Abstract
Defective apoptosis contributes to tumorigenesis, although the critical molecular targets remain to be fully characterized. PUMA, a BH3-only protein essential for p53-dependent apoptosis, has been shown to suppress lymphomagenesis. In this study, we investigated the role of PUMA in intestinal tumorigenesis using two animal models. In the azoxymethane (AOM)/dextran sulfate sodium salt model, PUMA deficiency increased the multiplicity and size of colon tumors but reduced the frequency of β-catenin hotspot mutations. The absence of PUMA led to a significantly elevated incidence of precursor lesions induced by AOM. AOM was found to induce p53-dependent PUMA expression and PUMA-dependent apoptosis in the colonic crypts and stem cell compartment. Furthermore, PUMA deficiency significantly enhanced the formation of spontaneous macroadenomas and microadenomas in the distal small intestine and colon of APCMin/+ mice. These results show an essential role of PUMA-mediated apoptosis in suppressing intestinal tumorigenesis in mice.
Introduction
Colon carcinogenesis is a multistep process involving the accumulation of a series of genetic and epigenetic alterations in normal colonic epithelial cells (1). Some of the most frequent changes found in colon cancer include mutations in the tumor suppressors APC (adenomatous polyposis coli) and p53, and those in the oncogenes β-catenin, K-ras, BRAF, and PI3K, many of which are capable of suppressing apoptosis (1). Overexpression of the antiapoptotic proteins such as Bcl-2, Bcl-xL, and survivin are also common in cancer (2). Apoptosis is a major mechanism regulating the turnover of intestinal epithelial cells, and is progressively inhibited in colon carcinogenesis (3, 4). Decreased apoptosis is a hallmark of cancer, leading to increased risk of clonal expansion of cells containing critical alterations (4), and subsequently driving other tumorigenic events, including extension of life span, accumulation of further genetic mutations, growth under stress conditions, and tumor angiogenesis (5). In vivo studies directly examining the role of apoptosis in colon tumorigenesis are still largely lacking.
We and others have shown that the Bcl-2 family protein PUMA (p53 up-regulated modulator of apoptosis) is a major mediator of p53-dependent apoptosis through the mitochondrial pathway in multiple cell and tissue types, including intestinal epithelial cells (6–10). PUMA expression is transcriptionally induced by apoptotic stimuli through both p53-dependent and p53-independent pathways (7, 11, 12). Several lines of evidence suggest the potential role of PUMA in tumor suppression. p53 deficiency abrogated the induction of PUMA by radiation and various chemotherapeutic drugs (7, 13, 14). PUMA shRNAs suppressed p53-dependent apoptosis, promoted oncogenic transformation of primary murine fibroblasts by E1A/ras, and dramatically accelerated Eμ-myc–induced lymphomagenesis (15, 16). Reduced expression of PUMA has been reported in subcutaneous melanomas and Burkitt lymphomas, and was correlated with worsened prognosis (16, 17). However, its role in solid tumors has not been firmly established.
In the current study, we investigated the role of PUMA in suppressing intestinal carcinogenesis using two mouse models, the carcinogen-induced tumor model [azoxymethane (AOM) and dextran sulfate sodium salt (DSS), (AOM/DSS)] and the APCMin/+ spontaneous tumor model. We found that PUMA deficiency enhances tumor formation in both models. p53-dependent PUMA induction is largely responsible for an acute apoptotic response induced by AOM at the bottom of colonic crypts where the colonic stem cells reside. PUMA deficiency increases the incidence of precursor lesion aberrant crypt foci (ACF) induced by AOM in the colon, or that of microadenomas in the small intestine of APCMin/+ mice. These data show that PUMA-mediated apoptosis suppresses tumor initiation in the gut.
Materials and Methods
Mice and Treatment
The procedures for all animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Detailed information on the generation of PUMA+/+, PUMA−/− (F6 on C57BL/6 background), APCMin/+/PUMA−/−, and p53−/− mice is described in Supplementary Materials and Methods.
AOM and DSS treatment
Six- to 10-wk-old littermates were injected i.p. with a single dose of AOM (Sigma; 12.5 mg/kg). After 7 d, 2.5% DSS (MP Biomedicals) was given in the drinking water, followed by 14 d of regular water. The cycle was repeated twice. Following the last cycle, mice were given regular water for 2 mo and sacrificed (Fig. S1). Some mice were injected i.p. with 100 mg/kg of bromodeoxyuridine (BrdUrd) 2 h prior to sacrifice to analyze crypt proliferation.
