Abstract
The endocannabinoids anandamide (AEA) and 2-arachidonylglycerol (2-AG) have opposing effects on cholangiocarcinoma growth. Implicated in cancer, Notch signaling requires the γ-secretase complex for activation. The aims of this study were to determine if the opposing effects of endocannabinoids depend on the differential activation of the Notch receptors; and to demonstrate that the differential activation of these receptors are due to presenilin 1 containing- and presenilin 2-containing- γ-secretase complexes. Mz-ChA-1 cells were treated with AEA or 2-AG. Notch receptor expression, activation and nuclear translocation was determined. Specific roles for Notch 1 and 2 on cannabinoid-induced effects were determined by transient transfection of Notch 1 or 2 shRNA vectors prior to stimulation with AEA or 2-AG. Expression of presenilin 1 and 2 was determined after AEA or 2-AG treatment and the involvement of presenilin 1 and 2 in the cannabinoid induced effects were demonstrated in cell lines with low presenilin 1 or 2 expression. Antiproliferative effects of AEA required increased Notch 1 mRNA, activation and nuclear translocation, whereas the growth-promoting effects induced by 2-AG required increased Notch 2 mRNA expression, activation and nuclear translocation. AEA increased presenilin 1 expression and recruitment into the γ-secretase complex whereas 2-AG increased expression and recruitment of presenilin 2. The development of novel therapeutic strategies aimed at modulating the endocannabinoid system, or mimicking the mode of action of AEA on Notch signaling pathways would prove beneficial for cholangiocarcinoma management.
Keywords: presenilin, γ–secretase complex, biliary cancer, anandamide, 2-arachidonyl glycerol
Introduction
Cholangiocarcinomas are devastating cancers of intrahepatic and extrahepatic origin that are increasing in both their worldwide incidence and mortality rates [1, 2]. The challenges posed by these often lethal biliary tract cancers are daunting, with conventional treatment options being limited and the only hope for long-term survival being that of complete surgical resection of the tumor [1, 2]. Conventional chemotherapy and radiation therapy are not effective in prolonging long-term survival [1]; therefore it is important to understand the cellular mechanisms of cholangiocarcinoma cell growth with a view to develop novel chemopreventive strategies.
We have recently demonstrated that the endocannabinoids anandamide (AEA) and 2-arachidonyl glycerol (2-AG) exert opposing effects on cholangiocarcinoma cell growth in vitro via cannabinoid receptor-independent mechanisms [3]. The antiproliferative effects of AEA are via the stabilization of lipid rafts and recruitment of death receptor complexes into the lipid rafts, whereas the disruption of lipid raft integrity by 2-AG, leads to increased cholangiocarcinoma cell proliferation [3]. The downstream mechanisms of the antiproliferative effects of AEA on cholangiocarcinoma are largely unknown, however, we have also shown that AEA can activate the non-canonical Wnt pathway that contributes to the antiproliferative effects [4].
The Notch proteins constitute an evolutionarily conserved family of transmembrane receptors that play a pivotal role in cellular differentiation, proliferation and apoptosis [5, 6]. When Notch binds to its ligands, Delta or Jagged, it goes through two consecutive cleavages at specific sites, the first occurs just outside the cell membrane and is performed by ADAM-like proteases [7]. The second cleavage is conducted by a γ-secretase complex containing, presenilin enhancer 2 (Pen-2), nicastrin, Anterior-pharynx-defective protein 1 (APH-1) and either presenilin 1 or presenilin 2 [8–12], and results in the activation of the Notch protein by releasing the intracellular part of the molecule (Notch intracellular domain; NICD) [13]. NICD is then transported to the nucleus where it binds to the transcription factor RBP-Jκ [14, 15], releasing it from its co-repressors and allowing the binding of transcriptional co-activators such as Mastermind, thus initiating the transcription of Notch target genes [16].
The diversity in the outcomes of the Notch signaling pathway may be attributed to cell type, signal strength, duration of the signal and context [17, 18]. However, because mammals possess 5 different ligands (Jagged 1 and 2, and Delta-like ligand −1, −3 and −4) as well as 4 different Notch receptors (Notch 1–4), and many variations of the γ-secretase complex, it is highly likely that specificity of ligand/receptor/secretase interaction may play a role in this diversity. Indeed, Notch 1 and Notch 2 have been shown to exert opposing actions on embryonal brain tumor growth [19] and have contrasting roles in breast cancer tumor differentiation [20]. The precise factors controlling this ligand/receptor/secretase specificity and the resulting cellular effects are poorly understood.
The aims of this study were 1) to determine if the opposing effects of endocannabinoids could be attributed to the differential activation of Notch 1 and Notch 2; 2) to determine if the differential activation of Notch 1 and Notch 2 can be associated with presenilin 1 containing- and presenilin 2-containing- γ-secretase complexes respectively.
MATERIALS AND METHODS
Reagents
For immunoblotting, antibodies against activated Notch 1 was purchased from Abcam (Cambridge, MA) and activated Notch 2 was purchased from Millipore (Temecula, CA), and antibodies against the c-terminal fragments of Notch 1 and 2 (used in immunofluorescence) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Specific Pen-2, APH-1, nicastrin, presenilin 1 and presenilin 2 antibodies were purchased from Zymed Laboratories (San Francisco CA). Primers, shRNA constructs and real time PCR reagents were obtained from SABiosciences (Frederick, MD).
Cell culture
All in vitro experiments were performed in Mz-ChA-1 cells which is a human cholangiocarcinoma cell line derived from gall bladder [21]. These cells were a kind gift from Dr. G. Fitz (University of Texas Southwestern Medical Center, Dallas, TX) and cultured as previously described [3]
Realtime PCR
RNA was extracted from Mz-ChA-1 cells after treatment with AEA or 2-AG (both at 10−5 M), for various time points and from tumor tissue treated chronically with AEA or vehicle, using the RNeasy Mini Kit (Qiagen Inc, Valencia, CA) according to the instructions provided by the vendor and reverse transcribed using the Reaction Ready™ First Strand cDNA synthesis kit (SABiosciences, Frederick, MD). These reactions were used as templates for the PCR assays using a SYBR Green PCR master mix (SABiosciences, Frederick, MD) in the real-time thermal cycler (Mx5000P, Agilent Technologies, Santa Clara, CA) using commercially available primers designed against human Notch 1, Notch 2, presenilin 1, presenilin 2, PEN2, APH-1, nicastrin, and GAPDH (SABiosciences, Frederick MD). A ΔΔCT analysis was performed [4, 22, 23] using the control treated samples as the reference sample. Data are expressed as relative mRNA levels ± SEM (n=3).
Immunoblotting
Following trypsinization, Mz-ChA-1 cells (1×106 cells), or lysates from tumors tissue taken from nude mice, chronically treated with either AEA or vehicle as described [4], were resuspended in lysis buffer as described [24] and sonicated. Immunoblots to detect activated Notch 1 and 2, the N-terminal fragments of both presenilin 1 and presenilin 2, APH-1, nicastrin, Pen-2 and β-actin were performed as previously described [24] using specific antibodies against each protein. Data are expressed as fold change (mean ± SEM) of the relative expression after normalization with β-actin.
Immunofluorescence
Immunofluorescence was performed as previously described [3]. Briefly, cells were seeded into six-well dishes containing a sterile coverslip on the bottom of each well and incubated with AEA (10 µM) or 2-AG (10 µM) for 4 hr. Subcellular location of Notch 1 and Notch 2 was then determined using Notch 1 and 2 specific primary antibodies followed by immunofluorescent detection using cy3-conjugated secondary antibodies (Jackson Immunochemicals, West Grove, PA). Coverslips were mounted onto microscope slides with Antifade gold containing 4V,6-diamidino-2- phenylindole (DAPI) as a counterstain (Molecular Probes, Eugene, OR). Negative controls were done with the omission of the respective primary antibodies. Sections were visualized using an Olympus IX-71 inverted confocal microscope.
In vivo xenograft model of cholangiocarcinoma
In vivo experiments were performed as described previously. Male balb/c 8-week-old nude (nu/nu) mice were kept in a temperature-controlled environment (20–22°C) with a 12-hour light–dark cycle and with free access to drinking water and standard mouse chow. Mz-ChA-1 cells (3 × 106) were suspended in 0.25 mL of extracellular matrix gel (ECM) and injected subcutaneously into the back flanks of these animals. After the establishment of tumors, mice received AEA or 2-AG (both at 10 mg/kg/day [4] ip dissolved in 1:4 tocrisolve™: 0.9% NaCl) or vehicle injected 3 times per week. Tumor parameters were measured twice a week by an electronic calliper and volume determined as: tumor volume (mm3) = 0.5 × [length (mm) × width (mm) × height (mm)]. After approximately 1 month, mice were anaesthetized with sodium pentobarbital (50 mg/kg ip) according to institutional guidelines and tumors and serum collected. Serum was analyzed for liver enzymes AST and ALT as described previously [4, 25] and tumors were processed for immunohistochemistry. Statistical significance of tumor volumes was assessed using a 2-way ANOVA followed by a Bonferroni post hoc analysis.
Immunohistochemistry
Immunohistochemical staining for proliferating cellular nuclear antigen (PCNA) was performed in paraffin embedded tumor tissue taken from nude mice, chronically treated with either AEA, 2-A or vehicle as described [4]. Briefly, sections were mounted on glass slides coated with 0.1% poly-L-lysine. After deparaffination, endogenous peroxidase activity was blocked by a 20-minute incubation in methanolic hydrogen peroxide (2.5%). Sections were hydrated in graded alcohol and rinsed in phosphate-buffered saline (PBS, pH 7.4) before applying the primary antibody. Sections were incubated overnight at 4°C with an antibody for PCNA (Santa Cruz Biotechnn ology, Santa Cruz CA). Samples were then rinsed with PBS for 5 minutes, incubated for 60 minutes at room temperature with secondary biotinylated antibody (VectaElite kits; Vector Laboratories, Burlingame, CA), incubated with ABC reagent (Vectastain ABC kit; Vector Laboratories, Burlingame, CA), and finally developed with 3–3’diaminobenzidine (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin prior to dehydration and mounting with coverslips. In parallel, apoptosis was assessed in these tumors using the TUNEL apoptosis detection kit according to the manufacturers instructions (Chemicon, Millipore, Temecula, CA). In each case, PCNA-positive and TUNEL-positive nuclei were assessed in 10 non-overlapping fields from 3 different tumors and expressed as a percentage of the total nuclei in each field.
Establishment of stable transfected Mz-ChA-1 cells
The role of presenilin 1 and presenilin 2 expression in the actions of AEA and 2-AG were demonstrated using cells that have the expression of each of these genes stably knocked down. These cell lines were established using SureSilencing shRNA (SABiosciences, Frederick, MD) plasmids for human presenilin 1 and 2, containing a marker for neomycin resistance for the selection of stably-transfected cells following the methodology described previously [4]. A number of subsequent clones were then assessed for the relative knockdown of each specific gene using real time PCR and a single clone with the greatest degree of knockdown was selected for subsequent experiments and were subsequently designated Mz-presenilin 1, Mz-presenilin 2 and Mz-neo neg (mock transfected control).
MTS assays
Mz-ChA-1 cells were seeded into 96 well plates (10,000 cells/well) in a final volume of 200 µl of growth medium and allowed to adhere to the plate overnight. Because serum binds to and sequesters cannabinoids [26], cells were then changed to serum free media immediately prior to the addition of either AEA (10 µM), or 2-AG (10 µM) in the presence or absence of the γ-secretase inhibitor DAPT[27] (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester; 1 µM) for 48 hr. In parallel, cells were transiently transfected with either Notch 1 shRNA or Notch 2 shRNA expression vectors (5 µg/ 1×106 cells) using the basic nucleofector solution kit (Lonza Inc; Walkersville, MD) followed by electroporation using the AMAXA Nucleofector device (Lonza Inc; Wackersville, MD) for 24 hr prior to the addition of AEA or 2-AG for 48 hr. Furthermore, stable transfected cell lines Mz-neo neg, Mz-presenilin 1 and Mz-presenilin 2 were also treated with AEA or 2-AG for 48 hr. Cell proliferation was assessed using a colorimetric cell proliferation assay (CellTiter 96AQueous; Promega Corp., Madison, WI) and absorbance measured at 490 nm by a microplate spectrophotometer (Versamax; Molecular Devices, Sunnyvale, CA). In all cases, data are expressed as the fold change of treated cells as compared to vehicle treated controls.
Bromodeoxy uridine staining
BrdU assays were performed as described previously [3] using Mz-ChA-1 cells pretreated with DAPT (1 µM) for 1 hr prior to the addition of AEA (10 µM) or 2-AG (10 µM) for 48 hr. In parallel, stable transfected cell lines Mz-neo neg, Mz-presenilin 1 and Mz-presenilin 2 were also treated with AEA (10 µM) or 2-AG (10 µM). The number of BrdU-positive nuclei was counted and expressed as a percentage of total cells in 5 random fields for each treatment group. Data are average ± SEM of 5 fields in 3 independent experiments.
