Circular dichroism (CD) is a powerful tool for assignment of configuration in natural products,[1] however, its use in acyclic molecules is limited by motional averaging that may reduce or eliminate Cotton effects (CE's). Recently, we reported application of liposomal exciton coupled CD (LECCD) for determination of both relative and absolute configuration of 1,n-acyclic diols (n >5)[2] which exploited two properties: dual chromophores with very large electronic charge-transition dipole moments and ordering of the long-chain carbon backbones within uniform unilamellar liposomes. This report now describes a sensitive technique – liposomal circular dichroism (LCD) – for assignment of configurations at remote methyl-branched stereocenters in long-chain natural products at sub-µmole levels by exploiting a single chromophore appended to the chain terminus. LCD reveals a general principle: simple Cotton effects arising from perturbation of single chromophores may be amplified by constraining molecules within lipid bilayers. Application of LCD is applied here to an outstanding problem: configurational assignment of the remote stereocenters in methyl-branched in polyketide peroxides (e.g. 1 and 2) from marine sponges of the genera Plakortis and Plakinastrella.
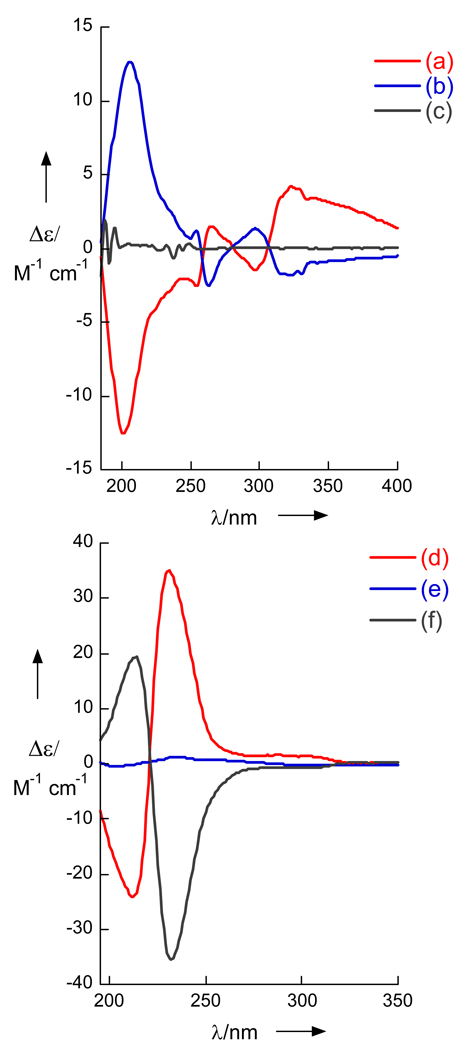
The ‘remote stereocenter problem’ is illustrated with the enantiomeric naphthamides (S)- and (R)-3. 2-Naphthamides exhibit strong charge transfer bands that have been exploited in CD studies of chiral aminoalcohols.[1b] Despite the presence of a chiragenic center at C2, (−)-(S)-3 and (+)-(R)-3[3] showed essentially flat-line CD spectra in MeOH (Figure 2) due to conformational averaging. In contrast, when the compounds were formulated in highly uniform unilamellar liposomes from 1,2-distearoyl-sn-glycero-3-phosphocholine (pressure extrusion through a 100 nm pore nylon membrane, DSPC= 2 mg/mL, lipid: naphthamide mole ratio 20:1, mean diameter, ϕ~30 nm)[2], strong Cotton effects appeared for (+)- and (−)-3 (e.g. (S)-3, λ 206 nm, Δε +12.6). Most important, the two spectra were mirror images of each other and the effect was reproducible.
