Abstract
Background
HIV replication and immune activation may increase inflammation and coagulation biomarkers. Limited data exist comparing such biomarkers for those with and without HIV infection.
Methods
For those aged 45–76, high sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), D-dimer, and cystatin C were compared for 494 HIV-infected individuals in the Strategies for Management of Anti-Retroviral Therapy (SMART) study with 5,386 participants in the Multi-Ethnic Study of Atherosclerosis (MESA). For those aged 33–44, hsCRP and IL-6 were compared for 287 participants in SMART with 3,231 participants in the Coronary Artery Development in Young Adults (CARDIA) Study.
Results
hsCRP and IL-6 were 55% (p<0.001) and 62% (p<0.001) higher among HIV-infected participants compared to CARDIA. Compared to MESA, hsCRP, IL-6, D-dimer and cystatin C were 50%, 152%, 94%, and 27% higher (p<0.001 for each). For HIV-infected participants on ART with HIV-RNA levels ≤400 copies/mL, levels were higher (p<0.001) than the general population for all biomarkers (by 21% to 60%).
Conclusions
hsCRP, IL-6, D-dimer and cystatin C are elevated with HIV infection and remain so even after HIV-RNA levels are suppressed with ART. Further research is needed on the pathophysiology of HIV-induced activation of inflammatory and coagulation pathways in order to guide potential interventions.
Keywords: Inflammation, coagulation, renal function, HIV infection, cardiovascular disease
INTRODUCTION
There is a growing body of data indicating that the risk of serious non-AIDS conditions such as cardiovascular disease (CVD), kidney disease, liver disease and non-AIDS defining malignancies is increased in individuals with HIV infection compared to the general population.1 Reasons for this are not clear. HIV-induced activation of inflammatory and coagulation pathways could explain the increased risk.
In the Strategies for Management of Anti-Retroviral Therapy (SMART) trial, episodic use of antiretroviral treatment (ART) guided by CD4+ count was compared with the current practice of continuous ART. The episodic ART strategy was also associated with an 84% (p=0.007) increased risk of all-cause mortality. Most of the deaths were attributed to serious non-AIDS diseases.2 A study using stored plasma specimens for SMART participants was undertaken to explore reasons for the greater risk of death from non-AIDS diseases in the episodic ART group. Interleukin-6 (IL-6) and D-dimer were found to increase significantly in the first month following the interruption of ART. Furthermore, levels of hsCRP, IL-6, and D-dimer measured at study entry were strongly related to mortality, and the association with mortality was evident in participants who were assigned to both the episodic and continuous ART groups.3 Taken together, the findings from these three reports support the hypothesis that HIV induces activation of inflammatory and coagulation pathways, and suggest that ART might dampen the effects.
To further understand these findings, we compared levels of hsCRP, IL-6, D-dimer and cystatin C for participants in the SMART study with values for similar-aged participants in two large population-based studies: 1) The Multi-Ethnic Study of Atherosclerosis (MESA);4 and 2) The Coronary Artery Risk Development in Young Adults (CARDIA) Study.5 Our hypothesis was that SMART participants, all of whom are HIV-infected, have higher values of each of these markers than either the CARDIA or MESA participants, who were recruited from the general population and were unlikely to be HIV-infected.
METHODS
The design, methods and results of the SMART trial and the MESA and CARDIA studies have been published.2,4,5,6
SMART study sample
In SMART, 5,472 men and women > 13 years of age and with CD4+ counts >350 cells/mm3 were enrolled by 318 sites in 33 countries between January 2002 and January 2006.2 Fifty percent of participants were aged 33 to 76 years and were enrolled by sites in the U.S. hsCRP, IL-6, D-dimer and cystatin C were measured at baseline in a subset of the randomized participants.3,7 SMART participants with prior CVD and participants enrolled by sites outside of the United States (U.S.) were excluded from the analyses. A total of 287 Black and non-Hispanic white SMART participants aged 33 to 44 years had levels of hsCRP, IL-6 or D-dimer and 172 of these participants also had cystatin C measurements. Four hundred and ninety-four non-Hispanic white, Black or Hispanic SMART participants aged 45 to 76 years had levels of hsCRP, IL-6 or D-dimer and 231 of these participants also had cystatin C measurements.
The SMART study, including consent for stored specimens, was approved by the institutional review board (IRB) at each site and at the University of Minnesota which served as the Statistical and Data Management Center. The IRB at the University of Minnesota also approved plans for analysis of stored specimens for consenting participants.