Immunoprecipitation
Mz-ChA-1 cells were treated with either AEA or 2-AG (both 10 µM) for 6 hr. Protein lysates were prepared using ice-cold CHAPS immunoprecipitation buffer (50 mM HEPES (pH 7.4), containing 2% CHAPS, 200 mM NaCl, 0.02% (w/v) sodium azide), containing protease inhibitors, sodium orthovanadate and phenylmethylsulphonyl fluoride as per the manufacturers instructions (Santa Cruz Biotechnology, Santa Cruz, CA). Total protein lysates (30 µg) were immunoprecipitated with 2 µL Rabbit anti-nicastrin antibody (Sigma Aldrich, St Louis. MO) attached to protein A/G sepharose beads (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. The immunoprecipitation reaction was washed in RIPA buffer and the resulting pellet was resuspended in 50 µL of SDS-PAGE denaturing buffer and denatured for 5 min at 95°C. 30µL of each sample was loaded onto an SDS-PAGE gel and processed for immunoblotting using PEN-2, presenilin 1 and presenilin 2 specific antibodies as described above.
Statistical Analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by the Student unpaired t-test when two groups were analyzed and ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test. A p value of less than 0.05 was used to indicate statistical significance.
RESULTS
The opposing actions of AEA and 2-AG on cell proliferation are associated with differential activation of Notch 1 and Notch 2
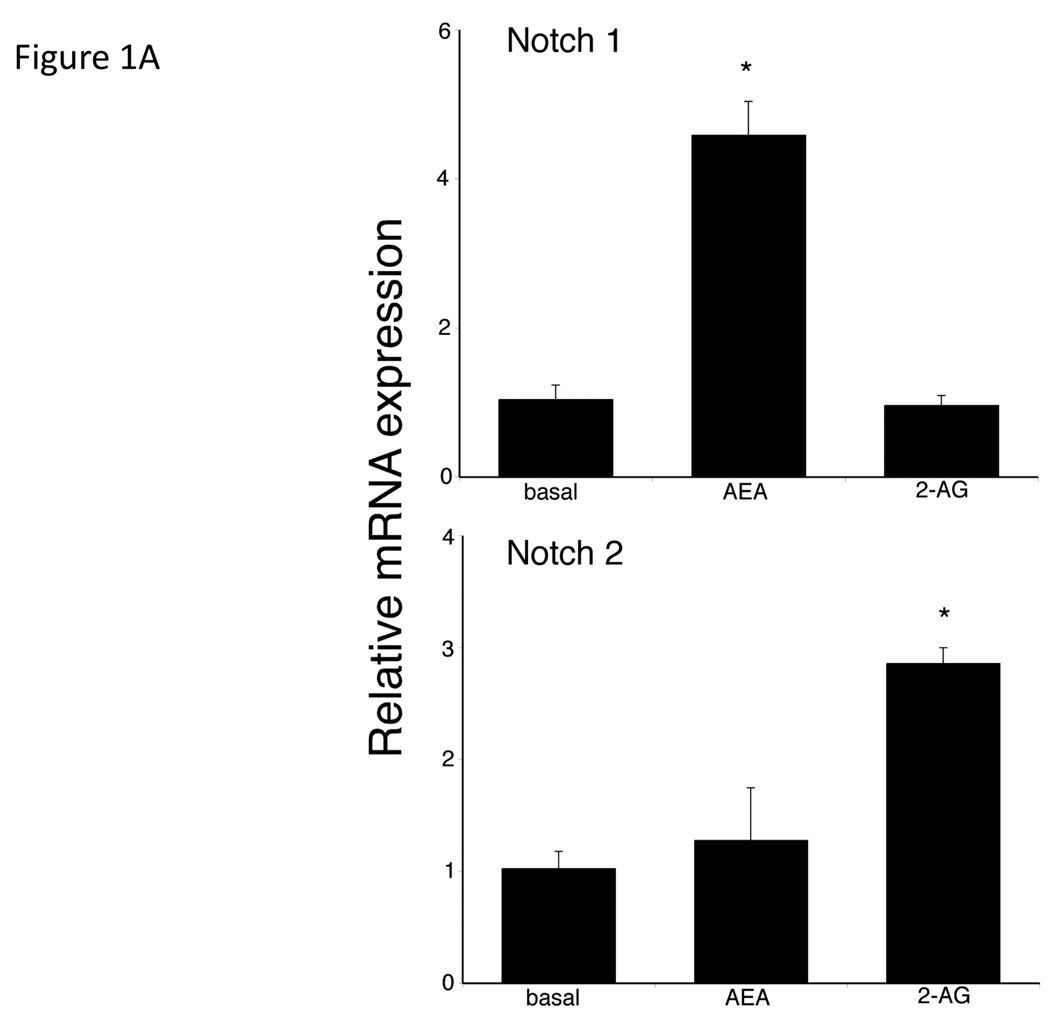
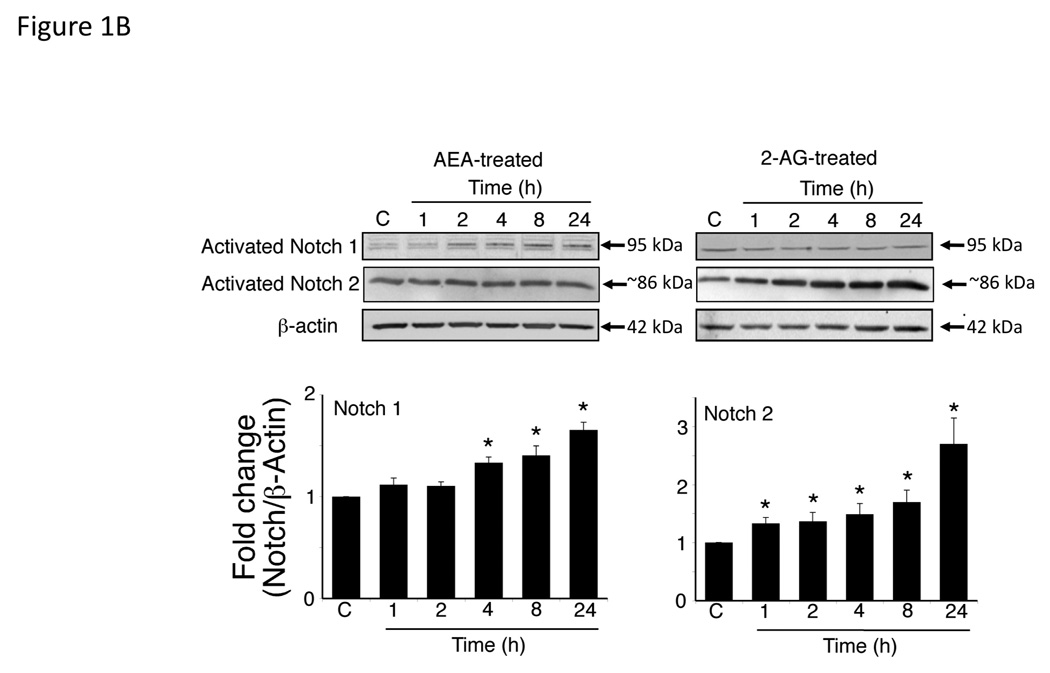
We have previously shown that AEA and 2-AG exert opposing effects on cholangiocarcinoma growth with AEA having anti-proliferative effects and 2-AG promoting growth [3]. We analyzed the effects of these endocannabinoids on the Notch signaling pathways. Treatment of Mz-ChA-1 cells with AEA increased Notch 1 mRNA expression with no effects on Notch 2 (Figure 1A), whereas 2-AG increased Notch 2 mRNA without affecting Notch 1 mRNA expression (Figure 1A). Immunoblot analysis using antibodies directed against the activated Notch 1 and Notch 2, confirmed the mRNA results. AEA treatment increases Notch 1 activation, with no effect on Notch 2 (Figure 1B) whereas 2-AG increased the activation of Notch 2, without effecting Notch 1 (Figure 1B). No changes were observed in Notch 3 or Notch 4 expression after either treatment (data not shown).
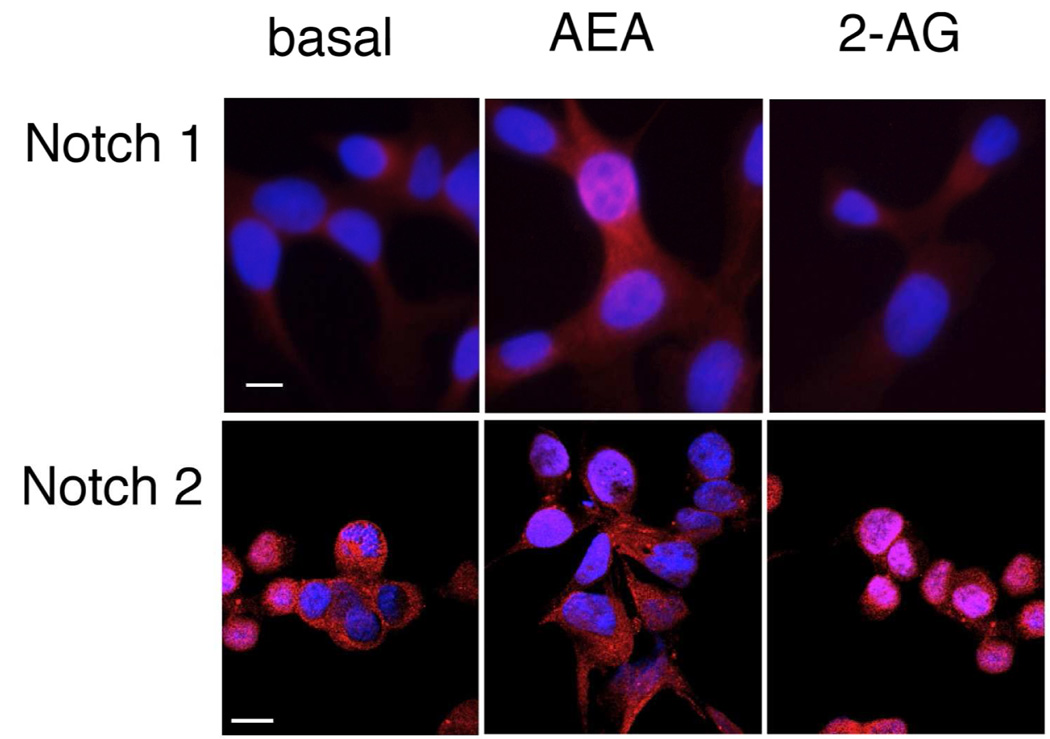
Figure 1.
AEA treatment increases the expression and activation of Notch 1 and 2-AG, the expression and activation of Notch 2 in cholangiocarcinoma cells in vitro. Mz-ChA-1 cells were treated with AEA (10 µM) or 2-AG (10 µM) for various time points. Notch 1 and 2 expression was assessed by real-time PCR (A), immunoblotting, using specific antibodies for activated Notch 1 and 2 (B) and immunofluorescence using antibodies against the intracellular domain of Notch 1 or 2 (red) and counterstained with DAPI (blue) (C) in vitro. Data are expressed as average expression ± SEM after correction for GAPDH (A) or β-Actin (B) and * denotes significance (p<0.05). Scale bar = 10 µm.
Activation of Notch signaling involves the nuclear translocation of the activated Notch proteins. Immunofluorescence analysis of the subcellular location of Notch 1 and 2 indicated that AEA treatment induced the nuclear translocation of Notch 1, whereas Notch 2 remains predominantly in the cytoplasm (Figure 1C). Conversely, 2-AG treatment resulted in the nuclear translocation of Notch 2, and Notch 1 remained in the cytoplasm (Figure 1C).