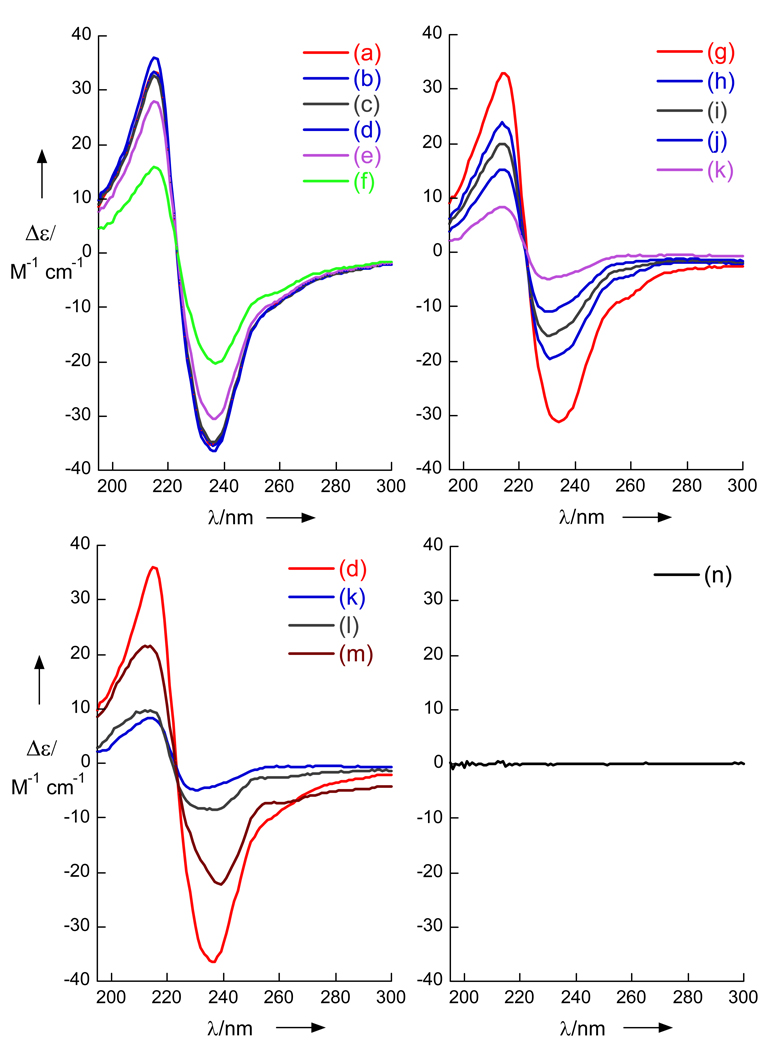
Figure 2.
Circular dichroism (CD) spectra of naphthamides (c= 0.23 mM, T = 23 °C). (a) Liposomal CD (LCD) of R-(+)-3, and (b) S-(−)-3, DSPC c= 2 mg/mL. (c) CD of (S)-3 in MeOH. (d) LCD spectra of synthetic (S)-8 (>99% ee). (e) (±)-8 and (f) LCD of (R)-8 from derived from 1. See Supporting Information for preparation of liposomes
The antipodal CD curves suggested that the remote methyl branch induces asymmetric perturbation of the Np chromophore as a consequence of liposomal ordering of the chains, not as a result of diastereomeric interactions with chiral polar head groups of DSPC. Consequently, LCD appeared be attractive for interrogation of remote stereocenters in acyclic natural products.
With a method for CD amplification in hand, we turned our attention to plakinic acids I (1) and J (2), two new ω-phenyl polyketide peroxides isolated from Plakortis halichondroides collected in the Bahamas. Compounds 1 and 2 are related to plakortin (4),[4a] also from P. halichondriodes with sub-micromolar activity against the malaria parasite Plasmodium falciparum,[4b] and the cytotoxic plakinic and epiplakinic acids.[4c] Peroxides 1 and 2 showed differential inhibition of paired haplodeficient lag1Δ/LAG1 strains of S. cerevisae,[5] suggesting interdiction of the yeast phosphoinositide pathway.
The absolute configurations of stereocenters around the 1,2-dioxane ring of 1 and the 1,2-dioxalane ring of 2 were solved conventionally by integrated 1H NMR analysis including NOESY spectra and, for 1, the Mosher's ester[6] of a secondary alcohol obtained by hydrogenolysis (Pd-C, H2) of 1 (for full characterization, see Supporting information).