MESA study sample
In MESA, 6,814 men and women aged 45 to 84 years from four American racial/ethnic groups (non-Hispanic white, Black, Hispanic, and Chinese) without clinical CVD were enrolled from the general population in six U.S. communities between July 2000 and August 2002.4 hsCRP, IL-6, D-dimer and cystatin C were measured from samples collected at the time of enrollment and findings from 5,386 participants of non-Hispanic white, Black or Hispanic race/ethnicity, aged 45 to 76 are used in analyses in this report. HIV infection was not assessed but there have been few deaths attributed to HIV/AIDS, and HIV infection was assumed to be rare.
CARDIA study sample
In CARDIA, 5,115 Black and non-Hispanic white men and women were enrolled in four U.S. communities between March 1985 and June 1986 (year 0).5 Following enrollment six additional examinations have been carried out. hsCRP and IL-6 were measured at year 15 (in years 2000 and 2001); all covariate values were also taken from the year 15 examination. Findings from 3,231 participants aged 33 to 44 are included in this analysis. Only 2 of the 4 sites measured IL-6. Data comparing cystatin C between CARDIA participants and HIV-infected participants have already been reported and CARDIA data in that report are used to make comparisons with SMART participants (raw data were not available).8 As in MESA, HIV-infection status was not assessed and assumed to be rare.
Biomarker methods
In SMART and MESA, for consenting participants, specimens stored at baseline were used to measure the biomarkers. In CARDIA, samples obtained during follow-up were used. EDTA plasma specimens were collected and were shipped frozen to a central repository where they were stored at −70° Centigrade.
The biomarkers for SMART, MESA and CARDIA were measured by the Laboratory for Clinical Biochemistry Research at the University of Vermont. hsCRP was measured using the BNII nephelometer (N High Sensitivity CRP; Siemens Healthcare Diagnostics, Deerfield, IL). The lower limit of detection was 0.16 μg/mL. IL-6 was measured by ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN). The lower limit of detection was 0.16 pg/mL. For D-dimer, an immuno-turbidimetric assay (Liatest D-DI; Diagnostica Stago, Parsippany, NJ) was used on a Sta-R analyzer (Diagnostica Stago, Parsippany, NJ). The lower limit of detection of the assay was 0.01 μg/mL. A BNII nephelometer (Siemens Healthcare Diagnostics, Deerfield, IL) that utilized a particle-enhanced immunonephelometric assay (N Latex Cystatin-C) was used to assay cystatin C. The assay range is 0.195 to 7.330 mg/dl. The biological and laboratory variability of these assays has been described. Intra-assay coefficients of variation (CV) for IL-6, hsCRP, D-dimer are 6.3%, 8.9%, and 12.2%. For cystatin-C, the CV ranges from 2.0–2.8%.9,10,11,12,13 Based on 3 to 4 controls per assay, inter-assay CVs range from 7–15% for IL-6, 3–6% for hsCRP, 5–14% for D-dimer and 2–6% for cystatin C (personal communication, Elaine Cornell).
Statistical methods
This study utilizes baseline measurements of hsCRP, IL-6, D-dimer and cystatin C in two age groups from SMART. hsCRP and IL-6 levels in participants in SMART aged 33 to 44 years were compared with participants in the same age range in CARDIA. Levels of hsCRP, IL-6, D-dimer and cystatin C for those 45 to 76 years in SMART were compared with MESA participants in the same age range. Comparisons were made for all SMART participants, for those not on ART, and for those on ART and with HIV RNA levels ≤ 400 copies/mL. Median and inter-quartile ranges for biomarkers are cited. Biomarker differences between HIV-infected participants in SMART and participants in MESA and CARDIA were compared using analysis of variance after loge transformation. Other characteristics between participants in the different studies are compared using Student’s t-test and Fisher’s exact test.
Log-transformed biomarker levels were compared using 3 models with: 1) no covariates; 2) covariates corresponding to age, race, and gender; and 3) covariates corresponding to age, race, gender, body mass index (BMI), smoking status, ratio of total/HDL cholesterol, diabetes history, use of lipid lowering drugs and use of blood pressure lowering drugs. The percent difference between SMART and CARDIA and between SMART and MESA were obtained using the loge transformed biomarker by exponentiating the difference in means. Adjusted percent differences derived from the three models described above are displayed graphically.
