Abstract
Background
Healthful dietary patterns, including eating fruits and vegetables (F&V) and avoiding obesity may decrease the risk of cancer and other chronic diseases. In addition to promoting health for the general population, a cancer diagnosis may provide a “teachable moment,” facilitating the adoption of more healthful eating habits and leading to lower risk of chronic disease and better overall health.
Purpose
This study was designed to test the effectiveness of two health communication interventions in increasing F&V consumption and physical activity in a sample of older adults (average age 66 years), including both colorectal cancer (CRC) survivors and non-CRC-affected (N-CRC) individuals.
Methods
Colorectal cancer survivors and N-CRC individuals were recruited from a population-based case-control study and randomly assigned to four conditions using a 2 × 2 design. We tested two different methods of communicating and promoting health behavior change alone or in combination: tailored print communication (TPC) and brief telephone-based motivational interviewing (TMI).
Results
A significant increase in F&V consumption was found for the combined intervention group in the entire sample (p<0.05). When stratified by cancer survivor status, the effect was concentrated in the N-CRC subset (p<0.01) versus CRC survivors. The combined intervention was also found to be most cost-effective for the N-CRC group, with TPC more cost-effective than TMI. For physical activity, none of the interventions produced statistically significant improvements.
Conclusions
This study indicates that combining tailoring and motivational interviewing may be an effective and cost-effective method for promoting dietary behavior change among older healthy adults. More research is needed to identify the optimal dose and timing for intervention strategies to promote dietary and physical activity change among both CRC survivors and the general population.
Introduction
Colorectal cancer (CRC) is the second most common cancer diagnosed each year in the United States. Current figures indicate approximately 108,070 new cases of colon cancer and 40,740 new cases of rectal cancer annually in the United States, and about 49,960 deaths (1). The average cost of colorectal cancer treatment is estimated at $41,134 (2) resulting in $6.1 billion in annual health costs. Although the death rate from colorectal cancer has been decreasing over the past 15 years, incidence and mortality rates remain disproportionately high for African Americans (1).
Improvements in health behaviors such as diet, physical activity, and CRC screening have the potential to reduce risk of a primary cancer (3,4). In terms of diet, much epidemiologic evidence suggests a protective effect of high fruit and vegetable intake for several cancers, including CRC (5), although intervention studies to date, such as the Polyp Prevention Trial, have not demonstrated that dietary changes affect disease precursors (6). According to recent data from the Behavioral Risk Factor Surveillance Survey, however, only about one-quarter of U.S. adults consumed at least five servings a day of fruits and vegetables (7). The rise in early detection as well as advances in disease treatment have led to improved survival, with roughly two-thirds of colon cancer patients surviving five or more years after diagnosis and higher percentages for those diagnosed in early stages. This growing population of survivors has created a need to develop accurate and relevant health information that may help in maintaining a good quality of life post-treatment, as well as reducing risk of chronic disease, including recurrence of cancer (8,9). Health behaviors such as eating well, exercising, and avoiding obesity are important for aging populations with or without a cancer diagnosis. However, relatively little is known about how CRC survivors compare to demographically similar non-cancer-affected adults in terms of their health information needs and responsiveness to lifestyle interventions. This type of information is essential in order to tailor health interventions to be most effective among various populations.
A number of previous interventions to increase fruit and vegetable (F&V) consumption have been implemented in healthy populations in a variety of settings including worksites, faith organizations, schools, and other community settings, using multiple strategies (10–12). These studies have generally shown increases in F&V consumption ranging from 0.2–1 serving per day, depending on the population and intervention strategy employed. Specifically, tailored home-based approaches, including providing telephone feedback and mailings to Cancer Information Services callers and sending printed tailored feedback to individuals in their homes, have shown promise for promoting dietary change (13,11).
A cancer diagnosis can be a “teachable moment” (14) to motivate individuals to make general health promotion changes that can improve overall health and well-being and reduce risk of other health problems, such as diabetes and heart disease (15). For example, studies of women with breast cancer and men with prostate cancer have shown that patients can make substantial dietary changes after cancer diagnosis (15–18). Kuhn and colleagues’ (19) review of the literature on the prevention of cancer through changes in diet (e.g., increased fruits and vegetables, reduced saturated lipids) identified only six intervention studies. All the studies reviewed focused on women with breast cancer and found that dietary counseling can lead to significant changes in vegetable, fruit, fiber, and fat intake. The Women’s Healthy Eating and Living study showed that, among women with breast cancer, an intensive clinic-based intervention with frequent counselor contact for those in the intervention arm resulted in large increases in daily intake of fruits and vegetables at 12-month follow-up (18). Demark-Wahnefried and colleagues showed changes resulting from a multiple cancer site survivor-focused intervention, “Fresh Start,” consisting of monthly tailored newsletters and motivational telephone calls (20). Motivational interviewing interventions also have been effective for encouraging a variety of health behavior changes including increasing F&V consumption and physical activity (21,12). There is a need for more research testing appropriate population-based strategies to intervene with colorectal cancer survivors.
The NC STRIDES (North Carolina Strategies for Improving Diet, Exercise, and Screening) study was a randomized trial of health communication strategies aimed at health promotion (diet, physical activity, and CRC screening) among both N-CRC members of the general population and CRC survivors. The study sample was drawn from an existing population-based case-control study of risk factors for CRC (22,23). NC STRIDES tested two different methods, tailored print communications (TPC) and telephone-based motivational interviewing (TMI), in terms of effectiveness and cost-effectiveness in promoting increases in fruit and vegetable consumption and regular moderate to vigorous physical activity. The two-by-two research design allowed for comparison of each intervention and a combined intervention against a no-intervention control condition. In addition, cost-effectiveness analysis provided a basis for translating the findings to recommendations for future research and dissemination. This paper presents results for the primary study outcomes of change in F&V consumption and change in physical activity.
Methods
Conceptual framework
The present study was designed to test two different methods of communicating and promoting health behavior change: tailored print communication (TPC) and brief telephone-based motivational interviewing (TMI). Both the TPC and TMI interventions were based on widely-used health behavior theories, the stages-of-change Transtheoretical Model (24) and Social Cognitive Theory (25,26), as well as on the principles of motivational interviewing (27). From these theories, we identified key constructs for the individualization of tailored messages and MI calls, including perceived barriers to change, motivation, stage of readiness, self-efficacy, social support, knowledge/awareness, core values, and demographic and health characteristics of participants. The TPCs provided expert feedback driven by baseline data, whereas the MI calls encouraged participants to overcome ambivalence and identify their own strategies for change. Both interventions aimed to enhance self-efficacy and motivation, leading to behavior change.
Recruitment
Study participants were recruited from the North Carolina Colon Cancer Study (NCCCS), a population-based case-control study of incident colon and rectal cancer patients in a 33-county area of central and eastern North Carolina. Counties were selected based on their mix of urban and rural areas as well as the proportion of the county population who were African American. Inclusion criteria for NCCCS cases were: persons with adenocarcinomas of the colon, ages 40–79 and of African American or European American ethnicity, who were being treated in one of 38 non-federal hospitals. Cases were identified using the rapid ascertainment component of the North Carolina Central Cancer Registry. Population-based controls in the NCCCS had been recruited from two sources: those under age 65 came from the NC Department of Motor Vehicles roster and those over age 65 came from the Health Care Financing Administration register. The cases were sampled to provide approximately equal numbers of African Americans and whites, and the controls were sampled to provide a group of similar age, race, and gender as the cases.
