and asked to choose if one or both probes matched the encoded targets, response times slowed, and cerebellar activation increased. Thus, preliminary evidence suggested that a substantial increase in executive working memory demands during the retrieval phase did, in fact, elicit cerebellar activity. Moreover, results from the retrograde tracing studies previously described provided a possible neuroanatomical route to support these activations (Akkal, Dum et al., 2007; Dum and Strick, 2003; Hoover and Strick, 1999; Middleton and Strick, 2000, 2001).
The present study aimed to 1) elicit cerebellar activity by systematically increasing executive verbal working memory demands, and 2) characterize the effects of executive load across the three phases of a Sternberg task. Characterization of cerebellar activity during the encoding, maintenance, and retrieval phases of the Sternberg task was revealed using event-related functional magnetic resonance imaging (fMRI) with a whole-brain FOV. We hypothesized that cerebro-cerebellar activity would increase as a function of executive load, especially during the retrieval phase, based on our preliminary findings described above. We also expected to see spatially dynamic changes in cerebro-cerebellar activity across the three phases of the task because the cognitive demands differed among the three phases. The encoding phase refers to the acquisition of information and rapid conversion of visual to phonological code by creating an articulatory trajectory. The maintenance phase includes articulatory rehearsal and phonological storage. The retrieval phase involves the utilization of the maintained information and decision processes.
A second aim of the study was to investigate whether cerebro-cerebellar function was an intrinsic component of executive verbal working memory that determined successful performance. To find out, we asked whether the state of brain activation prior to response (i.e., during the maintenance phase) in regions that were recruited during the retrieval phase of the task was predictive of subsequent accuracy (Pessoa, Gutierrez et al., 2002). We reasoned that neural processes occurring during the maintenance phase would be more robust on correct trials than on incorrect trials, thereby predicting task performance. An advantage of using an event-related fMRI design is that we were able to examine BOLD changes on a trial-by-trial basis for each subject. First, we identified regions of the circuit that were responsive to increased executive load during the retrieval phase. Then, we retrospectively examined activation in those same regions during the maintenance phase trial-by-trial and compared the activity between correct versus incorrect trials. Therefore, we hypothesized that greater cerebro-cerebellar activity during maintenance would be associated with successful probe recognition.
2. Materials and Methods
2.1 Subjects
Sixteen healthy male (n=6) and female (n=10) subjects were recruited from the Baltimore community. All subjects were native English speakers, self-reported right-handeders, with no known psychological or neurological conditions, or history of head trauma. The subjects’ mean age was 23.69 years (range: 19–28), and mean educational attainment was 16.44 years (range: 12–20). This research was approved by the Johns Hopkins Hospital Institutional Review Board. All subjects gave their written informed consent prior to inclusion in the study, and all were paid for their participation.
2.2 Task Procedures
Subjects performed two test conditions: “match” and “executive”. In both conditions, subjects were presented with two or six black uppercase consonant letters centered on a white background, as depicted in Figure 1. When only two letters were presented, four “#” symbols were also presented as placeholders. Letters and symbols were shown in two rows of three. Target stimuli were displayed for two seconds while subjects studied them (encoding phase). The stimuli disappeared for four or six seconds while subjects held them in mind (maintenance phase). Then, a single lowercase letter was presented as a probe for one second (retrieval phase). In the match condition, subjects indicated whether the probe matched any of the target letters. In the executive condition, subjects indicated whether two alphabetical letters forward of the probe matched any of the target letters. For example, if the probe was “f”, then a subject thought ahead two alphabetical letters (f → g → h) and then decided whether the new probe “h” matched any of the target letters. Subjects were instructed to respond as quickly and accurately as possible. They were given four seconds to respond with a button press (for all subjects, yes = right index finger; no = right middle finger) following probe onset. Failure to respond did not inhibit the start of the next trial. Trials were jittered with an inter-trial interval (ITI) that lasted six to nine seconds. Response time (RT) and accuracy were recorded for each trial. In order to familiarize subjects with the rules of the task, subjects practiced 10 trials of each condition prior to entering the MRI environment.
Figure 1.
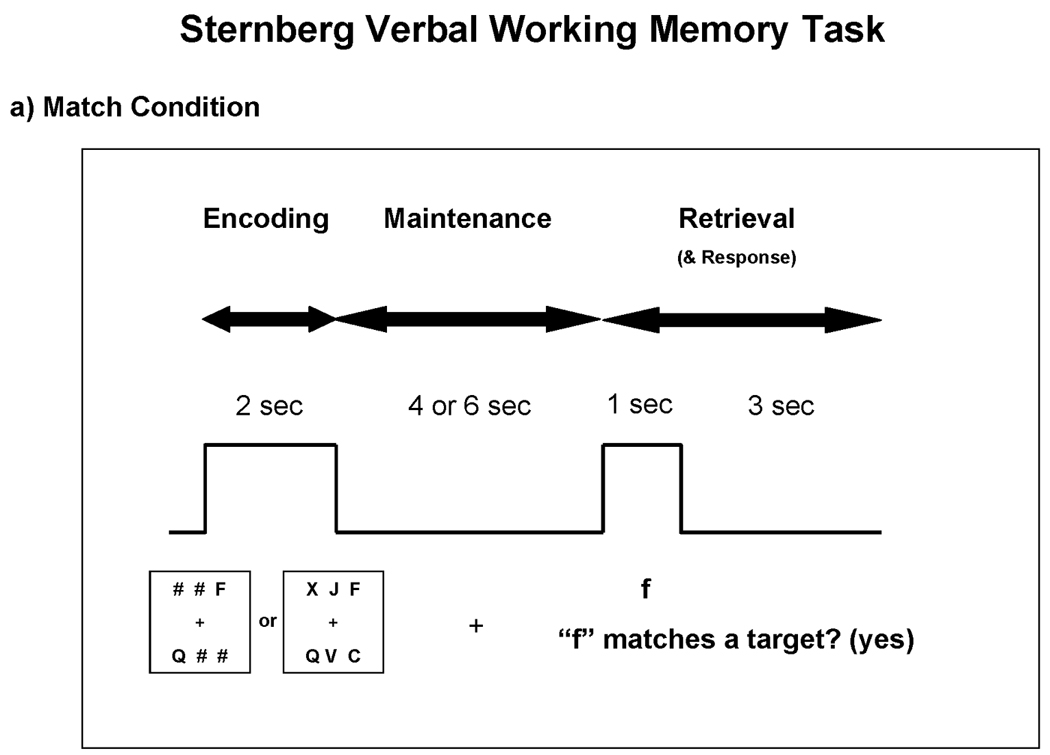
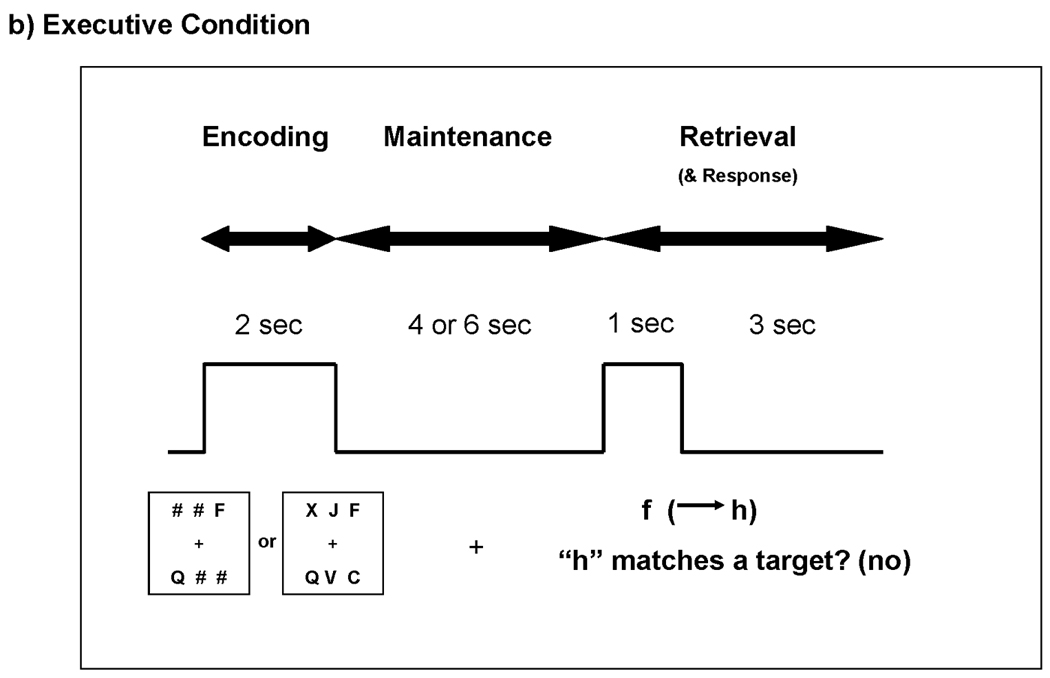
Task parameters for the match and executive conditions. Subjects studied two or six targets during the encoding phase and then held those targets in mind during the maintenance phase. a) During the retrieval phase of the match condition, subjects simply decided whether the probe matched any of the targets. b) During the retrieval phase of the executive condition, subjects decided whether two alphabetical letters forward of the probe matched any of the targets. Subjects responded yes/no with a button press.