In order to assess the effects of HIV infection and use of HIV treatment at study entry on the biomarkers, SMART participants were further divided according to HIV-RNA level and/or whether they were using ART. Within SMART, Pearson correlation coefficients between log10 HIV RNA and loge biomarker levels were calculated for participants with HIV-RNA > 400 by baseline ART status. In a multiple regression model for each biomarker, we assessed whether levels varied by class of ART -- non-nucleoside reverse transcriptase inhibitors (NNRTIs) with or without a protease inhibitor (PI), PI, no NNRTI, and nucleoside reverse transcriptase inhibitors (NRTIs), no PI or NNRTI – and by type of NRTI used -- abacavir, no didanosine (ddI), ddI, and other NRTI, no abacavir or ddI. These analyses were explored because we previously reported that participants receiving abacavir had higher inflammatory marker levels compared to those using NRTIs other than abacavir or ddI14. The comparisons by class of drug and type of NRTI are not protected by randomization. In addition to the ART indicators, the baseline covariates mentioned above for comparisons with MESA and CARDIA were included in the regression models.
Co-infection with hepatitis C was common in SMART.15 Thus, separate analyses for SMART participants who were not co-infected with hepatitis C were also performed. An earlier study that compared hsCRP levels for HIV-infected participants with those for participants in CARDIA found that HIV infection without hepatitis C co-infection was associated with higher hsCRP levels in men but not in women.16 Thus, we explored possible interactions with gender.
Statistical analyses were performed using SAS (Version 9.1). P-values are 2-sided and 95% confidence intervals are cited.
RESULTS
HIV-infected participants in SMART were more likely to be male and Black than similar aged participants in CARDIA and MESA. HIV-infected participants were also more likely to smoke cigarettes and take lipid and blood pressure-lowering drugs; they had higher total cholesterol/HDL ratios and lower body mass index than participants in CARDIA and MESA (Table 1).
Table 1.
Characteristics of SMART, CARDIA and MESA Participants Used for Biomarker Comparisons
| Participants aged 33–44 years |
Participants aged 45–76 years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SMART N=287 |
CARDIA N=3231 |
P-value for difference |
SMART N=494 |
MESA N=5386 |
P-value for difference |
|||||
| Mean or % |
SD |
Mean or % |
SD |
Mean or % |
SD |
Mean or % |
SD |
|||
| Age (years) | 40.0 | 3.1 | 39.5 | 3.3 | 0.04 | 53.0 | 6.6 | 60.3 | 8.9 | <0.001 |
| Female (%) | 27.5 | 55.5 | <0.001 | 26.5 | 53.0 | <0.001 | ||||
| Race/ethnicity (%) | <0.001 | <0.001 | ||||||||
| Black | 55.4 | 47.6 | 49.2 | 31.9 | ||||||
| Latino | - | - | 12.3 | 24.9 | ||||||
| White | 44.6 | 52.4 | 38.5 | 43.3 | ||||||
| BMI (kg/m2) | 27.4 | 5.7 | 28.7 | 6.9 | 0.002 | 27.4 | 5.7 | 29.1 | 5.5 | <0.001 |
| Total/HDL cholesterol | 4.7 | 2.0 | 3.9 | 1.4 | <0.001 | 5.1 | 2.7 | 4.1 | 1.3 | 0.004 |
| Total cholesterol (mg/dl) | 190.7 | 41.7 | 184.2 | 35.7 | 0.004 | 197.4 | 47.4 | 194.7 | 36.4 | 0.12 |
| LDL cholesterol (mg/dl) | 111.7 | 32.5 | 112.6 | 32.4 | 0.62 | 111.1 | 34.7 | 117.8 | 31.9 | <0.001 |
| HDL cholesterol (mg/dl) | 45.0 | 15.9 | 50.7 | 14.5 | <0.001 | 43.9 | 15.7 | 51.0 | 15.1 | <0.001 |
| Triglycerides (mg/dl) | 179.6 | 138.4 | 104.1 | 86.6 | <0.001 | 244.8 | 229.3 | 131.1 | 91.1 | <0.001 |
| Smoker (%) | 42.5 | 22.2 | <0.001 | 36.4 | 15.0 | <0.001 | ||||
| Diabetes (%) | 5.6 | 4.2 | 0.26 | 13.6 | 9.7 | 0.46 | ||||
| BP Lowering drugs (%) | 13.6 | 7.1 | <0.001 | 38.9 | 32.6 | 0.006 | ||||
| Lipid lowering drugs (%) | 11.1 | 2.1 | <0.001 | 24.9 | 16.0 | <0.001 | ||||
| Hepatitis C (%) | 14.3 | 24.3 | ||||||||
| CD4+ cell count (cells/mm3) | 623 | 235 | 657 | 260 | ||||||
| HIV RNA ≤ 400 copies/mL (%) | 51.4 | 62.5 | ||||||||
| On ART (%) | 72.5 | 82.4 | ||||||||
Fifty-one percent of SMART participants aged 33 to 44 and 62 percent aged 45–76 had HIV RNA levels ≤ 400 copies/mL (Table 1). The majority of participants in SMART were taking ART. Among these participants on ART, 68 percent for those aged 33 to 44 years and 72 percent for those aged 45 to 76 years had HIV RNA levels ≤ 400 copies/mL. Among those not taking ART, 29 percent were ART-naïve and 32 percent had not used ART for 6 months in the younger age group. Corresponding percents for those 45 to 76 years were 20 percent and 28 percent. CD4+ cell counts averaged over 600 cells/mm3 at study entry.