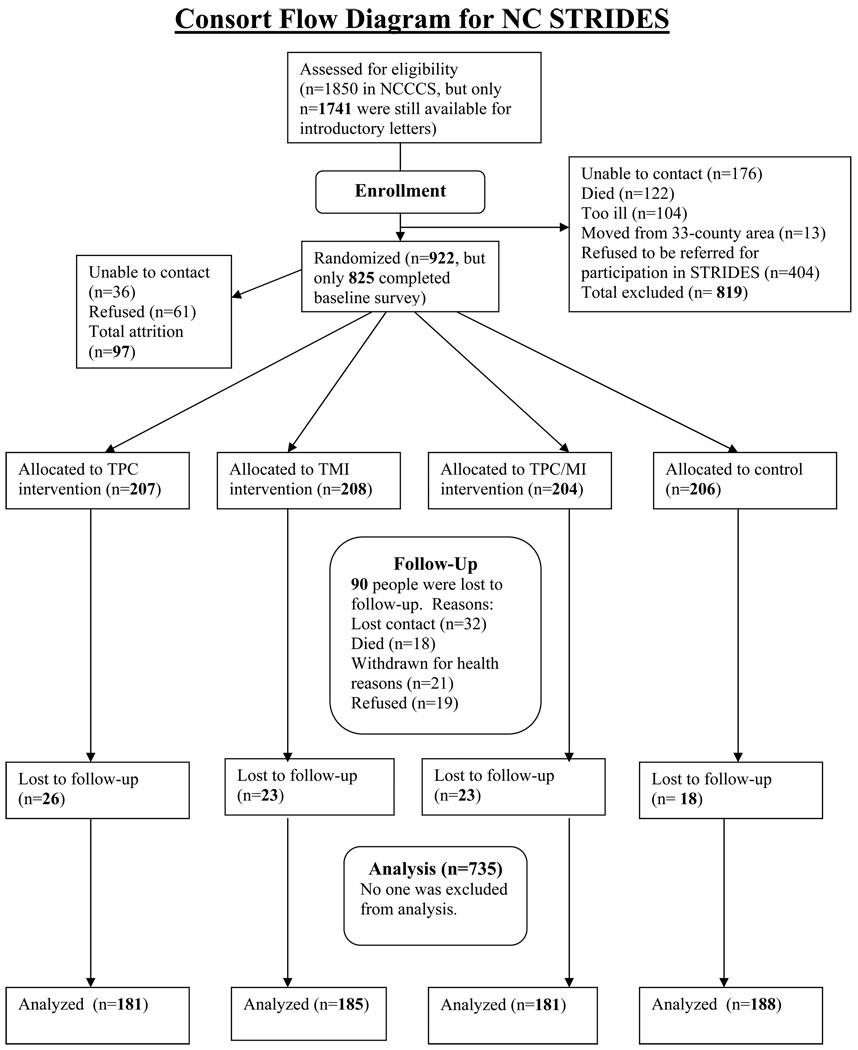
Previous NCCCS participants were recruited to participate in the NC STRIDES study, conducted from 2001 through 2004. Most of the participants had been recruited into the NCCCS approximately two years previously; therefore, the cancer survivors were generally 2.5 years post-diagnosis. All NCCCS participants were mailed a letter and brochure explaining the NC STRIDES study. NCCCS research staff then contacted potential participants via telephone to solicit their consent to participate in NC STRIDES. Only after informed consent was received were the names of potential participants shared with NC STRIDES staff, thereby protecting the confidentiality of NCCCS participants. NC STRIDES successfully recruited approximately 50% (n=922) of the entire NCCCS sample of 1850 cases (38%) and controls (62%). The high number of elderly persons in the sample increased the likelihood that people would decline to participate due to ongoing issues such as illness or difficulty hearing. In addition, CRC survivors were considered for recruitment only if they reported being healthy enough to make lifestyle changes and participate over the course of the year-long study.
Participants who consented were randomized by NCCCS into one of four groups, stratified by CRC or N-CRC status. One group (TPC only) received a series of four individually-tailored, printed newsletters. Another group (TMI only) received a series of four brief (approximately 20 minutes) telephone motivational calls. The third group (Combined group) received four individually-tailored, printed newsletters and four motivational calls. The TPC and MI interventions were designed to focus equally on each targeted health behavior in terms of newsletter content and telephone call topics. The fourth group served as the control group and received two “generic” health education mailings during the intervention period and four individually-tailored print newsletters after the final survey. Information provided to the control group focused on other cancer-related topics (e.g., skin, breast, or prostate cancer) and did not focus on colon cancer prevention or early detection. All TPC and control participants were called at six months (mid-intervention) to update contact information and to ask additional brief survey questions. For TMI and combined groups, the six-month update was done during MI call #3.
The intervention took place over a one-year period. Surveys and motivational interviews were administered on the telephone by trained interviewers. This protocol was approved by the IRB committee at the School of Public Health at the University of North Carolina at Chapel Hill.
Of those recruited to NC STRIDES (n=922), 825 (89.5%) completed the baseline survey. Participation by cancer status was very similar to the NCCCS study: baseline surveys were obtained from 37% CRC and 63% N-CRC individuals. A total of 735 (89.1% of baseline) individuals completed the one-year follow-up survey. Non-responses to the follow-up survey were due to 18 deaths, 21 people who withdrew from the study for health reasons, 19 refusals, and 32 lost contacts. Survey responses rates were equivalent among CRC and N-CRC participants.
Measures
Trained research staff collected baseline and follow-up data for NC STRIDES using a telephone-administered self-report survey. On average, surveys took 30 to 40 minutes to complete. Data were collected regarding socio-demographic information, self-rated health, health status information, fruit and vegetable intake, and psychosocial factors related to these behaviors. Approximately six months into the study, a truncated version of the survey was administered, also by telephone. This shorter survey took, on average, 10 minutes to complete, and was used to update contact information, aid with study retention, and obtain brief updated information on fruit and vegetable consumption and other psychosocial factors in order to re-tailor the information included in the fourth newsletter for the TPC and combined groups. One year after baseline, participants were asked to complete a third telephone survey. This survey lasted about 45 minutes, asked the same health, behavioral, and psychosocial questions as the baseline survey, and included process evaluation questions. In addition to the survey data, a subset of participants provided a biomarker sample at follow-up in order to validate the fruit and vegetable self-report measure. Although NC STRIDES interviewers were blind to the intervention group assignment, they were aware of cancer status, as there were slightly different versions of the survey for the CRC and N-CRC groups.
Demographic and health variables
Information on age, race/ethnicity (African American, white), gender, and CRC status (case/control) had been collected during the NCCCS. Additional socio-demographic information was collected as part of our survey, including education (7 categories), employment status (yes/no), annual income (5 categories), and marital status. Health-related variables included self-rated health (5 categories: excellent, very good, pretty good, fair, and poor), chronic conditions that might affect diet (heart disease, hypertension, diabetes, arthritis, other cancers) and health insurance coverage.
Fruit and vegetable consumption
Average daily fruit and vegetable consumption was measured using a 36-item modified version of the Block food frequency question (FFQ). The modified FFQ was developed and validated by Resnicow and colleagues for their work with a diverse Southern population (28). Resnicow’s tool was modified to query how often food was consumed in the last month, as opposed to the last week, and to omit food items that were not fruits or vegetables. Also, the item “French fries, fried potatoes, or home fries” was eliminated from analysis calculations; thus the F&V intake totals were based on 35 items. Fruit and vegetable item frequencies were converted to servings/day and then summed to provide total daily consumption values for fruit, vegetables, and total F&V. In addition to the FFQ, participants were asked to estimate their intake of fruit and vegetables per day using a brief 2-item screener: “How many servings of vegetables or vegetable juices/fruit or fruit juices do you usually have during a single day?” The 35-item and the 2-item F&V measures were moderately correlated: r= 0.43.