AOM treatment
Six- to 10-wk-old mice were injected i.p. with AOM (10 mg/kg) or saline (control) once a week for 6 wk. Animals were sacrificed 4 wk after the last AOM injection (18). To determine the levels of apoptosis induced by AOM, mice were injected i.p. with 15 mg/kg of AOM and sacrificed 8 h later. BrdUrd (100 mg/kg) was i.p. injected into the mice 2 h prior to euthanasia, or 2 h before AOM treatment in some experiments (72-h tracing experiments) to analyze crypt proliferation.
Total RNA Extraction and Real-time Reverse Transcriptase-PCR
Total RNA and cDNA were prepared from the freshly scraped intestinal mucosa as described (9). Real-time PCR was performed on a Mini Opticon Real-time PCR System (Bio-Rad) with SYBR Green (Invitrogen). The primers used for PUMA and β-actin have been described (9). Three mice were included in each group. Melting curve and agarose gel electrophoresis of the PCR products were used to verify the specificity of PCR amplification.
Western Blotting
Total protein extracts were purified and analyzed by NuPage gel (Invitrogen) electrophoresis as previously described (10). Primary antibodies included those for PUMA (Abcam) and β-actin (Sigma). Three mice were used in each group.
RNA In situ Hybridization
The protocol has been previously described (10). Digoxigenin-labeled PUMA and Lgr5 antisense RNA probes were generated by PCR amplification with incorporation of a T7 promoter (5′-CTAATACGACTCACTATAGGGAGA) into the antisense primer as previously reported (ref. 10; Table S1).
Tumor Histology
Macroadenomas (polyps; >0.5 mm) induced by AOM/DSS were scored under a stereomicroscope using fixed colons. Histology grading was done by a board-certified pathologist according to established criteria using 5 μm H&E sections (19). Macroadenomas in APCMin/+ mice on the AIN93G diet (Dyets, Inc.) were scored at 22 wk as described above. The microadenomas in APCMin/+ mice were scored at 5 wk using H&E sections of “Swiss-rolled” tissue under a light microscope (20). ACF were identified using methylene blue–stained colons (18). Details are described in the Supplementary Materials and Methods.
Immunostaining
Terminal nucleotidyl transferase–mediated nick end labeling (TUNEL) staining, BrdUrd, and Ki67 immunohistochemistry were performed on 5 μm sections as previously described (10). The TUNEL or BrdUrd-positive cells were scored in 100 crypt sections and reported as mean ± SE. Three mice were used in each group.
Statistical Analysis
Data were analyzed by ANOVA in which multiple comparisons were performed using the method of least significant difference. Data on the frequency of β-catenin mutations were analyzed by χ2 test. Differences were considered significant if the probability of the difference occurring by chance was less than 5 in 100 (P < 0.05).
Results
PUMA deficiency increased colon tumor formation in the AOM/DSS model
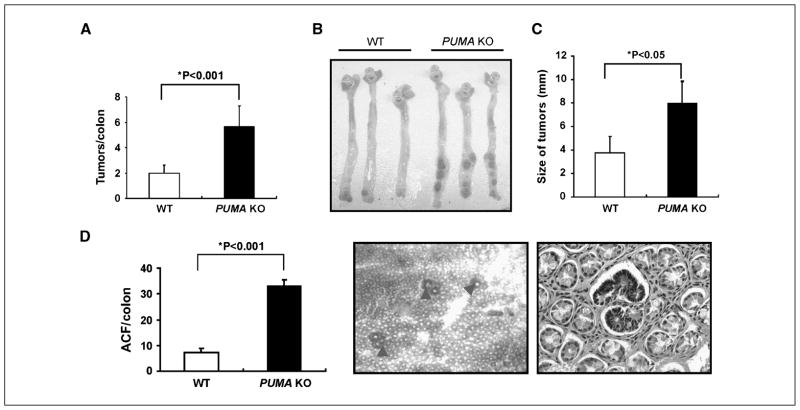
AOM and DSS are two commonly used colonic carcinogens in rodents. Both can induce tumors alone in the distal colon of rodents, although the incidence and multiplicity in some commonly used inbred mouse strains such as C57BL/6 are rather low even after an extended time (8–12 months; refs. 21, 22). Recently, DSS has been shown to promote AOM-induced colon cancer in C57BL/6 mice within 4 months (23, 24). To determine whether PUMA plays a role in colon carcinogenesis, we compared the tumor incidence, size, and grade in wild-type (WT) and PUMA-knockout (PUMA KO) littermates using a modified AOM/DSS model (Fig. S1A; ref. 24). All the mice in this protocol developed tumors in 16 weeks following the initial exposure to AOM (Fig. 1A; Fig. S1A). The tumor incidence in PUMA KO mice increased by 1.5-fold compared with WT mice (5.7 ± 1.6 versus 2.1 ± 1.1) with the majority of tumors located in the middle and distal colon (Fig. 1A and B). The size of tumors also significantly increased in PUMA KO mice compared with that in WT mice (Fig. 1C). Histopathologic evaluation indicated that the tumors developed in PUMA KO mice had higher grades (Table 1). For example, 91% of tumors in WT mice were adenomas with low-grade dysplasia, and none (0%) were adenocarcinomas (Table 1; Fig. S1B). In contrast, 86% of tumors in PUMA KO mice were adenomas with high-grade dysplasia, and nearly 10% were adenocarcinomas (Table 1; Fig. S1B).
