Abstract
Objectives
To describe the burden experienced by family caregivers of older adults with depression and to examine the positive effects on caregivers of treating late-life depression.
Design
Two-phase treatment study for major depressive disorder (MDD) that included 6 weeks of open treatment with antidepressant medication for all older patients followed by 16 weeks of randomized treatment for patients who were partial responders, comparing a combination of medication and interpersonal psychotherapy with medication alone.
Setting
Primary care and university late-life mental health research clinic.
Participants
Adults aged 60 and older participating in a randomized trial for treatment of MDD who enrolled in a family caregiver study and their caregiver (N = 244 dyads).
Measurements
Improvement in patient symptoms during open treatment (lower scores on the Hamilton Rating Scale for Depression (HRSD)) and remission of depression during randomized treatment (3 consecutive weekly HRSD scores of ≤7) were examined as predictors of lower general caregiver burden and burden specific to patient depression.
Results
Caregivers reported a moderate to high level of general caregiver burden on average. Change in patient depression during open treatment was associated with significantly decreased depression-specific burden (β = −0.22, P =.001) and a trend toward lower general burden (β = − 0.08, P =.08). Caregivers of patients who remitted showed significantly decreased depression-specific burden (F (1,76) = 4.27, P =.04).
Conclusion
Treatment of late-life depression has benefits that extend to the family members on whom patients depend. Caregiver education and support may strengthen these effects.
Keywords: depression, caregiver, family, burden, treatment
There is substantial evidence that late-life depression can be successfully treated with antidepressant medication.1–3 The benefits of treatment extend beyond patients' improved mood to include lower physical disability,4 better social adjustment,5 and lower healthcare costs.6 Another important but unexamined outcome of depression treatment is less burden on the family caregiver. Although older adults with depression rely on family for assistance and support,7 the burden of providing this care is associated with poorer health and greater risk for mortality in caregivers.8,9 Therefore, alleviating caregiver burden is imperative from a public health perspective and, if achieved through treatment of an elderly person's depression, may also provide a more-complete picture regarding the efficacy of depression treatment.
This study was designed to address this issue with family caregivers who were recruited from a study of partial treatment response in late-life major depressive disorder (MDD). The treatment study included 6 weeks of low-dose antidepressant medication for all patients with depression followed by 16 weeks of randomized treatment for patients who had only a partial response to medication at the end of the 6 weeks. Partial response to antidepressant medication at 6 weeks is associated with poorer likelihood of eventual symptom remission in older adults.10,11 The randomized phase of treatment examined whether an increased dose of antidepressant medication combined with interpersonal psychotherapy (IPT) was superior to an increased dose of antidepressant medication alone. Patients showed decreases of approximately 30% in depression ratings during open treatment, but remission rates during randomized treatment were similar in the two randomized groups12 (cumulative response rates of 48–61%).
In the current study, it was predicted that decreased depressive symptomatology during open treatment with antidepressant medication would be associated with significantly lower caregiver burden, based on findings from dementia studies showing that cholinesterase inhibitors or antidepressant medication for patients resulted in lower caregiver burden.13,14 The second prediction focused on the randomized treatment phase for partial responders, in which the goal was remission of patient symptoms. It was predicted that caregivers of patients who remitted during randomized treatment would show greater alleviation of burden than caregivers of patients who did not remit. Whether caregivers of patients who received IPT and medication during the randomized phase would show even less burden than caregivers of partial responders who received medication alone was also examined. Greater improvement in caregiver burden may occur as the result of targeting patients' interpersonal problems, which have been shown to hinder family members' efforts to provide care and support when it is needed.15
The focus of this study was on change in two related but unique types of caregiver burden: depression-specific burden and general burden. It was expected that the largest decreases would be found in caregiver burden that was specific to the patients' depressive symptoms and behaviors; the depression subscale from the Revised Memory and Behavior Problems Checklist was used to assess how bothered or upset caregivers were about specific depressive behaviors.16 However, the burden of these family members may also stem from the effects of patient illness on social life, other family responsibilities, and finances. Therefore, the effects of patient improvement on this more general type of caregiver burden were also examined using the Burden Interview.17
Methods
Participants
Participants were a subsample of patients in a treatment study for MDD who enrolled in an ancillary family caregiver study and their identified caregiver. All patients were aged 60 and older and met criteria for current nonpsychotic MDD as established by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders;18 had a baseline score of 15 or higher on the Hamilton Rating Scale for Depression (HRSD19); and scored at least 17 on the Mini-Mental State Examination (MMSE20). The patient identified the caregiver as the family member or friend who currently provided the most support or assistance, consistent with previous research in the area of family and mood disorder.21,22 All caregivers were required to be aged 18 and older, living independently in their own homes (i.e., not in assisted living or a nursing home), and cognitively intact (no more than 3 errors on the 10-item Short Portable Mental Status Questionnaire23).