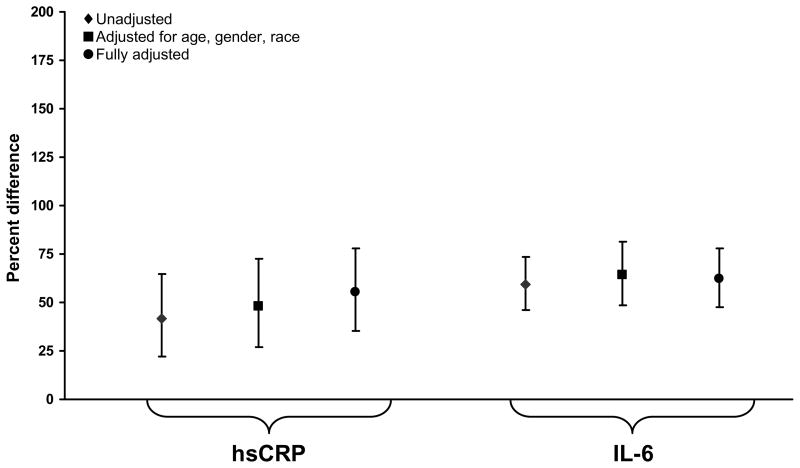
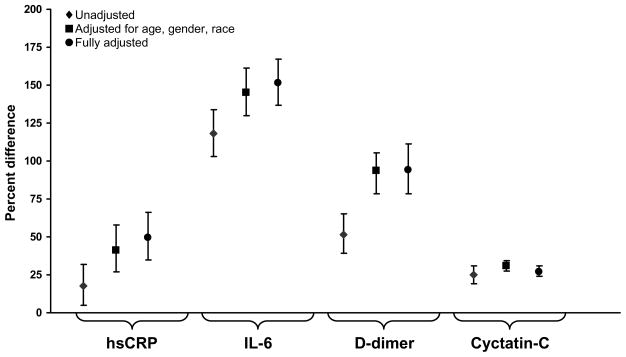
Unadjusted levels of hsCRP and IL-6 were 42% (p=0.002) and 59% (p<0.001) higher in HIV-infected participants compared to participants in CARDIA (Figure 1a). hsCRP, IL-6, D-dimer and cystatin C were 18% (p=0.003), 118% (p<0.001), 52% (p<0.001) and 25% (p<0.001) higher for HIV-infected participants in SMART compared to participants in MESA (Figure 1b). With adjustment, percent differences increased and all were significant (p<0.001) (Table 2, Figures 1a and 1b).
Figure 1.
Figure 1a: Percentage Difference (HIV-infected versus General Population for hsCRP and IL-6): Participants Aged 33–44 Years.
Figure 1b: Percentage Difference (HIV-infected versus General Population for hsCRP, IL-6, D-dimer and Cystatin C): Participants Aged 45–76 Years.
Table 2.
Median Levels and Interquartile Range (IQR) of Biomarkers for SMART, CARDIA and MESA Participants
| Participants aged 33–44 years |
Participants aged 45–76 years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SMART (N=287*) |
CARDIA (N=3231†) |
% Diff.‡ (P-value) |
SMART (N=494§) |
MESA (N=5386) |
% Diff.‡ (P-value) |
|||||
| Median |
IQR |
Median |
IQR |
Median |
IQR |
Median |
IQR |
|||
| hsCRP (μg/mL) | 2.12 | 0.80–4.77 | 1.36 | 0.53–3.78 | 55.2 (<0.001) | 2.68 | 1.07–6.01 | 2.17 | 0.94–4.70 | 49.6 (<0.001) |
| IL-6 (pg/mL) | 2.09 | 1.26–3.49 | 1.29 | 0.80–2.07 | 62.1 (<0.001) | 2.63 | 1.63–4.18 | 1.23 | 0.79–1.92 | 151.6 (<0.001) |
| D-dimer (μg/mL) | 0.29 | 0.17–0.55 | NA | NA | NA | 0.34 | 0.20–0.61 | 0.20 | 0.13–0.35 | 94.3 (<0.001) |
| Cystatin C (mg/dl) | 0.94 | 0.82–1.07 | NA | NA | NA | 1.00 | 0.88–1.17 | 0.85 | 0.76–0.96 | 27.2 (<0.001) |
NA = not available
172 had cystatin C measurements.