Biomarker validation of FFQ data
Plasma carotenoids were measured on a subsample of 269 volunteer participants who completed the study. Participants were asked at the end of the final survey if they would be willing to give a small amount (4 ml) of blood that would be collected from them at their home by a trained phlebotomist. Those who agreed were visited at home by appointment within four weeks by a trained phlebotomist who obtained written consent to draw the blood. The participants were given $25 as an incentive for the blood draw. Current smokers, heavy drinkers, and those on blood thinners were excluded from providing a biomarker, because of concerns about reduced validity of measured carotenoids or risk to the participant. The blood was immediately placed on ice and transported within 24 hours for processing, freezing, and storage at the University of North Carolina at Chapel Hill. Carotenoid analyses included assessment of plasma alpha-carotene, beta-carotene, lycopene, lutein, and beta-cryptoxanthin by high-performance liquid chromatography. For the purpose of validating the FFQ measure of total F&V, we focused on total carotenoids as the biomarker measure representing the largest number of F&V (29). The correlation between total carotenoids and the 35-item FFQ was 0.30 (p<.001) and between total carotenoids and the 2-item FFQ was 0.27 (p<.001). These correlations are similar to other published studies comparing multiple 24-hour food recalls with carotenoids (30) and comparing the 35-item and 2-item instruments with carotenoids (31).
Physical activity
Weekly physical activity was measured using a modified version of a validated measure, the 7-Day Physical Activity Recall (10,32), selected to be consistent with that of the parent NCCCS study. Minutes per week spent in very hard, hard, and moderate intensity activity were assessed, multiplied by a Metabolic Equivalent (MET) factor (33) to adjust for energy expenditure, and were summed to form a moderate-to-vigorous physical activity (MVPA) score. The square root of this value was then taken, to account for non-normality of the data (34).
Psychosocial measures
Data were collected on several psychosocial factors that may be mediators of health behavior change. Self-efficacy for eating F&V and for engaging in physical activity was measured using one item for each behavior, e.g.,: “If you decided to, how sure are you that you have the ability to succeed in eating five or more servings of fruits and vegetables every day for the next six months?” This item had a five-point Likert-type response, from very sure to very unsure (25). Social support was measured using four items based on previous research: “If you tried to eat healthier foods/exercise more, how much could you count on the people close to you to: encourage you, tell you about healthier foods and how to prepare them, prepare healthier food with or for you, eat healthier foods with you?” (35). A social support index was calculated by summing responses to the four items (0 = not at all or not applicable, 1 = some, 2 = a lot); Cronbach’s alpha = .84 for F&V, .82 for physical activity (34). Perceived barriers to behavior change for each behavior were measured through a series of statements that “would make it hard” to achieve the behavior change. Barrier statements were based in part on pre-survey focus groups and on previous research (36,37). Response options were measured on a 4-point scale ranging from “disagree a lot” to “agree a lot.” Barrier scores were created by summing the scores and obtaining the average value across the six items. The barriers scale had adequate consistency, Cronbach’s alpha= .66 for F&V, .62 for physical activity (34). Knowledge of the recommendations (e.g., how many servings of fruits and vegetables should a person eat each day) was tested using single-item multiple-choice questions based on previous research (10).
Process measures
On the final survey, additional questions were asked about intervention exposure, recall, personal relevance, and reactions to each intervention component.
Measures collected for cost-effectiveness analysis (CEA)
CEA is widely advocated as a tool for decision makers to allocate resources to programs that will have maximal benefit for society. Costs tracked for the present study included intervention-specific costs for the program and for the participants, as well as participant costs related to the intervention outcome. We excluded fixed or developmental costs for program development, such as the costs for developing the tailoring software program and for developing the training for the TMI intervention, because they would not need to be replicated by future users of the interventions. Program intervention-specific costs included: production, proofing, and mailing of TPCs and generic brochures to participants, and time spent producing the TPCs and TMI tailored sheets for the calls. We estimated costs for delivery of MI calls (e.g., interviewer cost for making/recording the calls, telephone charges) as well as time spent by interviewers conducting TMI calls. We estimated time costs as $12 per hour for staff and $15 per hour for phone counselors, plus telephone call and mailing costs. Participant intervention-specific costs included time spent reading, discussing, or using the TPCs or the generic brochures, and time spent participating in TMI calls, estimated at $12 per hour. Outcome-specific costs were the participant costs of increasing F&V, determined by pricing all items on the 35-item FFQ monthly over the intervention period and averaging the prices seasonally. Effect was measured as the change for each intervention group on the outcome variable of F&V consumption using the 35-item instrument described above.
Intervention
Tailored Print Communication (TPC) intervention
Computerized individual tailoring for health promotion is a technique that combines health behavior change theory, communication theory, social marketing principles, and computer-based programs and algorithms to produce personally relevant health messages for each project participant. Information obtained from each individual's survey data is accessed and assembled using specially-created software to generate customized messages for each person that are designed to promote healthy behavior changes (38). The information is tailored to be particularly relevant, interesting, culturally appropriate, and credible to the message recipient (36).
The tailoring framework for NC STRIDES incorporated demographic, psychosocial, behavioral, and community-specific resource information in a newsletter format. The TPC intervention included four personalized computer-tailored newsletters focused on fruit and vegetable consumption, physical activity, and CRC screening for N-CRC participants non-adherent at baseline. Newsletters for CRC survivors included follow-up surveillance as recommended by the participant’s physician. The first three newsletters were mailed to participants’ homes bi-monthly after baseline data collection (months 1, 3, and 5); the fourth mailing occurred 9 months post-baseline. Newsletters 1–3 were tailored using the baseline survey data; the fourth newsletter incorporated additional feedback on participant progress obtained from an update telephone call at 6 months post-baseline. Participants were also asked at baseline to select the health behavior topic of highest importance to them.
Pre-intervention focus groups conducted online or face to face with colorectal cancer survivors (33) were used in combination with pertinent literature and project team expertise to develop appropriate tailoring variables, message content, language, literacy level (approximately 8th grade), and graphic design. Each 8-page newsletter, sized 8 1/2 × 11 inches, was created from two 11 × 17 sheets pre-printed in two colors. Tailored content was laser printed in black onto both sides of the paper, which was then folded in half.
An extensive message library of approximately 400 text files, graphics, and photographs was developed to correspond with each survey question selected for tailoring and its possible response option combinations. The message library layout and format and the number of messages and graphics per newsletter were consistent across the health behaviors. The newsletters were targeted to appeal to an older (over age 40) and diverse (white and African American) audience through choices of graphic design, photographs, stories, and layout (larger font and adequate white space for those with reading or vision problems). Computer-based tailoring algorithms were used to link individual survey data to the appropriate messages and graphics.
Newsletters were personalized with participant names and provided behavioral feedback based on participants’ diet, physical activity, adherence to CRC screening recommendations, stages of change, social support, barriers to change, knowledge, and demographics (race and gender). Additional message elements included county-specific resources for each of the 33 counties in which participants resided, and testimonials written by CRC survivors and people who had not had CRC but had made changes in lifestyle factors such as diet and exercise. Photos allowed the testimonials to be tailored for race and gender. Message content was tailored for CRC status based on formative research (33).