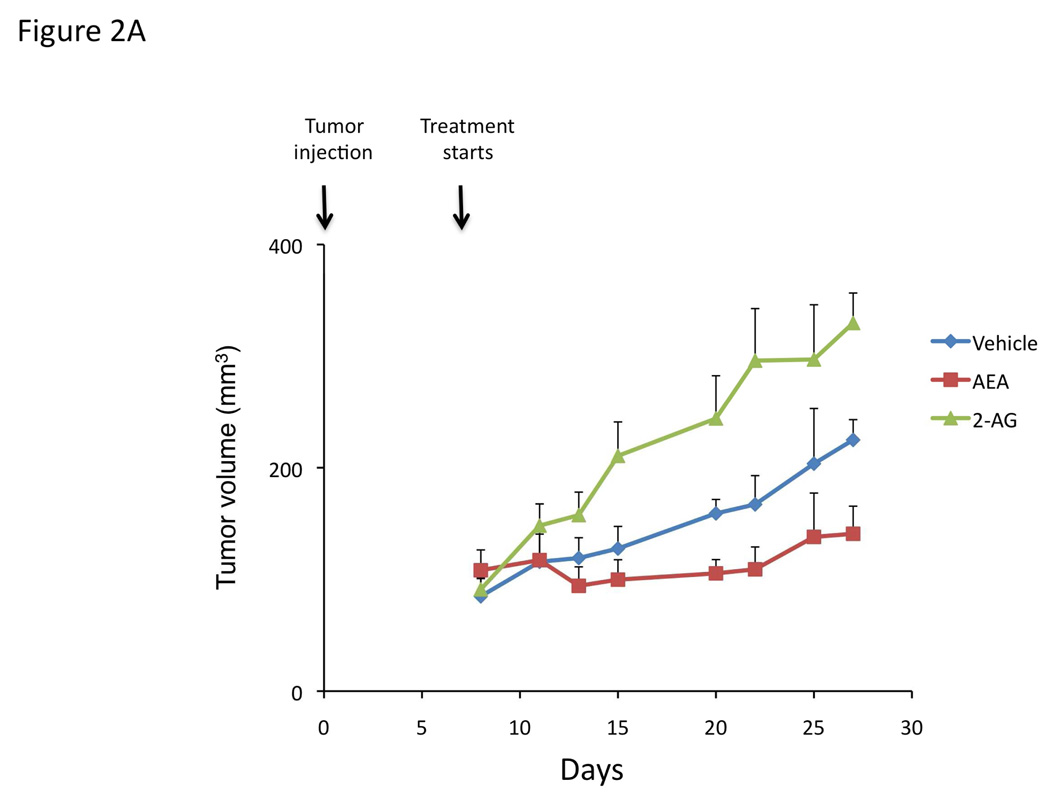
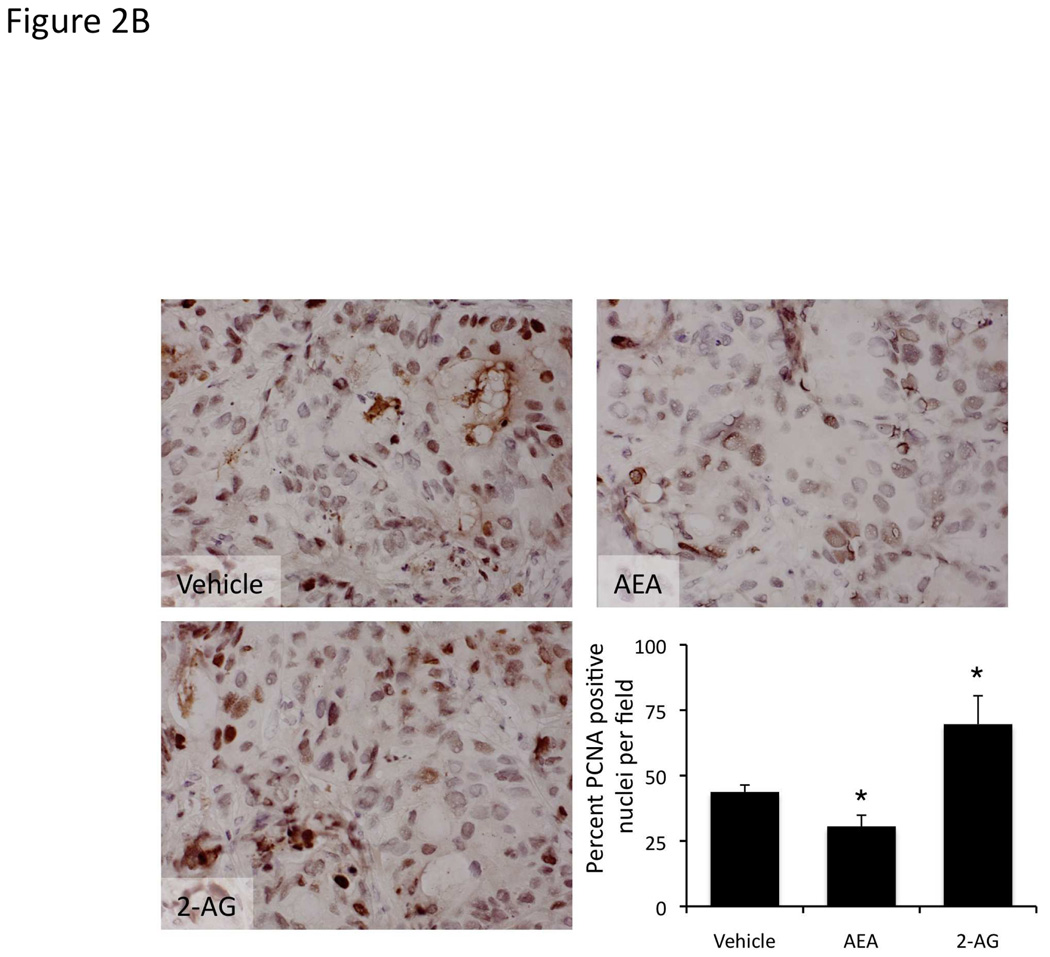
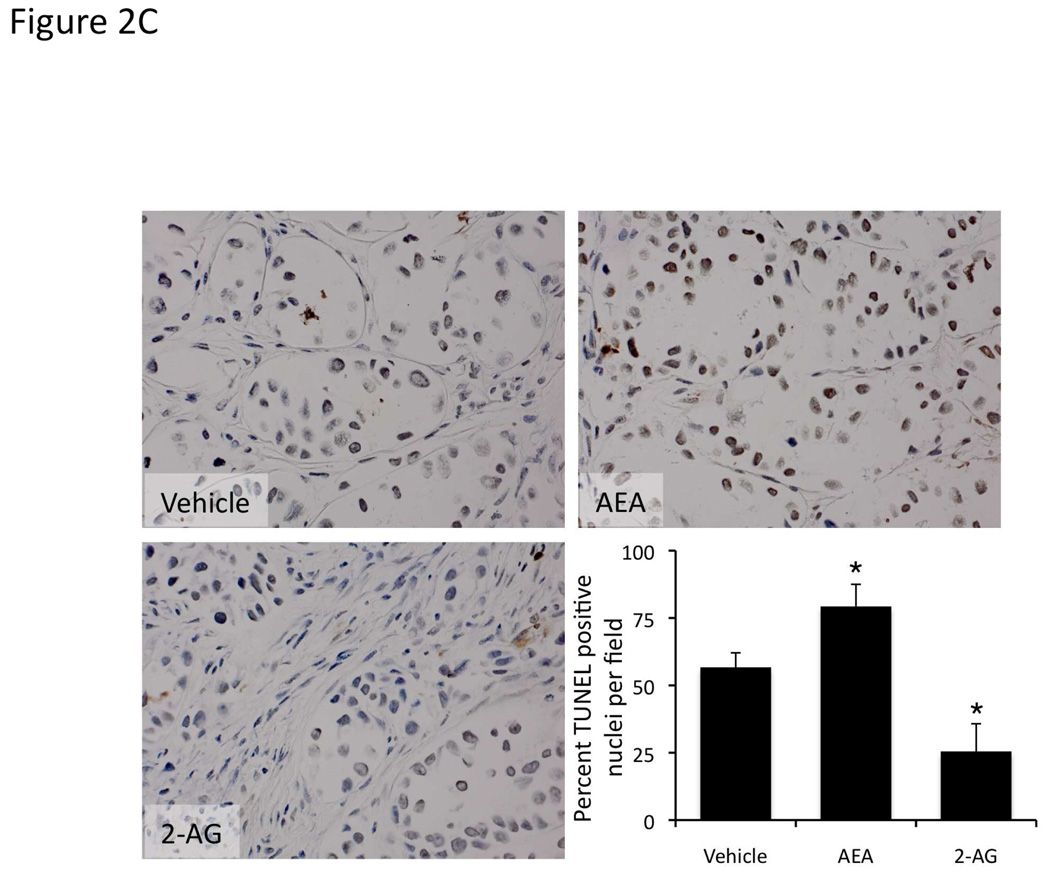
We have previously shown that chronic treatment of nude mice containing cholangiocarcinoma xenografts, with AEA reduced the tumor size [4]. To confirm the antiproliferative actions of AEA (shown previously [4]) and to demonstrate the growth promoting effects of 2-AG in our xenograft model of cholangiocarcinoma, we treated nude mice injected with Mz-ChA-1 cells chronically with AEA or 2-AG. Indeed, AEA treatment inhibited tumor growth whereas 2-AG treatment enhanced tumor growth (Figure 2A) over a period of 1 month. Analysis of liver enzymes in the serum revealed that there was no significant difference in AST (Vehicle, 82 ± 3.0 U/L; AEA, 110 ± 51.5 U/L; 2-AG, 98 ± 36.2 U/L) and ALT (Vehicle, 71 ± 15.0 U/L; AEA, 77.6 ± 23.3 U/L; 2-AG, 75.6 ± 33.5 U/L) levels between the treatment groups, all of which fell within normal range (AST, 59–247 U/L; ALT 28–132 U/L). Using PCNA immunoreactivity as a marker of proliferative capacity, AEA treatment decreased the number of PCNA positive nuclei compared to vehicle treatment, whereas 2-AG treatment increased the number of PCNA positive nuclei (Figure 2B). In contrast, the number of TUNEL positive nuclei was increased in the AEA-treated tumors versus vehicle treated, whereas the number of TUNEL-positive nuclei was decreased in 2-AG-treated tumors (Figure 2C).
Figure 2.
Opposing effects of the endocannabinoids AEA and 2-AG on cholangiocarcinoma growth also occur in an in vivo xenograft model of cholangiocarcinoma. Mz-ChA-1 cells were injected into the flank of athymic mice. After tumors were established, mice were treated with 10 mg/kg/day (ip) AEA, 2-AG or vehicle, three days per week for 28 days and tumor volume assessed (A). PCNA expression was assessed in tumor tissue from AEA, 2-AG and vehicle-treated mice by immunoreactivity (B). Semi-quantitative analysis of PCNA immunoreactivity was performed and data were expressed as average (± SEM) PCNA positive nuclei per field and the asterisk denotes significance (p<0.05) compared to vehicle-treated tumors. Apoptosis was also assessed in these tumors by TUNEL staining (C). Semi-quantitative nalysis of apoptosis was performed and data were expressed as average (± SEM) of TUNEL-positive nuclei per field and the asterisk denotes significance (p<0.05) compared to vehicle-treated tumors.
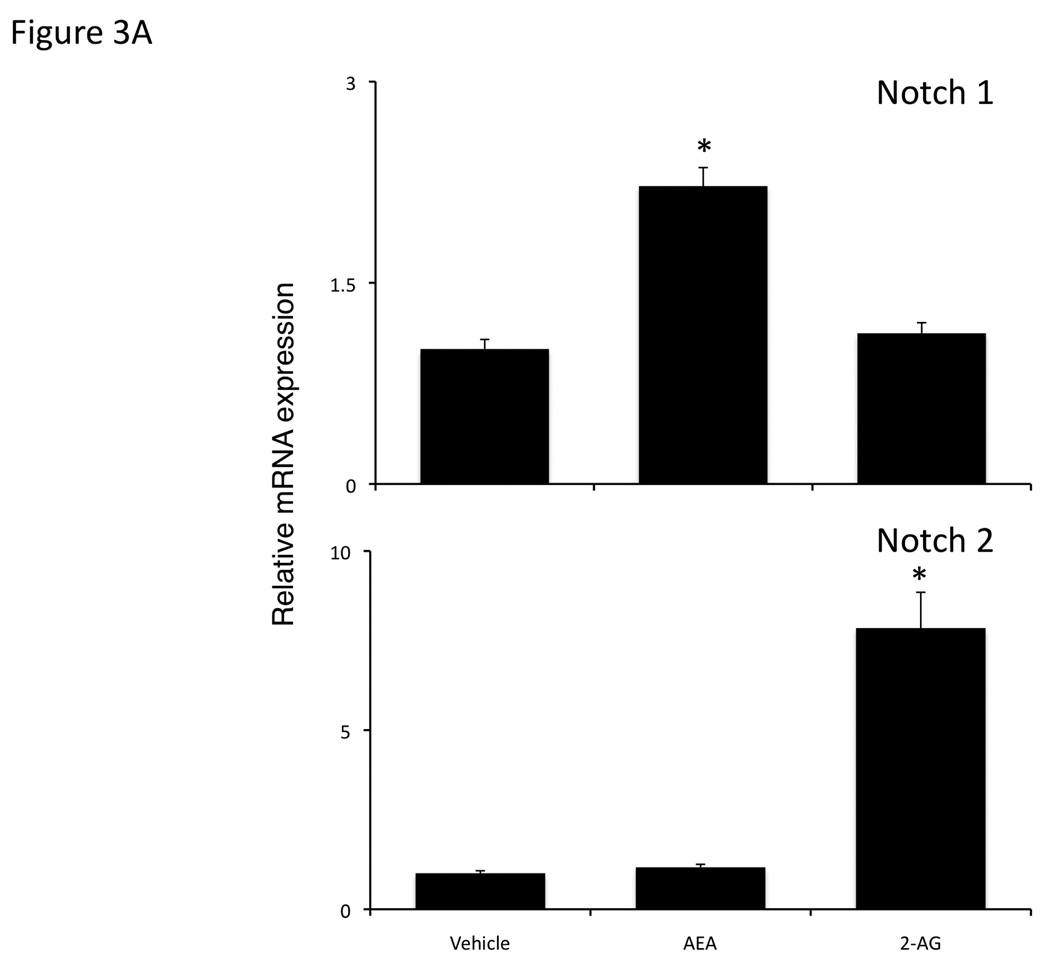
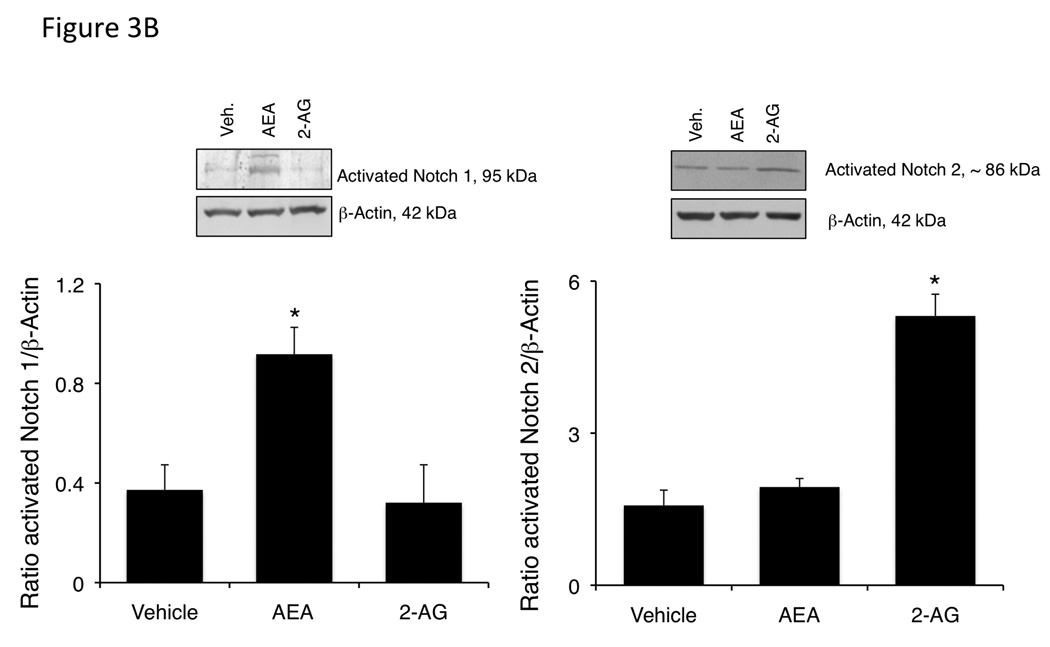
We analyzed the expression of the Notch proteins in the excised tumors. Notch 1 mRNA expression was significantly increased in the AEA-treated tumors compared to the vehicle and 2-AG-treated tumors (Figure 3A), whereas Notch 2 mRNA expression was increased in the 2-AG-treated, but not the AEA-treated tumors compared to vehicle (Figure 3A). Furthermore, activated Notch 1 was increased in AEA-treated tumors, with no change in activated Notch 2 as shown by immunoblotting, whereas activated Notch 2 was increased in 2-AG-treated tumors and Notch 1 remained unchanged (Figure 3B).
