SUMMARY
Objective
To evaluate the effect of maintenance Interpersonal Psychotherapy (IPT) on recurrence rates and time to recurrence of major depression in elderly patients with varying levels of cognitive function.
Methods/Design
Two-year maintenance study of monthly maintenance IPT vs supportive clinical management (CM) in remitted depressed elderly who were participants in a previously reported placebo-controlled study of maintenance paroxetine and IPT (Reynolds et al., 2006). We used Cox regression analysis to test interactions between cognitive status (Dementia Rating Scale score) and treatment (IPT, CM) with respect to recurrence of major depression.
Results
We observed a significant interaction between cognitive status and treatment: lower cognitive performance was associated with longer time to recurrence in IPT than in CM (58 weeks vs 17 weeks) (HR = 1.41 [95% CI=1.04, 1.91], p = 0.03). Subjects with average cognitive performance showed no effect of maintenance IPT vs CM on time to recurrence (38 vs 32 weeks, respectively).
Conclusion
Monthly maintenance IPT confers protection against recurrence of major depression in elders with lower cognitive functioning.
Keywords: late-life depression, cognitive functioning, interpersonal psychotherapy, recurrence prevention
INTRODUCTION
Interpersonal Psychotherapy (IPT) (Klerman et al., 1984) has demonstrated efficacy as a maintenance treatment for late-life depression (LLD) in cognitively normal ‘young old’ patients (Reynolds et al., 1999). Depressed elders, however, have a high prevalence of cognitive impairment both during depressive episodes and after their symptoms have remitted (Bhalla et al., 2006; Steffens et al., 2006). Psychotherapy, whether as monotherapy or in combination with medication, is important for those who cannot or will not take antidepressant medication. However, we have recently reported that maintenance IPT lacks efficacy, used as either monotherapy or in combination with paroxetine, in maintaining wellness in depressed elders 70 years of age and older (Reynolds et al., 2006). We also found in the same study no evidence of a moderating effect for cognition, anxiety, or sleep when examining data from all four maintenance cells. A review of the literature revealed no research specifically examining the use of maintenance IPT for depressed older adults with depression and comorbid cognitive dysfunction. In this paper, we further explore whether level of cognitive performance could influence the strength of any maintenance IPT effects on recurrence. We hypothesized that cognitive functioning would be associated with diminished efficacy of maintenance IPT, reasoning that impairments in attention, memory, of or abstraction could lessen ability to engage in IPT.
METHODS
This analysis was conducted on a subgroup of older depressed participants enrolled in a randomized placebo-controlled clinical trial comparing the efficacy of maintenance paroxetine, monthly IPT, and their combination as a maintenance therapy for LLD (Reynolds et al., 2006). This analysis is limited to data from the subgroup of participants not receiving maintenance paroxetine (but on pill placebo) in order to examine the effects of monthly maintenance IPT (versus supportive clinical management) in the context of varying levels of cognitive function. This study took place in a university geropsychiatry clinic specializing in late-life depression. The 52 participants whose data were analyzed for this report (17 men and 35 women ≥ 70 years of age) met SCID/DSM-IV requirements for current major depression and had scores of ≥ 15 on the 17-item Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960) and ≥ 17 on the Mini-Mental State Examination (Folstein et al., 1975). Written informed consent was obtained after a complete description of the study was given to subjects and caregivers.
In the acute phase of the study, the entire study group (n = 195) received open treatment with paroxetine (10–40ml/day) and weekly IPT sessions. Subjects who achieved HRSD-17 scores of ≤ 10 for three consecutive weeks entered into a 16-week continuation phase (n = 116) in which paroxetine was continued and IPT sessions were reduced to bimonthly. Patients who remained stable throughout continuation treatment were eligible to enter maintenance treatment, in which they were randomly assigned to paroxetine and monthly maintenance IPT, paroxetine and clinical management (CM), monthly maintenance IPT and placebo, or CM and placebo.