Telephone Motivational Interviewing (TMI) intervention
This intervention consisted of four brief (20-minute) motivational interviewing calls delivered over a nine-month period. Participants in the TMI-only group received three bi-monthly calls for the first six months (months 2, 4, and 6) and one call at nine months post-baseline. For the combined intervention group, the calls were timed to occur one month after receipt of each TPC in order to stagger the receipt of intervention components and to allow participants to discuss any questions regarding the TPCs they had received. To facilitate comparison of the TMI and combined groups, we timed all TMI calls on the same schedule. Each call followed a counseling protocol based on MI principles. These included using a client-centered, collaborative decision-making approach, giving non-judgmental feedback, allowing for resistance, and encouraging the participant to make the argument for change (27). Interviewers relied on using open-ended questions and reflections to draw out participants’ motives and desires about behavior change. Calls were conducted by research team members who had received a minimum of 20 hours of training on MI principles and techniques. In addition, weekly one-hour training meetings were held to address problem areas and expand skill levels. Practice interviews were conducted until the interviewers were comfortable following the protocol. In addition, each interviewer made two tapes that were reviewed for MI integrity by an MI consultant at the outset of the study. The calls were additionally “tailored” by incorporating information derived from participant baseline and follow-up surveys regarding priorities for change, baseline behavior, and cancer status. For the combined group, the MI interviewers asked participants if they wished to discuss any of the information in the TPC they had received.
To assess fidelity to MI principles, average scores for each of the dimensions of the MITI (Motivational Interviewing Treatment Integrity) coding scheme (39) were calculated for a random subsample of 77 coded interviews. The tapes were coded by members of our research team who did not conduct the interviews, and the five tapes coded with the highest scores were independently coded by a MITI coding expert at the University of New Mexico to determine whether the UNC coders were inflating scores. The independent coding was consistent overall with the UNC coding. The range of possible scores for the MI dimensions of empathy (the extent to which the interviewer understands and/or makes an effort to grasp the client’s perspective) and spirit (the overall competence of the interviewer in using motivational interviewing, explicitly focusing on the three characteristics of evocation, collaboration and autonomy) (40) was 1–7. Our interviewers’ scores ranged from 5–7, with an average empathy score of 6.1 and an average spirit score of 5.8, indicating that the MI calls were generally high in MI integrity.
Generic printed health information (CONTROL)
Participants in the control group received two mailings of generic (non-tailored) health information that was not related to the primary study outcomes. The purpose of these mailings was to provide cancer-relevant information and to maintain the group’s sense of connection to the study in order to encourage retention. The information consisted of brochures obtained from the Cancer Information Service and other agencies on prostate cancer (men), breast cancer (women), and skin cancer (all). The cost-effectiveness analysis required that the control group’s costs and likelihood of study-related effects be kept as minimal as possible without compromising study retention rates. After the follow-up data collection was completed, control group participants received four TPCs tailored to their follow-up survey data.
Statistical Analysis
All analyses were conducted using SAS software (41). Descriptive statistics were utilized to compare demographics, health, and health behaviors at baseline across the intervention groups. The unit of randomization and analysis in this study was the individual. Models were first analyzed including the whole sample. Next, separate models were run according to survivorship status (N-CRC and CRC). Fruit and vegetable consumption and physical activity were assessed as continuous outcome variables. For F&V, we calculated servings per day based on the 35-item, 2-item, and averaged fruit and vegetable FFQs. Because of the typical right-skewed distribution of F&V, we used a natural log transformation to improve normality of the data. For physical activity, frequency (minutes per week) and MET-hours per week of moderate to vigorous exercise were calculated based on the 7-Day Physical Activity Recall data.
We used multiple regression analysis, including generalized linear modeling and logistic regression analysis, to estimate intervention effects. To model the primary outcome measures of F&V and physical activity, we adjusted for baseline measures and demographic covariates of gender and race. For multiple comparisons, we divided p=.05 by the number of comparisons to determine statistical significance.
To test for potential psychosocial mediators of the F&V intervention effect, we used standard criteria based on Baron and Kenny and others (42) which outline steps to determine mediation. We first examined associations at baseline and follow-up between four potential mediators and F&V consumption: self-efficacy, social support, barriers, and knowledge. We also examined the cross-sectional association between mediators and intervention status separately at baseline and follow-up using regression models. Mediator variables were treated as continuous variables, except for knowledge, which was a binary variable. We further tested whether change in mediator candidates was associated with change in F&V intake. We used linear regression models to test the regression coefficients associated with change in F&V as a function of change in the mediator candidates, adjusting for demographic covariates. Further steps in assessing mediation were not performed based on the results of these analyses.
The cost effectiveness analysis (CEA) used the incremental cost-effectiveness ratio (ICER) given by the difference in costs between two health care interventions divided by the difference in outcomes between the programs with the same patient group (43). We compared the costs associated with each of the interventions, including both the research team and the participant costs, compared to the effect size achieved. For the cost-effectiveness analyses, we computed incremental cost-effectiveness ratios (ICERs) for each intervention condition compared to control, and for the comparison of the combined intervention to TPC or TMI alone (43). We conducted cost-effectiveness analysis only for the F&V outcome, because the intervention did not produce a significant effect on physical activity (see Results).
Results
Descriptive analyses
Among participants completing the baseline survey (n=735), 49% of participants were female and 35% were African American. The average age was 66.5 years (SD = 9.95), 78% had at least a high school education, and 53% had annual incomes of $30,000 or greater. Only 36% were actively employed on at least a part-time basis, and many were retired. Most people rated their health as at least “pretty good,” and 42% rated their health as “very good” or “excellent.” Most participants were overweight, with an average body mass index of 29. Thirty-seven percent of participants were CRC survivors (n=266). Few (7.6%) were diagnosed less than a year before the baseline survey; 29.4% were diagnosed 1 to 2 years before and 57.4% were diagnosed between 2 and 5 years before the survey. Among CRC survivors, 19% reported another cancer, and 14% of our comparison group reported being diagnosed with a cancer other than CRC. A very high percentage of participants were up-to-date with CRC testing. Over 80% of the N-CRC group had had a CRC screening test within the recommended time period.
Outcome Analyses
Fruit and vegetable consumption
Data analyses compared study groups’ F&V intake at one-year follow-up, stratified by cancer survivorship status and adjusted for baseline consumption, race, and gender. We conducted analyses using the 35-item and 2-item measures separately and as one averaged measure.
Results using the 35-item measure showed significant effects for the combined intervention in the N-CRC group. As shown in Table 2, we observed an increase of 1.0 serving in the combined intervention group, compared to approximately 0.5 serving for each independent intervention and little change for controls (p<.01). The effects of each independent intervention were not found to be statistically significant compared to the control group. No significant intervention effects were found for CRC survivors, however. The greatest increase in consumption among CRC survivors was seen in the group that received the TPC-only intervention, which increased an average of 1.0 daily servings. This difference, however, was not statistically significant compared to the control group.
Table 2.