Match and executive conditions were performed during separate blocks. The order of block condition was counterbalanced across subjects. Each block contained 64 trials and lasted approximately 16 minutes. Target letters were randomly generated so that letters were unique within a trial and randomly placed across all six possible spatial positions. Probes corresponded to a target on half of all trials. In such trials, the location of the target letter was counterbalanced across spatial positions. The number of studied letters (two or six), length of the maintenance phase (four or six seconds), expected response (yes or no), and duration of ITI (six to nine seconds) were pseudorandomized so that the presentation of identical parameters was limited to three consecutive trials.
Stimuli were delivered using E-Prime 1.1 software (Psychology Software Tools, 2002) on a Hewlett Packard xw4300 workstation running Windows XP Pro. The computer-generated visual display was rear-projected onto a screen situated behind the participant’s head. The display was then reflected onto a mirror directly within the participant’s line of view just outside the head coil. Responses were collected using two velcro-connected fiber optic button boxes (MRA, Inc., Washington, PA) that were held in the subject’s right hand.
2.3 MRI Data Acquisition
All MRI data were acquired using a 3.0T Philips Intera scanner. The structural MRI protocol consisted of a T1-weighted MPRAGE (TR = 7.0 ms; TE = 3.3 ms; flip = 8°, inplane resolution = 0.833 mm; slice thickness = 1 mm; 170 sagittal slices; FOV = 240 mm; 1 NEX). fMRI data were collected using a T2*-weighted gradient echo EPI pulse sequence (TR = 1000 ms; TE = 30 ms; flip = 61°; inplane resolution = 3.75 mm; slice thickness = 6 mm skip 1mm; 20 oblique-axial slices; FOV = 240 mm; 1 NEX). For the fMRI, T2*-weighted images were acquired in the oblique-axial plane rotated 25° clockwise with respect to the AC-PC line in order to optimize imaging of the cerebellum and neocortex. The start of the fMRI scan was triggered by E-prime software at the beginning of each block.
2.4 Functional Data Analysis
The SPM2 software package (Friston, Holmes et al., 1995) was used for preprocessing and statistical computations. In a previous study, Chen and Desmond (2005b) discovered subtle timing differences between the cerebellar and motor cortical hemodynamic response functions (HRFs). In order to maximize the accuracy of characterizing phase-specific BOLD responses, we used the averaged neocortical and cerebellar HRFs from the prior study by Chen and Desmond to convolve with reference waveforms for the encoding (2 s), maintenance (4 or 6 s) and retrieval (4 s) events to create regressors for each subject.
Standard image preprocessing steps were performed, including slice timing and motion correction, anatomical coregistration, normalization to the Montreal Neurological Institute (MNI) stereotaxic space, and spatial smoothing (FWHM = 5 mm). Individual statistical maps were computed for each subject using the general linear model approach as implemented in SPM2, with high pass filtering of 234 seconds. A random effects analysis was then performed to map the average responses to encoding, maintenance, and retrieval phases of the task. This analysis was performed by computing one contrast volume per subject and using these volumes to calculate one-sample t-test values at every voxel. Of particular interest were contrasts comparing executive- versus match-elicited brain activation during the encoding, maintenance, and retrieval phases of the task. Accurate trials only were included in the analyses.
Additional analyses were conducted in order to identify areas of activation that predicted accuracy within a trial. First, areas were identified in which the signal was greater for the executive condition relative to the match condition during the retrieval phase of the task. Activity during the maintenance phase for these selected areas was examined on a trial-by-trial basis and compared to each trial’s accuracy. We decided to focus on the maintenance phase, rather than the encoding phase, for accuracy prediction because properly encoded information had the potential to be lost during the maintenance phase. Therefore, we reasoned that trial success would be more closely tied to activity during maintenance than to encoding. The statistics for accuracy prediction were accomplished in two steps. The first step involved contrasting the beta parameter estimate of the retrieval phase-locked hemodynamic response on 6-target trials of the executive condition with the same parameter estimate for 6-target trials of the match condition, thresholded at p < .001 (uncorrected). The result of this step yielded voxels that reflected significantly greater BOLD signal for the retrieval phase during high executive demand. The second step assessed BOLD correlates of subsequent accuracy on this reduced set of voxels by creating an averaged BOLD signal on correctly answered 6-target trials for the executive condition at each voxel. A similar average was created at each voxel for incorrectly answered 6-target trials. BOLD signal on each trial was time-locked to the onset of the encoding phase of the task. Baseline was established based on the BOLD signal that occurred one time bin (1 TR) prior to the presentation of the encoding stimuli (i.e., during the final TR of the ITI). BOLD signal for each trial was thereby expressed as the percent signal change from baseline. The percent signal change for each of the first four seconds of the maintenance phase for correct and incorrect trials represented a total of 8 values per subject. These values were entered into an SPM repeated-measures analysis of variance (ANOVA) with non-sphericity correction, and a contrast of the accurate versus inaccurate condition was performed. Voxels surviving the first step at p < .001 were eliminated if the accurate-inaccurate contrast in the second step did not satisfy a p < .05 threshold. Putting these criteria together, a voxel was identified if it was preferentially involved in responding to the probe during the retrieval phase of the executive task, and its activity prior to the presentation of the probe (i.e., during the maintenance phase) predicted subsequent accuracy.
For the cerebrum, Montreal Neurological Institute (MNI) coordinates were transformed into the coordinate system of the Talairach and Tourneaux stereotaxic atlas (Talairach and Tournoux, 1988) using the MNI to Talairach transformation described by Lancaster et al. (Lancaster, Rainey et al., 1997). Anatomical determinations of activations were made by referencing the Talairach atlas and an automated coordinate-based Talairach labeling system (Talairach Daemon) (Lancaster, Rainey et al., 1997). For the cerebellum, MNI coordinates were used and referenced with the cerebellar atlas of Schmahmann et al.(Schmahmann, Doyon et al., 2000) and with a supplemental probabilistic atlas of human cerebellar nuclei (Dimitrova, Zeljko et al., 2006).
3. Results
3.1 Behavioral Results
Mean RT and accuracy scores were calculated for the following trial types: 2-target match condition, 6-target match condition, 2-target executive condition, and 6-target executive condition. Mean accuracy for all trials are graphed in Figure 2a. A 2 (condition: match vs. executive) × 2 (load: 2 targets vs. 6 targets) analysis of variance (ANOVA) yielded two main findings. First, there was a main effect of condition, F(1,15) = 20.79, p < .001, revealing that subjects committed more errors during the executive condition relative to the match condition. Second, there was a main effect of load, F(1,15) = 21.07, p < .001, indicating that subjects committed more errors during 6-target trials than 2-target trials. There was no interaction of condition × load, F(1,15) = 2.52, p = .13. Mean RTs were computed for accurate trials only, and are graphed in Figure 2b. A 2 (condition) × 2 (load) ANOVA on RT scores yielded two main findings. A main effect of condition, F(1,15) = 218.35, p < .001, indicated that subjects responded more quickly to trials in the match condition relative to the executive condition. A main effect of load, F(1,15) = 36.53, p < .001, indicated that subjects responded faster during 2-target trials than 6-target trials. There was no interaction of condition × load, F(1,15) = 1.48, p = .24. Thus, participants were fastest and most accurate in the 2-target match condition, and they were slowest and least accurate in the 6-target executive condition.
Figure 2.
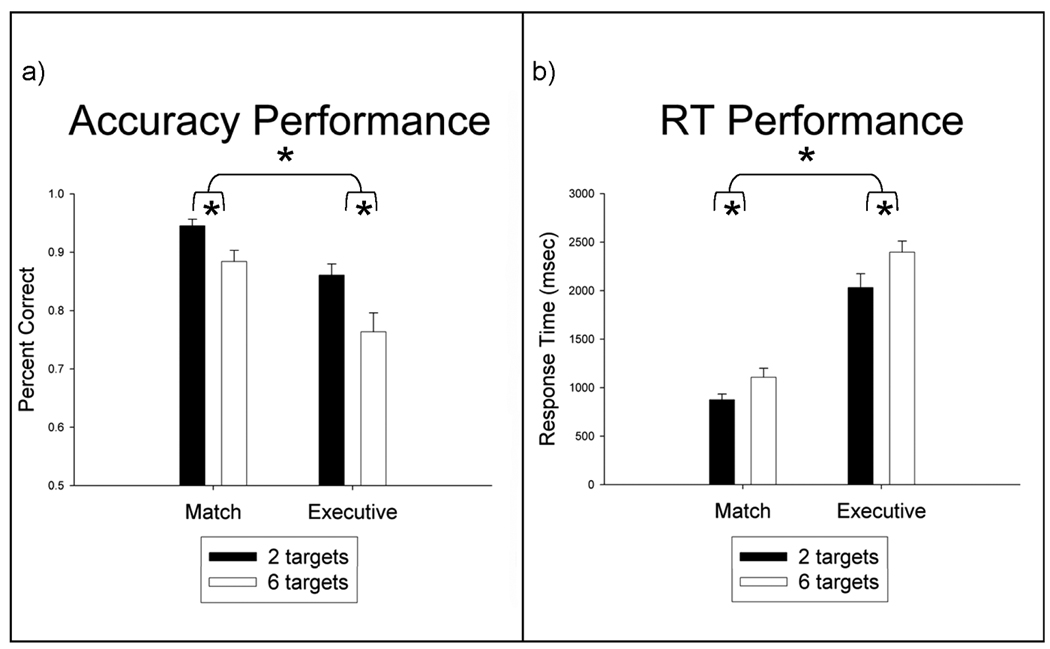
a) Mean accuracy for all trials as a function of condition (match vs. executive) and load (2 vs. 6 targets). b) Mean response times for correct responses as a function of condition and load. Error bars of 1 standard error are plotted for each bar in the graphs. Asterisks denote p-value < .01.