Design
Treatment Study
Patients received depression care management (DCM) and an open trial of escitalopram (i.e., 10 mg/d) for 6 weeks as the initial step of the treatment algorithm and were then reassessed for depression severity by a staff member who did not participate in treatment delivery or caregiver assessment. DCM was supportive and educational, with an emphasis on encouraging treatment adherence, managing risk for suicide, and working with family members to elicit their support of the treatment plan. After 6 weeks, patients meeting criteria for response (HRSD score ≤10) or nonresponse (HRSD score > 14) exited the study. Partial responders (HRSD score 11–14) had the escitalopram increased to 20 mg/d and were randomly assigned to receive 16 weekly sessions of IPT with DCM or DCM alone.
Caregivers were often in contact with clinic staff for the purpose of arranging patient appointments and managing medication-related issues but received no psychiatric treatment in this study. In addition, caregivers were not involved in patient IPT sessions with the exception of one session conducted with one patient. This research was conducted in primary care sites and in a university-based mental health research clinic.
Ancillary Caregiver Study
Recruitment of caregivers proceeded in two steps. First, patients who were eligible and interested in participating provided consent to contact their identified caregiver. Second, this individual was then contacted for screening and to request his or her participation. Baseline interviews were conducted with caregivers during the same time period of patients' baseline assessment and initiation of treatment, and caregivers were interviewed again at the time of patients' Week 6 assessment. Caregivers of patients who were randomized at Week 6 completed a final assessment at the end of the randomized treatment phase (Week 22).
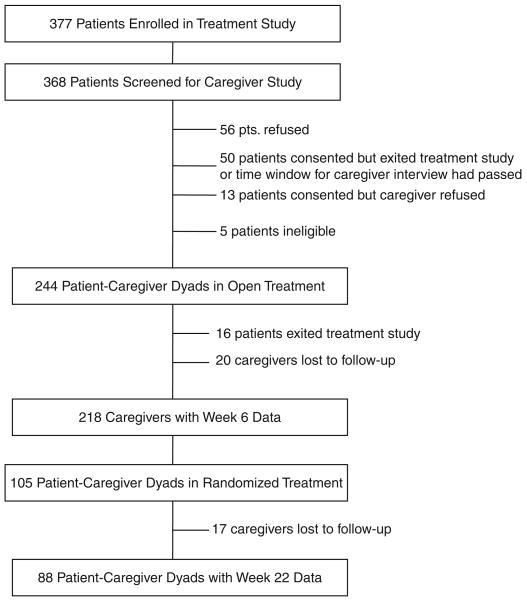
Three hundred seventy-seven patients were enrolled in the treatment study, and 368 of these were screened for participation in the caregiver study. Figure 1 provides information on accrual and retention of the sample during the open-treatment and randomized-treatment phases. Patients who participated in the caregiver ancillary study (n = 244) were compared with patients who did not (n = 124) on demographic and clinical characteristics. Patients in the caregiver study were more likely to be white than those who did not participate (chi-square (1, N = 365) = 9.67, P =.002). No other differences were observed between the two groups.
Figure 1.
Flow chart for participation in caregiver study.
One hundred twenty-four patients were randomized at Week 6, and 105 of these were enrolled in the caregiver study. Caregivers who were lost to follow-up during randomized treatment (n = 17) were compared with caregivers of patients who remained in the study (n = 88) on demographic characteristics and Week 6 burden. No significant differences were found between the two groups.
Measures
Patient Depression Severity
As described above, severity of the patients' depressive episode was assessed using the 17-item HRSD,19 an interviewer-administered rating scale that has been validated extensively in older patients (range 0–52, higher scores signifying worse depression). Independent raters blind to treatment assessed depression severity.