Results for Two-item and 35-item Fruit and Vegetable FFQs among General Population and CRC Survivors
| Study Group | N | Baseline Fruit/ Vegetable Intake Mean (SD) |
Follow-up Fruit/ Vegetable Intake Mean (SD) |
Difference† |
|---|---|---|---|---|
| General Population | 35-item measure | |||
| Control | 122 | 5.7 (2.6) | 5.8 (2.4) | +0.1 |
| TPC1 | 111 | 5.4 (2.1) | 5.8 (2.1) | +0.4 |
| TMI2 | 113 | 5.4 (2.4) | 6.0 (2.7) | +0.6 |
| Combined** | 123 | 5.4 (2.8) | 6.4 (2.8) | +1.0 |
| CRC Survivors | 35-item measure | |||
| Control | 66 | 5.1 (2.4) | 5.8 (2.9) | +0.7 |
| TPCa | 70 | 5.3 (2.2) | 6.3 (3.0) | +1.0 |
| TMIb | 72 | 5.8 (2.8) | 5.9 (2.8) | +0.1 |
| Combined | 58 | 5.7 (3.1) | 6.0 (3.2) | +0.3 |
| General Population | Two-item measure | |||
| Control | 120 | 4.4(2.0) | 4.7(2.1) | +0.3 |
| TPCa | 110 | 4.4(1.9) | 5.1(1.9) | +0.7 |
| TMIb | 109 | 3.9(1.8) | 4.7(1.9) | +0.8 |
| Combined* | 122 | 4.4(2.1) | 5.2(2.0) | +0.8 |
| CRC Survivors | Two-item measure | |||
| Control | 64 | 4.1(2.0) | 4.3(2.0) | +0.2 |
| TPCa * | 68 | 4.3(1.8) | 4.9(1.6) | +0.6 |
| TMIb ** | 70 | 3.9(1.8) | 5.0(2.0) | +1.1 |
| Combined** | 58 | 4.0(1.5) | 5.2(2.4) | +1.2 |
| General Population | Average of Two-item and 35-item measures | |||
| Control | 120 | 5.1.(2.0) | 5.3 (2.0) | +0.2 |
| TPCa | 110 | 4.9 (1.7) | 5.5 (1.6) | +0.6 |
| TMIb | 109 | 4.6 (1.8) | 5.3 (2.0) | +0.7 |
| Combined** | 122 | 4.9 (2.2) | 5.8 (2.1) | +0.8 |
| CRC Survivors | Average of Two-item and 35-item measures | |||
| Control | 64 | 4.6(1.8) | 5.1 (2.0) | +0.5 |
| TPCa | 68 | 4.8(1.8) | 5.7 (2.0) | +0.9 |
| TMIb | 70 | 4.8(2.0) | 5.5 (2.1) | +0.7 |
| Combined | 58 | 4.9(1.9) | 5.6 (2.4) | +0.7 |
p<=.05
p<.01 for results of multiple regression analysis, adjusted for gender and race.
Tailored print communication
Telephone motivational interviewing
Difference computed from columns 3 and 4 (follow-up minus baseline values)
Results using the 2-item F&V measure produced similar findings in the N-CRC sample, such that the combined intervention was associated with a statistically significant increase in consumption of F&V (0.8 daily servings). Among the CRC survivor subgroup, results using the 2-item brief measure differed from those based on the longer 35-item instrument. We saw statistically significant increases for all three intervention groups compared to the control group, with both the TMI-only and combined groups showing an increase of more than one daily serving. The three intervention groups did not differ statistically from each other, however.
We then created a third outcome variable by averaging the 35-item and 2-item measures (31). Using this averaged consumption measure, results were similar to the 35-item measure alone (see Table 2), demonstrating a significant effect of the combined intervention in the N-CRC group, but no significant treatment differences among CRC survivors.
Mediator and moderator analyses
Among the N-CRC subgroup, we conducted further analyses examining possible mediators and moderators of intervention effect using the 35-item F&V outcome measure. Men generally made more changes in F&V consumption compared to women, and African Americans made greater improvements compared to whites. However, these differences were not statistically significant except among the subgroup of African American men (see Table 3).
Table 3.
Results for Fruit and Vegetable Consumption According to General Population Demographic Subgroups
| Demographic sub-group |
N** | Baseline Fruit/ Vegetable Intake Mean (SD) |
Follow-up Fruit/ Vegetable Intake Mean (SD) |
Difference† |
|---|---|---|---|---|
| Black females | ||||
| Control | 29 | 6.2 (2.9) | 6.4 (2.8) | +0.2 |
| TPCa | 19 | 6.0 (2.4) | 6.5 (2.8) | +0.5 |
| TMIb | 24 | 5.5 (2.4) | 6.2 (3.5) | +0.7 |
| Combined | 24 | 6.4 (4.3) | 6.9 (2.9) | +0.5 |
| Black males * | ||||
| Control | 15 | 6.6 (3.7) | 5.6 (2.3) | − 1.0 |
| TPCa | 12 | 5.3 (2.3) | 6.8 (2.4) | +1.7 |
| TMIb | 21 | 5.8 (2.8) | 7.0 (2.6) | +1.2 |
| Combined | 16 | 4.0 (1.7) | 5.8 (3.2) | +1.8 |
| White females | ||||
| Control | 36 | 5.8 (2.4) | 5.9 (1.8) | +0.1 |
| TPCa | 41 | 5.3 (2.1) | 5.3 (1.9) | 0 |
| TMIb | 25 | 5.8 (2.6) | 6.2 (2.8) | +0.4 |
| Combined | 37 | 5.9 (2.4) | 6.4 (2.7) | +0.5 |
| White males | ||||
| Control | 42 | 5.0 (1.9) | 5.4 (2.7) | +0.4 |
| TPCa | 39 | 5.3 (1.9) | 5.7 (1.6) | +0.4 |
| TMIb | 43 | 4.9 (2.2) | 5.2 (2.2) | +0.3 |
| Combined | 46 | 5.4 (2.4) | 6.4 (2.7) | +1.0 |
Tailored print communication
Telephone motivational interviewing
Difference computed from columns 3 and 4 (follow-up minus baseline values)
p=0.05 difference between combined intervention and control, for the Black male subgroup only
N= numbers completing the follow-up survey in the general population subgroup.
We also examined four possible mediators of dietary change: self-efficacy, social support, barriers, and knowledge of the recommendations. Based on the criteria of Baron and Kenny, none of these variables was found to be a mediator of the intervention effect. Higher self-efficacy, for example, was associated with greater F&V consumption at both baseline and follow-up, but increase in self-efficacy did not predict more change in F&V consumption.
Physical activity
Baseline levels of physical activity were similar between the N-CRC and CRC groups, and there were no baseline differences according to study condition. At baseline, average self-reported MVPA was approximately 290 minutes per week with 54% of CRC survivors and 57% of N-CRC affected participants meeting the Centers for Disease Control recommendation of at least 150 minutes per week of MVPA (44). Results showed that none of the interventions produced significant effects on increasing physical activity among either the N-CRC or CRC groups. At follow-up, participants in all study conditions reported less physical activity compared to baseline, and there were no differences between groups. In addition, there was no change in BMI during the study period.
Cost-effectiveness
Modeling indicated that the combined intervention (TPC + TMI) had the highest cost, but also had the greatest effect on F&V intake. Lower Incremental Cost Effectiveness Ratios (ICERs) are generally indicative of greater cost-effectiveness. Table 4 shows the cost-effectiveness results for the general population participants using the effect size from the 35-item F&V measure. We did not compute cost-effectiveness ratios for the CRC subgroup because there was insufficient evidence of differential intervention effectiveness. It is clear from Table 4 that the TPC-only intervention was least costly, but it also produced the least amount of change compared to the control group. TMI produced somewhat more effect, but the cost was nearly twice as high as the TPC intervention. Comparisons of the combined intervention to TPC and TMI indicated that greater cost-effectiveness was attained by combining the two approaches.
Table 4.
Cost-effectiveness Results for General Population Participants
| Incremental effectiveness |
Incremental cost |
ICER* | Cost-effective | |
|---|---|---|---|---|
| General population | ||||
| Combined vs. Control | 92.313 | 10,479.090 | 113.5169 | 0.992 |
| Combined vs. TPCa | 63.321 | 6,763.096 | 106.8065 | 0.946 |
| TMIb vs. Control | 50.357 | 5,798.392 | 115.1457 | 0.915 |
| Combined vs. TMIb | 41.956 | 4,680.694 | 111.5620 | 0.848 |
| TPCa vs. Control | 28.992 | 3,715.990 | 128.1729 | 0.822 |
Tailored print communication
Telephone motivational interviewing
Estimates are derived using the 35-item FFQ. Costs include program-specific intervention costs, participant-specific intervention costs, and participant-specific outcome costs. Developmental costs of the interventions are excluded because they are fixed or sunk costs that would not need replication in future dissemination.