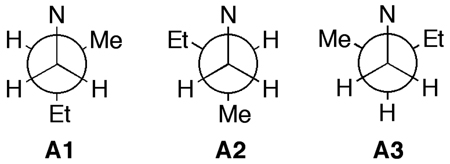
The methyl branched center C-8 is effectively insulated from the rest of the molecule by the C-6 quaternary center. Force field calculations of the staggered conformers around C-6–C-7 show they are equally populated.[7] Lack of conformational constraints between C-6 and C-8 compromises assignments of the C-8 configuration based 2,3JCH and NOE’s, but LCD analysis bypassed this limitation as follows.
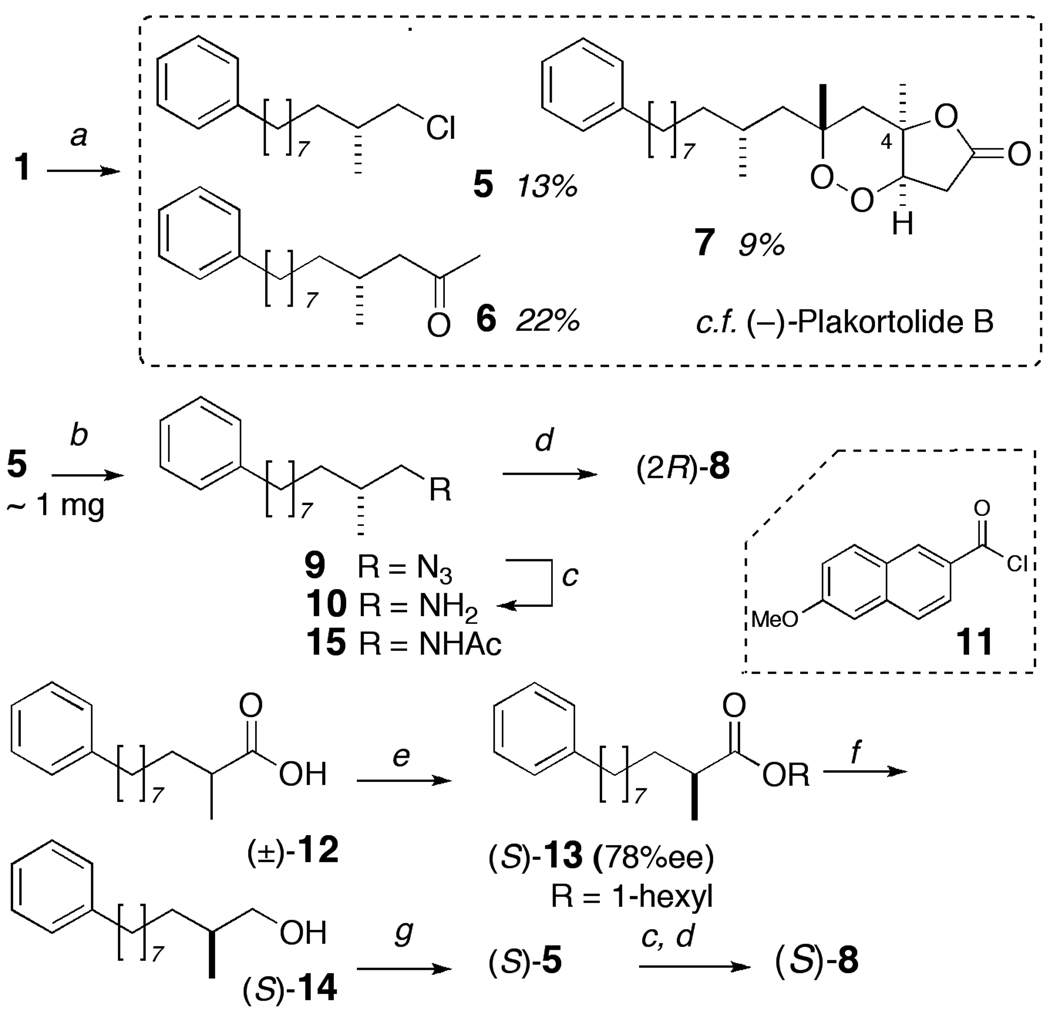
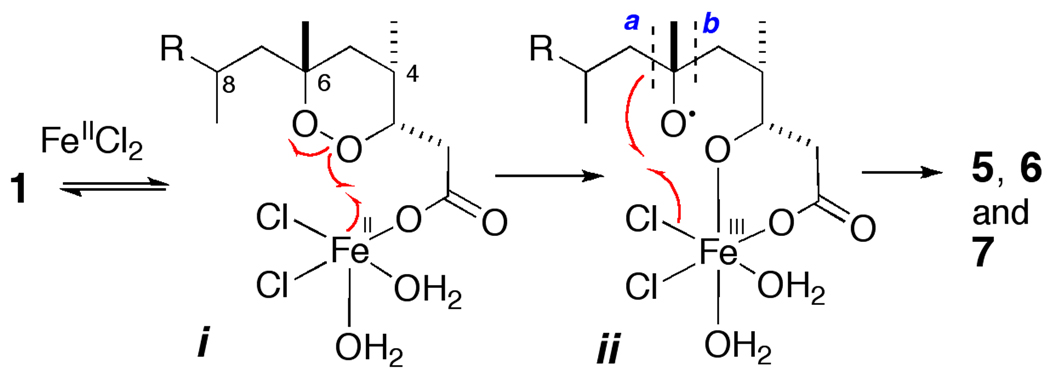
In order to segregate the C-8 stereocenter, we first cleaved the C-6–C-7 bond using a ligand-directed Fe(II) promoted fragmentation of 1 to give three products (Scheme 1); 5 (13% yield), 6 (22%) and a (−)-7 (9%).[8] The formation of 5 is rationalized in Figure 1. Carboxylato-Fe(II) species i promotes homolytic reduction of the O-O bond by single electron transfer and the incipient tert-alkoxy radical ii collapses by β-scission along two paths, a and b. Compound 5 is formed by a 'chloro-Fenton' reaction[9] in which cleavage of the C–C bond along path a, is followed by rebound and abstraction of Cl at the Fe center. Ketone 6 arises from the alternate β-fragmentation path b while (−)-7 is formed from a different radical reaction.[10] The relative configuration of (−)-7 was secured from NOESY experiments.
Scheme 1.
Degradation of 1 synthesis of authentic standards. Reagents and Conditions: a) FeCl2, CH3CN/H2O (degassed), rt, 45 min; b) NaN3, DMF, 100 °C; c) H2, Pd-C (hexane/EtOH); d) 11, Et3N, CH2Cl2; e) Candida rugosa lipase, 1-hexanol, cyclohexane, 50 h; f) LiAlH4, Et2O, rt; g) PPh3, CCl4.
Figure 1.
Proposed mechanism of the intramolecular ‘chloro-Fenton’ reaction[9] of peroxide 1 with FeIICl2 in CH3CN aq to give 5–7. For clarity, an axial H2O ligand has been removed from Fe in i.
Alkyl chloride 5 (~1 mg) was transformed by a three-step sequence: SN2 displacement of the chloride by NaN3 to give 9, which was hydrogenolysed to primary amine 10 that was N-acylated with 6-methoxy-2-naphthoyl chloride (11) to give 8 (purified by HPLC, ~140 µg,). Standard (S)-8 was prepared as follows. Kinetic resolution of racemic 2–methy-10-phenyldecanoic acid ((±)-12) by esterification with 1-hexanol in the presence of Candida rugosa lipase[12] gave the (S)-n-hexyl ester 13 (78%ee), which was reduced (LAH) to the corresponding alcohol (S)-14 and sequentially transformed into (S)-5 (PPh3, CCl4) and, finally (S)-8 as described above. Optically pure naphthamides, (S)- and (R)-8 (>99% ee) were also prepared from (±)-11 via enantiopure amines (S)- and (R)-10 using a modification of a method described earlier.[3]