Depression-Specific Caregiver Burden
Depression-specific caregiver burden was assessed with items from the Revised Memory and Behavioral Problems Checklist (RMBPC) depression burden subscale, which was developed for caregivers or people with dementia and includes subscales assessing burden associated with memory problems and disruptive behaviors.16 This subscale was chosen because it directly assesses burden associated with depressive behaviors such as sadness and expression of hopelessness, whereas other measures of family burden associated with psychiatric disorders often use a lengthy semistructured interview or assess extraneous constructs.
Caregivers were asked about problems that they had experienced in the previous week while caring for the patient in terms of nine depressive behaviors (e.g., appeared sad or depressed, made comments about being a failure, cried, made comments about loneliness). Patient behavior scores were calculated by summing the number of behaviors endorsed by the caregiver. Caregivers who indicated that a behavior occurred were then asked how bothered or upset they were by the behavior using a 5-point scale ranging from 0 (not at all) to 4 (extremely). Burden scores were calculated by summing bother/upset ratings across all behaviors and assigning 0 (not at all bothersome or upsetting) to behaviors that did not occur (range 0–36; higher scores signifying greater burden).
General Caregiver Burden
The 22-item Burden Interview was used to measure general caregiver burden17 (e.g., How often do you feel stressed between caring for him/her and trying to meet other responsibilities? How often do you feel that he/she is dependent on you?). The total score ranges from 0 to 88, with higher values indicating greater burden. The bivariate correlation coefficient (r) between depression-specific and general caregiver burden was 0.50 (P<.001).
Covariates
Patients were assessed at baseline for physical illness and cognitive impairment using the Cumulative Illness Rating Scale for Geriatrics (CIRS-G24) and the MMSE.20 The CIRS-G assesses the presence and severity of 13 illness conditions (e.g., heart disease, respiratory problems, musculoskeletal disease); scores range from 0 to 52. The MMSE consists of 13 questions that assess orientation to place and time, learning and memory, ability to copy a simple design, attention, and calculation skills (range 0–30). Caregivers were assessed at baseline for physical health and depressive symptoms, using the one-item perceived health question from the Medical Outcomes Study 36-item Short-Form Survey (SF-36)25 and the Centers for Epidemiologic Studies Depression Scale (CES-D).26
Statistical Analysis
The first hypothesis focused on the open-treatment phase and was tested using hierarchical ordinary least-squares regression analysis, controlling for patient baseline depression severity and caregiver baseline burden. To identify additional control variables, zero-order correlations of the caregiver burden measures were examined at Week 6 with baseline patient characteristics (i.e., demographics, duration of current episode, CIRS-G, MMSE) and caregiver characteristics (i.e., demographics, relationship to patient, assistance to patient with activities of daily living (ADLs) and instrumental activities of daily living (IADLs), perceived health, current psychotropic medication, and CES-D). The following four caregiver characteristics were significantly related to greater burden of either type (P<.05) and were entered into the regression analyses with baseline patient depression severity and caregiver burden: greater age, lower income, higher CES-D score, and being an adult child of the patient. Change in patient depression severity during open treatment (i.e., a difference score representing change in HRSD score from baseline to Week 6) was entered in the second block of this analysis to examine its independent effect on caregiver burden.
The second and third hypotheses focused on the randomized-treatment phase and were tested using repeated-measures analysis of covariance (RM ANCOVA) to determine whether there were significant interactions (P<.05) between change in caregiver burden from Week 6 to Week 22 and patient remission status or randomization group. These analyses controlled for patient depression severity at Week 6 and caregiver characteristics (age, income, CES-D, adult child status). Change in patient depression severity during randomized treatment (i.e., a difference score representing change in HRSD score from Week 6 to Week 22) was also examined as a predictor of caregiver burden at Week 22.
Results
Table 1 provides background information for the sample. Patients exhibited the levels of physical and cognitive functioning typically seen in late-life depression.27,28 The average score of 10 on the CIRS-G indicates a moderate level of chronic medical illness, and virtually all (98%) of the patients reported at least two comorbid chronic illness conditions. The average MMSE score was normal but ranged from 18 to 30. Approximately half of the caregivers were adult sons or daughters of the patient, and the remainder were spouses (n = 103, 42%) or other family members (e.g., siblings; n = 32, 13%). Most caregivers (88%) were assisting the patient with at least one IADL or ADL.