Incremental Cost-effectiveness ratios calculated as the ratio of incremental costs/incremental effectiveness based on standard formulas. Lower ICERs are indicative of more favorable cost effectiveness.
Discussion
NC STRIDES evaluated the relative efficacy of individually computer-tailored newsletters and telephone-delivered motivational counseling to promote behavior change in a population-based sample of older adults. The study findings demonstrated the efficacy of the combination of two relatively low-intensity home-based interventions (TPC and TMI) to promote change in F&V consumption. This result was confirmed in the general population subgroup, such that the combined intervention produced the greatest impact, with approximately half the effect for each individual intervention and little or no effect for the control condition. We had hypothesized that differences might emerge: for example, that there might be different responses among women versus men to receiving telephone calls versus tailored printed newsletters. We did not find substantial evidence of differential effectiveness of interventions among demographic subgroups, suggesting that the combination of TPC plus TMI was appropriate regardless of demographic differences. We did not find any significant intervention effects for physical activity promotion.
Among the CRC survivor sample, we did not find strong evidence for differential dietary changes according to treatment condition. No significant differences were found using the 35-item measure, although we did see significant intervention effects based on the brief 2-item F&V measure. Timing of intervention may have played a role, in that the majority of survivors were 2–5 years post-diagnosis when they began participating in NC STRIDES, and had already made changes in F&V consumption between the immediate post-treatment time point of the previous NCCCS study and the NC STRIDES baseline data collection period (23). The NCCCS study had provided participants with general dietary advice via written non-tailored materials, such as study newsletters, which may have triggered such changes. The Fresh Start study did find significant effects of tailored print interventions on dietary change among breast and prostate cancer survivors. However, in that study patients were enrolled shortly after the time of diagnosis (20). An alternative explanation for the difference in effects seen with the two different measures could be that consumption was better reported by one measure versus the other. For example, participants may have responded more accurately to the brief measure as opposed to the longer 35-item measure, possibly due to fatigue, response burden, or cognitive processing issues. The F&V questionnaire items were positioned early in the survey; however, overestimation of consumption has been documented with longer FFQ instruments (45). A third possibility is that cancer survivors were generally motivated to make dietary changes and that simply being contacted and interviewed as part of another study was sufficient to initiate change. Findings from the averaged analysis indicate that survivors generally increased F&V on the order of .5–.9 servings per day, regardless of study group. Further research is necessary to determine the best intervention approaches and timing, as well as assessment methods, for dietary change interventions among CRC survivors.
The lack of effect on physical activity was disappointing and may point to the need for more intensive interventions to enable older adults to become more physically active. Our data suggest that participants may have over-reported their level of physical activity at baseline, given that the mean reported MVPA values were higher than would be expected based on national studies (34). Other studies have shown over-reporting using the 7-Day Physical Activity Recall (46). Another study of CRC survivors also has shown a decline in physical activity over time (47). This may be due in part to the age of this population, or to other co-morbidities and/or late effects of treatment. There also is evidence that brief motivational interviewing interventions may not be effective in promoting physical activity in healthy populations (28). Clearly, more research is needed in order to find effective intervention strategies for physical activity promotion in these populations.
In addition to greater efficacy, evidence showed that the combined intervention was more cost-effective for promoting F&V consumption. Clearly, decisions based on cost-effectiveness data must factor in the availability of resources and personnel to deliver interventions. Whereas the combined intervention was more cost-effective, it also cost approximately three times as much as delivering tailored newsletters without the addition of the motivational interviewing component. Thus, in situations where resources are limited and small changes are acceptable, the choice of TPC may be reasonable. A recent meta-analysis by Noar and colleagues has evaluated the relative effects of printed, tailored interventions over a range of behaviors (48). Findings demonstrated small but statistically significant effect sizes (r<.10). Velicer and colleagues have argued that even if tailored interventions produce relatively small effects compared to more intensive clinic-based programs, the impact on population-wide health may be as or more substantial because of the potential for wide reach and low cost of dissemination (49). We chose to tailor newsletters on the baseline survey data, except for limited update data from the 6-month update call. Whereas re-tailoring for each TPC might be hypothesized to increase the effect by increasing perceived relevance and timeliness of the feedback, it also substantially increases cost and respondent burden. Two recent studies with callers to the National Cancer Institute Cancer Information Service hotline showed that re-tailoring of TPCs did not produce significant increases in F&V consumption compared to providing multiple TPCs tailored to baseline data, whereas it did improve abstinence rates among smokers trying to quit (50,51). More research is needed; however, the utility of re-tailoring may in part depend upon the health behavior of interest and the need for re-tailoring to address relevant determinants, such as factors related to relapse in smoking cessation.
A possible reason for greater efficacy of the combined intervention may be the higher dose of intervention received by participants in the combined group. In our study, combined group participants received the full dose of four TPCs plus four TMI calls, which was double the number of intervention contacts for each separate intervention. In our study we were unable to disentangle the dose from the combination of strategies in assessing reasons for greater efficacy of the combined intervention. However, we have since collaborated on a larger replication study in the Netherlands that has tested the same TPC and TMI interventions (52). The Dutch “Vitalum” study standardized the intervention dose by giving the combined intervention group two TPCs and two MI calls, with TPC-only group receiving four mailings and the TMI group receiving four calls. Results indicate that all three intervention groups showed similar effects at follow-up, consuming significantly more F&V compared to the control group (van Keulen, personal communication, 2009). Thus, future research might examine the effect of doubling or optimizing the dose of either TPC or TMI alone or in combination, in order to achieve a similar effect size to the combined intervention in our present study. Given the relative cost-effectiveness of TPC versus TMI, providing more frequent TPCs or interspersing TPCs with less-frequent TMI calls might prove to be a feasible option for translating to dissemination.
This study had a number of limitations, including use of self-report data as the primary method of assessing F&V. The biomarker data on a sub-sample indicated significant correlation between the self-report measure and the objective blood measure of carotenoids. However, assessment of dietary consumption is difficult, and biases and errors are impossible to avoid. In addition, the study sample was recruited from a previous epidemiological study. Whereas this approach provided benefits in terms of recruiting participants from a population-based sample representative of a large geographic area and diverse in composition, it also resulted in several possible biases. First, participants had already been part of a study about CRC risk factors, and, especially among the survivor group, had made substantial lifestyle change prior to enrolling in our intervention study (23). It is likely that participants were more highly motivated than the average person, having completed interviews and provided blood samples for the previous study. The high level of motivation, however, enabled us to achieve a very high study retention rate of close to 90% in this older population.
The decision to conduct the study among both cancer survivors and the general population led to several limitations. We were limited in our ability to enroll a portion of the cancer survivor group from the previous NCCCS study due to the higher rates of health conditions, cancer recurrence, and deaths associated with having had a previous diagnosis of a serious cancer. Recruiters were instructed to ask participants if they were healthy enough to make lifestyle changes such as diet and exercise, and we excluded those who did not feel they would be able to participate due to health problems. In addition, as noted above, our study began enrolling participants approximately two years after the NCCCS ended, and we may therefore have missed the “teachable moment” for the survivor group.