Figure 3.
Chronic AEA treatment increases Notch 1 expression and activation, whereas chronic 2-AG treatment increases Notch 2 expression and activation in an in vivo model of cholangiocarcinoma. Notch 1 and 2 expression was assessed by real-time PCR (A), and immunoblotting using antibodies against activated Notch 1 or 2 (B) in tumors. Data are expressed as average expression ± SEM after correction for GAPDH (A) or β-Actin (B) and * denotes significance (p<0.05).
Activation of Notch signaling is required for the effects of the endocannabinoids on cell proliferation
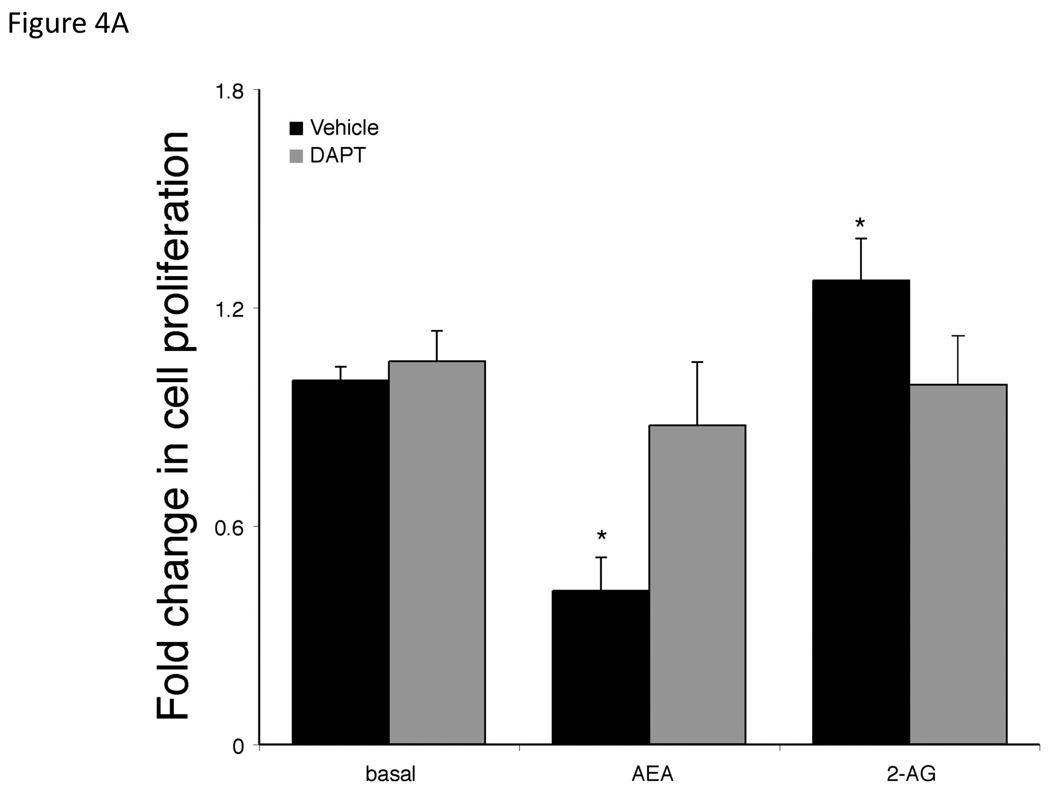
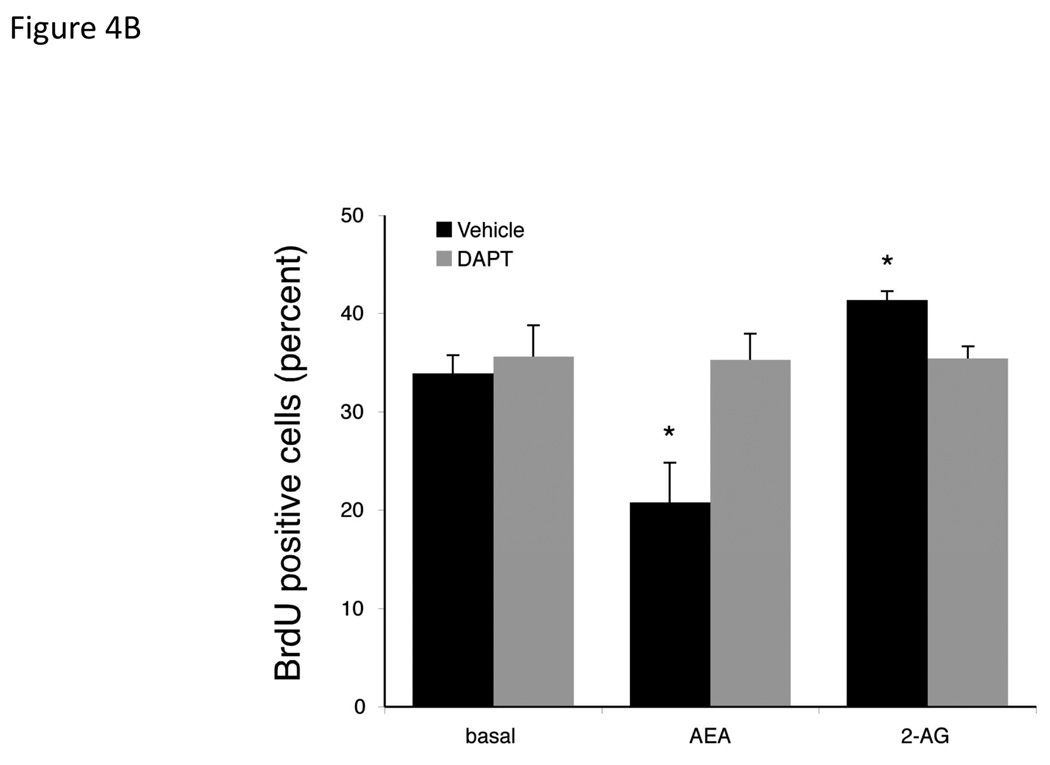
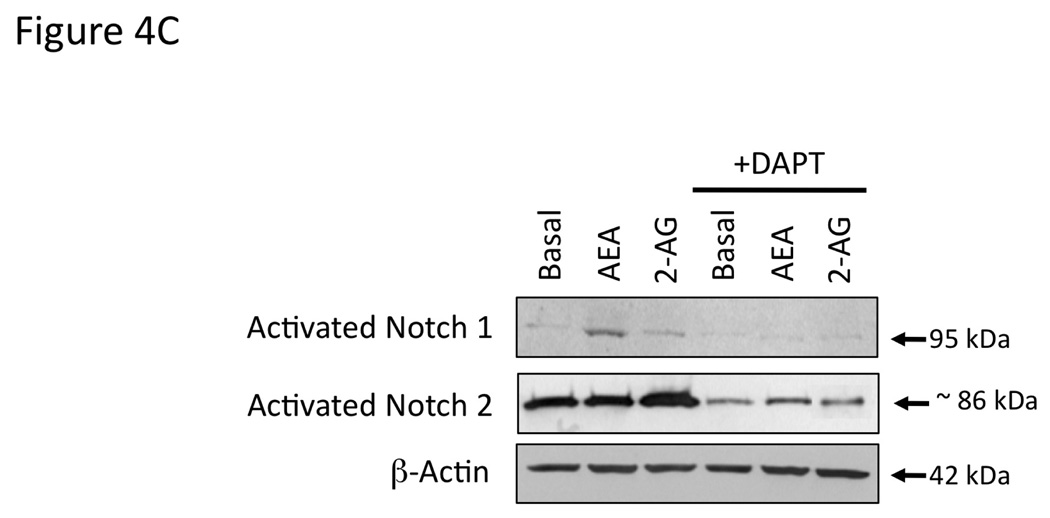
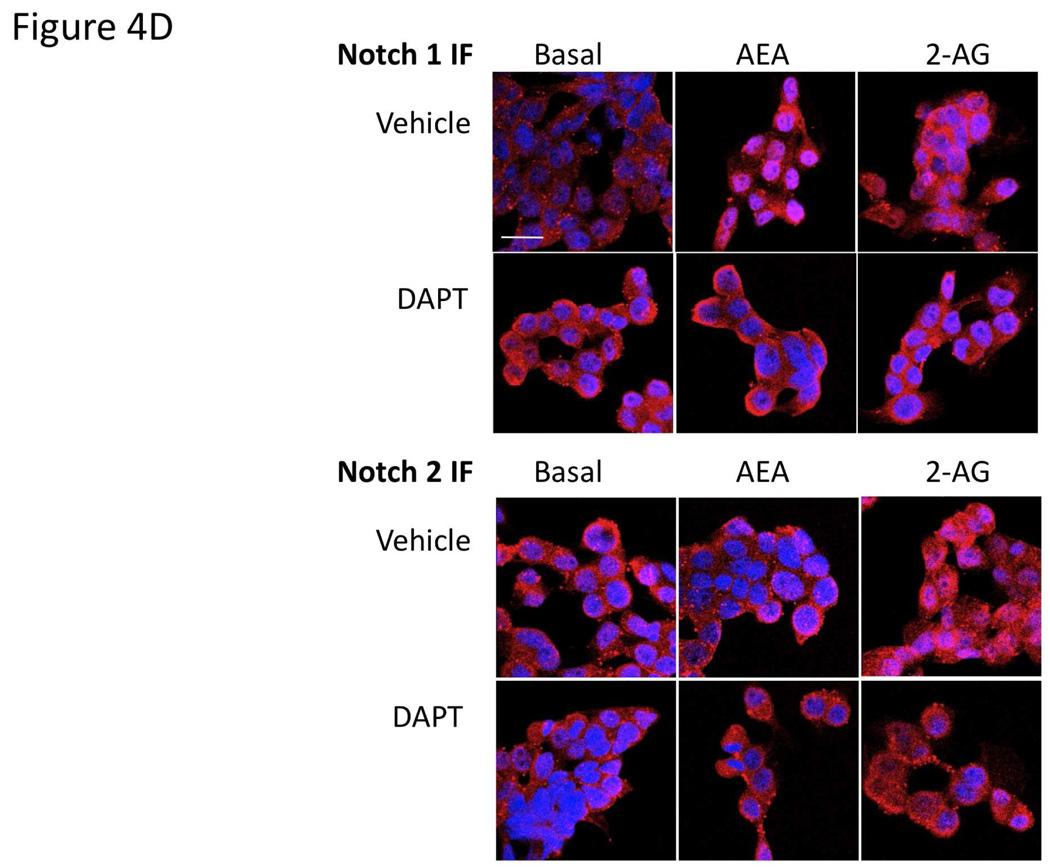
Gamma secretase is required for the activation of all Notch receptors [28]. Therefore we used the γ-secretase inhibitor, DAPT [27] to determine the involvement of the Notch signaling pathways in the endocannabinoid-mediated effects on cell growth. Pretreatment of cholangiocarcinoma cells with DAPT effectively prevented the antiproliferative effects of AEA as well as the growth promoting effects of 2-AG (Figure 4A). Furthermore, DAPT prevented the inhibitory effects of AEA on cell cycle progression (as shown by BrdU incorporation) and reversed the stimulatory effects of 2-AG (Figure 4B). Taken together, these data indicate a role of the γ-secretase complex in both the antiproliferative effects of AEA and the growth promoting effects of 2-AG. In addition, DAPT treatment prevented the activation and nuclear translocation of Notch 1 after AEA treatment (shown by immunoblotting; Figure 4C, and immunofluorescence; Figure 4D) and prevented the activation and nuclear translocation of Notch 2 after 2-AG treatment (Figure 4C and 4D).