The CD spectra of 8, derived from either 1 or 2, and standard (S)-8 are shown in Figure 2. Whereas, CD spectra of (S)-8 and (±)-8 measured in MeOH (see Supporting Information), or (±)-8 in DSPC liposomes gave only baseline, the CD spectra of natural product-derived 8 and synthetic (S)-8 in DSPC liposomes gave strong bi-signate Cotton effects (λ213 nm, Δε +20; 232, −36, peak-to-trough, A = 56) of essentially equal magnitudes but opposite signs. Note, 10 and 5 have no significant dichroism in isotropic media and very weak rotations (e.g. synthetic (S)-5, [α]D −1.3 (c 10.2, hexane). Therefore, the complete configurations of 1 and 2 are 3S,4S,6R,8R and 3R,5R,7R respectively.[13]
The liposomes used in these LCD experiments were very stable at room temperature; the Cotton effects of freshly prepared DSPC liposomes of (S)-8 were evident within 20 minutes of sample preparation and unchanged after 44 days at room temperature. In order to better understand the origin of LCD signals, the temperature dependence of LCD was examined (Figure 3) by measuring the CD spectra of liposomal preparations of (S)-8 at T = 4–90 °C which spans the gel phase transition temperature of DSPC liposomes (Tc = 54.5 °C).[14] The LCD spectra was largely unchanged from 4 °C to 40 °C, but above 40 °C significant decreases in the magnitude of the CE's were observed. At 90 °C, the CE's had diminished in magntitude (λ 213 nm, Δε+8.32; (λ 232, Δε −4.72) to less than 10% of their values at 23 °C (Figure 3b). The LCD spectrum of (S)-8 was largely restored upon cooling the sample to room temperature (23 °C, Figure 3c). These results are consistent with a reversible transition from a gel phase to a liquid phase in the liposome bilayer and attendant disruption of liposomal ordering of the embedded naphthamide methyl-branched alkyl chain of (S)-8.
Figure 3.
Liposomal circular dichroism (LCD) spectra of (S)-8 (a) t = 4 °C. (b) 10 °C (c) 20 °C (d) 23 °C (e) 30 °C (f) 30 °C (g) 40 °C. (h) 50 °C (i) 60 °C (j) 70 °C (k) 80 °C. (l) 90 °C. CD with annealing: (l) sample (k), cooled to 23 ° over 30 min (m) 23 °C, after 14h. (n) (S)-15 (78% ee).
