Abstract
The aim of this study was to examine the association between initial subjective effects from cigarettes and the rate of progression from first cigarette to regular smoking. Latent class analysis (LCA) was applied to subjective effects data from 573 offspring of twins ranging in age from 14 to 32 years. LCA revealed four classes: 1) High on both pleasurable and physiological respondeses, 2) Cough only response, 3) High on physiological, low on pleasurable respondses, and 4) High on pleasurable, low on physiological respondses. Classes of responses were then used to predict time from first cigarette to the onset of regular smoking in a Cox proportional hazards model. Time-varying covariates representing relevant psychiatric and psychosocial factors as well as dummy variables representing the offspring-of-twins design were included in the model. Members of classes 1 and 4 transitioned more rapidly to regular smoking than the classes characterized as low on the pleasurable response dimension. Our findings provide evidence that previously reported associations between pleasurable initial experiences and progression to regular smoking hold true as well for the rate at which that transition occurs. Furthermore, the fact that profiles of responses did not fall into global categories of exclusively pleasurable vs. exclusively negative (physiological) responses suggests the importance of considering both dimensions in combination to characterize risk for smoking-related outcomes.
Keywords: smoking, subjective effects, offspring of twins
1. Introduction
Nearly 50% of the U.S. population has experimented with cigarette smoking (Centers for Disease Control and Prevention, 2001) and about a third to half of those who try smoking become habitual smokers (McNeill, 1991). Identifying the possible determinants of progression to regular smoking is a major public health goal. One line of thinking is that sensitivity to the first smoking experiences plays an important role in subsequent smoking behavior (Pomerleau, Collins, Shiffman, & Pomerleau, 1993; Silverstein, Kelly, Swan, & Kozlowski, 1982). Sensitivity to the first cigarettes is commonly assessed by self-report of subjective effects that are routinely grouped into ‘positive’ (e.g., relaxed) and ‘negative’ (e.g., dizzy, lightheaded) responses, and this division has been supported by factor analytic approaches (Hu, Davies, & Kandel, 2006). Various hypotheses have been proposed regarding the link between initial sensitivity to cigarettes and subsequent smoking behavior. The most straightforward hypothesis is that initial sensitivity to the positive effects contributes to continued smoking whereas initial sensitivity to the negative effects is associated with a reduced likelihood of continued smoking.
In a review of studies of first tobacco use, Eissenberg and Balster (2000) reported that even though positive subjective effects were more rarely experienced relative to negative reactions such as nausea, coughing, or dizziness, of those who continued smoking, a greater number reported positive initial effects and fewer reported negative initial effects to smoking. Consistent with these findings, in adolescents from the Add Health Survey, a higher positive reactions factor score (on which items such as pleasant sensations, pleasurable rush or buzz, and feeling calm or relaxed had high loadings) was associated with progression to daily smoking whereas a higher negative subjective reactions factor score (on which items such as unpleasant sensations, nausea, coughing, difficulty inhaling, or heart pounding had high loadings) was associated with lower risk of daily smoking (Hu et al., 2006). Further, the positive, but not the negative factor, was also associated with increased levels of lifetime nicotine dependence. Similar findings were reported by Kandel, Hu, Griesler, & Schaffran (2007): rates of nicotine dependence were elevated in individuals endorsing pleasant subjective experiences, but there was no evidence that unpleasant subjective experiences predicted nicotine dependence. In two other studies of adolescents, retrospective report of experiencing relaxation was associated with progression to weekly or monthly smoking and nicotine dependence (DiFranza et al., 2004; DiFranza et al., 2007; Riedel, Blitstein, Robinson, Murray, & Klesges, 2003). Along these same lines, in Chen et al.’s (2003) study of 15 to 17 year old students, retrospective recall of relaxation, pleasurable buzz or rush, and cigarette smell were associated with smoking 100 or more cigarettes and with smoking persistence. Experiencing irritation (coughing, pain in the chest, irritated eyes, bad taste in the mouth), by contrast, has been found to protect against progression to later stages of smoking (DiFranza et al., 2004). Clearly, there is support for the hypothesized association of positive subjective effects with continued smoking and negative subjective effects with not progressing beyond experimentation, but there is also evidence suggesting that the association may not be so clear-cut.
Pomerleau (1995) found that sensitivity to negative effects is not necessarily protective, and can in fact be associated with continued smoking. Findings from some of the same studies reviewed above also indicate that the direction of the association between negative subjective effects and smoking outcomes is not consistent. For example, although DiFranza et al. (2004) found that irritation predicted lower rates of monthly smoking and nicotine dependence, in this same study, both nausea and dizziness were linked to higher risk for the two outcomes. Chen et al. (2003) also reported an association between dizziness and both progression to regular smoking and smoking persistence and Hu et al. (2006) produced evidence that dizziness predicts daily smoking. Of note as well, in many of these studies, the reported associations between subjective effects and smoking outcomes did not hold true for all smoking-related behaviors examined in the study (Chen et al., 2003; Hu et al., 2006; Riedel et al., 2003).
In this paper, we investigate the relationship between subjective reactions to the first cigarettes and the rate of progression to regular smoking. Three aspects of our study distinguish it from much of the prior work in this area. First, our examination of the speed of transition from first cigarette to regular smoking reflects a relatively underutilized approach to studying progression through stages of substance use. In focusing on timing of this transition, we gain more information about the pathway from first use to later outcomes than we can by addressing the more commonly studied question of whether or not a particular stage of substance use is reached. Second, we undertake a novel methodological approach to classifying individuals according to their patterns of subjective reaction endorsement, which may help explain some of the inconsistencies in the presence and directions of associations between subjective reactions and smoking outcomes reported in the literature. Finally, we make use of an offspring-of-twins design to adjust for genetic risk for smoking (Heath et al., 1993; Kendler et al., 1999; Lessov et al., 2004; Li, Cheng, & Swan, 2003; Madden, Pedersen, Kaprio, & Koskenvuo, 2004; True et al., 1997) and the possible overlap between genetic risk and patterns of subjective effects from initial cigarette use, thus providing a stringent test of the association between subjective effects and the rate of progression to regular smoking.