689 had IL-6 measurements.
% difference in means of log transformed value, adjusted for age, race, gender, BMI, smoking, total/HDL cholesterol, diabetes, lipid-lowering therapy and blood pressure lowering therapy.
231 had cystatin C measurements.
Differences According to Use of ART and HIV RNA Level
With the exception of D-dimer, the biomarkers did not vary for HIV-infected participants according to use of ART. Among HIV-infected participants 33 to 44 years, D-dimer was 62% (95% CI: 27 to 110; p<0.001) higher for participants not taking ART compared to those taking ART. For those aged 45 to 76 years, D-dimer levels were 63% (95% CI: 31 to 101; p<0.001) higher for those not taking ART compared to those on ART. For both those not taking ART and taking ART, D-dimer levels were significantly higher compared to MESA participants by 127% for those not on ART and by 39% for those taking ART (p<0.001 for both).
Table 3 gives median biomarker levels for those on ART and with HIV RNA ≤ 400 copies/mL. These levels were also higher than in the two general population cohorts (see Table 2). Adjusted percent differences of hsCRP and IL-6 for those aged 33 to 44 in this subgroup of HIV-infected participants were 40% and 39% higher compared to the general population (p<0.001 for both comparisons). For those aged 45 to 76 years, hsCRP, IL-6, D-dimer and cystatin C were 38%, 60%, 49% and 21% higher than the general population, respectively (p<0.001 for all comparisons).
Table 3.
Median Levels and Inter-quartile Range (IQR) of Biomarkers in SMART for Participants on Antiretroviral Treatment and with HIV RNA ≤ 400 copies/mL and Percent Difference from CARDIA and MESA Levels cited in Table 2
| Age (years) |
||||||||
|---|---|---|---|---|---|---|---|---|
| 33 – 44 |
45 – 76 |
|||||||
| Biomarker |
No. |
Median |
IQR |
% Diff. (P-value) |
No. |
Median |
IQR |
% Diff. (P-value) |
| hsCRP (μg/mL) | 140 | 2.13 | 0.77 – 5.20 | 40.2 (<0.001) | 293 | 2.83 | 1.07 – 6.80 | 37.8 (<0.001) |
| IL-6 (pg/mL) | 139 | 1.89 | 1.15 – 3.42 | 39.0 (<0.001) | 291 | 2.64 | 1.55 – 4.14 | 60.1 (<0.001) |
| D-dimer (μg/mL) | 140 | 0.21 | 0.15 – 0.46 | NA | 293 | 0.29 | 0.17 – 0.57 | 49.1 (<0.001) |
| Cystatin C (mg/dl) | 86 | 0.90 | 0.78 – 0.97 | NA | 130 | 1.00 | 0.86 – 1.16 | 20.9 (<0.001) |
SMART participants were further subdivided according to use of ART and HIV RNA level (see Appendix Table). P-values corresponding to adjusted differences between those on ART with HIV RNA ≤ 400 copies/mL and those on ART with HIV RNA > 400 copies/mL for loge transformed levels of hsCRP, IL-6, D-dimer and cystatin-C are 0.27, 0.63, 0.93, and 0.27, respectively.
Appendix Table.