Neither the chiral head-groups of DSPC or the terminal phenyl group appear to be strongly involved in the observed CE's in LCD measurements of 3 and 8, however the presence of the naphthamide was critically important. For example, the LCD spectrum of (S)-N-(2-methyl-10-phenyldecyl)acetamide 15, prepared by acetylation of (S)-10 (Ac2O, pyridine. See Supporting Information) was essentially a baseline, even after repeated sonication and annealing at 60 °C (Figure 3d). Similarly, the LCD spectrum of an N-6-methoxy-2-naphthamide of an achiral long-chain C14 amine (N-myristyl-6-methoxynaphthamide, See Supporting Information, S11) showed only baseline under the same conditions. For example, the LCD spectrum of (S)-N-(2-methyl-10-phenyldecyl)acetamide 15, prepared by acetylation of (S)-10 (Ac2O, pyridine. See Supporting Information) showed essentially a baseline spectrum, even after repeated sonication and annealing at 60 °C (Figure 3d). Similarly, the LCD spectrum of the-naphthamide of an achiral long-chain C14 amine (N-myristyl-6-methoxy-2-naphthamide, See Supporting Information, S11) showed only a baseline spectrum under the same conditions.
The origin of amplified Cotton effects in LCD is more complex than simple intramolecular perturbation of the chromophore in 3 and 8. Although it is clear that the LCD CE originates in asymmetric perturbation of the naphthoamide π-π* transitions by the remote stereogenic center bearing a β-methyl group, long-range intramolecular interactions are also operative.
The CE's arising from liposomal ordering of extended long-chains appear also to be modulated by intermolecular π-π interactions of naphthamide chromophores in higher-order J-aggregates within the bilayer. Evidence for delocalized (Frenkel) excitons[15] was most apparent in the LCD spectra of (+)-3 and (−)-3 which revealed weaker, red-shifted transitions (e.g. λ = 260, 290, 320 nm; Δε <±5). The simplest interpretation of the LCD would be that the major CE bands arise from 1,n pairwise exciton coupling of paired nearest-neighbor naphthamide groups (n = 2), held close by weak π-π interactions, however, quantitative analysis must await a more detailed photophysical description of LCD.