2. Material and Methods
2.1 Sample
The sample for the current study consisted of 573 offspring of male twins who were members of the Vietnam Era Twin Registry (VETR). The VETR is a national registry of same sex male twin pairs who served in the military during the Vietnam Era (1965–1975). Construction of the registry and method of determining zygosity (monozygotic [MZ] vs. dizygotic [DZ]) have been previously reported (Eisen, Neuman, Goldberg, Rice, & True, 1989; Eisen, True, Goldberg, Henderson, & Robinette, 1987; Henderson et al., 1990). Additional information regarding the VETR is available at: htttp://www.eric.seattle.med.va.gov/vetr/home.html.
Families were selected for participation in the study based on information reported by the fathers (i.e., the twins) in 1987 and 1992. In 1987, a mailed questionnaire sent to the twins was used to obtain birth dates of their children. In 1992, the Diagnostic Interview Schedule (DIS) (Robins, 1989) was administered by telephone to obtain their histories of DSM-III-R drug dependence and other psychiatric diagnoses. In 2002, we began an offspring-of-twins (OOT) study focused on understanding genetic and environmental influences on the inter-generational transmission of drug dependence. Criteria for selection of twin pairs into the OOT study included that both twins completed the 1987 questionnaire as well as the 1992 interview and at least one member of the pair had a child born between 1973 and 1987. Twin pairs were invited to participate if both of these criteria were met and at least one member of the pair met DSM-III-R lifetime criteria for drug dependence (DD) in the 1992 interview. A control group, in which both members of the twin pair completed the 1987 questionnaire and the 1992 interview, one or both had a child born between 1973 and 1987 and neither met DSM-III-R lifetime criteria for DD, was also recruited into the study.
Offspring were eligible for participation in this study if they reported ever having smoked a cigarette (n=573; 68.3% of the 839 offspring interviewed). The sample ranged in age from 14 to 32 years, with a mean age of 23.4 years (S.D.=3.9). Male offspring comprised 53.2% of the sample; 92.5% of participants’ fathers identified as non-Hispanic Caucasian and 42.7% of participants reported that both parents completed 12 or more years of education.
2.2 Procedures
Data were collected by experienced staff from the Institute for Survey Research at Temple University. Interviewers were blind to twins’ drug dependence status and gave equal effort to recruitment of all respondents. Prior to being interviewed, all participants gave verbal consent and in cases where offspring were under the age of 18, at least one parent provided written consent. Study procedures were approved by the Institutional Review Boards at the participating universities and the Seattle VA, where the VETR is maintained.
Details of paternal, maternal and offspring interviews have been previously reported (Duncan et al., 2008; Scherrer et al., 2008). In brief, telephone interviews, conducted from 2003–2004, were first administered to fathers. Fathers’ interviews covered family composition, demographic information, and lifetime histories of smoking (including diagnostic criteria for DSM-IV nicotine dependence [ND]), alcohol use, and illicit drug use. Permission to contact the biological mother of his offspring as well as the offspring themselves was obtained during the father interview. The maternal interview was also administered over the telephone, using an adaptation of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994; Hesselbrock, Easton, Bucholz, Schuckit, & Hesselbrock, 1995) to assess lifetime smoking behaviors (from which a heavy smoking index (HSI) was derived), DSM-IV drug abuse and dependence, and other psychiatric disorders. Mothers also provided diagnostic information for offspring attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD). Offspring interviews, also based on a modification of the SSAGA, were used to gather a detailed history of smoking, alcohol use, and illicit drug use that included DSM-IV diagnostic criteria for alcohol abuse and dependence, drug abuse and dependence, and nicotine dependence. The assessment of smoking behaviors included a 9-item measure of subjective reactions to the first few cigarettes smoked (described in more detail in 2.3.1). DSM-IV diagnoses of major depressive disorder, conduct disorder, generalized anxiety disorder, panic disorder, and social phobia were also assessed. Age at onset was reported for all DSM-IV disorders. Age at first use of alcohol and cigarettes was available for all participants who reported any lifetime use and age at onset of use for a given illicit drug was available for those who reported use of that drug more than five times.
2.3 Outcome and Predictors
2.3.1 Smoking-related Variables
The outcome of interest in the current study was the transition time from first cigarette to onset of regular smoking. Regular smoking was defined as smoking cigarettes at least 3–4 times per week for 3 weeks or more, totaling at least 21 cigarettes over the lifetime. (A lower threshold than the standard 100 cigarettes used with adult samples was chosen given the substantial number of participants under the age of 18. This intensity of smoking is associated with loss of control over smoking, ND, and withdrawal in young smokers (DiFranza et al., 2007). Of note, all participants meeting frequency criteria reported having smoked more than 21 cigarettes.) Time from first cigarette to the start of regular smoking was measured by subtracting age at first cigarette from age at onset of regular smoking. For those cases in which initiation and regular smoking were reported as occurring at the same age, transition time was estimated at 6 months. Mean age at first cigarette was 14.2 years (S.D.=3.3). A total of 315 participants (55.0% of ever smokers) met criteria for regular smoking. Mean age at onset of regular smoking was 16.2 years (S.D.=2.5).
The predictor of interest was the profile of initial subjective effects from cigarettes, which was derived through latent class analysis using the following items: 1) How much did you enjoy smoking your first cigarettes?; [While smoking your first cigarettes..]: 2) how much did you like the taste of the cigarette? 3) how often did you experience any other pleasant physical reaction? 4) how much did you cough? 5) how often did you feel dizzy or light-headed? 6) how often did you get a headache? 7) how often did you feel your heart racing? 8) how often did you feel nauseated, like vomiting? 9) how often did you experience any other unpleasant physical reaction? Respondents answered on a 4-point scale: none, a little, some, or a lot. Due to the skewness of the data, categories were collapsed into dichotomous indicators of presence vs. absence of a given experience.
2.3.2 Design Variables
To adjust for sampling design, variables representing genetic (G) and environmental (E) drug dependence (DD) risk were included in the models. Offspring were classified into one of four groups based on paternal DD status, DD status of father’s co-twin and zygosity of the twin pair. DD risk group 1 consisted of offspring of drug dependent fathers (high G and high E risk; n=362). DD risk group 2 consisted of offspring of unaffected MZ twins whose co-twins were positive for DD (high G, low E risk; n=49) and DD risk group 3 consisted of unaffected DZ twins whose co-twins were positive for DD (moderate G, low E risk; n=43). DD risk group 4 was comprised of offspring of unaffected fathers with unaffected co-twins (low G and low E risk; n=119).