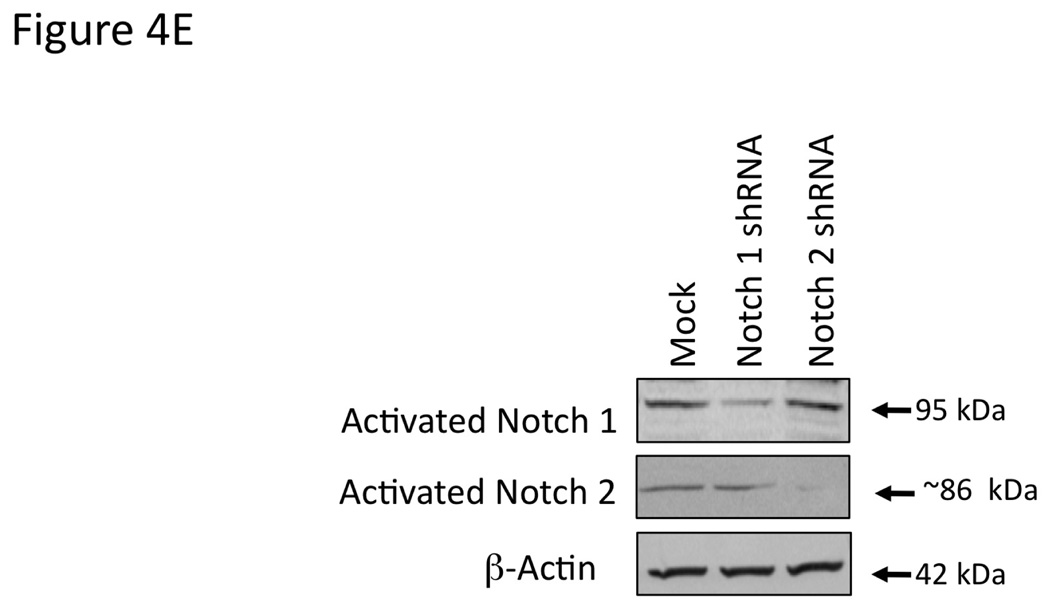
Figure 4.
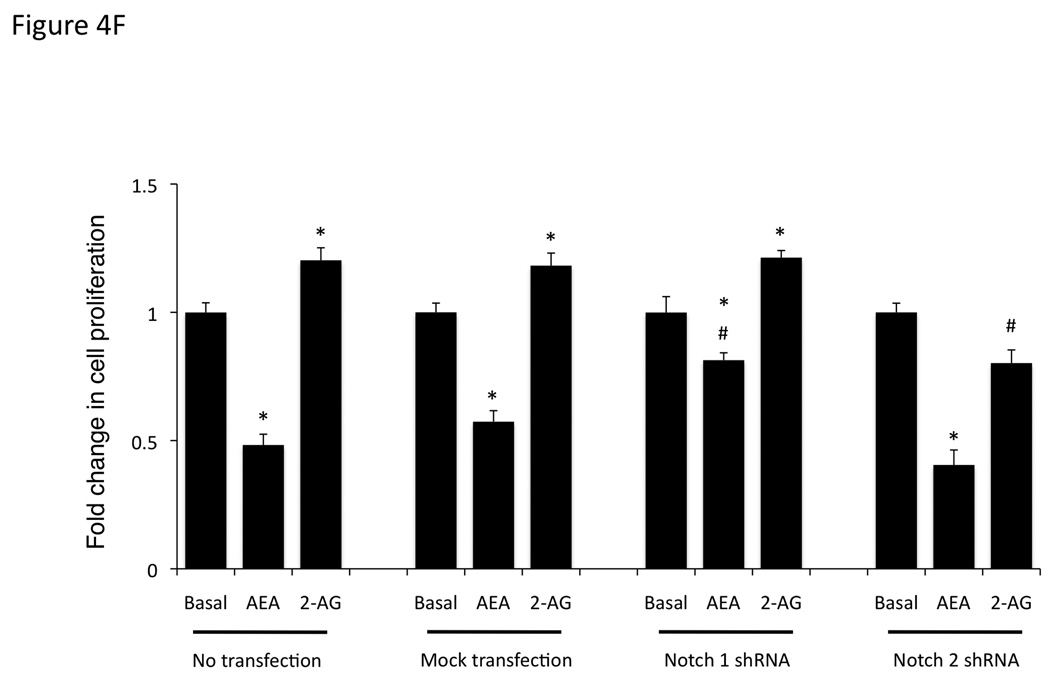
Blocking Notch 1 and 2 activation prevents the effects of AEA and 2-AG, respectively on cell proliferation. Mz-ChA-1 cells were pretreated with the γ-secretase inhibitor DAPT (1 µM) for 1 hour prior to the addition of AEA (10 µM) or 2-AG (10 µM) for 48 hrs. Cell proliferation was assessed by MTS assays (A), data are expressed as fold change in proliferation (average ± SEM, n=7) and * denotes p<0.05 compared to basal treatment. Cell cycle progression was assessed by BrdU incorporation assays (B). The number of BrdU-positive cells was expressed as a percentage of total cells. Data are expressed as the average ± SEM from 5 random fields from 3 independent experiments. * denotes significance (p<0.05) when compared to basal treatment. In parallel, Mz-ChA-1 cells were treated with AEA or 2-AG in the presence or absence of DAPT as described above and Notch 1 and 2 activation was assessed by immunoblotting using specific antibodies (C) and Notch 1 and 2 nuclear translocation was assessed by immunofluoresence (scale bar represents 20 µm; D). In addition, Mz-ChA-1 cells were transiently transfected with Notch 1 or Notch 2 shRNA and the efficiency and specificity of target gene knock down was assessed by immunoblotting (E). In parallel, transient transfection of Notch 1 or Notch 2 shRNA verctors was performed prior to the addition of AEA (10 µM) or 2-AG (10 µM) for 48 hrs. Cell proliferation was then assessed by MTS assays (F). Data are once again expressed as fold change in proliferation (average ± SEM, n=7) and * denotes p<0.05 compared to basal treatment and # denotes p<0.05 compared to the similar treatment in the mock transfected controls.
To further dissect the role of the Notch molecules in the endocannabinoid effects on cell growth, we transiently transfected Notch1 and Notch 2 shRNA-containing vectors or the empty vector. The efficiency and specificity of these shRNA constructs were analyzed by immunoblotting (Figure 4E). Treatment of cells after mock transfection with AEA resulted in decreased cell proliferation (Figure 4F). This could be partially blocked by the transient transfection of the Notch 1 shRNA construct, but not the Notch 2 shRNA construct (Figure 4F). Conversely, 2-AG administration to mock transfected cells resulted in an increase in cell proliferation that was blocked by the transfection of Notch 2 shRNA, but not Notch 1 shRNA (Figure 4F).
Changes in the composition of the γ-secretase complex associated with the opposing effects of the endocannabinoids
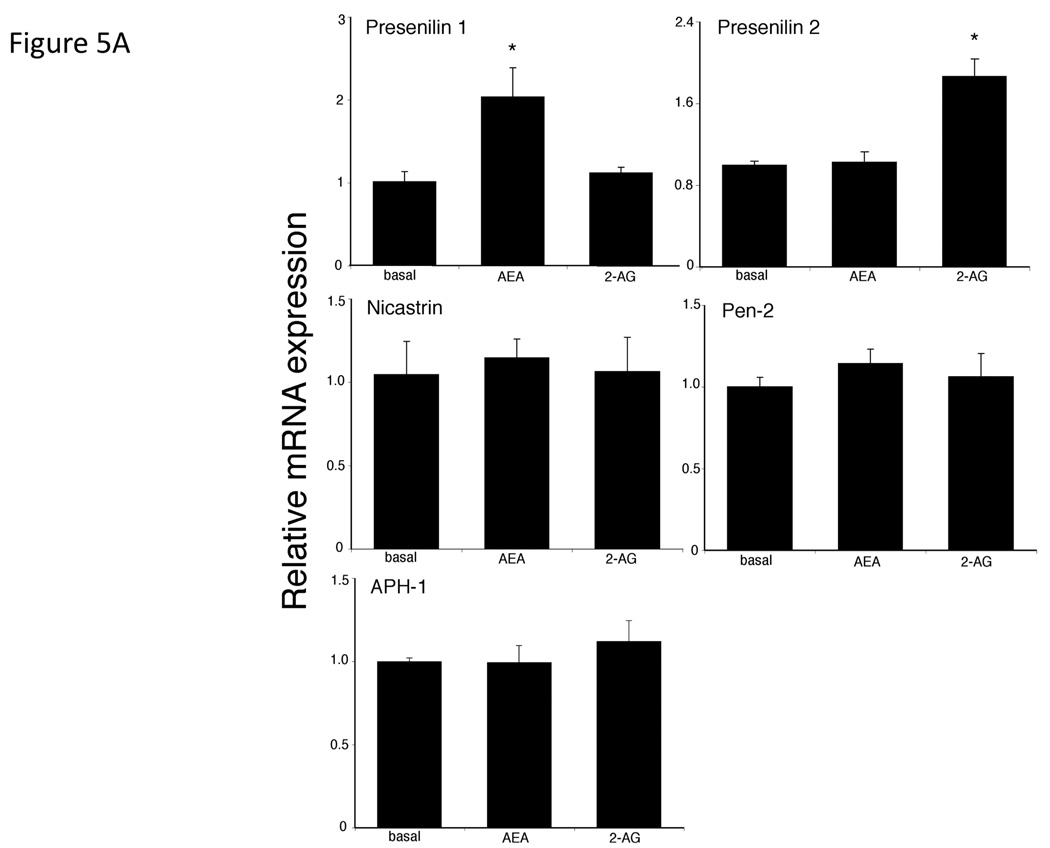
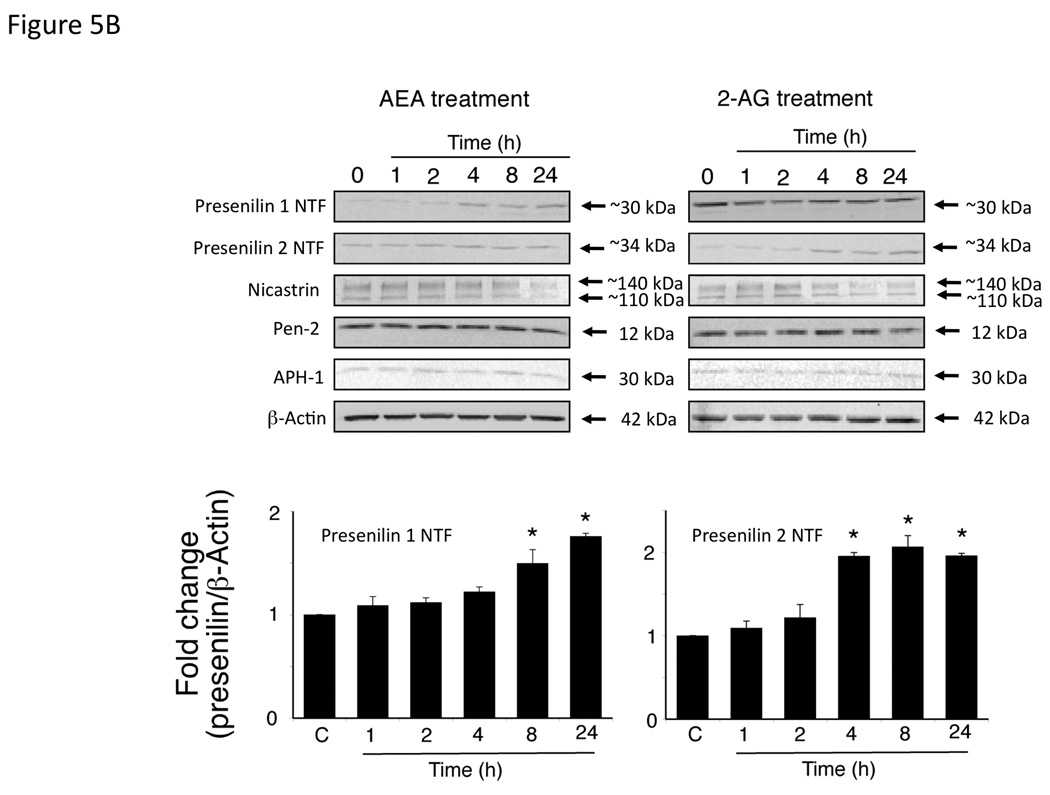
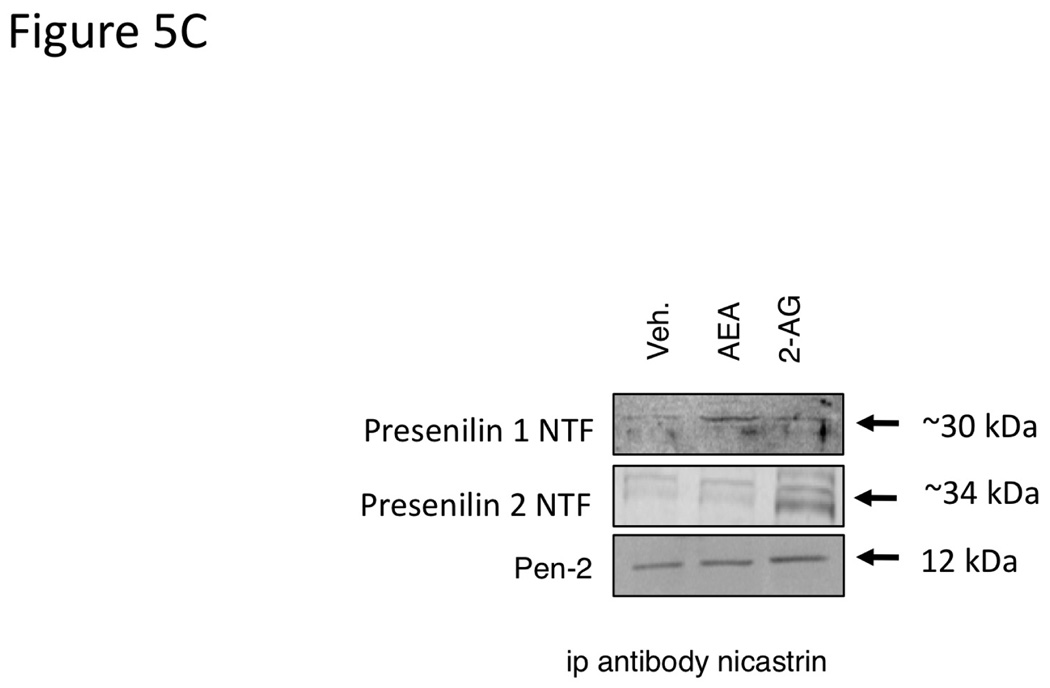
Treatment of Mz-ChA-1 cells with AEA resulted in an increase in presenilin 1 mRNA and protein expression with no change in any of the other protein components of the γ-secretase complex (presenilin 2, Nicastrin, Pen-2 or APH-1; Figure 5A and 5B). Alternatively, 2-AG administration increased the mRNA and protein expression of presenilin 2 without changing the expression of any other γ-secretase-related proteins (Figure 5A and 5B). Both the presenilin 1 and presenilin 2 antibodies detected the cleaved N-terminal fragment portion of their respective proteins. Taken together with the increase in mRNA transcript, we can assume that both the expression and cleavage of presenilin 1 is increased after AEA treatment, and that of presenilin 2 is increased after 2-AG treatment. The differential effects of endocannabinoids on presenilin 1 and 2 expression led us to hypothesize that the γ-secretase complex contains predominantly presenilin 1, in addition to Nicastrin, Pen-2 and APH-1, after AEA treatment, whereas after 2-AG treatment it contains mainly preseniln 2. To test this, we immunoprecipitated the γ-secretase complex after AEA and 2-AG treatment with an anti-nicastrin antibody. Indeed, after AEA treatment, an increased amount of presenilin 1 was co-immunoprecipitated with nicastrin, whereas after 2-AG treatment there was an increase in the amount of presenilin 2 that was co-immunoprecipitated with nicastrin (Figure 5C). Once again, the presenilin antibodies used only detected the cleaved N-terminal fragment of the respective proteins. As a control, the amount of Pen-2 immunoprecipitated did not change after any treatment (Figure 5C).