The evidence for efficacy and cost-effectiveness of the combined NC STRIDES intervention to promote F&V change argues for further research to study dissemination and replication in other populations and settings. Our research group has partnered with the US Department of Veterans’ Affairs VA National Center for Health Promotion in order to determine whether this model combining tailored communication and motivational interviewing can be adapted and implemented within a large health care system. Pilot results from a small study in two VA sites replicated the effect size of one daily serving increase in F&V (Campbell, presentation at the 2007 Society for Behavioral Medicine annual meeting, Washington, DC), and we are currently engaged in a larger dissemination and implementation research study (NCI R01 CA124400-01) in partnership with the VA health system to examine organization and intervention characteristics and approaches for disseminating tailoring and motivational interviewing interventions to achieve public health impact.
Figure 1.
Table 1.
Demographics and Treatment Group Status (N and Percent of Total)
| Control | TPCa | TMIb | Combined | Totals | |
|---|---|---|---|---|---|
| N of All participants | 188 (25.6) | 181 (24.6) | 185 (25.2) | 181 (24.6) | 735 (100.0) |
| Age (mean, SD) | 66.6 (10.1) | 66.2 (10.5) | 67.1 (9.5) | 65.9 (9.8) | 66.5 (10.0) |
| N of Cases/Controls | |||||
| Cases | 66 (9.0) | 70 (9.5) | 72 (9.8) | 58 (7.9) | 266 (36.2) |
| General population | 122 (16.6) | 111 (15.1) | 113 (15.4) | 123 (16.7) | 469 (63.8) |
| Gender | |||||
| Male | 95 (12.9) | 83 (11.3) | 105 (14.3) | 89 (12.1) | 372 (50.6) |
| Female | 93 (12.7) | 98 (13.3) | 80 (10.9) | 92 (12.5) | 363 (49.4) |
| Race | |||||
| White | 124 (16.9) | 128 (17.4) | 107 (14.6) | 116 (15.8) | 475 (64.6) |
| African American | 64 (8.7) | 53 (7.2) | 78 (10.6) | 65 (8.8) | 260 (35.4) |
| Education | |||||
| ≤ High school* | 70 (9.6) | 89 (12.2) | 79 (10.8) | 73 (10.0) | 311 (42.7) |
| Some college* | 47 (6.5) | 43 (5.9) | 67 (9.2) | 49 (6.7) | 206 (28.3) |
| ≥College degree* | 69 (9.5) | 49 (6.7) | 37 (5.1) | 57 (7.8) | 212 (29.1) |
| Relationship status | |||||
| Married/partnered* | 134 (18.3) | 120 (16.4) | 114 (15.6) | 124 (17.0) | 492 (67.5) |
| Divorced/widowed* | 48 (6.6) | 54 (7.4) | 64 (8.8) | 48 (6.6) | 214 (29.4) |
| Never married* | 6 (0.8) | 5 (0.7) | 6 (0.8) | 6 (0.8) | 23 (3.2) |
| Employment status | |||||
| Employed* | 63 (8.6) | 70 (9.6) | 65 (8.9) | 77 (10.5) | 275 (37.5) |
| Retired/unemployed* | 125 (17.1) | 111 (15.1) | 119 (16.2) | 103 (14.1) | 458 (62.5) |
| Income | |||||
| Income < $30,000* | 70 (10.4) | 82 (12.2) | 89 (13.2) | 62 (9.2) | 303 (45.0) |
| Income ≥ $30,000* | 102 (15.2) | 83 (12.3) | 83 (12.3) | 102 (15.2) | 370 (55.0) |
| Insurance status | |||||
| Any health insurance | 141 (19.2) | 128 (17.4) | 145 (19.7) | 142 (19.3) | 556 (75.7) |
| No health insurance | 47 (6.4) | 53 (7.2) | 40 (5.4) | 39 (5.3) | 179 (24.4) |
| Weight | |||||
| Baseline | 181.0(36.5) | 177.1(37.3) | 184.0(37.5) | 182.3(49.8) | 181.4(40.6) |
| Follow-up | 179.2(35.9) | 177.6(37.2) | 183.1(38.1) | 183.0(53.8) | 180.7(41.8) |
| BMI | |||||
| Baseline | 29.1(5.1) | 28.6(5.4) | 29.3(5.4) | 29.3(6.7) | 29.1(5.7) |
| Follow-up | 28.9(5.1) | 28.7(5.4) | 29.2(5.3) | 29.3(7.3) | 29.0(5.8) |
Tailored print communication
Telephone motivational interviewing
Percents are of non-missing values: 2 missing for employment; 6 missing for both education and relationship status; 62 missing for income.
Acknowledgments
This project was supported by a grant from the National Cancer Institute, RO1-CA81914, and by additional grants from the National Institutes of Health: DK034987, RO1-CA66635, and DK056350.
References
- 1.American Cancer Society: Cancer Statistics Combined. [Accessibility verified August 3, 2009]; Available at http://www.cancer.org/docroot/PRO/content/PRO_1_1_Cancer_Statistics_2007_Presentation.asp.
- 2.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prentice RL, Thomson CA, Caan B, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99:1534–1543. doi: 10.1093/jnci/djm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle VC. Nutrition and colorectal cancer risk: a literature review. Gastroenterol Nurs. 2007;30:178–183. doi: 10.1097/01.SGA.0000278165.05435.c0. [DOI] [PubMed] [Google Scholar]
- 5.Bianchini F, Vainio H. Isothiocyanates in cancer prevention. Drug Metab Rev. 2004;36:655–667. doi: 10.1081/dmr-200033468. [DOI] [PubMed] [Google Scholar]
- 6.Schatzkin A, Mouw T, Park Y, et al. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2007;85:1353–1360. doi: 10.1093/ajcn/85.5.1353. [DOI] [PubMed] [Google Scholar]
- 7.Behavioral Risk Factor Surveillance Survey. [Accessibility verified August 3, 2009]; Available at http://apps.nccd.cdc.gov/brfss/display.asp?cat=FV&yr=2005&qkey=4415&state=UB.