3.2 Imaging Results
The inclusion of two different levels of target load allowed us to confirm previous imaging findings (Chen and Desmond, 2005a, b; Kirschen, Chen et al., 2005). Behaviorally, this design also helped to establish that there was an increasing load effect. Given that the characterization of load-dependent effects has been described at length previously, the present study focused on the direct comparison of the 6-target executive versus 6-target match conditions for accurate trials only. We also directly compared the effects of high and low working memory demands within target load conditions (i.e., 2-target executive versus 2-target match and 6-target executive versus 6-target match). The findings within conditions revealed that activations in the 6-target comparison were larger, and with higher peak values, relative to those in the 2-target comparison. Moreover, certain activations were revealed only in the 6-target comparison. Therefore, we focused our analysis on the 6-target executive versus 6-target match conditions for the encoding, maintenance, and retrieval phases of the task. We used a cluster threshold of 17 voxels, based on a criterion that corresponded to a .05 cluster significance level for structures that are relatively spatially large. Activations smaller than 17 voxels were excluded from the results unless such activations resided in anatomically small regions that were particularly relevant, such as part of the hypothesized cerebro-cerebellar circuit.
3.2.1 Encoding
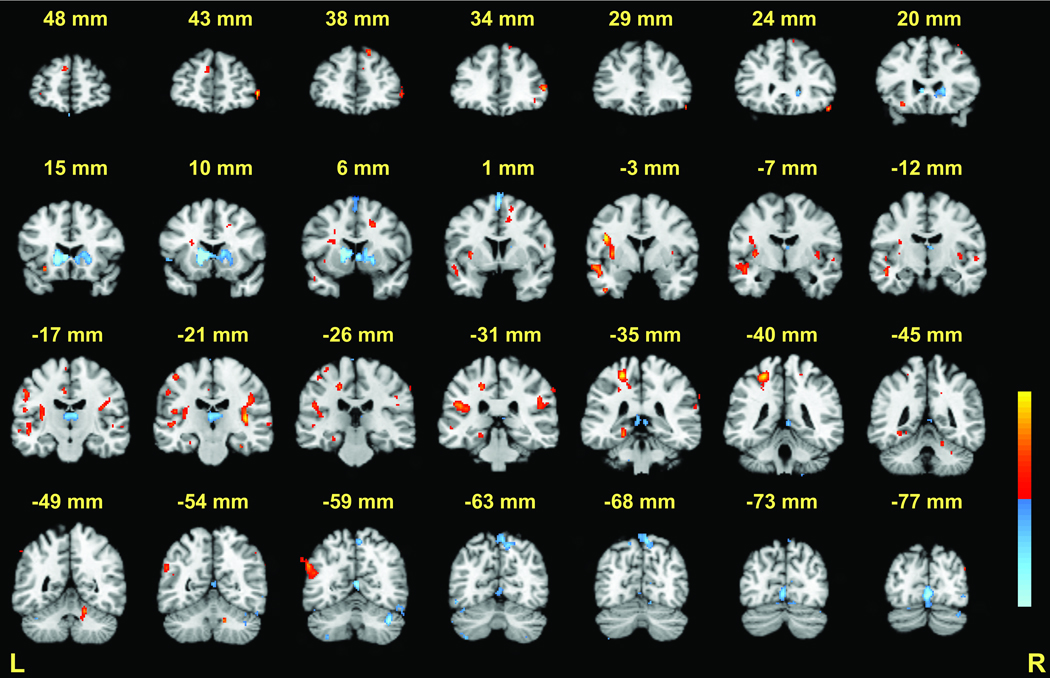
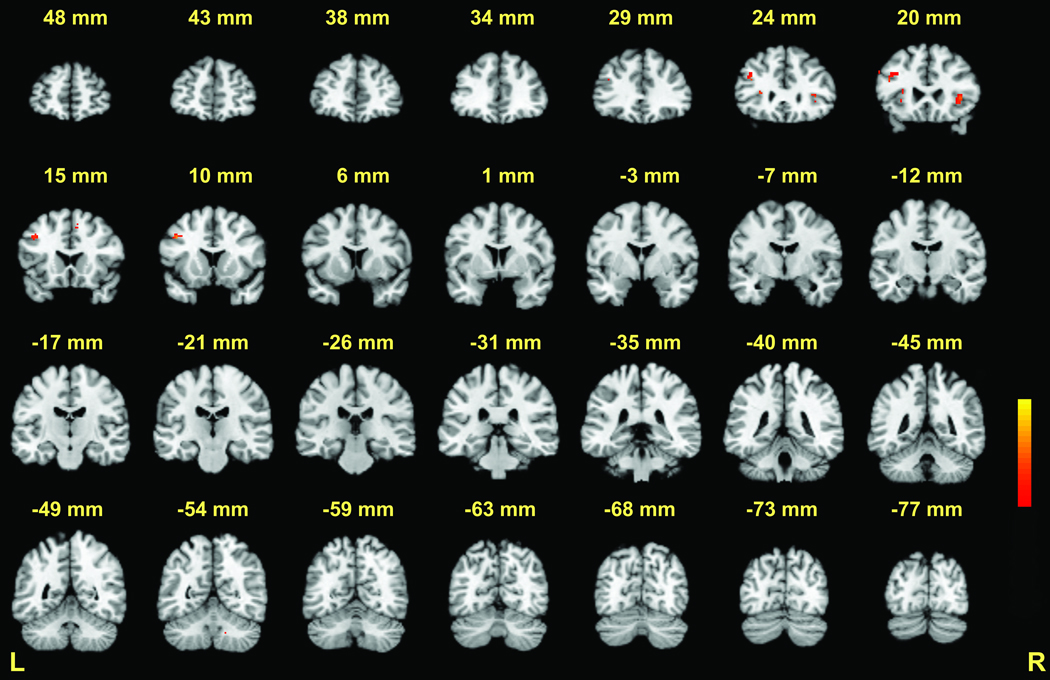
During the two seconds in which targets were presented for encoding, BOLD signal changes in the executive relative to match condition were observed in several brain regions (Table 1, Figure 3 and Figure 4). Notably, BOLD signal increased in the dorsal aspect of the cerebellar dentate. Additional areas of activation included the bilateral insula/claustrum and the left middle temporal gyrus. There were several large negative activations, which reflected increased activation in the match condition relative to the executive condition. Negative activations were confirmed by visual inspection of additional contrasts, including 6-target versus 2-target comparisons, and 6-target conditions locked to the encoding phase (e.g., 6-target executive encoding phase versus the “rest” of the trial). These included the left caudate/putamen, left medial frontal gyrus (BA 6), the dorsal medial nucleus of the thalamus, the right precuneus (BA 7), and right superior cerebellum.
Table 1.
Brain regions activated during the encoding phase of the executive condition, p < .001.