During maintenance treatment subjects were seen monthly by the same clinician as during acute and continuation treatment in order to avoid an effect due to withdrawal of therapist. Clinicians conducted both IPT and supportive clinical management (CM) sessions, depending upon randomized treatment assignment. They were blinded as to whether participants were receiving pill placebo or paroxetine (in the parent study). Sessions were audiotaped and evaluated for treatment fidelity by an independent rater blind to treatment assignment. IPT sessions lasted 45 min while CM sessions lasted 30 min. CM sessions contained no specific psychotherapy; instead, patients were encouraged to report any symptoms and adverse (side) effects. Subjects remained in the maintenance phase for 2 years or until a recurrence of a SCID-defined major depressive episode, whichever occurred first. As noted in the report of the parent study (Reynolds et al., 2006), the four maintenance treatment groups did not differ in sociodemographic, and clinical measures, Mattis scores, or extent of coexisting medical burden.
The Dementia Rating Scale (DRS; Mattis, 1988), which was used as the primary cognitive measure, is an extended screening instrument designed to assess cognitive functioning across five separate cognitive domains (Attention, Conceptualization, Construction, Initiation/Perseveration, and Memory) in dementia. The test consists of 36 items including: repeating digit strings, following one and to-step commands, counting target letters embedding in a random array of letters, generating abstract concepts common to series of two items presented verbally and three items presented visually, copying designs, name writing, naming supermarket items, repeating series of rhymes, performing double-alternating hand movements, copying rows of alternating symbols, answering orientation items, delayed recall of two sentences and recognition memory for series of word pairs and design pairs. The study used DRS age- and education-corrected Total Scaled Scores which have a mean of 10 and Standard Deviation of 3. We defined cognitive impairment as a DRS Total Scaled Score ≤ 7. (As shown in Table 1, the two groups (IPT, CM) did not differ in total or domain scores on the Mattis DRS, either raw or scaled). A cut-off scaled score of 7 is one standard deviation below the mean, which translates into the 17th percentile of subjects from the norm group of similar age and with similar levels of education.
Table 1.
Mattis Dementia Rating Scale Scores
| IPT (n = 35) | CM (n = 18) | |
|---|---|---|
| Mattis DRS total | 133.2 (10.2) [93,144] | 135.6 (5.2) [122,142] |
| Mattis DRS scaled score | 9.7 (3.5) [1,16] | 9.6 (2.4) [4,15] |
| Domain scaled score Attention | 10.5 (2.3) | 11.1 (1.8) |
| Conceptualization | 10.3 (2.4) | 9.6 (2.3) |
| Construction | 9.1 (1.9) | 9.6 (1.0) |
| Initiation | 9.9 (2.8) | 10.5 (2.5) |
| Memory | 9.0 (3.4) | 9.5 (2.9) |
Time to recurrence was examined with a Cox proportional hazard model using treatment (IPT/CM) and scaled DRS score at randomization as covariates. We specifically limited the analysis to the IPT + placebo and CM + placebo groups. A significant interaction would indicate the moderating effect of cognition on treatment. To illustrate the survival curve of the model, trajectory curves for each treatment at two levels of cognition were generated, using scores of 10 and 7 (1 SD below the mean; Jurica et al., 2001) for normal and impaired cognition, respectively. Actuarial recurrence rates were calculated for subjects grouped by level of cognition. The Breslow-Day test for homogeneity of the odds ratio was used to compare the rates of recurrence across cognitive level.
RESULTS
The Cox model showed a significant interaction between cognitive performance and treatment on time to recurrence, HR = 1.41 (1.04, 1.91) p = 0.03 (Figure 1). Thus, the trajectory for impaired subjects in clinical management (CM) was characterized by shorter median time to recurrence compared to the trajectory for impaired subjects randomized to IPT (17 weeks vs 58 weeks), while for average cognitive performance the median time was similar for IPT and CM (38 vs 32 weeks, respectively). Actuarial recurrence rates, with 95% Confidence Intervals, are shown in Table 2. The Breslow-Day test was significant X2 = 4.54, df = 1 p = 0.033, indicating that the odds ratio for treatment effect on rates of recurrence differed by level of cognition. Similar outcomes were obtained using raw Mattis scores as were obtained with age- and education-adjusted scores. Elsewhere we have reported that close to half of elderly depressed people treated to remission have mild cognitive impairment, both amnestic and multiple cognitive domain subtypes (Bhalla et al., under review).