In conclusion, the Cotton effects induced by liposomal circular dichroism (LCD) of a single naphthamide chromophore – amplified by lipid ordering and second-order intermolecular interactions – were used to assign the C-8 configuration of plakinic acids I (1) and J (2). The method is sensitive; the limit of detection (LOD) for 8 is ~16 nmol and suitable for 'nanomole-scale' structure elucidation of natural products,[16] including other plakinic acids.[17]
The worked presented here demonstrates a specific case in application of LCD – utilization of liposomes to amplify the CD spectrum of an acyclic chiral long-chain naphthoamide for configurational assignment. LCD should find general utility in natural products chiroptical analysis of acyclic methyl-branched long-chain polyketides where Cotton effects appear weak or even below the limits of detection.
Experimental Section
Experimental details, complete characterization of all synthetic products and general procedures can be found in the Supporting Information.
The sponge Plaktortis halichondroides was collected from reef habitat in the Bahamas (lat 24° 25.163', long 75° 58.435', accession number, 07-26-171) at a depth of −27 m during the June 2007 cruise of the RV Seward Johnson. The sponge was identified by Micha Ilan (Department of Zoology, University of Tel Aviv), and frozen immediately until used.
Extraction and Isolation
Frozen sample of the sponge Plakortis halichondroides (07-26-171; 200 g) was extracted with MeOH:CH2Cl2 overnight at rt. (100 mL × 2), the combined extracts were filtered and concentrated under reduced pressure. The methanol extract was fractionated using sequential solvent-solvent partitioning with adjustment of the H2O content at each step: 0% v/v H2O, hexane (100 mL, Fraction A), 40% v/v H2O, CHCl3 (100 mL × 2, Fraction B). The MeOH was removed under reduced pressure and the aqueous residue extracted with n-BuOH (100 mL × 2, Fraction D). Fraction B (1.41 g) was subjected to silica flash chromatography (2 × 12.5 cm, 0 to 100% MeOH, stepwise 20% increment in CHCl3) to yield fractions #1–8. Fraction #2 (320 mg) was further purified by silica gel flash chromatography (Analogix, RS-4 cartridge, 4g 50 µm 60Å) using mixtures of hexane and ethyl acetate in increasing polarity (0–100%). Fractions were pooled according to their TLC profiles. Fraction 1 (153 mg) was purified by reversed phase HPLC (C18 Luna Phenomenex, 250 × 10 mm) under gradient conditions (70:30 CH3CN:H2O to 100% CH3CN, 3 mL/min, UV detection λ = 254 nm) to give pure plakinic acid I (1, 58 mg, 0.029 % wet weight) and plakinic acid J (2, 47 mg, 0.023%).
Plakinic acid I (1), colorless oil. [α]D24 −113 (c 4.37, CHCl3), UV (MeOH) λmax 260 nm (ε 286), 268 (200), FTIR (ATR, neat) ν 2921, 2854, 1712, 1452, 1374, 1291, 1026, 738, 691 cm−1. 1H and 13C NMR data (see Table S1, Supporting Information). HREIMS m/z 404.2928 [M]+, calcd. 404.2921 for C25H40O4.
Plakinic acid J (2). colorless oil; [α]D24 = −43.4 (c 4.42, CHCl3); UV (MeOH) λmax 261 nm (ε183), 261 (260); FTIR (ATR, neat) ν 2920, 2850, 1715, 1452, 1371, 1305, 1218, 743, 697 cm−1; 1H and 13C NMR (see Table S3, Supporting Information). HREIMS m/z 390.2773 [M]+, calcd. 390.2765 for C24H38O4.
FeIICl2-Promoted Fragmentation of 1 and 2
FeIICl2•4H2O (AR grade, purified by washing with 6 M HCl) was prepared as a stock solution (1 M) in degassed, distilled H2O. A solution of 1 (7.0 mg, 17.3 µmol) in CH3CN/H2O (8:2, 1.0 mL, de-aerated, N2 purge, 40 min) was treated with FeIICl2 stock solution (74 µL, 51.9 µmol) and stirred under an atmosphere of N2 for 30 min, before quenching with 4 drops of aqueous citric acid (1.0 M). The mixture was vortexed with hexane (4 volumes) for 1 min, and centrifuged to separate the organic layer. The aqueous layer was washed twice with hexane and the combined hexane layers were concentrated under reduced pressure. The residue was purified on a short pipet column (silica, 1:9, 2:8 and 3:7 EtOAc/hexanes) to give (R)-5 as a colorless oil (0.89 mg, 13%), followed by 6 (1.5 mg, 22%) and (−)-7 (0.59 mg, 9%). See Supporting Information for characterization of 5-7. Treatment of 2 under the same conditions also gave (R)-5 (19%), however no 5 was formed by treatment of the methyl ester of 2, with FeCl2.