- 8.Bines J, Gradishar WJ. Primary care issues for the breast cancer survivor. Compr Ther. 1997;23:605–611. [PubMed] [Google Scholar]
- 9.Talamini R, Franceschi S, Favero A, Negri E, Parazzini F, La Vecchia C. Selected medical conditions and risk of breast cancer. Br J Cancer. 1997;75:1699–1703. doi: 10.1038/bjc.1997.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell MK, Demark-Wahnefried W, Symons M, et al. Fruit and vegetable consumption and prevention of cancer: The Black Churches United for Better Health Project. Am J Public Health. 1999;89:1390–1396. doi: 10.2105/ajph.89.9.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell MK, James A, Hudson MA, et al. Improving multiple behaviors for colorectal cancer prevention among African American church members. Health Psychol. 2004;23:492–502. doi: 10.1037/0278-6133.23.5.492. [DOI] [PubMed] [Google Scholar]
- 12.Resnicow K, Jackson A, Wang T, et al. A motivational interviewing intervention to increase fruit and vegetable intake through Black churches: Results of the Eat for Life trial. Am J Public Health. 2001;91:1686–1693. doi: 10.2105/ajph.91.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus AC, Heimendinger J, Wolfe P, et al. A randomized trial of a brief intervention to increase fruit and vegetable intake: a replication study among callers to the CIS. Prev Med. 2001;33:204–216. doi: 10.1006/pmed.2001.0873. [DOI] [PubMed] [Google Scholar]
- 14.McBride CM, Clipp E, Peterson BL, Lipkus IM, Demark-Wahnefried W. Psychological impact of diagnosis and risk reduction among cancer survivors. Psychooncology. 2000;9:418–427. doi: 10.1002/1099-1611(200009/10)9:5<418::aid-pon474>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W, Rock CL. Nutrition-related issues for the breast cancer survivor. Semin Oncol. 2003;30:789–798. doi: 10.1053/j.seminoncol.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 18.Pierce JP, Newman VA, Flatt SW, et al. Telephone counseling intervention increases intakes of micronutrient- and phytochemical-rich vegetables, fruit and fiber in breast cancer survivors. J Nutr. 2004;134:452–458. doi: 10.1093/jn/134.2.452. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn KG, Boesen E, Ross L, Johansen C. Evaluation and outcome of behavioural changes in the rehabilitation of cancer patients: A review. Eur J Cancer. 2005;41:216–224. doi: 10.1016/j.ejca.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 21.Elliot DL, Goldberg L, Kuehl KS, Moe EL, Breger RK, Pickering MA. The PHLAME (Promoting Healthy Lifestyles: Alternative Models’ Effects) firefighter study: outcomes of two models of behavior change. J Occup Environ Med. 2007;49:204–213. doi: 10.1097/JOM.0b013e3180329a8d. [DOI] [PubMed] [Google Scholar]
- 22.Kinney AY, Harrell J, Slattery M, Martin C, Sandler RS. Rural-urban differences in colon cancer risk in blacks and whites: The North Carolina Cancer Study. J Rural Health. 2006;22:124–130. doi: 10.1111/j.1748-0361.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- 23.Satia JA, Keku T, Galanko JA, et al. Diet, lifestyle, and genomic instability in the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev. 2005;14:429–436. doi: 10.1158/1055-9965.EPI-04-0486. [DOI] [PubMed] [Google Scholar]
- 24.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promotion. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 25.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44:1175–1184. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 26.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 27.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. New York: Guilford Press; 2002. [Google Scholar]
- 28.Resnicow K, Jackson A, Braithwaite R, et al. Healthy Body/Healthy Spirit: a church-based nutrition and physical activity intervention. Health Educ Res. 2002;17:562–573. doi: 10.1093/her/17.5.562. [DOI] [PubMed] [Google Scholar]
- 29.Drewnowski A, Rock CL, Henderson SA, et al. Serum beta-carotene and vitamin C as biomarkers of vegetable and fruit intakes in a community-based sample of French adults. Am J Clin Nutr. 1997;65:1796–1802. doi: 10.1093/ajcn/65.6.1796. [DOI] [PubMed] [Google Scholar]
- 30.Dixon LB, Subar AF, Wideroff L, Thompson FE, Kahle LL, Potischman N. Carotenoid and tocopherol estimates from the NCI diet history questionnaire are valid compared with multiple recalls and serum biomarkers. J Nutr. 2006;136:3054–3061. doi: 10.1093/jn/136.12.3054. [DOI] [PubMed] [Google Scholar]
- 31.Resnicow K, Odom E, Wang T, et al. Validation of three food frequency questionnaires and 24-hour recalls with serum carotenoids in a sample of African American adults. Am J Epidemiol. 2000;152:1072–1080. doi: 10.1093/aje/152.11.1072. [DOI] [PubMed] [Google Scholar]
- 32.James AS, Hudson MA, Campbell MK. Demographic and social correlates of physical activity among African Americans. Am J Health Behav. 2003;27:421–431. doi: 10.5993/ajhb.27.4.14. [DOI] [PubMed] [Google Scholar]
- 33.Campbell MK, Meier A, Carr C, Reedy J, James A, Zheng B. Health behavior changes after colon cancer: Comparison of face-to-face and on-line focus group findings. Fam Community Health. 2001;24:88–103. doi: 10.1097/00003727-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 34.James A, Campbell MK, DeVellis B, Reedy J, Carr C, Sandler R. Health behavior correlates among colon cancer survivors: NC STRIDES baseline results. Am J Health Behav. 2006;30:720–730. doi: 10.5555/ajhb.2006.30.6.720. [DOI] [PubMed] [Google Scholar]
- 35.Kelsey KS, DeVellis BM, Begum M, Belton L, Hooten EG, Campbell MK. Positive affect, exercise and self-reported health in blue-collar women. Am J Health Behav. 2006;30:199–207. doi: 10.5555/ajhb.2006.30.2.199. [DOI] [PubMed] [Google Scholar]
- 36.Brug J, Glanz K, Van Assema P, Kok G, van Breukelen GJ. The impact of computer-tailored feedback and iterative feedback on fat, fruit, and vegetable intake. Health Educ Behav. 1998;25:517–531. doi: 10.1177/109019819802500409. [DOI] [PubMed] [Google Scholar]
- 37.Skinner CS, Strecher VJ, Hospers H. Physicians' recommendations for mammography: Do tailored messages make a difference? Am J Public Health. 1994;84:43–49. doi: 10.2105/ajph.84.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreuter MW, Farrell D, Olevitch L, Brennan L. Tailoring Health messages: Customizing Communications Using Computer Technology. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. [Google Scholar]
- 39.Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Substance Abuse Treatment. 2005a;28:19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Moyers TB, Miller WR, Hendrickson SM. How does motivational interviewing work? Therapist interpersonal skill predicts client involvement within motivational interviewing sessions. J Consult Clin Psychol. 2005b;73:590–598. doi: 10.1037/0022-006X.73.4.590. [DOI] [PubMed] [Google Scholar]
- 41.SAS Institute Inc. 100 SAS Campus Drive. Cary, NC: 27513-2414. [Google Scholar]
- 42.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 43.Gold MR, Siegel JE, Russel LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 44.Centers for Disease Control and Prevention. [Accessibility verified August 3, 2009]; Available at http://www.cdc.gov/cancer/minorityawareness/2000nhistext.htm.
- 45.Krebs-Smith S, Heimendinger J, Subar A, Patterson B, Pivonka E. Using food frequency questionnaires to estimate total fruit and vegetable intake: Association between the number of questions and total intake. J Nutr Educ. 1995;27:80–85. [Google Scholar]
- 46.Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. [Accessed May 28, 2009];Int J Behav Nutr Phys Act [serial online] 2006 3:7. doi: 10.1186/1479-5868-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lynch BM, Cerin E, Newman B, Owen N. Physical activity, activity change, and their correlates in a population-based sample of colorectal cancer survivors. Ann Behav Med. 2007;34:135–143. doi: 10.1007/BF02872668. [DOI] [PubMed] [Google Scholar]
- 48.Noar S, Benac C, Harris M. Does Tailoring Matter? Meta-Analytic Review of Tailored Print Health Behavior Change Interventions. Psychol Bull. 2007;133:673–693. doi: 10.1037/0033-2909.133.4.673. [DOI] [PubMed] [Google Scholar]
- 49.Velicer WF, Prochaska JO, Redding CA. Tailored communications for smoking cessation: Past successes and future directions. Drug Alcohol Rev. 2006;25:49–57. doi: 10.1080/09595230500459511. [DOI] [PubMed] [Google Scholar]
- 50.Heimendinger J, O'Neill C, Marcus AC, et al. Multiple tailored messages are effective in increasing fruit and vegetable consumption among callers to the Cancer Information Service. J Health Communication. 2005;10:65–82. doi: 10.1080/10810730500263646. [DOI] [PubMed] [Google Scholar]
- 51.Strecher VJ, Marcus A, Bishop K, et al. A randomized controlled trial of multiple tailored messages for smoking cessation among callers to the cancer information service. J Health Communication. 2005;10:105–118. doi: 10.1080/10810730500263810. [DOI] [PubMed] [Google Scholar]
- 52.van Keulen HM, Mesters I, Brug J, et al. Vitalum study design: RCT evaluating the efficacy of tailored print communication and telephone motivational interviewing on multiple health behaviors. BMC Public Health. 2008;8:216. doi: 10.1186/1471-2458-8-216. [DOI] [PMC free article] [PubMed] [Google Scholar]