Offspring were also categorized into one of four ND risk status groups based on paternal reports of lifetime ND in the 1992 assessment. Inclusion of ND risk group status in analyses allowed us to assess whether the association between initial subjective effects and rate of progression from first cigarette to regular smoking was accounted for by genetic risk for ND. Similar to the DD risk groups, ND risk group 1 (n=374) was comprised of offspring whose fathers met ND criteria (high G, high E); ND risk group 2 (n=51) consisted of offspring of unaffected MZ twins whose co-twins were positive for ND (high G, low E); ND risk group 3 (n=47) was comprised of offspring of unaffected DZ twins whose co-twins were positive for ND (moderate G, low E); and ND risk group 4 (n=119) consisted of offspring whose fathers and fathers’ co-twins were unaffected (low G, low E). (See Jacob et al. (2001) for additional information on risk group design).
2.3.3 Covariates
Demographic information and psychiatric conditions associated with smoking were tested as possible correlates of transition time from first cigarette to regular smoking. Covariates represented four domains. The first was demographics: paternal and maternal education, race (Caucasian vs. non-Caucasian) and parental marital status (i.e., divorced or not). The second was externalizing disorders: ADHD, ODD, and conduct disorder. The third domain was internalizing disorders: major depressive disorder, panic disorder, generalized anxiety disorder, and social phobia. Substance use and substance use disorders comprised the fourth domain: alcohol use, cannabis use, alcohol abuse, cannabis abuse, and alcohol dependence.
2.4 Data Analysis
2.4.1 Deriving Classes of Initial Subjective Responses to Cigarettes
Classes of responders were derived through latent class analysis (LCA) (McCutcheon, 1987). LCA is a parametric clustering and data reduction technique used to find mutually exclusive subsets (latent classes) of subjects based on their responses to a set of categorical items. The underlying assumption is that the observed association among the indicators is attributable to the existence of an unobserved latent construct with a finite number of mutually exclusive classes. LCA was chosen over other data reduction techniques, such as factor analysis, for two primary reasons. First, using ordinary factor analysis with categorical data is apt to lead to misleading parameter estimates and misleading goodness-of-fit indices (Vermont & Magidson, 2005). Second, applying LCA to the data allowed us to explore patterns of endorsement across the two constructs believed to be underlying the 9 items and to estimate the frequency with which these two constructs ‘travel together’ (i.e., how common it is for individuals to report high on both vs. low on both vs. high on one and low on the other).
There are two parameters of primary interest in a LCA model: 1) probability that a randomly chosen individual is a member of a given latent class (i.e., prevalence of each class) and 2) probability of endorsing an individual item (item endorsement probability, or IEP), given membership in a particular class. In the present analyses, participants were assigned to the class with the highest posterior probability of membership based on their item endorsement profiles. Since LCA models are not nested within each other, parsimony indices such as the Bayesian Information Criterion (BIC) are generally the preferred method for comparison of different models, so we used the BIC to determine the best fitting model. Analyses were conducted with Latent Gold v.4.0 (Vermont & Magidson, 2005).
2.4.2 4-Class Solution
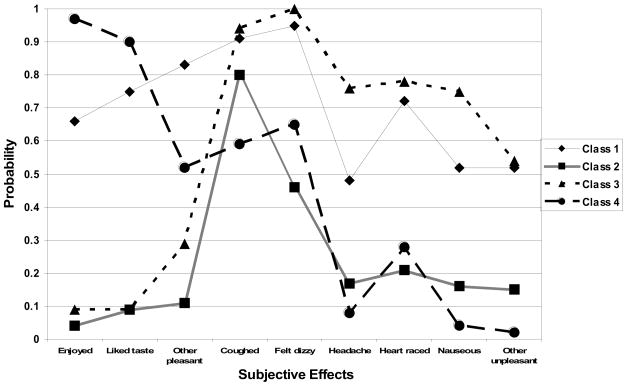
A 4-class solution gave the best fit to the data, as it had the lowest BIC value (−2594.90) and showed the greatest decrease in BIC value from the 1-class solution (Δ BIC = −539.49) when compared with other possible solutions. (Item endorsement probabilities for each class are available upon request from the authors.) As seen in Figure 1, response profiles were not suggestive of a severity continuum, but rather of qualitative distinctions between groups of respondents. Each class reflected a unique combination of the two dimensions of subjective effects: physiological responsivity (coughed, felt dizzy or light-headed, got a headache, heart raced, felt nauseous, other unpleasant reaction) and pleasurable response (enjoyed, liked taste, other pleasant reaction). Class 1 ‘High on pleasurable and high on physiological responses’ was characterized by high levels of endorsement (IEP >0.50) of 8 out of 9 subjective effects (the only exception being ‘got a headache,’ which had an IEP of 0.48). By contrast, in Class 2 ‘Cough only response’ the only IEP over 0.50 was ‘coughed.’ Thus, Class 1 was high on both dimensions and Class 2 was low on both dimensions. In classes 3 and 4, pleasurable response and physiological responsivity dimensions diverged. In Class 3, the three subjective effects that fell into the ‘pleasurable response’ category had IEPs of 0.09 (enjoyed), 0.09 (liked taste), and 0.29 (other pleasant reaction). IEPs for all of the physiological response items exceeded 0.50 (and for all but ‘other unpleasant reaction’ exceeded 0.75). The pattern was reversed in Class 4, in which IEPs ranged from 0.52–0.97 for pleasurable subjective effects and from 0.08–0.28 for 4 of the 6 physiological response items. Class 3 was therefore referred to as ‘High on physiological, low on pleasurable respondses’ and Class 4 as ‘High on pleasurable, low on physiological responses.’
Figure 1.