| Brain Region | Brodmann’s Area (BA) | X | Y | Z | SPM Z max | NVox |
|---|---|---|---|---|---|---|
| Positive Activations | ||||||
| R Inferior Frontal Gyrus | BA46 | 47 | 43 | 6 | 4.3 | 95 |
| L Medial Frontal Gyrus | BA6/9 | −7 | 43 | 32 | 3.7 | 32 |
| R Superior Frontal Gyrus | BA8 | 13 | 35 | 47 | 4.1 | 43 |
| R Inferior Frontal Gyrus | BA47 | 47 | 26 | −12 | 4 | 25 |
| L Inferior Frontal Gyrus | BA47 | −29 | 16 | −7 | 3.8 | 41 |
| R Cingulate Gyrus | BA24 | 17 | 2 | 39 | 3.6 | 67 |
| L Middle Temporal Gyrus | BA21 | −41 | 0 | −32 | 3.9 | 17 |
| L Middle Temporal Gyrus | BA21 | −53 | −4 | −8 | 4.2 | 332 |
| L Insula/Claustrum | BA13 | −40 | −7 | 23 | 4.8 | 386 |
| R Superior Temporal Gyrus | BA22 | 53 | −15 | 2 | 3.6 | 52 |
| L Cingulate Gyrus | BA31 | −9 | −22 | 36 | 3.6 | 18 |
| R Claustrum | 34 | −23 | 8 | 4.4 | 231 | |
| L Parahippocampal Gyrus | −27 | −24 | −15 | 3.8 | 19 | |
| L Postcentral Gyrus | BA2 | −42 | −29 | 53 | 4 | 56 |
| L Insula | BA13 | −48 | −31 | 19 | 4 | 268 |
| R Insula | BA13 | 43 | −32 | 20 | 3.7 | 75 |
| L Cingulate Gyrus | BA31 | −20 | −33 | 40 | 4 | 74 |
| R Inferior Parietal Lobule | BA40 | 58 | −34 | 40 | 3.6 | 18 |
| L Superior Parietal Lobule | BA40 | −24 | −42 | 52 | 4.4 | 206 |
| R Cerebellar Dentate (Dorsal) | 12 | −56 | −36 | 4.4 | 78 | |
| L Superior Temporal Gyrus | BA39 | −53 | −60 | 23 | 4 | 153 |
| Negative Activations | ||||||
| R Inferior Frontal Gyrus | BA Brodmann area 11 | 16 | 34 | −21 | −3.8 | 12 |
| L Caudate/Putamen | −7 | 13 | 5 | −5.9 | 961 | |
| L Superior Temporal Gyrus | BA Brodmann area 22 | −51 | 8 | 2 | −3.5 | 19 |
| L Medial Frontal Gyrus | BA Brodmann area 6 | −2 | −5 | 61 | −4.1 | 181 |
| L Thalamus− Medial Dorsal Nucleus | −5 | −21 | 9 | −3.9 | 208 | |
| Parahippocampal Gyrus | BA Brodmann area 30 | −7 | −37 | 2 | −3.5 | 40 |
| R Fusiform Gyrus | BA Brodmann area 37 | 45 | −58 | −17 | −3.4 | 32 |
| R Cerebellar Crus I | 40 | −64 | −36 | −4.4 | 84 | |
| L Cerebellar Crus II/VI IB | −46 | −70 | −52 | −3.7 | 46 | |
| R Precuneus | BA Brodmann area 7 | 4 | −72 | 48 | −4 | 299 |
| L Cerebellar Lobule VI | −10 | −74 | −20 | −3.6 | 30 | |
| L Lingual Gyrus | BA Brodmann area 17 | −9 | −88 | 1 | −3.8 | 18 |
Note. Peak activations within each cluster are reported in the anterior-posterior direction along the y-axis. Activations within the cerebrum are reported using Talairach coordinates, and activations in the cerebellum are reported using MNI coordinates to correspond with the cerebellar atlases referenced in the methods section.
Figure 3.
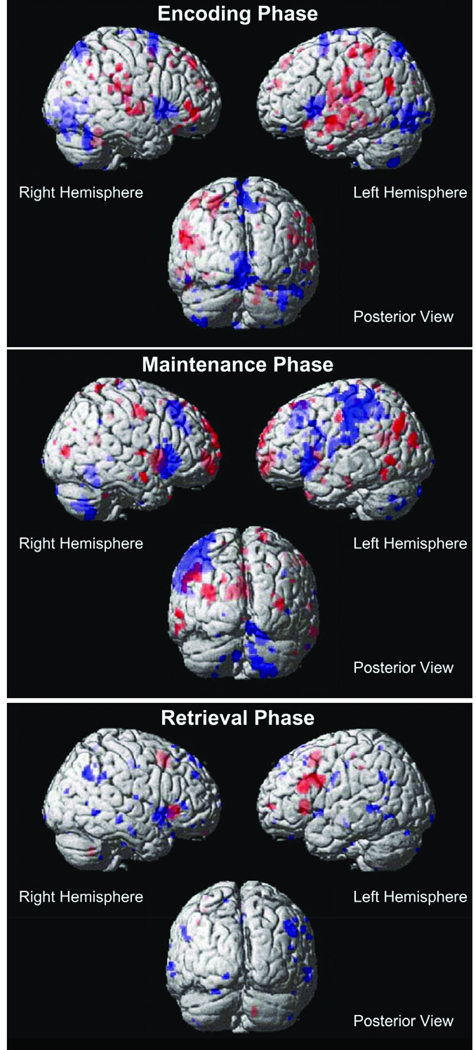
A summary of brain activations across the three task phases for the 6-target executive versus 6-target match comparisons. In all phases, red regions represent increased activity during the executive condition. In the encoding and maintenance phases, blue regions represent increased activity during the match condition. For the retrieval phase, blue regions represent deactivations during the executive condition. P < .001 for all activations.
Figure 4.
Encoding phase: 6-target executive versus 6-target match (see Table 1 for peak coordinates). Coronal slices from Talairach y = +48 to −77mm are depicted. Positive activations (executive > match) are shown in red; negative activations (match > executive) are shown in blue; p < .001 − .00001.
3.2.2 Maintenance
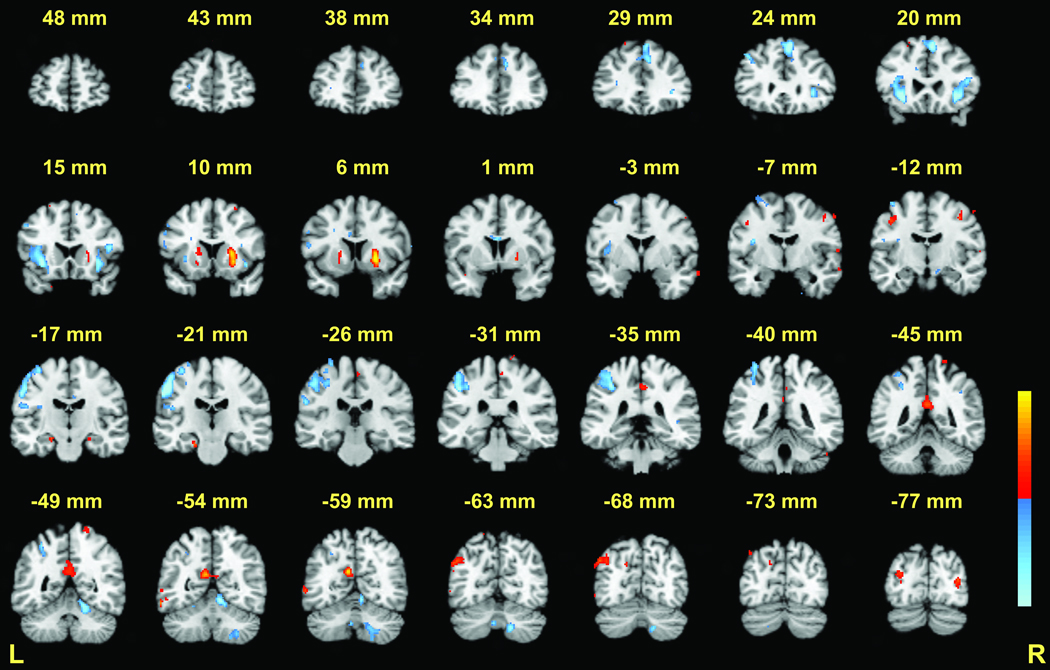
Positive BOLD signal changes during the maintenance phase of the executive condition relative to the match condition were observed in several brain regions (Table 2, Figure 3 and Figure 5) but did not involve the cerebellum. In the left hemisphere, positive BOLD signal changes included the superior frontal gyrus (BA 9/10), posterior cingulate and precuneus (BA 30/31), middle temporal gyrus (including angular gyrus) (BA 39), and middle occipital gyrus (BA 19). Positive changes were also observed in the medial frontal gyrus (BA 9/10), bilateral putamen (with greater activation on the right), and hippocampus. Negative BOLD signal changes were observed in the cerebellum, which reflected increased activity during the match condition. This was confirmed by visual inspection of of additional contrasts, including a 6- versus 2-target match condition contrast which elicited cerebellar activity that was not observed in a 6- versus 2-target executive condition contrast. Match-related cerebellar activations included right cerebellar lobules V and VIIIA. In addition, match-related activations were observed in the bilateral inferior frontal gyrus (BA 47), right superior frontal gyrus (BA 6), and left inferior parietal lobule (BA 40).
Table 2.
Brain regions that increased BOLD signal during the maintenance phase of the executive condition, p < 001.