Figure 1.
PUMA deficiency enhanced tumorigenesis induced by AOM/DSS in the mouse colon. A, quantification of colon tumor burden in WT and PUMA KO mice at 4 mo in the AOM/DSS model. Columns, mean; bars, SD (n = 12 in each group; *, P < 0.001). B, representative pictures of AOM and DSS-induced colon tumors in WT and PUMA KO mice. C, quantification of tumor size in WT and PUMA KO mice. Columns, mean; bars, SD (n = 12 in each group; *, P < 0.05). D, quantification of ACF in WT and PUMA KO mice at 10 wk after the initial AOM injection. Columns, mean; bars, SD (n = 4 in each group; *, P < 0.001). Arrowheads, examples of ACF visualized by methylene blue staining (left, magnification, ×40) and a typical ACF involving multiple crypts surrounded by normal crypts on an H&E section (right, magnification, ×400).
Table 1.
Histologic grades of AOM/DSS-induced colon tumors
| WT mice | PUMA KO mice |
|---|---|
| 3 LGD | 1 LGD, 1 HGD |
| 2 LGD | 3 HGD |
| 1 LGD | 3 HGD, 1 AC |
| 2 LGD | 4 HGD, 1 AC |
| 1 LGD | 1 LGD, 3 HGD |
| 1 LGD, 1 HGD | 3 HGD |
Abbreviations: LGD, adenoma with low-grade dysplasia; HGD, adenoma with high-grade dysplasia; AC, adenocarcinoma (n = 6 in each group).
We analyzed size-matched tumors from WT and PUMA KO mice for apoptosis and proliferation. Apoptosis was found to be reduced in the tumors from PUMA KO mice (Fig. S2A; data not shown). Reduction of apoptosis was even more pronounced in the center of larger tumors found in PUMA KO mice, suggesting resistance to nutrient deprivation and hypoxia (7, 25). Proliferation only increased slightly in the tumors from PUMA KO mice (Fig. S2B). These results suggest that PUMA suppresses AOM/DSS-induced colon tumorigenesis through apoptosis induction.
PUMA deficiency increased ACF formation induced by AOM
The elevated tumor incidence in PUMA KO mice suggests a role for PUMA in suppressing tumor initiation. Several studies suggested that ACF are likely to be precursor lesions and a risk factor for colon cancer in both human and rodents (26). We therefore analyzed the incidence of ACF induced by AOM in WT and PUMA KO mice. ACF incidence increased by ~4-fold in PUMA KO mice (33 ± 4.5 versus 7.3 ± 1.5; Fig. 1 D). These results indicate that PUMA potently suppresses tumor initiation induced by genotoxin.