Item Endorsement Probabilities by Class
2.4.3 Constructing Statistical Models
2.4.3.1 Base Model
A Cox proportional hazards model was chosen to conduct multivariate regression with time-to-event data (Cox, 1972), given that not all of the participants had lived through the period of risk for onset of regular smoking. Classes of subjective effects were represented in the model using three dummy variables, with Class 2 (‘Cough only’) as the reference group. The base model also included 3 dummy variables representing DD risk group (entered to adjust for sampling design) and 3 dummy variables representing ND risk group. In both cases group 4 (low G, low E risk) served as the reference group. In addition, two dummy variables representing mother’s HSI (low = 1–2; high = 3 or higher, with never smokers as the comparison group) were entered into the model to adjust for maternal transmission of risk for smoking. Indicators of age at first cigarette were included as well to adjust for variability in proximity of age at first cigarette to the risk period for development of regular smoking. Two dummy variables were used to reflect the lowest and highest 25% of the distribution for age at first cigarette, with ‘average age’ (13–16 years) as the reference group. ‘Early age at first cigarette’ was defined as initiating smoking at age 12 or younger and ‘late age at first cigarette’ as initiating smoking at age 17 or older. Age at the time of assessment and gender were also entered into the base model.
2.4.3.2 Model with Covariates
Demographic information and psychiatric conditions associated with smoking were tested in two stages with classes of subjective effects, maternal HSI, age at first cigarette, age at assessment, gender, and paternal DD and ND risk group status. First, covariates were tested by domain. For example, major depressive disorder was tested as part of an internalizing domain by combining it in a model with social phobia, generalized anxiety disorder, and panic disorder. If the hazard ratios were statistically significant (i.e., confidence intervals did not include 1.00), covariates were retained and then combined with significant covariates from the other domains assessed. The reduced model was derived through manual backward deletion. Covariates that were no longer significant in the context of other predictors were removed one at a time to produce the final model.
In an effort to ensure that the sequence of onset for a given risk factor and onset of regular smoking was modeled accurately (e.g., that ODD was treated as a risk factor only in those cases where the disorder manifested prior to onset of regular smoking) a ‘person year’ data set was created using SAS, version 9.1 (SAS Institute Inc., 2002) to represent time-varying covariates. Data were constructed such that each line of data represented a single year of life for every individual. For cases that were positive for a given covariate, that covariate was coded as absent in each year up to the age of onset for that condition and present from that year onward. In the present analyses, all variables in the externalizing disorder, internalizing disorder, and substance use/disorder domains were coded as time-varying covariates and demographics were coded as present/absent across all years. Analyses were conducted with Stata, version 8.2 (Statacorp, 2005).
Confidence intervals were adjusted for family clustering using Huber-White robust standard errors. The proportional hazards assumption that risk remains constant over time was assessed using the Grambsch and Therneau test of the Schoenfeld residuals (Grambsch & Therneau, 1994). In the base model, the proportional hazards assumption was violated for participant age at the time of interview. To adjust the model, the distribution for years at risk was split into 7 subdivisions (up to 3, 4–6, 7–9, 10–12, 13–15, and 16–18 years) and interaction terms were created between age and each of the divisions. Interaction terms were entered into the model one at a time to test for changes in the proportional hazards estimations. The addition of the interaction term representing age by the risk period 16–18 years resulted in a non-significant outcome in the test of proportional hazards assumptions. The same procedure was followed in the model containing covariates to adjust proportional hazards violations for ND risk group 2, early age at first cigarette, age, and low maternal HSI. Inclusion of the interaction terms reflecting ND risk group 2 by the risk period 13–15 years, early age at first cigarette by the risk periods 13–15 and 16–18 years, late age at first cigarette by the risk period 16–18 years, low maternal HSI by the risk periods 13–15 and 16–18 years, and DD risk group 3 by the risk period 10–12 years resolved all violations of the proportional hazards assumption.
3. Results
3.1 Family History of Smoking and Smoking Transitions by Class
Timing of smoking milestones and indicators of familial risk for smoking outcomes (paternal ND risk group and maternal HSI) are shown by class in Table 1. Distributions across paternal ND risk groups did not vary by class, nor did maternal HSI levels, suggesting that class membership was not purely a function of familial liability to smoking. Age at first cigarette also did not differ across classes, ruling out the possibility that distinctions in subjective responses were accounted for by differences in age at smoking initiation. By contrast, likelihood of becoming a regular smoker (χ2(3)=80.57; p<.001) and transition time from first cigarette to onset of regular smoking (χ2(6)=21.42; p<.01) differed significantly across classes. As seen in Table 1, members of ‘High on both pleasurable and physiological responses’ and ‘High on pleasurable, low on physiological responses’ classes were the most likely to progress to regular smoking (77.9% and 66.3%, respectively). These same two classes also had the highest rates of rapid progression to regular smoking. Just over 42% of regular smokers in Class 1 and 52% of regular smokers in Class 4 made the transition in one year or less.
TABLE 1.