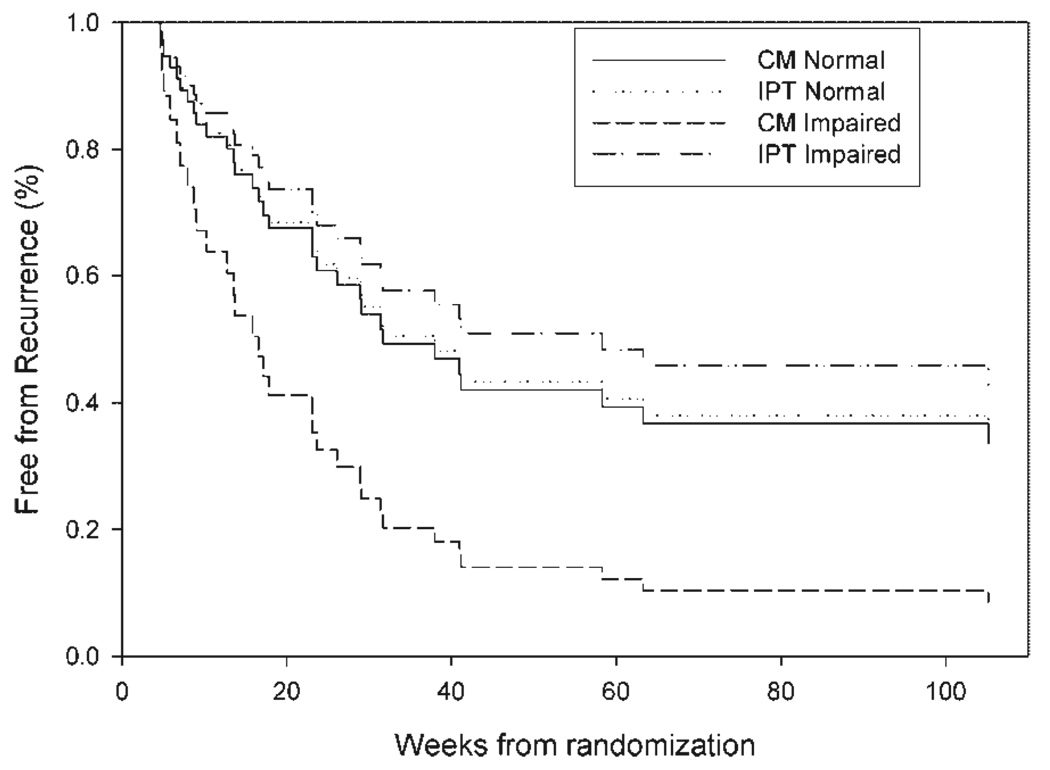
Figure 1.
A significant interaction between level of cognitive functioning and treatment on time to recurrence was present (HR = 1.41, 95% CI = 1.04, 1,91; p = 0.03) in the Cox regression. The simulated curves represent predicted survival at two levels of cognition functioning (normal: scaled DRS of ≥ 8; and impaired: scaled DRS of 7) with and without monthly maintenance IPT. The curve for monthly maintenance IPT in cognitive impairment had longer (median) time to recurrence than supportive clinical management (58 weeks vs 17 weeks). By contrast median time to recurrence was similar in those with average cognitive funtioning for IPT or CM (38 vs 32 weeks, respectively).
Table 2.