Biomarker levels by HIV-RNA Level and ART status in SMART at Study Entry
| On ART and HIV-RNA ≤ 400 copies/mL (N=433)* |
On ART and HIV-RNA >400 copies/mL (N=179)* |
Not on ART and HIV-RNA >400 copies/mL (N=144)* |
||||
|---|---|---|---|---|---|---|
| Mean (SD) |
Median (IQR) |
Mean (SD) |
Median (IQR) |
Mean (SD) |
Median (IQR) |
|
| hsCRP (μg/mL) | 5.48 (9.29) | 2.55 (0.97–6.07) | 4.25 (5.01) | 2.50 (1.14–5.06) | 3.74 (5.99) | 2.07 (0.78–4.26) |
| IL-6 (pg/mL) | 3.56 (6.05) | 2.44 (1.40–3.89) | 3.53 (3.39) | 2.63 (1.67–3.85) | 13.6 (125.2) | 2.41 (1.68–3.92) |
| D-dimer (μg/mL) | 0.46 (0.78) | 0.27 (0.17–0.51) | 0.42 (0.36) | 0.29 (0.20–0.53) | 0.64 (0.51) | 0.47 (0.29–0.87) |
| Cystatin C (mg/dl) | 1.11 (1.00) | 0.95 (0.82–1.10) | 1.10 (0.40) | 0.99 (0.88–1.14) | 1.10 (0.56) | 1.02 (0.91–1.13) |
Fewer patients had cystatin-C measurements:
216 on ART with HIV RNA ≤ 400, 86 on ART with HIV-RNA > 400, and 87 not on ART with HIV-RNA >400.
We also explored the correlation of log10 HIV RNA levels and loge biomarker levels for those on ART with HIV RNA levels > 400 copies/mL. A significant positive correlation was found between HIV RNA and D-dimer, but not the other markers. The correlation coefficients (p-values) were −0.03 (0.68), 0.10 (0.18), 0.25 (<0.001) and −0.01 (0.96), for hsCRP, IL-6, D-dimer and cystatin-C, respectively.
Differences According to Type and Duration of ART
Some differences were noted in biomarker levels according to type of ART in SMART. In all cases, however, median levels were higher for SMART participants than participants in MESA and CARDIA. Median levels of hsCRP were greater for those taking an NNRTI (2.81 μg/mL) versus a PI (2.14 μg/mL). In a regression model that adjusted for baseline covariates, hsCRP levels were 48% higher for those on an NNRTI (with or without a PI) versus a PI alone (p<0.001). Levels of IL-6 (p=0.46), D-dimer (p=0.12) and cystatin-C (p=0.13) did not differ significantly between these classes of drugs. Median levels of hsCRP and IL-6 were higher for those taking abacavir (3.07 μg/mL and 2.70 pg/mL) versus other NRTIs besides ddI (2.39 μg/mL and 2.36 pg/mL). After adjustment, hsCRP was 28% higher for those on abacavir compared to other NRTIs besides ddI (p=0.05) and IL-6 was 19% higher (p=0.03). Also, although median levels of D-dimer were similar for those taking abacavir and other NRTIs besides ddI (0.27 and 0.27 μg/mL), the adjusted percent difference was 21% (p=0.054). Cystatin-C levels were similar for those on abacavir and other NRTIs (1% difference, p=0.81).
We also examined whether biomarker levels varied according to total years of ART, years of PI and years of NNRTI use and these associations were not significant (data not shown).
Differences for Smokers and Non-Smokers
Because of the large differences in smoking status between HIV-infected participants and participants in CARDIA/MESA, separate analyses for non-smokers were carried out. Adjusted hsCRP and IL-6 levels were 50% (95% CI: 26 to 79) and 63% (95% CI: 46 to 83) higher in non-smoking HIV-infected participants compared to non-smoking participants in CARDIA. For non-smokers in SMART compared to non-smokers in MESA, levels of hsCRP, IL-6, D-dimer and cystatin C were 60% (95% CI: 41 to 81), 164% (95% CI: 146 to 184), 88% (95% CI: 70 to 108) and 29% (95% CI: 25 to 34) higher, respectively.
Differences According to Gender and Hepatitis C Infection
In all three studies, hsCRP levels were higher in women than men. In SMART, median (IQR) levels were 3.81 μg/mL (1.23–7.50) for women and 2.08 μg/mL (0.92–4.66) for men. There was evidence of an interaction between gender and HIV for hsCRP (p=0.0005 for CARDIA comparison and p=0.01 for MESA comparison) but not for the other biomarkers. Both men and women with HIV-infection had higher adjusted levels of hsCRP than participants of the same gender in CARDIA and MESA, but relative differences were larger for men [77% (p<0.0001) higher than CARDIA and 60% (p<0.0001) higher than MESA] as compared to women [9% (p=0.48) higher than CARDIA and 26% (p=0.02) higher than MESA].
Differences in hsCRP levels between HIV-infected participants and those in the CARDIA and MESA cohorts were greater when participants in SMART with hepatitis C co-infection were excluded (22% of men and 18% of women in SMART) (information to make a similar exclusion was not available for CARDIA and MESA but infection with hepatitis C was assumed to be low). hsCRP levels were 97% higher (p<0.0001) for HIV-infected men in both age groups (SMART versus CARDIA and SMART versus MESA). The corresponding percentage differences for women were 25% (p=0.11) (SMART versus CARDIA) and 62% (p<0.0001) (SMART versus MESA). For both age groups the interaction with gender persisted (p=0.001 for CARDIA comparison and p=0.05 for MESA comparison) after the exclusion of participants in SMART with hepatitis C infection.