PUMA deficiency reduced the frequency of β-catenin mutations in AOM/DSS-induced colon tumors
Mutations in β-catenin or APC lead to the stabilization of β-catenin and oncogenic transformation by interfering with the phosphorylation and degradation of β-catenin mediated by glycogen synthase kinase-3β (27). Correspondingly, frequent mutations at codons 33, 34, 37, and 41 of β-catenin, within the glycogen synthase kinase-3β phosphorylation motif, have been reported in AOM-induced colon tumors in rats and mice (21). We therefore sequenced exon 3 of β-catenin in the tumors from WT and PUMA KO mice using laser capture microdissected tumor tissues. We found that 44% (7 of 16) of tumors in WT mice had β-catenin mutations distributed at all four codons, whereas 12% (2 of 17) of those in PUMA KO mice had mutations in only codon 33 (Fig. S3; P < 0.05). No hotspot (codons 12 and 13) mutations in K-ras or p53 overexpression were found in the tumors from either group (Fig. S3; data not shown; ref. 28). We also analyzed β-catenin localization in the tumors that were negative for hotspot mutations by immunohistochemistry. Intense nuclear β-catenin staining was found in 2 out of 9 (~22%) tumors in WT mice, and 5 out of 15 (~33%) tumors in PUMA KO mice. These data suggest that preexisting apoptotic deficiency affects mutation frequency or spectrum in AOM/DSS-induced colon tumors.
AOM rapidly induced colonic PUMA expression
AOM is known to induce DNA damage (29). We therefore analyzed the expression of PUMA in the colonic mucosa of WT mice after AOM injection. Using quantitative reverse transcriptase-PCR, we found that PUMA mRNA was induced by 4-fold at 8 hours compared with untreated mice (Fig. 2A). PUMA protein levels were also substantially elevated in the mice after AOM treatment (Fig. 2B). RNA in situ hybridization was then used to identify the specific cell types in which PUMA expression was induced. PUMA mRNA was highly induced in the lower one-third of the crypts as early as 8 hours after AOM treatment, with the highest concentration at the bottom of the crypts (Fig. 2C). The basal levels of PUMA were undetectable (Fig. 2C). Recently, the orphan receptor Lgr5 was reported as a specific stem cell marker for the small intestine and colon (30). Using RNA in situ hybridization, we detected Lgr5 expression in the bottom of the crypts as reported (Fig. 2D; ref. 30), where robust PUMA induction was observed (Fig. 2C). These results indicate that AOM treatment rapidly induces PUMA in the colonic crypts and the stem cell compartment in particular.
Figure 2.
AOM treatment induced PUMA expression in the colonic crypts and stem cells. A, PUMA mRNA expression in the colonic mucosa of WT mice at 8 h following injection with either saline (Un) or 15 mg/kg of AOM was analyzed by quantitative real-time reverse transcriptase-PCR. Columns, mean; bars, SD (n = 3 in each group; *, P < 0.001). B, PUMA protein expression in the colonic mucosa of WT mice was determined by Western blotting 8 h after AOM injection. β-Actin was used as the control for loading (n = 3 mice in each group). C, PUMA mRNA (red) in situ hybridization in the colon of WT mice following indicated treatment (magnification, ×600). Right, an enlarged view of the selected area. D, Lgr5 mRNA (red) in situ hybridization in the colon of WT mice without any treatment (magnification, ×600).
PUMA deficiency abrogated apoptosis induced by AOM in the colonic crypts
To determine whether PUMA mediates AOM-induced apoptosis (31), we compared apoptosis in the colonic crypts of WT and PUMA KO mice (Fig. 3A). Apoptosis in the crypts markedly increased at 8 hours after AOM treatment in WT mice, but this increase was blocked by >90% in PUMA KO mice (Fig. 3B). In contrast, apoptosis in the submucosa was present at similarly low levels in both groups after AOM treatment (Fig. 3B). The basal level of apoptosis in these two groups of mice was negligible (<0.02 cells/crypt) and was not significantly different. Interestingly, AOM-induced apoptosis occurred primarily at the bottom of crypts where Lgr5-positive cells are located, resembling the patterns of PUMA induction (Figs. 2C, D and 3B). Active caspase-3 staining also confirmed the location of apoptotic cells (Fig. 3C). PUMA deficiency did not affect crypt proliferation measured by BrdUrd (Fig. S4) or Ki67 staining (data not shown) either at 8 or 72 hours after AOM treatment. These results show that PUMA is a major mediator of AOM-induced apoptosis in the colonic crypts and stem cell compartment.
Figure 3.
PUMA mediated AOM-induced apoptosis in the colonic crypts and stem cells. The colon tissue was harvested 8 h after 15 mg/kg of AOM injection. A, apoptotic index in the colonic crypts was measured by counting 100 crypts/mouse following TUNEL staining. Columns, mean; bars, SD (n = 3 in each group; *, P < 0.0001). B, examples of TUNEL staining (brown) in the colon of WT and PUMA KO mice (magnification, ×600). C, active caspase 3 immunohistochemistry (brown) in the colon of WT and PUMA KO mice (magnification, ×400).