Preparation of DPC Liposomes and Liposomal CD (LCD) Measurements
Liposomal naphthamides were prepared using a modification of the previously described method.[2] Briefly, a solution of 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC, 2 mg/mL in CHCl3) was added to a solution of naphthamide in CHCl3, contained in a 25 mL round bottom flask and the solution 'shell-evaporated' under reduced pressure using a rotatory evaporator. To the dried residue was added, HPLC grade H2O (2 mL) and the mixture was subjected to the following treatment: sonication for 2 min, heating (60 °C) and cooling (rt), repeated twice. Uniform liposomes were prepared from this mixture by repeated extrusion (×25) through a 100 nm polycarbonate membrane secured between two 0.5 mL gas tight syringes (Liposofast, Avestin, Toronto, Canada). CD measurements were carried out on the resulting clear preparations using the following parameters: T = 23 °C; sensitivity, 100 mdeg; scanning speed, 50 nm/min; wavelength, from 180 to 400 nm; N = 15 accumulations. The CD spectra were subtracted from the blank spectra measured on DSPC liposomes prepared without added naphthamide. Sample concentrations were determined from absorbance at λ 238 nm in MeOH and ε values. See Supporting information (Table S4) for tabulations of λ, Δε values for 3 and 8.
Footnotes
Financial support for this work was provided by the National Institutes of Health (CA1225601 and RO1 AI039987). The authors are grateful to E. Rogers and B. Morinaka for assistance with NMR spectra and MMFF calculations, A. Marcus (University of Oregon) for informative discussions and to J. Pawlik (University of North Carolina, Wilmington) and the crew of the RV Seward Johnson for logistics of sample collection. EI HRMS data were provided by Y. Su.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.(a) Nakanishi K, Berova N, Woody RW, editors. Circular Dichroism: Principles and Applications. New York: VCH; 1994. [Google Scholar]; (b) Ikemoto N, Lo L-C, Nakanishi K. Angew. Chem. Int. Ed. 1992;31:890–891. [Google Scholar]
- 2.(a) MacMillan JB, Molinski TF. J. Am. Chem. Soc. 2004;126:9944–9945. doi: 10.1021/ja047741a. [DOI] [PubMed] [Google Scholar]; (b) MacMillan JB, Linington RG, Andersen RJ, Molinski TF. Angew. Chem. Intl. Ed. 2004;43:5946–5951. doi: 10.1002/anie.200461158. [Angew. Chem. 2004, 116, 6072–6077] [DOI] [PubMed] [Google Scholar]
- 3.Nicholas GN, Molinski TF. Tetrahedron. 2000;56:2921–2927. [Google Scholar]
- 4.(a) Higgs MD, Faulkner DJ. J. Org. Chem. 1978;43:3454–3457. [Google Scholar]; (b) Fattorusso C, Campiani G, Catalanotti B, Persico M, Basilico N, Parapini S, Taramelli D, Campagnuolo DC, Fattorusso E, Romano A, Taglialatela-Scafati O. J. Med. Chem. 2006;49:7088–7094. doi: 10.1021/jm060899g. [DOI] [PubMed] [Google Scholar]; (c) Davidson BS. J. Org. Chem. 1991;56:6722–6724. [Google Scholar]
- 5.Giaever G, Shoemaker DD, Jones TW, Liang H, Winzeler EA, Astromoff A, Davis RW. Nature Genet. 1999;21:278–283. doi: 10.1038/6791. [DOI] [PubMed] [Google Scholar]
- 6.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J. Am. Chem. Soc. 1991;113:4092–4096. [Google Scholar]
- 7.Force field calculation of the relative energies of the three gauche conformers for a simple model of 1 (MMFF94, Spartan 04) show they differ by less than 0.3 kcal.mol−1 (E = 29.5, 29.6 and 29.8 kcal.mol−1). Similarly, minimized C1–C2 gauche conformers of 6-methoxy-N-(2-methylbutyl)-2- naphthamide (c.f. 3) have similar energies: A1 (12.8 kcal.mol−1), A2 (12.0) A3 (12.1).