DISCUSSION
We compared levels of four biomarkers between HIV-infected participants and participants in two large population-based studies. Each of the biomarkers has been associated with CVD in the general population,17,18,19,20,21,22,23,24 and three of the biomarkers (hsCRP, IL-6 and D-dimer) have recently been associated with all-cause mortality in SMART.3 Compared to participants from the general population in CARDIA and MESA, HIV-infected individuals in SMART had higher levels of inflammatory markers, as measured by hsCRP and IL-6, coagulation and fibrinolysis activity, as measured by D-dimer, and impaired renal function, as measured by cystatin C. Higher levels of these markers were evident even among HIV-infected participants on ART and with HIV RNA levels ≤ 400 copies/mL.
The elevation of these biomarkers among HIV-infected persons on effective ART as well as those not on ART may reflect ongoing immune activation even with successful suppression of HIV replication.25 Given the magnitude of the elevations and their relationship with CVD, renal disease and all-cause mortality in the general population as well as with all-cause mortality in SMART, treatments that target inflammatory and coagulation pathways and decrease hsCRP, IL-6 and D-dimer levels may warrant investigation among HIV patients.26,27 Among SMART participants, some differences in biomarker levels according to use of NNRTI and PI and according to type of NRTI were noted. For the latter, we found higher levels of hsCRP and IL-6 among participants taking abacavir compared to other NRTIs other than ddI. This is consistent with a previous report on the SMART cohort.14 Differences among ART drugs are best explored in randomized studies and these findings indicate that these markers should be considered in future trials of ART regimens.
Only a few studies have compared these biomarkers between participants with HIV-infection and uninfected controls. hsCRP has been the focus of several reports.16,28,29 In the largest study, the Fat Redistribution and Metabolic Change in HIV Infection (FRAM), hsCRP levels were elevated in HIV-infected men but not women compared to HIV-uninfected participants in CARDIA, and the differences between HIV-infected and HIV-negative participants varied according to co-infection with hepatitis C.16 Like FRAM, we found that HIV infection was associated with greater differences in hsCRP for men compared to women. Reasons for this are unclear. We also found that hsCRP differences between those with HIV infection and those in the general population were smaller for those with hepatitis C co-infection compared to those who were not. Reasons for differences in hsCRP levels by hepatitis C status are likely multi-factorial, though hsCRP is primarily synthesized by hepatocytes and differences in hepatic function among HIV/hepatitis C co-infected patients, compared to hepatitis C mono-infected patients may be a contributing factor to the hsCRP difference between co-infected and mono-infected participants.30
In the FRAM study, cystatin C levels were also compared to participants in CARDIA.8 Levels were higher in HIV-infected participants compared to participants in CARDIA after adjustment for demographic and clinical factors. Cystatin C may reflect, in part, ongoing inflammation as well as loss of kidney function. We recently showed that cystatin C increased rapidly following ART interruption and that cystatin C levels were correlated with inflammatory and coagulation markers.7
Strengths of this investigation include use of a single laboratory for the measurement of biomarkers in SMART, CARDIA and MESA. The age range and gender-ethnic distribution of SMART permitted comparisons across a broad spectrum of people with HIV. A weakness is that SMART did not exclude participants with more advanced HIV and included few ART naïve participants. Further, there were many differences between the participants in SMART and those in CARDIA and MESA. Although many factors were considered in the multivariate analyses, and did not have a substantial effect on the results, and several subgroup analyses were considered, it is possible that there are other differences that explain our findings. Finally, as these were cross-sectional comparisons, the temporal relationship between the biomarkers and some of the predictors considered is uncertain.
In summary, we found that markers of inflammation, coagulation and renal function were elevated in HIV participants on and off ART compared to participants in two large population-based studies. Further research on reasons for these elevations and interventions to lower them is needed.
Acknowledgments
We would like to acknowledge the SMART, MESA and CARDIA participants, the SMART study team (see N Engl J Med, 2006:355:2294-2295 for list of investigators), the MESA study team (see Am J Epidemiol 2002;156:871-881 for list of investigators), the CARDIA study team and the INSIGHT Executive Committee.