PUMA mediated p53-dependent apoptosis induced by AOM in the colonic crypts
Induction of apoptosis is an essential function of p53 as a tumor suppressor in response to DNA damage. Although a large number of proapoptotic genes can be regulated by p53, only a few have been shown to be required for p53-dependent apoptosis (32). To determine whether p53 is required for PUMA induction by AOM, we compared PUMA expression in WT and p53-knockout (p53 KO) mice at 8 hours after AOM treatment. The induction of PUMA mRNA and protein was completely abrogated in p53 KO mice, which was most evident at the bottom of the crypts (Fig. 4A and B). Apoptosis in the crypts was reduced by >90% in p53 KO mice, a level similar to that observed in PUMA KO mice (Figs. 3 and 4C, D). These results suggest that p53-dependent PUMA induction is primarily responsible for AOM-induced apoptosis in the colonic crypts and stem cell compartment.
Figure 4.
PUMA mediated p53-dependent apoptosis induced by AOM in the colonic crypts. A, PUMA mRNA expression in the colonic mucosa was evaluated by quantitative real-time reverse transcriptase-PCR in WT and p53 KO mice at the indicated time points after AOM treatment. Columns, mean; bars, SD (n = 3 in each group; *, P < 0.01). Right, PUMA protein expression in the colonic mucosa was determined by Western blotting at the indicated time points after AOM treatment. β-Actin was used as the control for loading. B, PUMA mRNA (red) in situ hybridization in the colon of WT and p53 KO mice 8 h after AOM treatment (magnification, ×600). C, apoptotic index was calculated by counting 100 crypts following TUNEL staining. Columns, mean; bars, SD (n = 3 in each group; *, P < 0.001). D, representative pictures of TUNEL staining in WT and p53 KO mice 8 h after AOM treatment (magnification, ×400).
PUMA deficiency increased tumor formation in APCMin/+ mice
APCMin/+ mice develop a large number of intestinal adenomas on a C57BL/6 background, mostly in the distal small intestine (33). To examine the potential role of PUMA in spontaneous intestinal tumorigenesis, we generated APCMin/+/PUMA KO mice. The sex- and age-matched cohorts of APCMin/+ and APCMin/+/PUMA KO mice were analyzed for tumor incidence at the age of 5 months on a high-fat diet (AIN93G). The number of macroadenomas (polyps ≥ 0.5 mm in diameter) in the colon of APCMin/+/PUMA KO mice (4.2 ± 1.0) was significantly elevated compared with that in APCMin/+ mice (2.0 ± 0.7; Fig. 5A and Fig. S5). A smaller, yet statistically significant increase (64.4 ± 4.0 versus 47.6 ± 6.3) in the overall number of macroadenomas in the small intestine of APCMin/+/PUMA KO mice was also found (Fig. 5B). Further anatomic stratification revealed that macroadenomas in the distal small intestine significantly increased in APCMin/+/PUMA KO mice (43.3 ± 5.0) compared with APCMin/+ mice (24.6 ± 4.3), whereas those in the proximal and middle small intestine did not (Fig. 5C). No significant difference in the size of polyps between these two groups was found either in the small intestine (Fig. 5C) or colon (data not shown). Consistent with the role of PUMA-mediated apoptosis in suppressing tumor initiation, microadenomas (20) in the small intestine of APCMin/+/PUMA KO mice at an age of 4 to 5 weeks increased compared with APCMin/+ mice (47 ± 2.5 versus 33 ± 3.6; Fig. 5D). These results indicate that loss of PUMA leads to increased susceptibility of developing early neoplastic lesions in the absence of acute DNA damage in the intestinal tract.
Figure 5.
PUMA deficiency enhanced tumorigenesis in APCMin/+ mice. A, polyps in the colon (≥0.5 mm in diameter) were scored under a stereoscope in age-matched (22 wk) and sex-matched APCMin/+ and APCMin/+/PUMA KO mice. B, polyps in the small intestine were scored as in A. C, left, polyps in the small intestine stratified by anatomic region. Right, the size distribution of polyps in the small intestine. Columns, mean; bars, SD (n = 14 in each group in A–C). D, the number of microadenomas scored on H&E sections in the small intestine and colon of mice at 5 wk of age (n = 5 in each group).