Family history of smoking and rate of transitions between smoking stages for each class
| Class 1 High on physiological and pleasurable responses | Class 2 Cough only | Class 3 High on physiological, low on pleasurable responses | Class 4 High on pleasurable, low on physiological responses | |
|---|---|---|---|---|
| n=172 (30.0%) | n=159 (27.8%) | n=141 (24.6%) | n=101 (17.6%) | |
| Age at first cigarette | ||||
| Early: ≤ 12 years | 30.2% | 25.8% | 29.8% | 20.8% |
| Average: 13–16 years | 50.6% | 49.7% | 50.3% | 51.5% |
| Late: ≥ 17 years | 19.2% | 24.5% | 19.9% | 27.7% |
| Regular smoker (n=315)a | 77.9% | 32.7% | 44.0% | 66.3% |
| Timing of first cigarette to onset of regular smoking (n=315)a | (n=134) | (n=52) | (n=62) | (n=67) |
| Rapid: ≤ 1 year | 42.5% | 28.9% | 35.5% | 52.2% |
| Average: 2–3 years | 39.6% | 26.9% | 27.4% | 26.9% |
| Slow: ≥ 4 years | 17.9% | 44.2% | 37.1% | 20.9% |
| Paternal nicotine dependence (ND) risk group status | ||||
| Group 1: high genetic (G), high environmental (E) | 64.5% | 57.9% | 66.7% | 64.4% |
| Group 2: high G, low E | 4.7% | 9.4% | 10.6% | 10.9% |
| Group 3: moderate G, low E | 9.9% | 9.4% | 5.0% | 4.0% |
| Group 4: low G, low E | 20.9% | 23.3% | 17.7% | 20.8% |
| Maternal heavy smoking index | ||||
| Non-smoker | 49.1% | 51.6% | 52.8% | 59.6% |
| Low: 1–2 | 14.6% | 16.4% | 13.6% | 13.1% |
| High ≥ 3 | 36.3% | 32.1% | 33.6% | 27.3% |
Statistically significant difference across classes, p <.01
3.2 Time from First Cigarette to Onset of Regular Smoking
3.2.1 Base Model
Results from the first Cox proportional hazards regression analysis using initial subjective response to cigarettes to predict rate of progression from first cigarette to regular smoking are shown in Table 2 (left side). In this base model, adjustments were made for risk conferred by paternal ND and maternal smoking history, age, sampling design (i.e., paternal DD risk group status), gender, and age at the time of first cigarette. Classes reflecting initial subjective responses were strongly associated with rate of transition from first cigarette to regular smoking. Compared to Class 2 (‘Cough only’), Classes 1 (‘High on pleasurable and physiological respondses’) and 4 (‘High on pleasurable, low on physiological respondses’) evidenced substantially higher rates of progression to regular smoking. Membership in Class 1 was associated with a two and a half fold increase in the rate of transition from initiation to onset of regular smoking (HR=3.55; CI: 2.57–4.92). Rate of progression in Class 4 was also greater than three times as rapid as in Class 2 (HR=3.41; CI: 2.27–5.12). Class 3 (which, in contrast to Classes 1 and 4, was characterized by the absence of pleasurable subjective effects) did not differ significantly from Class 2 in transition time from first cigarette to regular smoking (HR=1.20; CI: 0.81–1.78). Although not evident in unadjusted descriptive analyses (reported in Table 1), in the context of additional covariates, ND risk group status was a significant predictor of transition time to regular smoking. Both high genetic risk groups evidenced more rapid transitions than controls, with HR=1.73 (CI: 1.19–2.50) for ND risk group 1 and 2.19 (CI: 1.24–3.89) for ND risk group 2, but, as indicated by the significant hazard ratios for Classes 1 and 4, genetic risk for ND did not account for the association between initial subjective effects and rate of transition to regular smoking.
TABLE 2.
Cox proportional hazards models for time from first cigarette to onset of regular smoking
| Hazard Ratio (95% CI) | ||||
|---|---|---|---|---|
| Base Model | Model with Covariates | |||
| Classa | ||||
| 1: High on physiological and pleasurable responses | 3.55 (2.57–4.92) | 2.70 (1.88–3.88) | ||
| 3: High on physiological, low on pleasurable responses | 1.20 (0.81–1.78) | 0.98 (0.63–1.54) | ||
| 4: High on pleasurable, low on physiological responses | 3.41 (2.27–5.12) | 2.54 (1.66–3.89) | ||
| Paternal nicotine dependence risk group statusb | ||||
| Group 1: high G, high E | 1.73 (1.19–2.50) | 1.25 (0.83–1.87) | ||
| Group 2: high G, low E | 2.19 (1.24–3.89) | 0.99 (0.47–2.08) | ||
| Group 3: moderate G, low E | 1.40 (0.79–2.48) | 0.88 (0.47–1.64) | ||
| Maternal heavy smoking indexc | ||||
| Low: 1–2 | 0.99 (0.68–1.45) | 0.24 (0.05–1.11) | ||
| High ≥ 3 | 1.31 (0.99–1.72) | 1.26 (0.94–1.68) | ||
| Age at 1st cigarette | ||||
| Early: ≤ 12 years | 0.72 (0.52–1.00) | 3.80 (2.09–6.92) | ||
| Late: ≥ 17 years | 2.87 (1.89–4.37) | 0.09 (0.03–0.26) | ||
| Female gender | 1.28 (0.99–1.65) | 1.12 (0.84–1.49) | ||
| Age | 0.98 (0.95–1.01) | 0.99 (0.95–1.02) | ||
| Paternal drug dependence risk group statusb | ||||
| Group 1: high G, high E | 1.07 (0.72–1.58) | 1.51 (1.01–2.26) | ||
| Group 2: high G, low E | 1.26 (0.74–2.17) | 1.86 (1.09–3.18) | ||
| Group 3: moderate G, low E | 1.22 (0.69–2.16) | 2.11 (1.12–3.99) | ||
| Parental marital status (intact) | -------- | 0.74 (0.56–0.98) | ||
| Alcohol use | -------- | 1.88 (1.32–2.69) | ||
| Cannabis use | -------- | 2.22 (1.67–2.95) | ||
| Oppositional defiant disorder | -------- | 1.80 (1.23–2.63) | ||
Hazard Ratios relative to Class 2 (Cough only);
Hazard Ratios relative to Group 4 (low G, low E);
Hazard Ratios relative to non-smokers
3.2.2 Model with Covariates
Results from the second Cox proportional hazards regression analysis are also reported in Table 2 (right side). In addition to factors included in the base model, this second model incorporated demographic and psychiatric covariates that produced significant hazard ratios in the context of the base model variables, namely alcohol use, cannabis use, ODD, and parental marital status. Even after adjusting for these additional factors, initial subjective responses strongly predicted the rate of progression from first cigarette to regular smoking.
Hazard ratios for Class 1 and Class 4 were somewhat attenuated compared with estimates in the base model, but they remained highly significant: 2.70 (CI: 1.88–3.88) and 2.54 (CI: 1.66–3.89), respectively. As in the base model, Class 3 did not differ from Class 2 (HR=0.98; CI: 0.63–1.54). Intact parental marital status was protective against rapid transition to regular smoking (HR=0.74; CI: 0.56–0.98). By contrast, cannabis users progressed from initiation to onset of regular smoking (HR=2.24; CI: 1.67–2.95) at greater than twice the rate as non-users. Alcohol use and ODD were also associated with an elevated rate of transition from first cigarette to regular smoking (for alcohol use, HR=1.88; CI: 1.32–2.69 and for ODD, HR=1.80; CI: 1.23–2.63). After accounting for parental marital status, alcohol use, cannabis use, and ODD, paternally-transmitted genetic risk for ND status was no longer a significant predictor of transition time to regular smoking.