Recurrence rates (95% Confidence Intervals)
| IPT (n = 35) | CM (n = 17) | |
|---|---|---|
| Higher cognitive functioning: scaled score of 8 or better | 19/29 (66%) [48,83] | 7/14 (50%) [24,76] |
| Lower cognitive functioning: scaled score of 7 or less | 2/6 (33%) [0,71] | 3/3 (100%) [88,100] |
DISCUSSION
An unexpected finding emerged from this analysis: the subgroup of cognitively impaired depressed elders who received monthly maintenance IPT fared better than those receiving supportive clinical management (CM). In cognitively normal subjects, we did not detect a differential benefit for IPT over CM. The finding is surprising because we expected subjects who had cognitive impairment to be less able to utilize psychotherapy, for several reasons, including diminished attention during sessions, impaired memory across sessions and abstraction, which could affect insight. In fact, these subjects were able to remain free of a recurrence longer that those in CM. Our observation suggests that, despite mild to moderate cognitive impairment, depressed subjects can use IPT to negotiate interpersonal relationships more effectively, relationships that are often strained when caregivers of depressed individuals begin to see themselves as caregivers for someone with declining cognitive abilities. Because such tensions may be less salient in the absence of cognitive impairment, there may be less potential for benefit from IPT, which specifically deals with role or interpersonal conflict. We have reported elsewhere that maintenance effects of IPT, in preventing recurrence, are evident in patients with interpersonal or role conflict more than in bereavement or role transitions (Miller et al., 2003). Further exploration of the merits of working with cognitively impaired depressed elderly and with concerned family members in a more systematized fashion is the focus of subsequent modifications of IPT known as IPT-CI (Cognitive Impairment) (Miller et al., 2007; Miller and Reynolds, 2007).
In summary we have evidence that maintenance IPT protects cognitively impaired, remitted depressed elderly from recurrence of major depression, as compared with supportive clinical management. We speculate that IPT’s efficacy in the context may be related to ameliorating social role and interpersonal conflicts related to increasing care-giver burden.
ACKNOWLEDGEMENTS
Supported in part by NIMH grants P30 MH071944; R37 MH43832; the University of Pittsburgh Medical Center Endowment for Geriatric Psychiatry; and the John A. Hartford Center of Excellence in Geriatric Psychiatry.
Clinical Trial #NCT00178100.
GlaxoSmithKline provided paroxetine supplies for the conduct of the parent trial (MTLD-II; Reynolds et al., 2006).
The study was approved by the University of Pittsburgh Institutional Review Board.
Footnotes
CONFLICT OF INTEREST
None known.
REFERENCES
- Bhalla RK, Butters MA, Becker JT, et al. Patterns of MCI following treatment of depression in the elderly. Am J Geriatr Psychiatry. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SW, McHugh PR. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A Rating Scale For Depression. J. Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2: Professional Manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Klerman GL, Weissman MM, Rounsaville BJ, Chevron E. Interpersonal Psychotherapy Of Depression. New York: Academic Press, Basic Books Inc.; 1984. [Google Scholar]
- Mattis S. Dementia Rating Scale (DRS) Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Miller MD, Frank E, Cornes C, et al. The value of maintenance interpersonal psychotherapy (IPT) in older adults with different IPT foci. Am J Geriatr Psychiatry. 2003;11:97–102. [PubMed] [Google Scholar]
- Miller MD, Reynolds CF. Expanding the usefulness of interpersonal psychotherapy (IPT) for depressed elders with comorbid cognitive impairment. Int J Geriatr Psychiatry. 2007;22:101–105. doi: 10.1002/gps.1699. [DOI] [PubMed] [Google Scholar]
- Miller MD, Richards V, Zuckoff A, et al. A model for modifying interpersonal psychotherapy (IPT) for depressed elders with cognitive impairment. Clinical Gerontologist. 2007;30:79–101. [Google Scholar]
- Reynolds CF, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N. Engl J Med. 2006;354:1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA. 1999;281:39–45. doi: 10.1001/jama.281.1.39. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Otey E, Alexopoulos GS, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006;63:130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]