Discussion
Our studies indicate that the BH3-only protein PUMA suppresses intestinal tumorigenesis induced by either carcinogens or germ line mutation in the APC tumor suppressor gene. PUMA is induced by AOM and mediates apoptosis in a p53-dependent manner in the colonic crypts and stem cell compartment. Several earlier studies showed that an intact p53 pathway suppresses AOM or DSS-induced colon carcinogenesis (34–36). Our study suggests that this suppression is likely mediated by PUMA. On the other hand, p53 was reported to have little effect on the incidence or the progression of adenomas in APCMin/+ mice, or on their spontaneous apoptosis (37, 38). However, the role of p53 in this model cannot be ruled out due to genetic background or modifiers (39). Enhanced tumorigenesis found in APCMin/+/PUMA KO mice suggests a p53-independent function of PUMA in tumor suppression. Therefore, PUMA-mediated apoptosis through either p53-dependent (AOM/DSS model) or p53-independent mechanism (APCMin/+ mice) serves as a critical roadblock to tumorigenesis. Furthermore, our data suggest that PUMA-mediated apoptosis plays a more significant role in suppressing colonic carcinogenesis induced by DNA damage and tissue injury associated with inflammation. This is consistent with the prominent role of PUMA in mediating apoptosis in this tissue, as opposed to other members in the extended BH3-only protein family (6–10). These findings provide direct evidence supporting apoptosis induction as an important mechanism for tumor suppression in the intestinal tract (40).
Previous studies have established that p53-dependent induction of PUMA is required for apoptotic response to a wide range of stresses (6–10). However, the evidence for PUMA in tumor suppression, particularly in solid tumors, is still very limited. Unlike p53 KO mice, PUMA KO mice are not overtly tumor-prone (41, 42). Our data suggest that p53-mediated PUMA induction serves as a safeguard to inhibit tumorigenesis in response to DNA damage, which is mutagenic if left unrepaired, or may present as oncogenic stress following error-prone repair of either tumor suppressors or oncogenes (43). Interestingly, p21-deficient mice developed significantly higher numbers of ACF and showed less apoptosis in the colon after AOM treatment compared with WT mice (18). It is not clear how p21 deficiency inhibits apoptosis in this setting, as p21 deficiency in human colon cancer cells triggered a PUMA-dependent killing in response to DNA damage (7). Loss of PUMA does not affect crypt proliferation after AOM treatment, nor does loss of p53 or p21 (18, 35). These findings support a hypothesis that p53 suppresses colon carcinogenesis primarily through the induction of apoptosis in response to perhaps chronic DNA damage due to exposure to the mutagens in the colonic contents, and oncogenic mutations of APC, β-catenin, or K-ras as suggested (40). Therefore, loss of apoptotic response to DNA damage (35) or abnormal oncogenic stimulation might be the most important mechanism leading to tumorigenesis in this organ system. This might also explain why the small intestine is much less prone to carcinogenesis, perhaps due to the more limited life span of differentiated cells, and reduced carcinogen exposure in terms of both - concentration and duration.
The function or regulation of PUMA in response to DNA damage is likely to be severely impaired in most colon cancers given the prevalence of p53 mutations and the overexpression of anti-apoptotic protein Bcl-xL (1, 44, 45). This might explain why reduced expression or mutation of PUMA is not found in colon cancer. Unlike in human colon cancer (46), the mutations in K-ras and p53 are rare in rodent colon tumors induced by AOM (28). Most of the tumors found in APCMin/+ mice are in the small intestine. This might be explained by differences in development, diet, life span, environment, and carcinogen exposure between the mouse models and human. It is well established that the Wnt/β-catenin pathway is antiapoptotic and can stimulate the expression of Bcl-2 like proteins in colon cancer cells (27, 47). Therefore, PUMA deficiency might bypass the need of activating β-catenin mutations to inhibit apoptosis and facilitate tumor initiation, which might explain the reduced frequency of β-catenin mutations found in AOM/DSS-induced PUMA-deficient tumors. However, activation of the β-catenin pathway in AOM/DSS-induced tumors clearly involves alternative mechanisms that remain to be defined. It would be interesting to identify other genetic or epigenetic alterations that cooperate with PUMA deficiency through global genetic or genomic analysis. PUMA antagonizes all five known members of the antiapoptotic Bcl-2 family of proteins, several of which are overexpressed in colon (48). We would predict that tumors from the PUMA-deficient mice are less likely to overexpress these proteins. Such studies might reveal important pathways and interactions in tumor biology and therapy, and provide a better molecular understanding of tumor heterogeneity.