4. Discussion
Findings from the current study provide support for the association between initial subjective responses to cigarettes and the rate of progression from first cigarette to the onset of regular smoking. Three major aspects of this study distinguish it from other investigations of subjective effects and smoking-related outcomes. First, we focused on the timing of the transition to regular smoking, which better captures the pathway from initiation to regular smoking than the more commonly used dichotomous indicator of ever becoming a regular smoker. Second, through the use of an OOT design to adjust for paternally transmitted genetic liability to smoking and the inclusion of maternal smoking status we were able to characterize the magnitude of the association between transition time and initial subjective effects above and beyond what can be explained by familial risk for smoking. Third, we used latent class analysis to derive profiles of initial subjective effects rather than categorizing effects more globally as positive or negative.
Four profiles of initial subjective responses to cigarettes emerged in our analyses. Thus, individuals did not fall into exclusively pleasurable vs. exclusively physiological responders. Classifications also did not represent a severity continuum of overall reactivity. Class 1, characterized by pleasurable experiences and high responsivity to physiological effects, and Class 2, by the absence of pleasurable experiences combined with low levels of response to physiological effects, can be described simply as high on both and low on both dimensions. Classes 3 and 4, by contrast, reflected a divergence of the two dimensions, with members of Class 3 endorsing high levels of physiological response and an absence of pleasurable experiences and those in Class 4 endorsing a high degree of pleasurable response and minimal physiological responsivity. The emergence of Class 1 is of greatest interest with respect to predicting later smoking behaviors. The existence of this group highlights the fact that initial experiences with cigarettes can be evaluated as enjoyable even when they are coupled with physiological effects typically thought to be unpleasant. Although rarely seen in the smoking literature, a very similar approach to developing profiles of initial subjective effects has been applied to cannabis use and to cocaine use. Results were similarly suggestive of qualitative distinctions in subjective responses rather than either differing levels of overall responsivity or a simple dichotomy of positive vs. negative reactions (Grant et al., 2005; Scherrer et al., in press), and, like ours, these studies found that profiles of subjective effects varied in their associated risks for substance use outcomes.
Results from survival analyses indicate that pleasurable initial experiences with cigarettes – regardless of whether or not they are combined with strong physiological responses – are associated with rapid progression to regular smoking, as evidenced by the fact that members of Classes 1 and 4 were at similarly high risk for transitioning quickly to regular smoking. Previously reported associations between positive initial experiences and progression to regular smoking (Chen et al., 2003; Eissenberg & Balster, 2000; Hu et al., 2006) therefore appear to hold true as well for the rate at which that transition occurs. Thus, we can characterize the relationship between subjective effects and regular smoking onset more precisely. We can also draw on these results to address some discrepancies in the literature regarding initial physiological responses to cigarettes and later smoking behaviors.
Although a number of studies have found that individuals endorsing negative (i.e., physiological) initial subjective effects have a reduced likelihood of becoming regular smokers or developing nicotine dependence (DiFranza et al., 2004; DiFranza et al., 2007; Riedel et al., 2003), others have reported higher rates of these smoking outcomes in those endorsing such negative subjective effects as dizziness and nausea (DiFranza et al., 2004; Chen et al., 2003; Hu et al., 2006). Our findings may help to explain these inconsistencies. In our sample, pleasurable and physiological effects were not mutually exclusive. A subgroup of participants (Class 1) endorsed both and these individuals differed from those who endorsed physiological but not pleasurable subjective effects (Class 3) in their risk for making a rapid transition to regular smoking. Categorizing initial responses to cigarettes as one or the other results in the loss of important information about the range of subjective effects experienced and, consequently, the associations of those effects with smoking-related outcomes. Our results suggest that pleasurable subjective reactions may play a larger role than negative ones, but even more importantly, that considering the two dimensions in combination can provide a more accurate characterization of risk for rapid progression to regular smoking.
In addition to providing support for the role of initial subjective response to cigarettes, results point to a number of other risk and protective factors that (even after accounting for familial contributions to smoking behaviors, age, gender, and age at first cigarette) predict the rate at which individuals transition from first cigarette to regular smoking. When considered in combination with initial subjective effects, alcohol use, cannabis use, ODD, and parental marital status remained significant predictors of transition times. Alcohol use and cannabis use have both been linked consistently to higher rates of regular smoking (Degenhardt., Hall, & Lynskey, 2001; Dierker, Avenevoli, Merikangas, Flaherty, & Stolar, 2004; Grucza & Bierut, 2006; Reid, Lynskey, & Copeland, 2000; Rohde, Kahler, Lewinsohn, & Brown, 2004; Swift, Hall, & Teesson, 2001) and in a prior study by our group, they were associated with rapid progression from first cigarette to onset of regular smoking (Sartor et al., 2008). Our finding that ODD is associated with a more rapid rate of transition to regular smoking is consistent with an extensive literature documenting a strong link between externalizing disorders and smoking outcomes (Disney, Elkins, McGue, & Iacono, 1999; Galera, Fombonne, Chastang, & Bouvard, 2004; Kollins, McClernon, & Fuemmeler, 2005; Tercyak, Lerman, & Audrain, 2002). The protective role of intact marital status is also well established in the substance use literature more generally, with several studies providing evidence for smoking outcomes in particular (Jeynes, 2001; Patton et al., 1998; Wolfinger, 1998).
4.1 Conclusions
Findings from the current study have important implications for intervention in the pathway from exposure to cigarettes to regular smoking. Each day, 4,400 adolescents try their first cigarettes (Centers for Disease Control and Prevention, 2003), so in addition to reducing risk for ever trying cigarettes, a critical goal of prevention programs is to reduce risk for developing regular smoking habits after exposure has occurred. Initial subjective responses to cigarettes are powerful indicators of how quickly individuals are likely to progress to regular smoking, with pleasant reactions predicting rapid onset of regular smoking. In our sample, 42.5% and 52.2% of those individuals in the two classes endorsing pleasurable subjective effects (Classes 1 and 4, respectively) who went on to become regular smokers made that transition one year or less after trying their first cigarettes. For those who enjoy their first smoking experiences, it is especially important that efforts aimed at disrupting the progression to regular smoking focus on the period shortly after first cigarette use.