- 8.Compound 7 is diastereomeric with plakortolide B, from Plakinastrella onkodes Horton PA, Longley RE, Kelly-Borges M, McConnell OJ, Ballas LM. J. Nat. Prod. 1994;57:1374–1381. doi: 10.1021/np50112a006.
- 9.(a) Sawyer DT, Hage JP, Sobkowiak A. J. Am. Chem. Soc. 1995;117:106–109. [Google Scholar]; (b) Fattorusso E. CMDD Symposium; Seoul. 2007. Nov. 1–4, [Google Scholar]
- 10.Compound 2 also gave 5 under the same conditions. Evidence for ligand-directed free radical cleavage is found in treatment of the methyl ester of 2 with FeIICl2 which fails to give 5. The formation of the bicyclic peroxy-γ-lactone (−)-7 from 1 probably proceeds by a different radical pathway involving a complex of Fe(II) ligated to two molecules of 1. FeII-promoted scission of the O-O bond at one ligand 1 is followed by intra-molecular H-abstraction from C4 by the alkoxy radical from the second ligand. Rebound of the C-centered radical to carboxylato ligand gives (−)-7. Interestingly, C4 in (−)-7 is inverted with respect to 1. Since compound (−)-(7) is similar to plakortolides B (ref. 8) and G (ref. 13), formation of (−)-7 from 1 suggests a biomimetic transformation relevant to plakortolide biogenesis.
- 11.All new compounds were fully characterized by HRMS, FTIR, 1H & 13C NMR. See Supporting Information. Acid (±)-12 was prepared by a malonic acid synthesis as follows. Diethyl 2-methylmalonate was alkylated with (8-bromooct-1-ynyl)benzene (NaOEt) followed by hydrogenation (H2, Pd-C) saponificaiton (NaOH, H2O-EtOH) and decarboxylation (100 °C, H2SO4 aq). See Supporting Information.
- 12. Berglund P, Hölmquist M, Hedenström E, Hult K, Högberg H-E. Tetrahedron: Asymmetry. 1993;4:1869–1878. Assignment of the 2S configuration of the enriched ester follows from the known enantioselectivity of Candida rugosa lipase Type VII (Sigma-Aldrich). %ee was measured, after reduction (LAH) to the alcohol (−)-S-14, by 1H NMR integration of the corresponding (+)- and (±)- Mosher’s esters.
- 13.(+)-Plakortolide G, a peroxylactone similar to (−)-7, was assigned 2S,4S,6R,8S configuration – opposite at C-8 – by ab initio Hartree-Fock calculations of molar rotations. Perry TL, Dickerson A, Khan AA, Kondru RK, Beratan DN, Wipf P, Kelly M, Hamann MT. Tetrahedron. 2001;57:1483–1487. The absolute configuration of the 1,2-dioxolane ring in 2 was assigned by comparison of the [α]D with those of synthetic 'plakinates' Dai P, Trullinger TK, Liu X, Dussault PH. J. Org. Chem. 2005;71:2283–2292. doi: 10.1021/jo0522254.
- 14.Zein M, Winter W. Phys. Chem. Chem. Phys. 2000;20:4545–4551. [Google Scholar]
- 15.Fidder H, Knoester J, Wiersma DA. J. Chem. Phys. 1993;98:6564–6566. [Google Scholar]
- 16.Molinski TF. Curr. Opin. Drug Disc. Develop. 2009;12:197–206. [PubMed] [Google Scholar]
- 17.Sandler JS, Colin PL, Hooper JNA, Faulkner DJ. J. Nat. Prod. 2002;65:1258–1261. doi: 10.1021/np020228v. [DOI] [PubMed] [Google Scholar]