| Brain Region | Brodmann’s Area (BA) | X | Y | Z | SPM{Z) | NVox |
|---|---|---|---|---|---|---|
| Positive Activations | ||||||
| R Superior Frontal Gyrus | BA10 | 6 | 68 | 16 | 3.9 | 29 |
| L Superior Frontal Gyrus | BA9/10 | −9 | 62 | 30 | 4.4 | 341 |
| R Medial Frontal Gyrus | BA9/10 | 5 | 57 | 3 | 4.8 | 219 |
| L Superior Frontal Gyrus | BA6 | −24 | 27 | 57 | 3.5 | 20 |
| L Superior Temporal Gyrus | BA38 | −21 | 20 | −27 | 4.5 | 18 |
| L Putamen | −18 | 5 | 8 | 3.5 | 77 | |
| R Putamen | 19 | 4 | 3 | 4.5 | 277 | |
| R Middle Temporal Gyrus | BA21 | 60 | −6 | −10 | 4 | 36 |
| R Precentral Gyrus | BA43 | 56 | −8 | 10 | 4 | 19 |
| R Pre/Postcentral Gyrus | BA3/4 | 45 | −10 | 46 | 3.9 | 81 |
| L Precentral Gyrus | BA4 | −42 | −16 | 40 | 3.6 | 42 |
| R Hippocampus | 20 | −17 | −13 | 3.3 | 8 | |
| L Hippocampus | −21 | −20 | −21 | 3.7 | 30 | |
| Cingulate Gyrus | BA31 | 0 | −41 | 38 | 3.5 | 31 |
| L Fusiform Gyrus | BA37 | −58 | −54 | −16 | 3.8 | 29 |
| R Postcentral Gyrus | BA7 | 16 | −55 | 66 | 3.5 | 29 |
| L Inferior Temporal Gyrus | BA37 | −57 | −59 | −4 | 3.6 | 43 |
| L Posterior Cingulate/Precuneus | BA 30/31 | −11 | −61 | 18 | 4.1 | 335 |
| L Middle Temporal Gyrus | BA39 | −50 | −70 | 26 | 3.7 | 175 |
| R Middle Occipital Gyrus | BA19 | 32 | −79 | 8 | 3.7 | 52 |
| L Middle Occipital Gyrus | BA19 | −27 | −84 | 16 | 3.7 | 119 |
| Negative Activations | ||||||
| L Precentral Gyrus | BA9 | −40 | 21 | 40 | −3.8 | 43 |
| L Inferior Frontal Gyrus | BA47 | −29 | 20 | −3 | −5 | 463 |
| R Inferior Frontal Gyrus | BA47 | 31 | 19 | −1 | −4.5 | 463 |
| R Superior Frontal Gyrus | BA6 | 4 | 18 | 53 | −4.7 | 463 |
| R Inferior Frontal Gyrus | BA9 | 62 | 11 | 24 | −3.9 | 18 |
| L Middle Frontal Gyrus | BA9 | −50 | 10 | 37 | −3.9 | 46 |
| L Inferior Frontal Gyrus | BA44 | −53 | 7 | 17 | −3.6 | 26 |
| L Cingulate Gyrus | BA24 | −5 | 0 | 24 | −4 | 62 |
| L Insula | BA13 | −36 | −9 | 19 | −4.1 | 112 |
| L Precentral Gyrus | BA6 | −31 | −15 | 62 | −3.3 | 33 |
| L Postcentral Gyrus/Inferior Parietal Lobule | BA 2/40 | −52 | −26 | 39 | −5 | 1520 |
| L Postcentral Gyrus | BA3 | −28 | −32 | 64 | −3.8 | 62 |
| R Cerebellar Lobule V | 12 | −56 | −14 | −4.7 | 225 | |
| L Cerebellar Vermis | −6 | −64 | −40 | −4.3 | 38 | |
| R Cerebellar Lobule VIIIA | 14 | −70 | −46 | −4.1 | 258 |
Note. Coordinates are reported as in Table 1.
Figure 5.
Maintenance phase: 6-target executive versus 6-target match (see Table 2 for peak coordinates). Coronal slices from Talairach y = +48 to −77mm are depicted. Positive activations (executive > match) are shown in red; negative activations (match > executive) are shown in blue; p < .001 − .00001.
3.2.3 Retrieval
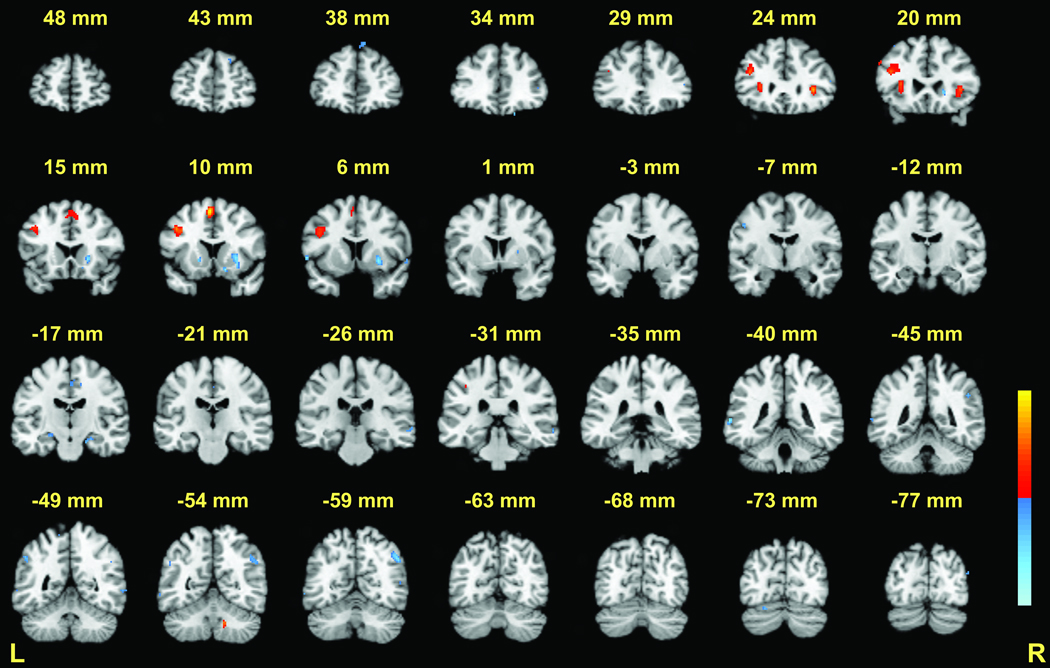
During the retrieval phase of the executive condition, BOLD signal increases relative to match condition were observed in the ventral portions of the right cerebellar dentate. Positive activations were also seen in the left middle frontal gyrus (BA 9/46), left medial frontal gyrus (pre-SMA) (BA 6), and the bilateral anterior insula (BA 13) (Table 3, Figure 3 and Figure 6). Negative activations in this comparison did not appear to be the result of greater activation during the match condition. This was deduced by examining the activity of the 6-target match and executive activations separately during the retrieval phase. Instead, most negative activations in this comparison reflected deactivations during the executive condition. Of these, the largest deactivation was observed in the right putamen.
Table 3.
Brain regions that increased BOLD signal during the retrieval phase of the executive condition, p < 001.
| Brain Region | Brodmann’s Area (BA) |
X | Y | Z | SPM{Z} | NVox |
|---|---|---|---|---|---|---|
| Positive Activations | ||||||
| R Insula | BA13 | 31 | 24 | 7 | 3.9 | 115 |
| L Insula | BA13 | −29 | 22 | 11 | 3.9 | 105 |
| L Middle Frontal Gyrus | BA 9/46 | −40 | 19 | 23 | 4.1 | 335 |
| L Medial Frontal Gyrus | BA6 | −5 | 5 | 53 | 4.2 | 159 |
| R Cerebellar Dentate (Ventral) | 12 | −57 | −40 | 4.0 | 18 | |
| Negative Activations | ||||||
| R Superior Frontal Gyrus | BA8 | 17 | 38 | 41 | −3.4 | 13 |
| R Superior Frontal Gyrus | BA6 | 8 | 34 | 56 | −3.4 | 25 |
| R Inferior Frontal Gyrus | BA46 | 42 | 29 | 11 | −3.7 | 24 |
| R Putamen | 23 | 6 | 0 | −3.8 | 162 | |
| L Precentral Gyrus | BA6 | −46 | −12 | 35 | −3.4 | 21 |
| R Parahippocampal Gyrus | BA28 | 20 | −15 | −17 | −3.4 | 27 |
| L Middle Temporal Gyrus | BA21 | −62 | −41 | 1 | −3.8 | 31 |
| R Superior Temporal Gyrus | BA39 | 43 | −50 | 29 | −3.4 | 18 |
| L Supramarginal Gyrus | BA40 | −52 | −53 | 33 | −3.5 | 29 |
| R Middle Temporal Gyrus | BA39 | 43 | −63 | 31 | −3.7 | 96 |
Note. Coordinates are reported as in Table 1.
Figure 6.
Retrieval phase: 6-target executive versus 6-target match (see Table 3 for peak coordinates). Coronal slices from Talairach y = +48 to −77mm are depicted. Positive activations (executive > match) are shown in red; negative activations (deactivations) are shown in blue; p < .001 − .00001.
3.2.4 Accuracy Prediction
Five brain regions elicited greater BOLD signal during the maintenance phase of the executive condition for correct versus incorrect trials (Table 4 and Figure 7): the left middle frontal gyrus (BA 9), bilateral anterior insula (BA 13), right medial frontal gyrus (pre-SMA) (BA 6/32), and right cerebellar dentate. In Table 4, all activations that survived the rigorous two-step conjunction analyses have been listed, including those smaller than 17 voxels in size. Figure 8 shows the mean change in BOLD signal from baseline (established as the final time point of the ITI) for all five brain regions. In each case, accurate trials exhibited a higher BOLD signal than did inaccurate trials. There was a general decline in BOLD signal from baseline. This may reflect a continued return to resting state that extended beyond the ITI and into the start of the trial.
Table 4.
Greater BOLD activity during the maintenance phase was associated with subsequent trial accuracy in the executive condition, p < .001
| Brain Region | Brodmann’s Area (BA) | X | Y | Z | SPM{Z} | NVox |
|---|---|---|---|---|---|---|
| L Middle Frontal Gyrus | BA9 | −42 | 9 | 35 | 4.0 | 106 |
| R Insula | BA13 | 31 | 22 | 7 | 3.8 | 72 |
| L Insula | BA13 | −29 | 22 | 11 | 3.9 | 17 |
| R Medial Frontal Gyrus | BA 6/32 | 6 | 11 | 45 | 3.3 | 7 |
| R Cerebellar Dentate (Ventral) | 14 | −58 | −42 | 3.7 | 1 |
Note. A 2-step conjunction analysis first identified voxels that showed significant BOLD signal during the retrieval phase of the executive task. Of these, voxels were then identified that showed significantly greater BOLD signal on correct trials versus incorrect trials during the maintenance phase. Coordinates are reported as in Table 1.