4.2 Limitations and Future Directions
Some possible limitations should be taken into consideration when interpreting findings from the current study. First, initial subjective effects were reported retrospectively and are therefore subject to the biases inherent in retrospective measures. We attempted to minimize possible forward telescoping biases in reports of age at first cigarette and age at onset of regular smoking (Johnson & Schultz, 2005) by adjusting for age at the time of report, but accuracy would likely be higher with prospective measurement. It is also possible that the greater exposure to cigarette use among regular smokers (compared with experimenters) resulted in more positive recall of initial experiences (Pomerleau, Pomerleau, Mehringer, Snedecor, & Cameron, 1998; Riedel et al., 2003). Second, our assessment of subjective effects did not cover the full range of pleasurable experiences queried in prior studies, although the consistency of our findings with previous reports suggests that our abbreviated measure captured the construct relatively well. Third, the vast majority of participants are non-Hispanic Caucasian, so to the extent that initial subjective responses to cigarettes or the association of subjective effects with risk for later smoking outcomes vary by ethnicity or race, findings may not generalize to members of other ethnic and racial groups. Fourth, the validity of retrospective reporting of initial subjective effects has only rarely been studied and merits further investigation. However, findings from one of the few studies to address this issue support the validity of retrospective recall of early responses to cigarettes (Pomerleau, 2005). Finally, although in prior studies using the same sample we did not find evidence for lower rates of participation in twin pairs in which one or both members were drug-dependent (and in fact found the opposite [Scherrer et al., 2008]), there is a possibility that drug-dependent offspring were less likely to take part in the study. It is also likely (as in most studies based on community samples) that the most severe cases of drug dependence are underrepresented in our study; thus our sample may not reflect the entire population of smokers.
Our findings suggest a number of possible directions for future investigations, including the extension of this approach to address the association of initial subjective effects with later stages of smoking behaviors. For example, do those individuals who initially experienced both physiological and pleasurable subjective effects remain habitual smokers as long as those who experienced (nearly) exclusively pleasurable effects? Do these groups differ in their rates of nicotine dependence, smoking cessation or relapse? In order to effectively intervene in the progression of smoking behaviors, it is critical as well that we gain a clearer understanding of the aspects of early smoking experiences that are perceived to be pleasant or reinforcing. Situational influences, for example, the presence of older peers or siblings, may contribute to the evaluation of these experiences as pleasurable, as might expectancies about smoking that were in place even before smoking the first cigarette. Identifying the factors that shape early smoking experiences is a critical step in developing the range of strategies needed for prevention and treatment across the course of smoking behaviors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report of the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Women and smoking: A report of the Surgeon General. U.S. Department of Health and Human Services; Rockville, MD: 2001. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Tobacco use among middle and high school students--United States, 2002. Morbidity and Mortality Weekly Report. 2003;52:1096–1098. [PubMed] [Google Scholar]
- Chen X, Stacy A, Zheng H, Shan J, Spruijt-Metz D, Unger JB, et al. Sensations from initial exposure to nicotine predicting adolescent smoking in China: A potential measure of vulnerability to nicotine. Nicotine and Tobacco Research. 2003;5:455–463. doi: 10.1080/1462220031000118603. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) Journal of the Royal Statistical Society. 1972;B34:187–220. [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use and other substance use in the general population. Drug and Alcohol Dependence. 2001;64:319–327. doi: 10.1016/S0376-8716(01)00130-2. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Merikangas KR, Flaherty BP, Stolar M. Association between psychiatric disorders and the progression of tobacco use behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1159–1167. doi: 10.1097/00004583-200110000-00009. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, DiFranza JR, Savageau JA, Fletcher K, et al. Recollections and repercussions of the first inhaled cigarette. Addictive Behaviors. 2004;29:261–272. doi: 10.1016/j.addbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, O’Loughlin J, Pbert L, Ockene JK, et al. Symptoms of tobacco dependence after brief intermittent use. The development and assessment of nicotine dependence in Youth-2 study. Archives of Pediatric and Adolescent Medicine. 2007;161:704–10. doi: 10.1001/archpedi.161.7.704. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Pbert L, O’Loughlin J, McNeill AD, et al. Susceptibility to nicotine dependence: The development and assessment of nicotine dependence in Youth 2 Study. Pediatrics. 2007;120:e974–e983. doi: 10.1542/peds.2007-0027. [DOI] [PubMed] [Google Scholar]
- Disney ER, Elkins IJ, McGue M, Iacono WG. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. American Journal of Psychiatry. 1999;156:1515–1521. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- Duncan AE, Sartor CE, Scherrer JF, Grant JD, Heath AC, Nelson EC, et al. The association between cannabis abuse and dependence and childhood physical and sexual abuse: evidence from an offspring of twins design. Addiction. 2008;103:990–997. doi: 10.1016/j.drugalcdep.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clinical Genetics. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Geneticae Medicae Gemellologiae. 1987;36:61–67. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug and Alcohol Dependence. 2000;59(Suppl1):S41–S60. doi: 10.1016/S0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Galera C, Fombonne E, Chastang J-F, Bouvard M. Childhood hyperactivity- inattention symptoms and smoking in adolescence. Drug and Alcohol Dependence. 2005;78:101–108. doi: 10.1016/j.drugalcdep.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Grambsch P, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika Trust. 1994;81:515–526. [Google Scholar]