Sources of support: National Institute of Allergy and Infectious Diseases, National Institutes of Health [grant numbers U01AI042170, U01AI46362] (SMART); National Heart, Lung and Blood Institute, National Institutes of Health [contracts N01-HC-95159 through N01-HC-95169] (MESA); and National Heart, Lung and Blood Institute, National Institutes of Health [contracts NO1-HC-48047, NO1-HC-48048, NO1-HC-48049, NO1-HC-48050 and NO1-HC-95095] (CARDIA).
Footnotes
Russell Tracy reports being a paid Consultant to Abbott and Merck. No other authors have a commercial or other association that might pose a conflict of interest.
Presented in part at 16th Conference on Retroviruses and Opportunistic Infections, Montreal, Canada, February 2009
References
- 1.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count – guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–22296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 3.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, et al. for the INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 5.Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, et al. Cardiovascular Risk Factors in Young Adults. The CARDIA Baseline Monograph. Cont Clin Trials. 1991;12(Supplement):1S–78S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 6.The SMART Study Group. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy. Ann Inter Med. 2008;149:289–299. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- 7.Mocroft A, Wyatt C, Szczech L, Neuhaus J, El-Sadr W, et al. Interruption of antiretroviral therapy is associated with increased plasma cystatin C; Results from the SMART Study. AIDS. 2009;23:71–82. doi: 10.1097/QAD.0b013e32831cc129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odden MS, Scherzer R, Bacchetti P, Szczech LA, Sidney S, et al. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection. Arch Intern Med. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenny NS, Tracy RP, Ogg MS, et al. In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:2066–2071. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 10.Sakkinen PA, Macy EM, Callas PW, Cornell ES, Hayes TE, et al. Analytical and biologic variability in measures of hemostasis, fibrinolysis, and inflammation: assessment and implications for epidemiology. Am J Epidemiol. 1999;149:261–267. doi: 10.1093/oxfordjournals.aje.a009801. [DOI] [PubMed] [Google Scholar]
- 11.Keller C, Katz R, Cushman M, Fried LF, Shlipak M. Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: a cross-sectional analysis from the Multi-Ethnic Study of Atherscelerosis (MESA) BMC Nephrology. 2008;9:9. doi: 10.1186/1471–2369-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 13.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in health subjects: implications for reference intervals and epidemiological applications. Clinical Chemistry. 1997;43:52–58. [PubMed] [Google Scholar]
- 14.SMART/INSIGHT and D:A:D Study Groups. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22:F17–F24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tedaldi E, Peters L, Neuhaus J, et al. for the SMART Study Group and International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the Strategic Management of Antiretroviral Therapy (SMART) study. Clin Infect Dis. 2008 doi: 10.1086/593102. epub 27 October. [DOI] [PubMed] [Google Scholar]
- 16.Reingold JS, Wanke C, Kotler DP, Lewis CE, Tracy R, et al. Association of HIV infection and HIV/HCV coinfection with c-reactive protein levels. J Acquir Immune Defic Syndr. 2008;48:142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested-case control study. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;144:537–547. doi: 10.1093/oxfordjournals.aje.a008963. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 20.Harris TB, Ferrucci L, Tracy RP, Corti MC, Washolder S, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5(4):e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzoulaki I, Murray GD, Lee AJ, et al. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke. The Edinburgh Artery Study. Circulation. 2007;115:2119–2127. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 23.Danesh J, Whincup P, Walker M, et al. Fibrin D-dimer and coronary heart disease. Prospective study and meta-analysis. Circulation. 2001;103:2323–2327. doi: 10.1161/01.cir.103.19.2323. [DOI] [PubMed] [Google Scholar]
- 24.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl H Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 25.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22:439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 26.van Gorp ECM, Suharti C, ten Cate H, Dolmans WM, van der Meer JW, et al. Review: Infectious diseases and coagulation disorders. J Infect Dis. 1990;180:176–186. doi: 10.1086/314829. [DOI] [PubMed] [Google Scholar]
- 27.Connolly NC, Riddler SA, Rinaldo CR. Proinflammatory cytokines in HV disease – a review and rationale for new therapeutic targets. AIDS Reviews. 2005;7:168–180. [PubMed] [Google Scholar]
- 28.Dolan SE, Hadigan C, Killilea, Sullivan MP, Hemphill L, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39:44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 29.Jones CY, Jones CA, Wilson IB, Know TA, Levey AS, et al. Cystatin C and creatinine in an HIV cohort: The Nutrition for Healthy Living Study. Am J Kidney Dis. 2008;51:914–924. doi: 10.1053/j.ajkd.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepys MB, Hirshcfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]