Figure 7.
Brain regions that predicted trial accuracy included the left middle frontal gyrus (BA 9), bilateral anterior insula (BA 13), medial frontal gyrus, including the pre-SMA (BA 6/32), and right ventral cerebellar dentate (See Table 4 for peak coordinates.) Coronal slices from Talairach y = +48 to −77mm are depicted. Because the conjunction analysis that was used to identify these brain regions was defined in terms of increased activations, only positive activations are shown, p < .001 − .00001.
Figure 8.
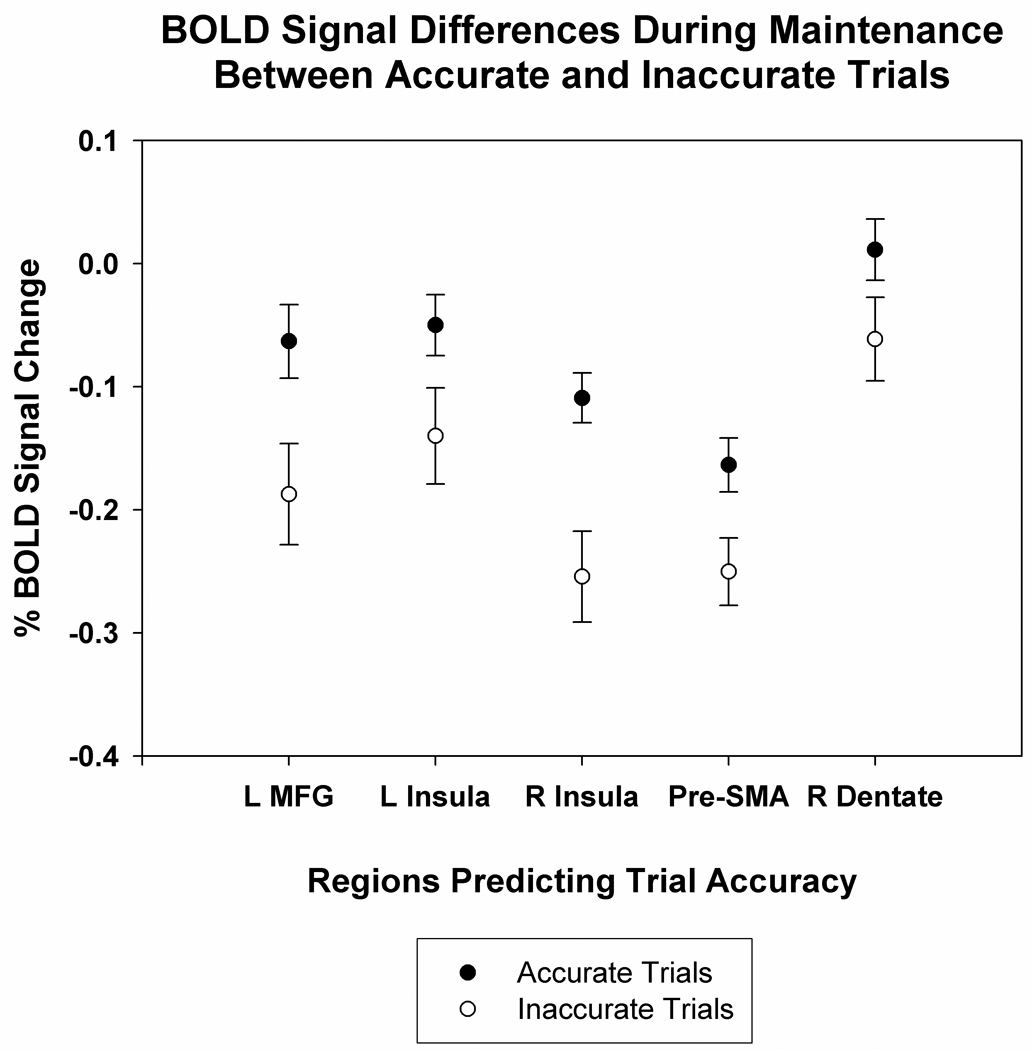
The mean BOLD signal for accurate versus inaccurate trials within the left middle frontal gyrus, left insula, right insula, pre-SMA, , and right ventral cerebellar dentate (See Table 4 for peak coordinates and Figure 7 for visualization of the activations.) For each area of interest, the BOLD signal during the maintenance phase was significantly greater for accurate trials (filled circles) relative to inaccurate trials (open circles). Error bars represent 1 standard error.
4. Discussion
This study examined cerebro-cerebellar activity during executive function using a Sternberg verbal working memory paradigm. Three main conclusions can be drawn from this study. First, cerebellar activity increased as a function of executive load. Activity in the cerebellar dentate, specifically, was related to encoding and retrieval under high executive working memory demands. Second, the topography of cerebellar activation varied throughout the task, presumably as each phase elicited a different cognitive mechanism. The dorsal dentate was related to encoding-phase processes, presumably reflecting rapid orthographic to phonological conversion and computation of an articulatory trajectory for subsequent rehearsal. By contrast, the ventral dentate was related to retrieval-phase processes, requiring the manipulation of stored information in the executive condition. Third, cerebro-cerebellar activity predicted performance success on the executive task on a trial-by-trial basis.
Behavioral data revealed that, as expected, an increase in target load and working memory demand slowed response times and reduced accuracy. Six targets were more difficult to encode and maintain in memory than were two targets. The executive condition, in which participants were required to manipulate the probe information by thinking two alphabetical letters ahead to create a new probe, was more difficult than the match condition. Overall, the most difficult combination of parameters involved the 6-target executive condition.
The neuroimaging analyses focused on the 6-target executive minus 6-target match conditions in order to examine trials that best reflected the neural underpinnings of executive working memory demands. Each of the three phases of the Sternberg task was analyzed separately. Our original expectation was that neural activations between the match and executive conditions would be similar for the encoding and maintenance phases and would differ only during the retrieval phase because that was the point at which the rules of the task differed between the two conditions. Yet, neuroimaging analyses revealed activation differences for all three phases of the task. This suggested that subjects applied a different cognitive approach to each test condition and recruited different neural systems for each. Exploring this further, we found that negative activations in the encoding and maintenance phases were consistent with match-related activity. The nature of this activity was generally consistent with at least two previous neuroimaging studies that used a straightforward matching Sternberg paradigm (i.e., without the additional working memory manipulation load that was unique to our study) and, importantly, included a phase analysis (Chang, Crottaz-Herbette et al., 2007; Chen and Desmond, 2005b). Therefore, it seems that subjects in our study used a typical approach to performing the Sternberg task during the match condition. However, for the executive condition, subjects developed a novel strategy, and this was incorporated into all three phases of the task.
4.1 Encoding
The right cerebellar dentate was activated during executive encoding. Activation in the right dentate, which is the output nucleus of the cerebellum, suggested that the cerebellum’s communication with other brain structures increased during the encoding phase of the executive condition. We examined this activation further. Our scan sequences were not optimized for fine resolution within the dentate on a subject-by-subject basis, but by averaging data across subjects, we were able to determine coordinate estimates, which were then compared to cerebellar nuclei maps (Dimitrova, Zeljko et al., 2006). This analysis revealed that cerebellar activity corresponded with the dorsal region of the dentate, as shown in Figure 9. The dorsal dentate is connected to motor regions of the frontal cortex, such as M1, the premotor cortex, and the SMA (Akkal, Dum et al., 2007; Dum and Strick, 2003; Hoover and Strick, 1999). We did not find increased activity in these regions during the encoding phase at the p < .001 threshold. We did, however, see an activation cluster in the left SMA (−9, −12, 53; BA 6) when the threshold was decreased to p < .005. The SMA influences the planning and initiation of movement sequences (Hoshi and Tanji, 2004). During encoding, this process would have been relevant to the subvocalization of target letters. Because we did not see activity in the M1 or premotor areas at the decreased threshold, it is unlikely that overt speech occurred at this phase and leads us to believe that the SMA activity was involved with creating a subvocal articulatory trajectory of the target letter sequence, and the dorsal dentate contributed to this process (Chen and Desmond, 2005b). An articulatory representation may serve to strengthen memory traces (Ravizza, McCormick et al., 2006) and, in this case, enabled an intense state of encoding that was required for the executive condition. Otherwise, if target letters were not strongly encoded, then the subsequent working memory processes involved with identifying a new probe would have diminished one’s ability to recall those targets at the end of the trial.
Figure 9.