Figure 5.
AEA treatment increases the expression of presenilin 1 and 2-AG treatment increases the expression of presenilin 2 in cholangiocarcinoma cells in vitro. Mz-ChA-1 cells were treated with AEA (10 µM) or 2-AG (10 µM) for various time points. The expression of presenilin 1, presenilin 2, Nicastrin, Pen-2 and APH-1 was assessed by real-time PCR (A), and immunoblotting, (B) in vitro. Where appropriate, data are expressed as average expression ± SEM (n=4) after correction for GAPDH (A) or β-Actin (B) and * denotes significance (p<0.05). The relative amount of presenilin 1 or 2 in the γ-secretase complex after AEA or 2-AG treatment of Mz-ChA-1 cells for 8 hours was determined by co-immunoprecipitation. The γ-secretase complex was precipitated using an anti-nicastrin antibody and the expression of presenilin 1, presenilin 2 and PEN-2 was determined by immunoblotting (C).
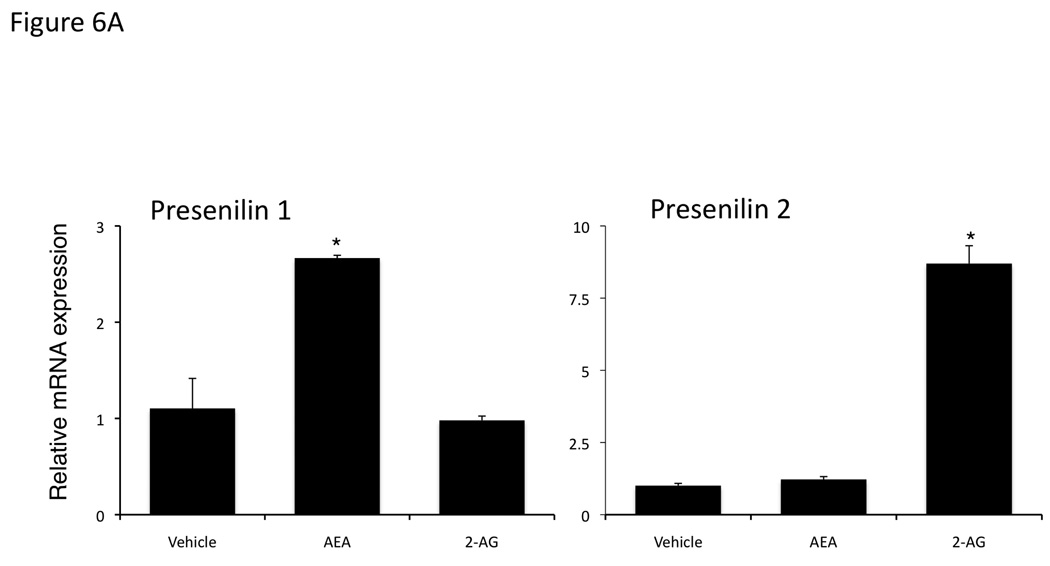
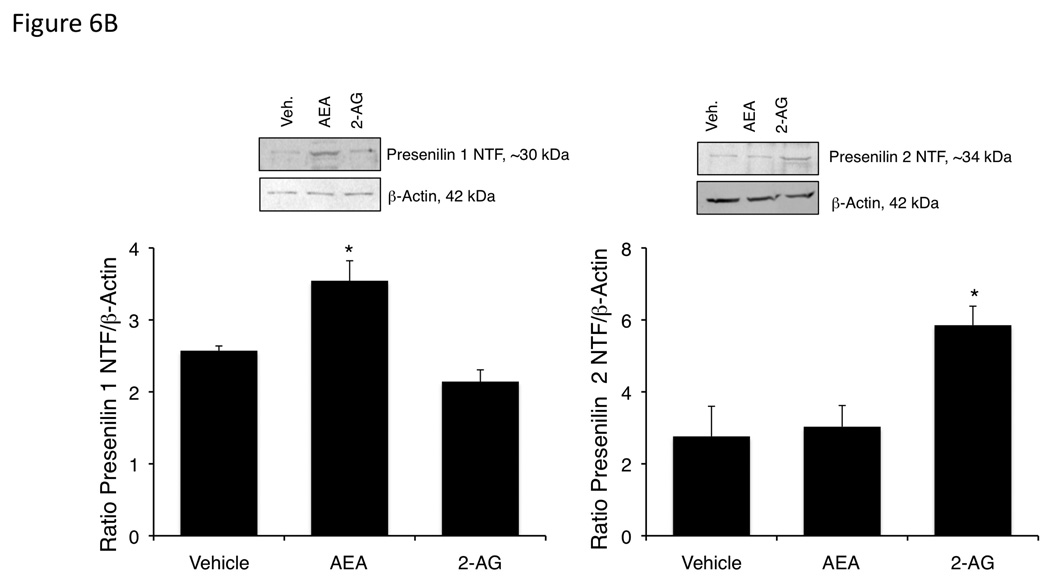
We confirmed the increase in presenilin 1 expression after AEA treatment and presenilin 2 expression after 2-AG treatment using our in vivo cannabinoid-treated tumors. Chronic AEA treatment increased presenilin 1 mRNA and protein expression with no observable change in presenilin 2 (Figure 6A and 6B). Whereas, chronic 2-AG treatment increased presenilin 2 mRNA and protein with no observable changes in presenilin 1 (Figure 6A and 6B).
Figure 6.
Chronic AEA treatment increases presenilin 1 expression, whereas chronic 2-AG treatment increased preseniln 2 expression in an in vivo model of cholangiocarcinoma. Mz-ChA-1 cells were injected into the flank of athymic mice. After tumors were established, mice were treated with 10 mg/kg/day (ip) AEA, 2-AG or vehicle three days per week for 28 days. Presenilin 1 and 2 expression was assessed by real-time PCR (A) and immunoblottng (B). Data are expressed as average expression ± SEM after correction for GAPDH (A) or β-Actin (B) and * denotes significance (p<0.05).
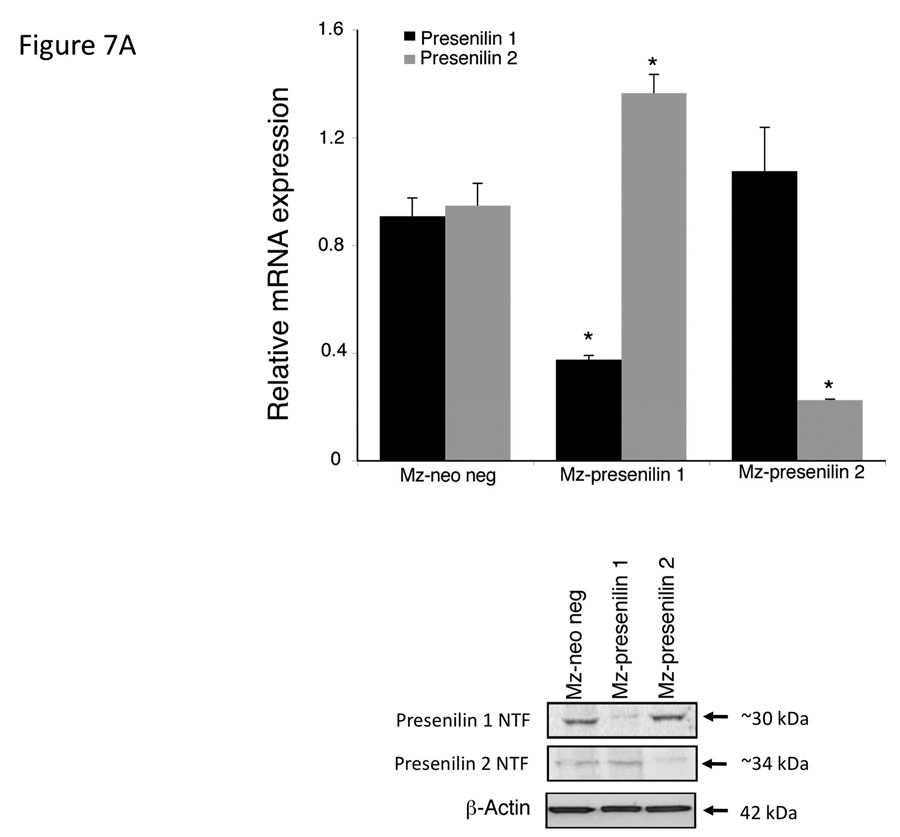
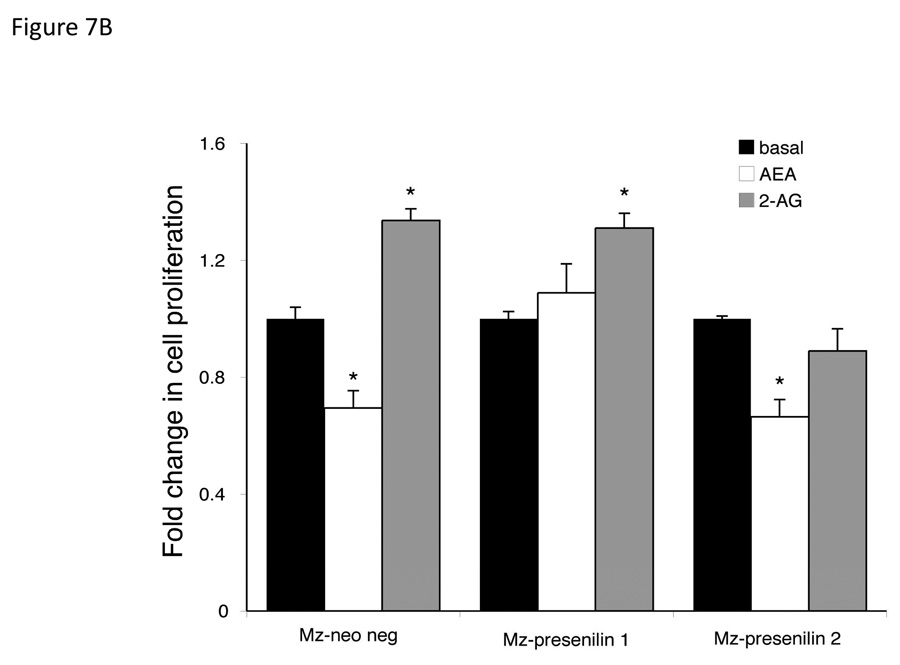
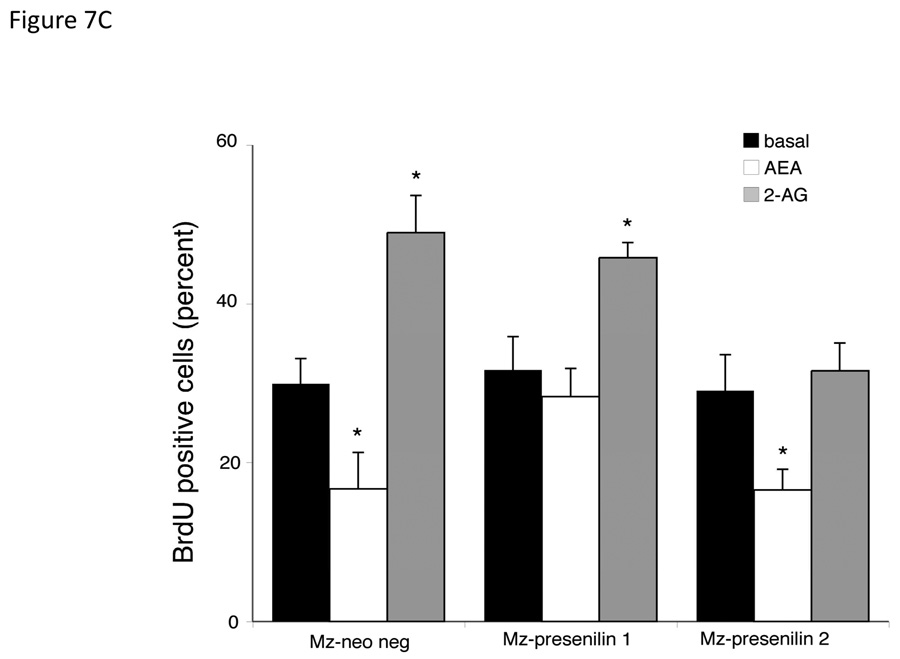
To confirm the association of the respective presenilin proteins in the effects of endocannabinoids on cell proliferation, we specifically knocked down the expression of each protein using shRNA. Stable transfection of Mz-ChA-1 cells with Presenilin 1 shRNA (Mz-presenilin 1) decreased the expression of presenilin 1 to 30% of the mock transfected cell line (Mz-neo neg; Figure 7A). Surprisingly, there was also an increase in the expression of Presenilin 2 in this cell line. Similarly, stable transfection of the presenilin 2 specific shRNA into Mz-ChA-1 cells (Mz-Presenilin 2) knocked down the expression of presenilin 2 to approximately 20% of Mz-neo neg cells, with no effect on presenilin 1 expression (Figure 7A). Using these cell lines in MTS cell proliferation assays, we could show that in the mock transfected Mz-neo neg cells, AEA exerted antiproliferative effect and 2-AG increased cell proliferation in a similar manner to the effects exerted on the parental cell line (Figure 7B). The antiproliferative effects of AEA were abolished in Mz-presenilin 1 cells, but 2-AG still increased cell proliferation (Figure 7B). Conversely, the growth promoting effects of 2-AG were abolished in Mz-presenilin 2 cells, with no effect on the antiproliferative effects of AEA (Figure 7B). These data were confirmed using BrdU incorporation assays (Figure 7C). Taken together, these data suggest that presenilin 1 expression is associated with the growth suppressing effects of AEA and that presenilin 2 expression is associated with the growth promoting effects of 2-AG.