- Grant JD, Scherrer JF, Lyons MJ, Tsuang M, True WR, Bucholz KK. Subjective reactions to cocaine and marijuana are associated with abuse and dependence. Addictive Behaviors. 2005;30:1574–1586. doi: 10.1016/j.addbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Bierut LJ. Co-occurring risk factors for alcohol dependence and habitual smoking: update on findings from the Collaborative Study on the Genetics of Alcoholism. Alcohol Research & Health. 2006;29:172–178. [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Cates R, Martin NG, Meyer J, Hewitt JK, Neale MC, et al. Genetic contribution to risk of smoking initiation: comparisons across birth cohorts and across cultures. Journal of Substance Abuse. 1993;5:221–246. doi: 10.1016/0899-3289(93)90065-j. [DOI] [PubMed] [Google Scholar]
- Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Reports. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA - A comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hu M-C, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. American Journal of Public Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob T, Sher KJ, Bucholz KK, True WT, Sirevaag EJ, Rohrbaugh J, et al. An integrative approach for studying the etiology of alcoholism and other addictions. Twin Research. 2001;4:103–118. doi: 10.1375/1369052012218. [DOI] [PubMed] [Google Scholar]
- Jeynes WH. The effects of recent parental divorce on their children’s consumption of alcohol. Journal of Youth and Adolescence. 2001;30:305–319. doi: 10.1023/A:1010440111698. [DOI] [Google Scholar]
- Johnson EO, Schultz L. Forward telescoping bias in reported age of onset: an example from cigarette smoking. International Journal of Methods in Psychiatric Research. 2005;14:119–129. doi: 10.1002/mpr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Hu M-C, Griesler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug and Alcohol Dependence. 2007;91:26–39. doi: 10.1016/j.drugalcdep.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological Medicine. 1999;29:299–308. doi: 10.1017/S0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon J, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Archives of General Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, et al. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychological Medicine. 2004;34:865–879. doi: 10.1017/S0033291703001582. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Madden P, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The epidemiology and genetics of smoking initiation and persistence: crosscultural comparisons of twin study results. Twin Research. 2004;7:82–97. doi: 10.1375/13690520460741471. [DOI] [PubMed] [Google Scholar]
- McCutcheon A. Latent class analysis. Newbury Park, CA: Sage Publications; 1987. [Google Scholar]
- McNeill AD. The development of dependence on smoking in children. British Journal of Addictions. 1991;86:589–592. doi: 10.1111/j.1360-0443.1991.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. The course of early smoking: a population-based cohort study over three years. Addiction. 1998;93:1251–1260. doi: 10.1046/j.1360-0443.1998.938125113.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behavior Genetics. 1995;25:161–177. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Collins AC, Shiffman S, Pomerleau CS. Why some people smoke and others do not: new perspectives. Journal of Consulting and Clinical Psychology. 1993;61:723–731. doi: 10.1037//0022-006x.61.5.723. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Cameron OG. Validation of retrospective reports of early experiences with smoking. Addictive Behaviors. 2005;30:607–611. doi: 10.1016/j.addbeh.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Reid A, Lynskey M, Copeland J. Cannabis use among Australian adolescents: findings of the 1998 National Drug Strategy Household Survey. Australian & New Zealand Journal of Public Health. 2000;24:596–602. doi: 10.1111/j.1467-842X.2000.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Riedel BW, Blitstein JL, Robinson LA, Murray DM, Klesges RC. The reliability and predictive value of adolescents’ reports of initial reactions to smoking. Nicotine and Tobacco Research. 2003;5:553–559. doi: 10.1080/1462220031000118658. [DOI] [PubMed] [Google Scholar]
- Robins N. Diagnostic grammar and assessment: translating criteria into questions. Psychological Medicine. 1989;19:57–68. doi: 10.1017/s0033291700011028. [DOI] [PubMed] [Google Scholar]
- Rohde P, Kahler CW, Lewinsohn PM, Brown RA. Psychiatric disorders, familial factors, and cigarette smoking: II. Associations with progression to daily smoking. Nicotine and Tobacco Research. 2004;6:119–132. doi: 10.1080/14622200310001656948. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS Statistical Software: Release 8.1. Cary, NC: 2002. [Google Scholar]
- Sartor CE, Xian H, Scherrer JF, Lynskey M, Duncan AE, Haber JR, et al. Psychiatric and familial predictors of transition times between smoking stages: Results from an offspring-of-twins study. Addictive Behaviors. 2008;53:235–251. doi: 10.1016/j.addbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer JF, Grant JD, Duncan AE, Pan H, Waterman B, Jacob T, et al. Measured environmental contributions to cannabis abuse/dependence in an offspring of twins design. Addictive Behaviors. 2008;33:1255–1266. doi: 10.1016/j.addbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer JF, Grant JD, Duncan AE, Pan H, Waterman B, Jacob T, True WR, Heath AC, Bucholz KK. Parent, sibling and peer influences on adolescent and young adult cannabis abuse/dependence after accounting for genetic vulnerability. Addictive Behaviors. 2008;33:1255–1266. doi: 10.1016/j.addbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer JF, Grant JD, Duncan AE, Sartor CE, Haber JR, Jacob T, et al. Subjective effects to cannabis are associated with use, abuse and dependence after adjusting for genetic and environmental influence. Drug and Alcohol Dependence. 2009;105:76–82. doi: 10.1016/j.drugalcdep.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein B, Kelly E, Swan J, Kozlowski LT. Physiological predisposition toward becoming a cigarette smoker: experimental evidence for a sex difference. Addictive Behaviors. 1982;7:83–86. doi: 10.1016/0306-4603(82)90030-2. [DOI] [PubMed] [Google Scholar]
- Statacorp. Stata Statistical Software: Release 8.2. College Station, TX: 2005. [Google Scholar]
- Swift W, Hall W, Teesson M. Cannabis use and dependence among Australian adults: results from the National Survey of Mental Health and Wellbeing. Addiction. 2001;96:737–748. doi: 10.1046/j.1360-0443.2001.9657379.x. [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Lerman C, Audrain J. Association of attention-deficit/hyperactivity disorder symptoms with levels of cigarette smoking in a community sample of adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:799–805. doi: 10.1097/00004583-200207000-00011. [DOI] [PubMed] [Google Scholar]
- True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, et al. Genetic and environmental contributions to smoking. Addiction. 1997;92:1277–1287. [PubMed] [Google Scholar]
- Vermont JK, Magidson J. Latent Gold 4.0 User’s Guide. Statistical Innovations, Inc; Belmont, MA: 2005. [Google Scholar]
- Wolfinger NH. The effects of parental divorce on adult tobacco and alcohol consumption. Journal of Health and Social Behavior. 1998;39:254–269. [PubMed] [Google Scholar]