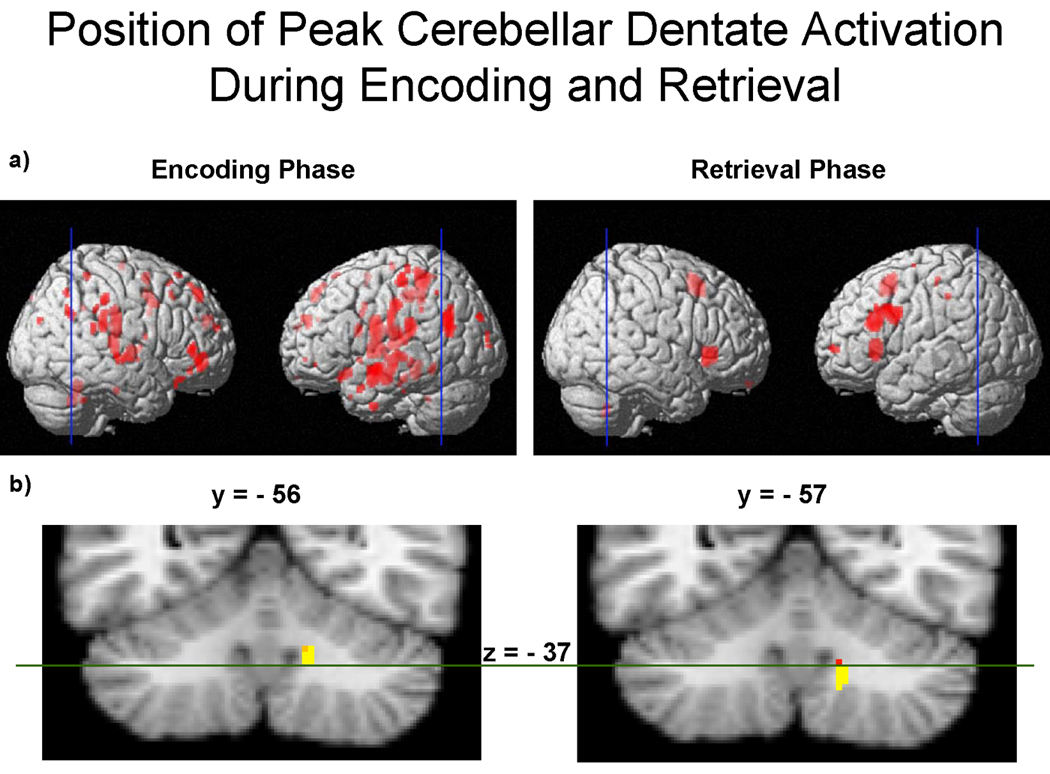
Peak activations within the right cerebellar dentate are shown for encoding and retrieval phases of the Sternberg task (6-target executive versus 6-target match), p < .001. a) The blue vertical lines drawn through the whole brains indicate the location of the cross-sectional slices shown below in part (b). b) The cross-sections correspond to the peak dentate activation in the y-plane for encoding (y = −56) (shown on the left) and retrieval (y = −57) (shown on the right). The position of activation during encoding extends dorsally from the dentate to Lobules IV, V, and VI, and is visible in z-planes −18 to −37. By comparison, the position of activation during retrieval extends ventrally from the dentate to Lobules VIIIB and IX, and is visible in z-planes −36 to −46. As can be seen, there is little spatial overlap between the two activations. A green line has been drawn at z = −37 to mark a functional division between the dorsal and ventral dentate, which divides approximately between z-planes −36 to −40 (Dimitrova, Zeljko et al., 2006). This functional division is consistent with a neuroanatomical pathway division, in which the dorsal dentate projects to motor regions of the frontal lobe, and the ventral dentate projects to cognitive regions of the frontal lobe (Akkal, Dum et al., 2007; Dum and Strick, 2003), implicating a “motor” dorsal dentate and a “cognitive” ventral dentate.
Previously, we reported increased cerebellar activity during the encoding phase in the right superior cerebellum during a Sternberg match paradigm (i.e., in the absence of additional working memory manipulation demands imposed by the present study) (Chen and Desmond, 2005b). Replicating those findings, we observed match-related right superior cerebellar activity during encoding (depicted in blue in Figure 4). Match-related activity was observed in the basal ganglia as well, which also replicates findings from previous reports (Chang, Crottaz-Herbette et al., 2007; Chen and Desmond, 2005b). However, executive-related activations were not observed in these brain regions. A dissociation in activation patterns between the executive and match conditions suggests that subjects used a typical strategy when completing the match condition, but used a novel strategy when completing the executive condition.
Rather large activations were seen within the insula. A middle region was activated on the left side and a more posterior region was activated bilaterally. It has been suggested that separate regions of the insula are differentially involved in orthographic and phonologic processing. Specifically, the posterior insula has been linked to whole-word pronunciation of a letter sequence (Borowsky, Cummine et al., 2006). Though we did not present whole words, it is possible that subjects in the executive condition initially, or automatically, grouped the target letters into “words” as a way to better encode the targets.
4.2 Maintenance
We observed cerebellar activity during the match condition (blue activations in Figure 5) that was generally consistent with previous studies. Specifically, match-related maintenance activity in the right-cerebellar lobes VI and VIII replicated findings from similar Sternberg studies that included a target load (encoding phase) but not a working memory manipulation load (retrieval phase) (Chang, Crottaz-Herbette et al., 2007; Chen and Desmond, 2005b). Net increases in activations, however, were not observed in the cerebellum for this comparison. This suggested that the executive and match condition requirements were similar to each other during the maintenance phase within the cerebellum. This contrasted with the cerebellum’s pattern of activity during the encoding phase and, as will be seen, during the retrieval phase.
Two other primary brain regions of interest exhibited greater activation for match relative to executive conditions, and these were consistent with previous reports using a Sternberg match paradigm. First, match-related activity was observed in the left inferior parietal lobe (IPL, BA 40). This region has been associated with phonological storage (Jonides, Schumacher et al., 1998) and is often co-activated with the contralateral inferior cerebellum (Chen and Desmond, 2005a, b; Kirschen, Chen et al., 2005). A second region of match-related activation was found in the anterior insula bilaterally. The anterior insula (approximately +/− 30, 22, 7) is consistently activated in tasks that involve making decisions about briefly encoded material, such as letters (Chang, Crottaz-Herbette et al., 2007; Chen and Desmond, 2005a, b; Kirschen, Chen et al., 2005; Wager, Sylvester et al., 2005), pseudowords (Fiez, Balota et al., 1999), colors (Chikazoe, Jimura et al., 2009; Wager, Sylvester et al., 2005), and spatial configurations (Pessoa, Gutierrez et al., 2002). Moreover, this region has been associated with a goal-directed “task mode”, which is the opposite of (or anticorrelated to) the default mode (Dosenbach, Visscher et al., 2006). Activity in the anterior insula during the maintenance phase of the match condition, therefore, suggests that this phase of the trial was particularly relevant to the task. Indeed, for the match condition, maintaining the target letters was necessary so that they could be directly compared to the subsequent probe. In the executive condition, however, anterior insula activity was shifted to the retrieval phase, which will be discussed in the next section.
Basal ganglia activation increased during the maintenance phase for the executive condition. Dynamic activation of the basal ganglia throughout different phases of the Sternberg task have been attributed to an adaptive process by which the basal ganglia distributes information to other brain regions to facilitate the transformation of sensory input and cognitive operations into behavior (Chang, Crottaz-Herbette et al., 2007). This function may have had particular relevance during the executive condition in order to strengthen the memory trace of the target information. Further discussion of this will be provided in the upcoming retrieval phase section when additional changes in the basal ganglia activity were observed.
Taken together, the pattern of activations depicted in Figure 5 during maintenance reflected two different strategies at work for the match versus executive conditions. Match-related activations in the cerebellum, IPL, and anterior insula were consistent with phonological storage processes typically used during the Sternberg match paradigm. Executive-related activations in the basal ganglia suggested that an alternative strategy was used to enhance the subject’s ability to process the target information and retain it throughout the maintenance phase.
4.3 Retrieval
Increased activity for the executive condition relative to the match condition was observed in the right cerebellar dentate during the retrieval phase. This area of activity was ventral to that observed during encoding. Figure 9 shows the dissociation between dorsal and ventral dentate activation during the executive encoding and retrieval phases, respectively. A line bisecting the cerebellum marks the plane (z = −37) at which the two activations overlapped, with the encoding activation superior to the dividing line, and the retrieval activation inferior to it. This z-plane coordinate corresponds with the dorsal/ventral midpoint range of the dentate, as mapped by Dimitrova et al. (2006) to be within the range of z = −36 to −40. Thus, the difference in location of functional activity within the dentate dissociated nicely along a dorsal (encoding) and ventral (retrieval) dentate division at z = −37.
Pre-supplementary area (pre-SMA) activity on the left side was also observed. This area has connections with the prefrontal cortex and is thought to be involved in cognitive, rather than motor, functions, such as the anticipation of behavioral events (Bates and Goldman-Rakic, 1993; Hoshi and Tanji, 2004). By contrast, the more caudal SMA that was activated during encoding is thought to be more directly involved in motor planning and execution. This pre-SMA/SMA distinction is relevant to the results at hand because each area has topographically distinct connections with the cerebellar dentate. The dorsal dentate communicates with the SMA, whereas the ventral dentate communicates with the pre-SMA (Akkal, Dum et al., 2007). In addition to communicating with the pre-SMA, the ventral dentate communicates with other frontal regions, such as BA 9 and 46 (Dum and Strick, 2003), which were also activated during the retrieval phase. We observed a large activation in the left middle frontal gyrus (BA 9/46), which has been linked to verbal tasks requiring executive function as well as storage (Smith and Jonides, 1999). It is unknown whether the ventral dentate was co-activated with BA 9/46 or the pre-SMA (or both), which are also connected to each other (Bates and Goldman-Rakic, 1993). However, in the general sense, it is noteworthy that during retrieval the “cognitive” portion of the cerebellar dentate was co-activated with cognitive frontal regions, and at the same time, neither the “motor” portion of the dentate nor motor frontal regions were activated. This pattern of activity contrasted with that which occurred during the encoding phase when the dorsal “motor” dentate co-activated with the SMA. Given that the prefrontal BA 9/46 is involved in executive functions and the pre-SMA is involved in anticipation of behavior, it seems that the ventral dentate’s role in the retrieval phase was related to computing (rather than executing) the correct response.