Table 1.
Sample Characteristics (N = 244 Dyads)
| Characteristic | Value |
|---|---|
| Patient | |
| Age, mean ± SD | 73.2 ± 7.9 |
| Female, n (%) | 169 (69.3) |
| White, n (%) | 217 (88.9) |
| Education, years, mean ± SD | 13.6 ± 2.9 |
| Duration of current major depressive disorder episode, weeks, mean ± SD | 170.9 ± 338.5 |
| Cumulative Illness Rating Scale for Geriatrics score, mean ± SD (range 0–52) | 10.3 ± 3.8 |
| Mini-Mental State Examination score, mean ± SD (range 0–30) | 28.1 ± .1 |
| Caregiver | |
| Age, mean ± SD | 57.8 ± 13.9 |
| Female, n (%) | 159 (65.2) |
| White, n (%) | 212 (86.9) |
| Adult child of patient, n (%) | 109 (44.7) |
| Education, years, mean ± SD | 14.7 ± 2.0 |
| Household income, $, range | 30,000–39,000 |
| Number of activity of daily living or instrumental activity of daily living provided assistance to patient with, mean ± SD | 2.7 ± 1.8 |
| Perceived health, mean ± SD (1 = poor, 5 = excellent) | 3.5 ± 1.0 |
| Current use of psychotropic medication, n (%) | 49 (20.1) |
| Centers for Epidemiological Studies Depression Scale score, mean ± SD (range 0–60) | 10.2 ± 10.1 |
SD = standard deviation.
Change in Caregiver Burden During Patients' Open Treatment
At baseline, patient depression severity was moderate to severe, with scores ranging from 15 to 27 (Table 2). Reflecting the design of the treatment study, scores for patients who continued after 6 weeks were in the partial response range (i.e., HRSD = 11–14). The average general caregiver burden score of 24 found in this sample has been characterized as a moderate to high level of burden in caregivers or people with dementia29 and is the cut point for risk for clinical depression in family caregivers to different patient populations.30 In terms of depression-specific burden, comparative data for subscales of the RMBPC are not readily available, although caregiver average score on the total RMBPC (13.4; not shown) is close to the average score of 16 for caregivers of people with dementia in the Resources for Enhancing Caregiver Health trial, whose elderly subjects were moderately to severely cognitively impaired.31
Table 2.
Change in Average Levels of Patient Depression and Caregiver Burden
| Mean ± Standard Deviation | |||
|---|---|---|---|
| Phase | Patient 17-Item Hamilton Rating Scale for Depression Score (Range 0–52) |
Depression Burden Subscale of the Revised Memory and Behavior Problems Checklist (Range 0–36) |
Burden Interview Score (Range 0–88) |
| Open Treatment (n = 244 dyads) | |||
| Baseline | 18.5 ± 3.0 | 8.4 ± 7.5 | 23.9 ± 15.3 |
| Week 6 | 11.5 ± 4.7 | 4.2 ± 5.7 | 19.2 ± 13.7 |
| Randomized Treatment (n = 105 dyads) | |||
| Week 6 | 12.4 ± 1.1 | 5.0 ± 5.7 | 20.9 ± 14.4 |
| Week 22 | 8.8 ± 4.9 | 3.7 ± 5.8 | 19.0 ± 14.4 |
Table 2 shows that there was a substantial drop in depression-specific caregiver burden during open treatment, with scores decreasing by half, and a smaller decrease during randomized treatment. General caregiver burden dropped by 4 points on average during open treatment but showed little change during randomized treatment.
The first two columns of Table 3 display findings from the last step of the hierarchical regression analyses examining the independent effects of change in depression severity during the open-treatment phase on each type of caregiver burden. As predicted, improvement in patient depression was associated with lower depression-specific caregiver burden at Week 6 (β = −0.22, P<.001; coefficient of determination (R2) change = 0.04). Improvement in patient depression also was associated with decreased general caregiver burden, but at a trend level (β = −0.08, P =.08; R2 change = 0.01).