Figure 7.
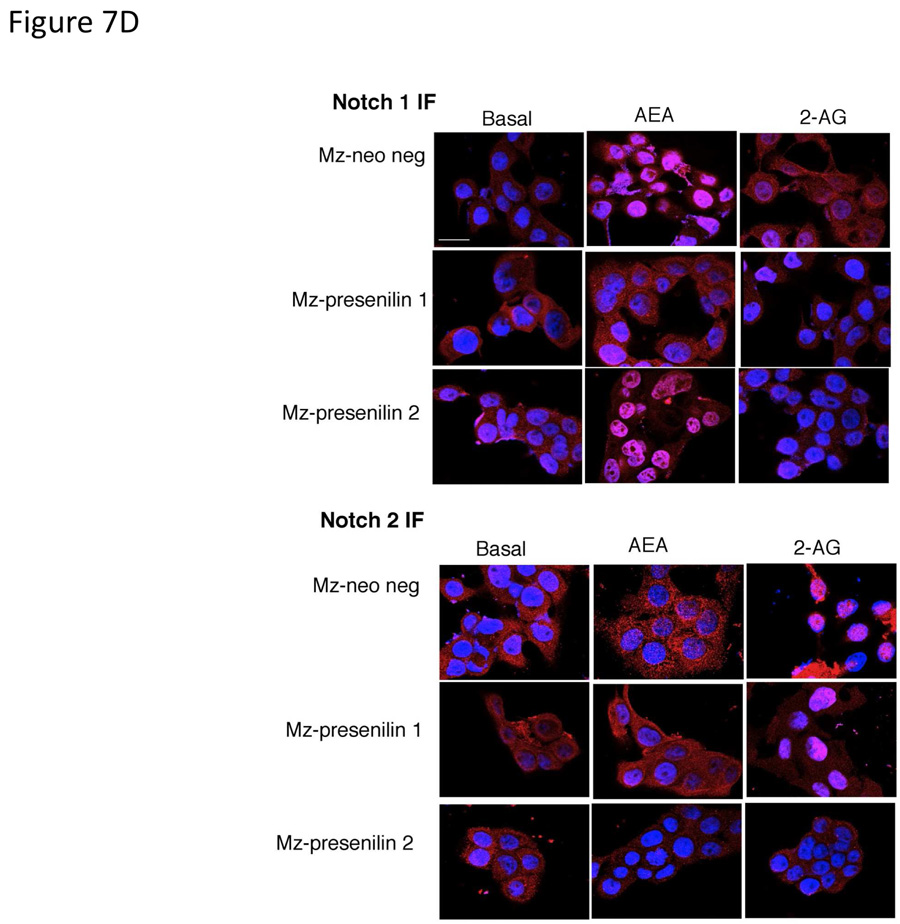
Presenilin 1 and 2 are required for the actions of AEA and 2-AG respectively. Mz-ChA-1 cells were stably-transfected with presenilin 1 or presenilin 2 shRNA vectors. The expression of presenilin 1 and 2 were assessed in the mock-transfected cell line (Mz-neo neg) and the cell lines containing the presenilin 1 shRNA (Mz-presenilin 1) and presenilin 2 shRNA (Mz-presenilin 2) by real time PCR and immunoblotting (A). Data are expressed as average ± SEM after correction for GAPDH expression. The effect of AEA treatment on cell proliferation was assessed by MTS assays (B). The three cell lines were treated with AEA (10 µM) or 2-AG (10 µM) for 48 hr and data are expressed as fold change in proliferation (average ± SEM, n=7). * denotes p<0.05 compared to basal treatment within each cell line. Cell cycle progression was assessed by BrdU incorporation assays (C). The number of BrdU-positive cells was expressed as a percentage of total cells. Data are expressed as the average ± SEM from 5 random fields from 3 independent experiments. * denotes significance (p<0.05) when compared to basal treatment. Nuclear translocation of the Notch 1 and Notch 2 intracelllular domains was also assessed by immunofluorescence (red) and counterstained with DAPI (blue) (D). Scale bar = 20 µm.
To confirm that presenilin 1 is associated with the AEA-mediated activation of Notch 1, we performed immunofluorescence to assess nuclear translocation in the stable transfected cell lines. Using Mz-neo neg cells, Notch 1 immunoreactivity was predominantly found in the cytoplasm under basal conditions, and translocated to the nucleus after AEA treatment but not 2-AG treatment (Figure 7D). As expected, this AEA-mediated effect was abolished in Mz-presenilin 1 cells, but did occur in the Mz-presenilin 2 cell line. Conversely, Notch 2 translocated to the nucleus after 2-AG treatment, but not AEA, in Mz-neo neg cells and in Mz-presenilin 1 cells, but was abolished in the Mz-presenilin 2 cell line (Figure 7D).
DISCUSSION
The findings of this study relate to the downstream signaling events associated with the antiproliferative and growth promoting effects of AEA and 2-AG respectively. We have shown that the antiproliferative actions of AEA are associated with the activation of Notch 1 and the presence of presenilin 1 in the γ-secretase complex, whereas the growth promoting effects of 2-AG are associated with the activation of Notch 2 and the presence of presenilin 2 in the γ-secretase complex. The opposing regulation of the Notch signaling pathways by endocannabinoids offers an intriguing target for the design of potential chemotherapeutic agents.
We have previously shown the differential effects of AEA and 2-AG on cholangiocarcinoma growth in vitro [3] using a number of cholangiocarcinoma cell lines. The growth promoting effects of 2-AG was found to be via a cannabinoid receptor-independent mechanism involving the disruption of lipid raft structures in the cell membrane [3]. Conversely, the antiproliferative actions of AEA were via a mechanism involving the stabilization of lipid rafts in the plasma membrane and the recruitment of death receptor complexes into these membrane microdomains [3]. More recently, we have shown that AEA suppresses tumor growth in vivo using a xenograft model of cholangiocarcinoma [4] and that there was a concomitant activation of the non-canonical Wnt pathway via upregulation of Wnt 5a [4]. The data presented here extends our findings in Mz-ChA-1 cells to include the differential activation of the Notch signaling pathways by these endocannabinoids and represents novel mechanisms by which the differential effects of endocannabinoids on cholangiocarcinoma cell growth can be regulated. However, because the current study was performed in only one cell line, we can not rule out the possibility that the effects of endocannabinoids on Notch signaling may only occur in a subset of cholangiocarcinoma cells. The dependence and recruitment of the γ-secretase complex to lipid raft structures has previously been shown to modulate γ-secretase activity [29, 30], therefore it is conceivable that agents that stabilize or disrupt lipid raft structures such as cannabinoids [3] may indeed also regulate the Notch signaling pathway. The involvement of lipid rafts in the differential activation of the Notch signaling pathways by endocannabinoids are a topic of ongoing research in our laboratory. Furthermore, activation of the Wnt signaling pathway has been shown to overlap and cross talk with the Notch signaling pathway [31–36]. Indeed, activation of Notch 1 has been shown to upregulate the expression of Wnt5a in a number of cell models [31, 32]
Consistent with our findings using AEA, cannabinoids of various origins (endogenous, plant-derived or synthetic analogues) have been shown to suppress cancer cell growth in vitro [37–40] as well as in vivo [41]. Indeed, a recent clinical pilot study to determine the safety of intracranial administration of the plant-derived, Δ9-tetrahydrocannabinol in patients with glioblastoma demonstrated a marked reduction in Ki67 staining within the tumor [42]. This observation, together with the very promising results observed in cell cultures and laboratory animals [37–41] suggest that administering cannabinoids alone or in conjunction with existing chemotherapeutic agents may be a promising treatment strategy for aggressive cancers of various origins. In the present study, we also observed that in contrast to AEA, 2-AG treatment resulted in an increase in cholangiocarcinoma tumor growth in vivo. The growth promoting effects of cannabinoids have also been previously demonstrated via the transactivation of the EGFR in a TACE/ADAM17 metalloprotease-dependent manner [43]. This was demonstrated in a number of cell lines from various origins including lung cancer, squamous cell carcinoma, bladder carcinoma, glioblastoma, astrocytoma, and kidney cancer [43].
In our study, we demonstrate that AEA activates Notch 1, whereas 2-AG activates Notch 2. A similar activation of the Notch signaling pathways by cannabinoids has recently been shown in dendritic cells with Δ9-tetrahydrocannabinol decreasing the expression of the Notch ligand Delta 4, and increasing the expression of Jagged 1 [44]. This is the first demonstration of an influence of cannabinoids on Notch signaling. However, this manuscript does not show any of the downstream consequences on Notch signaling that this differential expression of the Notch ligands causes [44].
Here we show opposing actions of Notch 1 and Notch 2 on cholangiocarcinoma cell proliferation. Such opposing actions of Notch receptors are not without precedent. Notch 1 is overexpressed in malignant mesothelioma, whereas Notch 2 is downregulated and is associated with a tumor suppressive role [45]. Similarly, opposite effects of Notch 1 and Notch 2 have also been found in breast cancer [46], multiple myeloma [47], with Notch 1 acting as to enhance proliferation and Notch 2 acting as the tumor suppressor in each case. Furthermore, the expression of Notch 2 can antagonize the effects of Notch 1 [48]. Conversely, and consistent with the data presented here, in embryonal brain tumor cells, proliferation, soft agar colony formation, and xenograft growth were all promoted by Notch2 and inhibited by Notch1 [19]. The downstream mechanism for these opposing actions in the current study is unclear, and is the topic of ongoing research in our laboratory. For example, we have preliminary data suggesting that AEA can induce the expression of Hairy/enhancer of Split-(Hes)-1 in cholangiocarcinoma cells (data not shown), which has previously been shown to have tumor suppressive actions in certain cellular contexts [49, 50]. Conversely, 2-AG may increase the expression of Hes-6 in cholangiocarcinoma cells (data not shown), a target that is overexpressed in other tumors such as hepatocellular carcinoma [51] and colon cancer [50].
A crucial step of Notch signaling is the proteolytic cleavage of the Notch receptors by the γ-secretase complex [28]. Very little has been reported concerning the composition of the γ-secretase complex (with respect to the presenilin proteins) and its association with differential activation of various Notch ligands and subsequent downstream consequences. A recent case report demonstrated presenilin 2 expression in a particular breast cancer [52].
Furthermore, novel presenilin 2 variants that confer a loss-of-function phenotype, have been reported to occur three times more frequently in breast cancer cases versus controls [53]. In addition, the requirement for presenilin 1 and 2 in the processing of truncated Notch oncoproteins has been demonstrated [54, 55]. Similarly, here we demonstrate an association for presenilin 1 expression in the processing of Notch 1 and presenilin 2 expression in the processing of Notch 2. These data may simply reflect that the γ-secretase complex that cleaves either Notch 1 or Notch 2 contains the predominant presenilin subtype expressed rather than a preferential substrate choice.