Executive-related anterior insula activity during retrieval was observed in the same location as was the match-related activity during maintenance. A surge in activity during the retrieval phase under executive demands, relative to match, indicated that the insula responded to the probe. Keeping in mind the proposal that the anterior insula supports a goal-directed task mode (Dosenbach, Visscher et al., 2006), we speculate that the anterior insula increased its activity during retrieval in the executive condition because this phase contained the relevant information that was necessary to successfully complete the trial. Specifically, this phase provided information that allowed the subjects to identify a new probe to compare to the targets held in memory. This could not be done until the probe had been presented. Therefore, a contrast between executive and match conditions revealed executive-related insula activity occurred during the retrieval phase, whereas match-related insula activity occurred during the maintenance phase. This difference in the pattern of activity between the match and executive conditions further supports the notion that subjects used two different strategies when performing each of the conditions.
Finally, the retrieval phase executive condition yielded negative activity in the basal ganglia. This appeared to be the result of de-activation. This region had increased its activity during the maintenance phase under executive conditions. The dynamics we observed in the basal ganglia across task phases between conditions may reflect the general ability of the basal ganglia to modulate cortical activity by inhibition and disinhibition (Parent and Hazrati, 1995). One mode of basal ganglia function is to serve as a “brake” on connected brain regions, thereby providing a means for controlling and updating working memory (Chang, Crottaz-Herbette et al., 2007). Basal ganglia activity, therefore, may have served as a cortical gating mechanism in our study that responded to the changing cognitive demands required by the three phases of the Sternberg task.
4.4 Accuracy Prediction
The BOLD response in five brain regions predicted trial success. Figure 8 illustrates the BOLD changes in these regions during executive maintenance for trials that were accurate versus inaccurate. These regions were selected if they showed increased activity during the executive retrieval phase and if their BOLD signal was greater for accurate versus inaccurate trials during the maintenance phase. These regions included the left prefrontal BA 9, bilateral anterior insula, the medial pre-SMA (BA 6/32), and the right ventral cerebellar dentate. The dentate activation, however, should be viewed with a degree of caution given that only one voxel survived the rigorous conjunction analysis. These areas have all been linked anatomically (Akkal, Dum et al., 2007; Dum and Strick, 2003; Hoover and Strick, 1999;Middleton and Strick, 2000, 2001) or via connectivity analysis (Habas, Kamdar et al., 2009), and their functions have been described in the preceding sections. It is interesting to note that all of these areas predicted trial success in another study that examined visual working memory (Pessoa, Gutierrez et al., 2002). This suggests that the functionality of these five regions is not specific to verbal working memory and may serve an analogous purpose during the storage and manipulation of non-verbalizable information.
Greater activity for accurate trials versus inaccurate trials during the maintenance phase implies that information was encoded correctly. Otherwise, an increased BOLD signal would have reflected both accurate and inaccurate information, with no relationship to successful recall. Similarly, increased BOLD signal for accurate trials suggests that target information was effectively stored and updated throughout the entire duration of the maintenance phase. If not, the information would have been lost. Thus, our conjunction analysis indicated that these five regions were tied to the utilization of information and provided essential contributions to verbal working memory prior to making a response. Another way to interpret these data is that deactivation of these regions, observed for inaccurate trials, reflected distractibility, or a lack of focus, which prevented a rise in signal that was otherwise present on accurate trials (Kelly, Uddin et al., 2008). Therefore, activation in these regions during a critical part of the trial— when proper functioning was essential—was associated with trial success, whereas deactivation was associated with trial failure.
4.5 Summary
This study examined the functional and temporal dynamics of cerebro-cerebellar activity within the context of executive verbal working memory. Although further high resolution imaging explorations of these findings is desirable, particularly for the small deep cerebellar nuclei, and localization and magnitude of the results are influenced by pre-processing settings such as width of the smoothing kernel, our results demonstrated that phase-changing activations within the cerebro-cerebellar circuit were sensitive to executive load. The results showed that encoding involved the SMA and dorsal cerebellar dentate. By contrast, during the retrieval phase a high executive load involved the pre-SMA and ventral cerebellar dentate. Performance success was predicted by several brain regions, including the pre-SMA/ventral dentate circuit, underscoring the cerebro-cerebellum’s relevance to executive verbal working memory. Acknowledging Baddeley’s assertion that the phonological loop is critical for language, there is a need for future studies to clarify the contribution of cerebro-cerebellar circuitry to language. Doing so will improve our understanding of language development and of language-related disorders.
Acknowledgments
The authors would like to thank Deborah Ellis for her assistance with subject recruitment and data collection, and Monica Faulkner for her assistance with manuscript preparation. MRI scans were obtained at the Kirby Center of the Kennedy Krieger Institute in Baltimore, MD. Parts of this research have been reported previously at the Society for Neuroscience Annual Meetings, 2006 and 2008. This research was funded by NIMH R01 MH060234.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: Targets of basal ganglia and cerebellar output. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. The Journal of Comparative Neurology. 1993;336:211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- Becker JT, MacAndrew DK, Fiez JA. A comment on the functional localization of the phonological storage subsystem of working memory. Brain and Cognition. 1999;41:27–38. doi: 10.1006/brcg.1999.1094. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Guediche S, Fiez JA. Cerebellar contributions to verbal working memory: Beyond cognitive theory. Cerebellum. 2007;6:193–201. doi: 10.1080/14734220701286195. [DOI] [PubMed] [Google Scholar]
- Borowsky R, Cummine J, Owen WJ, Friesen CK, Shih F, Sarty GE. FMRI of ventral and dorsal processing streams in basic reading processes: Insular sensitivity to phonology. Brain Topography. 2006;18:233–239. doi: 10.1007/s10548-006-0001-2. [DOI] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34:1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005a;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005b;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cerebral Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: An fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: Language, learning, and memory. Trends in Neuroscience. 1998;2:355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Marvel CL. Cognition: Cerebellum role. In: Squire LR, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. pp. 1079–1085. [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Dimitrova A, Zeljko D, Schwarze F, Maschke M, Gerwig M, Frings M, Beck A, Aurich V, Forsting M, Timmann D. Probabilistic 3DMRI atlas of the human cerebellar dentate/interposed nuclei. Neuroimage. 2006;30:12–25. doi: 10.1016/j.neuroimage.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. Journal of Neurophysiology. 2003;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115(Pt 1):155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME. Linguistic processing. International Review of Neurobiology. 1997;41:233–254. doi: 10.1016/s0074-7742(08)60354-2. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. The Journal of Neuroscience :The Official Journal of the Society for Neuroscience. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–510. [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. The Journal of Neuroscience :The Official Journal of the Society for Neuroscience. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. The Journal of Neuroscience :The Official Journal of the Society for Neuroscience. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential roles of neuronal activity in the supplementary and presupplementary motor areas: From information retrieval to motor planning and execution. Journal of Neurophysiology. 2004;92:3482–3499. doi: 10.1152/jn.00547.2004. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR. The role of parietal cortex in verbal working memory. The Journal of Neuroscience :The Official Journal of the Society for Neuroscience. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: An fMRI study. Neuroimage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Davis-Ratner MS, Milner MW, Chen SH, Schraedley-Desmond P, Fisher PG, Desmond JE. Verbal memory impairments in children after cerebellar tumor resection. Behavioural Neurology. 2008;20:39–53. doi: 10.3233/BEN-2008-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziota JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio MG, Silveri MC, Petrosini L, Molinari M. Phonological grouping is specifically affected in cerebellar patients: A verbal fluency study. Journal of Neurology, Neurosurgery, and Psychiatry. 2000;69:102–106. doi: 10.1136/jnnp.69.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Schwartz BL, Isaacs KL. Word production deficits in schizophrenia. Brain and Language. 2004;89:182–191. doi: 10.1016/S0093-934X(03)00366-3. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research. Brain Research Reviews. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. The Journal of Neuroscience :The Official Journal of the Society for Neuroscience. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Developmental dyslexia, learning and the cerebellum. Journal of Neural Transmission Supplementum. 2005:19–36. doi: 10.1007/3-211-31222-6_2. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16:1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, Hallett M. Procedural learning in parkinson's disease and cerebellar degeneration. Annals of Neurology. 1993;34:594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: FMRI amplitude predicts task performance. Neuron. 2002;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Psychology Software Tools I. E-prime v1.1. Pittsburgh: 2002. [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Petrides M, Evans AC, Toga AW. MRI Atlas of the Human Cerebellum. San Diego: Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal working memory: Evidence from neuroimaging. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain 3-D Proportional System, An Approach to Cerebral Imaging. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]