Table 3.
Effect of Change in Patient Depression on Caregiver Burden
| β (Standard Error) | ||||
|---|---|---|---|---|
| Open-Treatment Phase (N = 244 Dyads) | Randomized-Treatment Phase (N = 105 Dyads) | |||
| Predictor | Depression Caregiver Burden at Week 6 | General Caregiver Burden at Week 6 | Depression Caregiver Burden at Week 22 | General Caregiver Burden at Week 22 |
| Block 1, Covariates | ||||
| Baseline/Week 6 caregiver burden | 0.49 (0.06)*** | 0.78 (0.05)*** | 0.58 (0.08)*** | 0.73 (0.07)*** |
| Baseline/Week 6 patient Hamilton Rating Scale for Depression | 0.04 (0.06) | −0.03 (0.05) | 0.07 (0.08) | 0.16 (0.07)** |
| Caregiver age | −0.08 (0.07) | −0.12* (0.06) | −0.13 (0.11) | −0.02 (0.09) |
| Caregiver income | −0.11 (0.06) | 0.09* (0.05) | 0.10 (0.09) | 0.03 (0.07) |
| Caregiver Centers for Epidemiologic Studies Depression Scale score | −0.01 (0.06) | −0.06 (0.05) | 0.17 (0.08)* | 0.17 (0.07)** |
| Caregiver relationship: adult child | 0.01 (0.07) | 0.03 (0.06) | −0.11 (0.10) | −0.08 (0.08) |
| Block 2 | ||||
| Change in patient depression | −0.22 (0.06)*** | −0.08† (0.05) | −0.23 (0.08)** | −0.09 (0.07) |
| Total coefficient of determination | 0.38*** | 0.64*** | 0.48*** | 0.66*** |
P =.08,
P≤.05,
P≤.01,
P≤.001.
Change in Caregiver Burden During Randomized Treatment
Findings from analyses focused on change in HRSD scores during randomized treatment were similar to findings for the open-treatment phase (last two columns of Table 3). Improvement in patient depression was associated with less depression-specific caregiver burden (β = −0.23, P<.01; R2 change = 0.05). A similar effect was found for general caregiver burden, but it did not reach statistical significance (β = −0.09, P =.15).
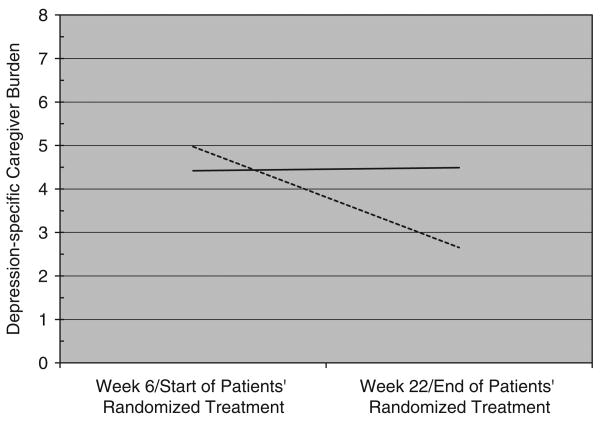
Fifty-two patients in this ancillary study met criteria for remission of depression during randomized treatment, whereas 53 patients did not remit. RM ANCOVA showed a significant interaction between change in depression-specific caregiver burden and patient remission status (F (1,76) = 4.27, P =.04). As depicted in Figure 2, caregivers of patients who remitted during treatment reported a significant decrease in burden, whereas caregivers of patients who did not remit experienced no alleviation of their burden.
Figure 2.
Patient remission status × time interaction for depression-specific caregiver burden. Dashed line = patient remitted (3 consecutive weekly scores of ≤7 on the Hamilton Rating Scale for Depression).
Solid line = patient did not remit.
Analyses conducted using the patient depressive behavior subscale of the RMBPC (i.e., number of behaviors occurring) showed findings similar to those conducted with the depression burden subscale of the RMBPC, for the open- and randomized-treatment phases. The analysis for general caregiver burden did not show an interaction with remission status, in that all caregivers experienced a small decrease in burden. Findings from the RM ANCOVA also did not indicate differential change in either type of caregiver burden according to patient randomization group (IPT vs medication).