In conclusion, we have demonstrated that the antiproliferative effects of AEA is associated with the presenilin 1-dependent proteolytic cleavage of Notch 1 and that the growth promoting effects of 2-AG is associated with the presenilin 2-dependent activation of Notch 2 signaling. We propose that the development of novel therapeutic strategies aimed at modulating the endocannabinoid system, or mimicking the mode of action of AEA on Notch signaling pathways would prove beneficial for the treatment of this devastating disease
Acknowledgements
This work was supported by an NIH K01 grant award (DK078532) and internal funds from the Department of Medicine, Scott & White Hospital, Texas A&M Health Science Center to Dr DeMorrow and a State Scholarship of China Scholarship Council (No 2009638036) to Dr Huang. We sincerely thank Professor Gianfranco Alpini for his assistance and expert advice in the development of this manuscript and the Texas A&M Health Science Center Microscopy Imaging Center for assistance with the confocal microscopy imaging.
Abbreviations
- 2-AG
2-arachidonyl glycerol
- AEA
anandamide
- APH-1
anterior-pharynx defective protein 1
- BrdU
bromodeoxy uridine
- DAPI
4V,6-diamidino-2-phenylindole
- NICD
notch intracellular domain
- Pen-2
presenilin enhancer 2
- SEM
standard error of the mean
- shRNA
short hairpin RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alpini G, Prall R, LaRusso N. The pathobiology of biliary epithelia. The Liver; Biology & Pathobiology. 2001;4E:421–435. [Google Scholar]
- 2.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 3.DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S, Alpini G. Opposing actions of endocannabinoids on cholangiocarcinoma growth: Recruitment of fas and fas ligand to lipid rafts. J Biol Chem. 2007;282:13098–13113. doi: 10.1074/jbc.M608238200. [DOI] [PubMed] [Google Scholar]
- 4.DeMorrow S, Francis H, Gaudio E, Venter J, Franchitto A, Kopriva S, Onori P, Mancinelli R, Frampton G, Coufal M, Mitchell BM, Vaculin B, Alpini G. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the non-canonical Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1150–G1158. doi: 10.1152/ajpgi.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allman D, Punt JA, Izon DJ, Aster JC, Pear WS. An invitation to T and more: notch signaling in lymphopoiesis. Cell. 2002;109 Suppl:S1–S11. doi: 10.1016/s0092-8674(02)00689-x. [DOI] [PubMed] [Google Scholar]
- 6.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 8.Kopan R, Goate A. Aph-2/Nicastrin: an essential component of gamma-secretase and regulator of Notch signaling and Presenilin localization. Neuron. 2002;33:321–324. doi: 10.1016/s0896-6273(02)00585-8. [DOI] [PubMed] [Google Scholar]
- 9.Lai EC. Notch cleavage: Nicastrin helps Presenilin make the final cut. Curr Biol. 2002;12:R200–R202. doi: 10.1016/s0960-9822(02)00749-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee SF, Shah S, Li H, Yu C, Han W, Yu G. Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-beta precursor protein and Notch. J Biol Chem. 2002;277:45013–45019. doi: 10.1074/jbc.M208164200. [DOI] [PubMed] [Google Scholar]
- 11.Li T, Ma G, Cai H, Price DL, Wong PC. Nicastrin is required for assembly of presenilin/gamma-secretase complexes to mediate Notch signaling and for processing and trafficking of beta-amyloid precursor protein in mammals. J Neurosci. 2003;23:3272–3277. doi: 10.1523/JNEUROSCI.23-08-03272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer LS, Sorbi S, Bruni A, Fraser P, St George-Hyslop P. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 13.Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa T, Maruyama S, Kawaichi M, Honjo T. The Drosophila homolog of the immunoglobulin recombination signal-binding protein regulates peripheral nervous system development. Cell. 1992;69:1191–1197. doi: 10.1016/0092-8674(92)90640-x. [DOI] [PubMed] [Google Scholar]
- 15.Schweisguth F, Posakony JW. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell. 1992;69:1199–1212. doi: 10.1016/0092-8674(92)90641-o. [DOI] [PubMed] [Google Scholar]
- 16.Petcherski AG, Kimble J. Mastermind is a putative activator for Notch. Curr Biol. 2000;10:R471–R473. doi: 10.1016/s0960-9822(00)00577-7. [DOI] [PubMed] [Google Scholar]
- 17.Rehman AO, Wang CY. Notch signaling in the regulation of tumor angiogenesis. Trends Cell Biol. 2006;16:293–300. doi: 10.1016/j.tcb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Weng AP, Aster JC. Multiple niches for Notch in cancer: context is everything. Curr Opin Genet Dev. 2004;14:48–54. doi: 10.1016/j.gde.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 20.Parr C, Watkins G, Jiang WG. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. Int J Mol Med. 2004;14:779–786. doi: 10.3892/ijmm.14.5.779. [DOI] [PubMed] [Google Scholar]
- 21.Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Meyer zum Buschenfelde KH. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579–596. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Coufal M, Invernizzi P, Gaudio E, Bernuzzi F, Frampton GA, Onori P, Franchitto A, Carpino G, Ramirez JC, Alvaro D, Marzioni M, Battisti G, Benedetti A, Demorrow S. Increased local dopamine secretion has growth promoting effects in cholangiocarcinoma. Int J Cancer. 2009 doi: 10.1002/ijc.24909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanno N, Glaser S, Chowdhury U, Phinizy JL, Baiocchi L, Francis H, LeSage G, Alpini G. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284–291. doi: 10.1016/s0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 25.Alpini G, Invernizzi P, Gaudio E, Venter J, Kopriva S, Bernuzzi F, Onori P, Franchitto A, Coufal M, Frampton G, Alvaro D, Lee SP, Marzioni M, Benedetti A, DeMorrow S. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008;68:9184–9193. doi: 10.1158/0008-5472.CAN-08-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bojesen IN, Hansen HS. Binding of anandamide to bovine serum albumin. J Lipid Res. 2003;44:1790–1794. doi: 10.1194/jlr.M300170-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Vandermeeren M, Geraerts M, Pype S, Dillen L, Van Hove C, Mercken M. The functional gamma-secretase inhibitor prevents production of amyloid beta 1–34 in human and murine cell lines. Neurosci Lett. 2001;315:145–148. doi: 10.1016/s0304-3940(01)02369-2. [DOI] [PubMed] [Google Scholar]
- 28.Shih Ie M, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;67:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 29.Gamerdinger M, Clement AB, Behl C. Effects of sulindac sulfide on the membrane architecture and the activity of gamma-secretase. Neuropharmacology. 2008;54:998–1005. doi: 10.1016/j.neuropharm.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125:1016–1026. doi: 10.1002/ijc.24375. [DOI] [PubMed] [Google Scholar]
- 31.Katoh M. Transcriptional mechanisms of WNT5A based on NF-kappaB, Hedgehog, TGFbeta, and Notch signaling cascades. Int J Mol Med. 2009;23:763–769. doi: 10.3892/ijmm_00000190. [DOI] [PubMed] [Google Scholar]
- 32.Koyanagi M, Bushoven P, Iwasaki M, Urbich C, Zeiher AM, Dimmeler S. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res. 2007;101:1139–1145. doi: 10.1161/CIRCRESAHA.107.151381. [DOI] [PubMed] [Google Scholar]
- 33.Shin M, Nagai H, Sheng G. Notch mediates Wnt and BMP signals in the early separation of smooth muscle progenitors and blood/endothelial common progenitors. Development. 2009;136:595–603. doi: 10.1242/dev.026906. [DOI] [PubMed] [Google Scholar]
- 34.Carlson ME, Silva HS, Conboy IM. Aging of signal transduction pathways, and pathology. Exp Cell Res. 2008;314:1951–1961. doi: 10.1016/j.yexcr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol. 2007;42:705–710. doi: 10.1007/s00535-007-2087-z. [DOI] [PubMed] [Google Scholar]
- 36.Collu GM, Brennan K. Cooperation between Wnt and Notch signalling in human breast cancer. Breast Cancer Res. 2007;9:105. doi: 10.1186/bcr1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Petrocellis L, Melck D, Palmisano A, Bisogno T, Laezza C, Bifulco M, Di Marzo V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci U S A. 1998;95:8375–8380. doi: 10.1073/pnas.95.14.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz L, Miguel A, Diaz-Laviada I. Delta9-tetrahydrocannabinol induces apoptosis in human prostate PC-3 cells via a receptor-independent mechanism. FEBS Lett. 1999;458:400–404. doi: 10.1016/s0014-5793(99)01073-x. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez C, de Ceballos ML, del Pulgar TG, Rueda D, Corbacho C, Velasco G, Galve-Roperh I, Huffman JW, Ramon y Cajal S, Guzman M. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001;61:5784–5789. [PubMed] [Google Scholar]
- 40.Sanchez C, Galve-Roperh I, Canova C, Brachet P, Guzman M. Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998;436:6–10. doi: 10.1016/s0014-5793(98)01085-0. [DOI] [PubMed] [Google Scholar]
- 41.Galve-Roperh I, Sanchez C, Cortes ML, del Pulgar TG, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6:313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- 42.Guzman M, Duarte MJ, Blazquez C, Ravina J, Rosa MC, Galve-Roperh I, Sanchez C, Velasco G, Gonzalez-Feria L. A pilot clinical study of Delta(9)-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95:197–203. doi: 10.1038/sj.bjc.6603236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart S, Fischer OM, Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004;64:1943–1950. doi: 10.1158/0008-5472.can-03-3720. [DOI] [PubMed] [Google Scholar]
- 44.Newton CA, Chou PJ, Perkins I, Klein TW. CB(1) and CB(2) Cannabinoid Receptors Mediate Different Aspects of Delta-9-Tetrahydrocannabinol (THC)-Induced T Helper Cell Shift Following Immune Activation by Legionella Pneumophila Infection. J Neuroimmune Pharmacol. 2009;4:92–102. doi: 10.1007/s11481-008-9126-2. [DOI] [PubMed] [Google Scholar]
- 45.Graziani I, Eliasz S, De Marco MA, Chen Y, Pass HI, De May RM, Strack PR, Miele L, Bocchetta M. Opposite Effects of Notch-1 and Notch-2 on Mesothelioma Cell Survival under Hypoxia Are Exerted through the Akt Pathway. Cancer Res. 2008;68:9678–9685. doi: 10.1158/0008-5472.CAN-08-0969. [DOI] [PubMed] [Google Scholar]
- 46.O'Neill CF, Urs S, Cinelli C, Lincoln A, Nadeau RJ, Leon R, Toher J, Mouta-Bellum C, Friesel RE, Liaw L. Notch2 signaling induces apoptosis and inhibits human MDA-MB-231 xenograft growth. Am J Pathol. 2007;171:1023–1036. doi: 10.2353/ajpath.2007.061029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nefedova Y, Cheng P, Alsina M, Dalton WS, Gabrilovich DI. Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood. 2004;103:3503–3510. doi: 10.1182/blood-2003-07-2340. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu K, Chiba S, Saito T, Kumano K, Hamada Y, Hirai H. Functional diversity among Notch1, Notch2, and Notch3 receptors. Biochem Biophys Res Commun. 2002;291:775–779. doi: 10.1006/bbrc.2002.6528. [DOI] [PubMed] [Google Scholar]
- 49.Jiao J, Qin Z, Li S, Liu H, Lu Z. Potential role of Notch1 signaling pathway in laryngeal squamous cell carcinoma cell line Hep-2 involving proliferation inhibition, cell cycle arrest, cell apoptosis, and cell migration. Oncol Rep. 2009;22:815–823. doi: 10.3892/or_00000504. [DOI] [PubMed] [Google Scholar]
- 50.Huang Q, Raya A, DeJesus P, Chao SH, Quon KC, Caldwell JS, Chanda SK, Izpisua-Belmonte JC, Schultz PG. Identification of p53 regulators by genome-wide functional analysis. Proc Natl Acad Sci U S A. 2004;101:3456–3461. doi: 10.1073/pnas.0308562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gramantieri L, Giovannini C, Lanzi A, Chieco P, Ravaioli M, Venturi A, Grazi GL, Bolondi L. Aberrant Notch3 and Notch4 expression in human hepatocellular carcinoma. Liver Int. 2007;27:997–1007. doi: 10.1111/j.1478-3231.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 52.Ai-Ping F, Yue Q, Yan W. A case report of remote cutaneous metastasis from male breast carcinoma. Int J Dermatol. 2007;46:738–739. doi: 10.1111/j.1365-4632.2006.02923.x. [DOI] [PubMed] [Google Scholar]
- 53.To MD, Gokgoz N, Doyle TG, Donoviel DB, Knight JA, Hyslop PS, Bernstein A, Andrulis IL. Functional characterization of novel presenilin-2 variants identified in human breast cancers. Oncogene. 2006;25:3557–3564. doi: 10.1038/sj.onc.1209397. [DOI] [PubMed] [Google Scholar]
- 54.Das I, Craig C, Funahashi Y, Jung KM, Kim TW, Byers R, Weng AP, Kutok JL, Aster JC, Kitajewski J. Notch oncoproteins depend on gamma-secretase/presenilin activity for processing and function. J Biol Chem. 2004;279:30771–30780. doi: 10.1074/jbc.M309252200. [DOI] [PubMed] [Google Scholar]
- 55.Weng AP, Nam Y, Wolfe MS, Pear WS, Griffin JD, Blacklow SC, Aster JC. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol. 2003;23:655–664. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]