Our data provide a model to test the provocative cancer stem cell hypothesis. Colonic epithelium is constantly renewed, with the loss of differentiated cells at the top of the crypts through apoptosis to maintain homeostasis (3). It is unclear whether colon cancer is derived from stem cells that have endured critical mutations in a certain sequence (1, 46), or from more differentiated cells that have acquired mutations conferring stem cell-like properties, and the relative contribution of both mechanisms. A recent study showed that selective targeting of APC in the intestinal stem cells, but not in rapidly proliferating progenitors, results in rapid intestinal tumorigenesis in mice (49). Our data suggest that PUMA-mediated apoptosis protects colonic stem cells against carcinogenesis. Using recently published stem cell marking mice (30), we will be able to trace the cellular origin of colon tumors. It is possible that a pre-existing deficiency in apoptosis can affect the types of cells that give rise to tumors as a result of a shifted mutation spectrum. Moreover, mutation and phenotype connection in the precursor lesions in humans is supported by a recent report, in which BRAF mutations are associated with serrated-ACF and polyps, but not K-ras mutations (50). Genetically engineered mice that are resistant to apoptosis make a great model system to further explore this possibility.
Supplementary Material
Acknowledgments
Grant support: NIH grants CA106348 and CA121105, American Cancer Society grant RSG-07-156-01-CNE (L. Zhang), and NIH grants CA129829, U19-A1068021 (Pilot Project), and from ACGT, FAMRI, and the Hillman Foundation (J. Yu).
We thank other members of our labs for helpful discussion and advice, Hongtao Liu for breeding mice, and Gerard P. Zambetti at St. Jude Children’s Research Hospital for providing PUMA KO mice.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Zhang L. Apoptosis in human cancer cells. Curr Opin Oncol. 2004;16:19–24. doi: 10.1097/00001622-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 4.Bedi A, Pasricha PJ, Akhtar AJ, et al. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–6. [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–82. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Wang P, Ming L, Wood MA, Zhang L. SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene. 2007;26:4189–98. doi: 10.1038/sj.onc.1210196. [DOI] [PubMed] [Google Scholar]
- 9.Wu B, Qiu W, Wang P, et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut. 2007;56:645–54. doi: 10.1136/gut.2006.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu W, Carson-Walter EB, Liu H, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–83. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P, Yu J, Zhang L. The nuclear function of p53 is required for PUMA-mediated apoptosis induced by DNA damage. Proc Natl Acad Sci U S A. 2007;104:4054–9. doi: 10.1073/pnas.0700020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ming L, Sakaida T, Yue W, Jha A, Zhang L, Yu J. Sp1 and p73 activate PUMA following serum starvation. Carcinogenesis. 2008;29:1878–84. doi: 10.1093/carcin/bgn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J, Yue W, Wu B, Zhang L. PUMA sensitizes lung cancer cells to chemotherapeutic agents and irradiation. Clin Cancer Res. 2006;12:2928–36. doi: 10.1158/1078-0432.CCR-05-2429. [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Sakaida T, Yue W, Gollin SM, Yu J. Chemosensitization of head and neck cancer cells by PUMA. Mol Cancer Ther. 2007;6:3180–8. doi: 10.1158/1535-7163.MCT-07-0265. [DOI] [PubMed] [Google Scholar]
- 15.Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci U S A. 2004;101:9333–8. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrison SP, Jeffers JR, Yang C, et al. Selection against PUMA gene expression in Myc-driven B cell lymphomagenesis. Mol Cell Biol. 2008;28:5391–403. doi: 10.1128/MCB.00907-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karst AM, Dai DL, Martinka M, Li G. PUMA expression is significantly reduced in human cutaneous melanomas. Oncogene. 2005;24:1111–6. doi: 10.1038/sj.onc.1208374. [DOI] [PubMed] [Google Scholar]
- 18.Poole AJ, Heap D, Carroll RE, Tyner AL. Tumor suppressor functions for the Cdk inhibitor p21 in the mouse colon. Oncogene. 2004;23:8128–34. doi: 10.1038/sj.onc.1207994. [DOI] [PubMed] [Google Scholar]
- 19.Boivin GP, Washington K, Yang K, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 20.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K. Frequent mutations of the β-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117–20. [PubMed] [Google Scholar]
- 22.Hibi T, Ogata H, Sakuraba A. Animal models of inflammatory bowel disease. J Gastroenterol. 2002;37:409–17. doi: 10.1007/s005350200060. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki R, Kohno H, Sugie S, Tanaka T. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004;95:721–7. doi: 10.1111/j.1349-7006.2004.tb03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greten FR, Eckmann L, Greten TF, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Ming L, Sakaida T, Yue W, Jha A, Zhang L, Yu J. Sp1 and p73 activate PUMA following serum starvation. Carcinogenesis. 2008;29:1878–84. doi: 10.1093/carcin/bgn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens RG, Swede H, Rosenberg DW. Epidemiology of colonic aberrant crypt foci: review and analysis of existing studies. Cancer Lett. 2007;252:171–83. doi: 10.1016/j.canlet.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–80. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinberg A, Zedeck MS. Production of a highly reactive alkylating agent from the organospecific carcinogen methylazoxymethanol by alcohol dehydrogenase. Cancer Res. 1980;40:4446–50. [PubMed] [Google Scholar]
- 30.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 31.Hirose Y, Yoshimi N, Makita H, Hara A, Tanaka T, Mori H. Early alterations of apoptosis and cell proliferation in azoxymethane-initiated rat colonic epithelium. Jpn J Cancer Res. 1996;87:575–82. doi: 10.1111/j.1349-7006.1996.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–8. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 33.Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–70. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 34.Nambiar PR, Giardina C, Guda K, Aizu W, Raja R, Rosenberg DW. Role of the alternating reading frame (P19)-p53 pathway in an in vivo murine colon tumor model. Cancer Res. 2002;62:3667–74. [PubMed] [Google Scholar]
- 35.Hu Y, Le Leu RK, Young GP. Absence of acute apoptotic response to genotoxic carcinogens in p53-deficient mice is associated with increased susceptibility to azoxymethane-induced colon tumours. Int J Cancer. 2005;115:561–7. doi: 10.1002/ijc.20876. [DOI] [PubMed] [Google Scholar]
- 36.Fujii S, Fujimori T, Kawamata H, et al. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–6. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fazeli A, Steen RG, Dickinson SL, et al. Effects of p53 mutations on apoptosis in mouse intestinal and human colonic adenomas. Proc Natl Acad Sci U S A. 1997;94:10199–204. doi: 10.1073/pnas.94.19.10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke AR, Cummings MC, Harrison DJ. Interaction between murine germline mutations in p53 and APC predisposes to pancreatic neoplasia but not to increased intestinal malignancy. Oncogene. 1995;11:1913–20. [PubMed] [Google Scholar]
- 39.Halberg RB, Katzung DS, Hoff PD, et al. Tumorigenesis in the multiple intestinal neoplasia mouse: redundancy of negative regulators and specificity of modifiers. Proc Natl Acad Sci U S A. 2000;97:3461–6. doi: 10.1073/pnas.050585597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rupnarain C, Dlamini Z, Naicker S, Bhoola K. Colon cancer: genomics and apoptotic events. Biol Chem. 2004;385:449–64. doi: 10.1515/BC.2004.053. [DOI] [PubMed] [Google Scholar]
- 41.Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–8. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 42.Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 43.Wood RD, Mitchell M, Lindahl T. Human DNA repair genes, 2005. Mutat Res. 2005;577:275–83. doi: 10.1016/j.mrfmmm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–92. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 45.Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA dissociates Bax and BCL-XL to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281:16034–42. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- 46.Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–41. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Dashwood WM, Zhong X, Nakagama H, Dashwood RH. Bcl-2 overexpression in PhIP-induced colon tumors: cloning of the rat Bcl-2 promoter and characterization of a pathway involving β-catenin, c-Myc and E2F1. Oncogene. 2007;26:6194–202. doi: 10.1038/sj.onc.1210438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labi V, Erlacher M, Kiessling S, Villunger A. BH3-only proteins in cell death initiation, malignant disease and anticancer therapy. Cell Death Differ. 2006;13:1325–38. doi: 10.1038/sj.cdd.4401940. [DOI] [PubMed] [Google Scholar]
- 49.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2008;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg DW, Yang S, Pleau DC, et al. Mutations in BRAF and KRAS differentially distinguish serrated versus non-serrated hyperplastic aberrant crypt foci in humans. Cancer Res. 2007;67:3551–4. doi: 10.1158/0008-5472.CAN-07-0343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.