Discussion
The effects of late-life depression on family caregivers have received little research attention despite the aging of the population and increasing rates of clinically significant depressive symptomatology in late life.32 The current study found that caregivers of people with depression experience moderate to high levels of burden on average, putting them at risk for physical and psychiatric morbidity, but benefit when their relative receives depression treatment. The finding of less burden during open treatment extends a previous finding that caregivers of patients with Alzheimer's disease and depression who responded to 6 weeks of sertraline show lower burden specific to patients' behavioral disturbances.14 The current study found that remission of patient depression during randomized treatment was associated with further decreases in caregiver burden, whereas caregivers of patients who did not remit experienced no alleviation of burden despite the fact that patient HRSD scores had improved.
Although the average level of depression-specific burden during open treatment decreased by half, improvement in patient depression accounted for a small decrease in caregiver burden. In addition, caregivers of patients in randomized treatment experienced less burden regardless of whether the patient received psychotherapy and medication or medication alone. This is consistent with the finding that the rate of patient remission in both groups was substantially better than rates commonly observed in usual care, but combined treatment was not superior to medication alone.12 One explanation is that depression care management was used in both groups and involved caregivers to some extent to elicit their support throughout the study. This suggests that additional improvement in caregiver outcomes beyond those observed in the current study might be achieved with a dyadic psychosocial treatment approach that systematically targets all caregivers.33 Specifically, an education and support intervention for caregivers such as that used successfully in adult bipolar disorder could supplement patient pharmacotherapy.34
These findings are important for the broader caregiving literature, which has given little attention to depression as an indicator of suffering of older people. Subsyndromal or syndromal depression often accompanies physical illness and cognitive impairment in late life35 and may be a unique source of caregiver burden. That is, recent research on older adults with general physical impairment found that depression was an independent predictor of family caregiver burden after controlling for the patient's level of cognitive impairment, disability, and medical illness burden.36,37 Findings of the current study also are consistent with those of a previous study that mere exposure to the psychological suffering of a significant other contributes to caregiver distress.38,39
The treatment study's enrollment of older adults from primary care, use of relatively few exclusion criteria, and use of a widely prescribed antidepressant with good efficacy and tolerability enhances the generalizability of these findings. Despite these strengths, it also is important to acknowledge potential sources of bias in the sample. First, caregiver availability for assessment was not required for enrollment in the treatment study, and therefore the sample is likely to include less-burdened family members, although this also means that the improvement in burden observed in this study may be an underestimate of what could be achieved. It is important for future research on burden in caregivers of people with depression to be conducted outside of clinical trials in which patients receive intensive care management and the care team encourages family members' support for treatment. Second, minority patients were less inclined than white patients to participate in the ancillary caregiver study, perhaps reflecting cultural differences in patients' privacy regarding mental health concerns or the unwillingness of their family caregivers to participate in research.40,41
National data indicate that older adults receive additional hours of physical assistance from their family members when they have depressive symptoms, with estimated costs equivalent to approximately $9 billion for this care.7 Thus, the findings of this study have implications that extend beyond caregiver psychological well-being and physical health to their ability to provide ongoing care to one of the most vulnerable populations of elderly people. The secondary benefits of successful depression treatment observed in this study strengthen the argument for greater attention to identification and treatment of late-life depression.
Acknowledgments
The authors thank the staff of the Clinical Trials Management Unit of the Advanced Center for Interventions and Services Research at the University of Pittsburgh Department of Psychiatry for their care of the research participants. The authors also thank the research participants themselves for participation in the study.
This research was supported by NIH grants K01 MH065547, R01 MH37869, R01 MH43832, K23 MH073772, K23 MH067710, KL2 RR024154, T32 MH19986, P30 MH071944; the University of Pittsburgh Medical Center Endowment in Geriatric Psychiatry; and the John A. Hartford Foundation Center of Excellence in Geriatric Psychiatry.
Footnotes
Conflict of Interest: Dr. Reynolds receives research support in the form of pharmaceutical supplies for his National Institutes of Health (NIH)–sponsored research from GlaxoSmithKline, Forest Laboratories, Pfizer, Lilly, Bristol Meyers Squibb, and Wyeth. Dr. Karp receives medication supplies from Eli Lilly for his NIH-sponsored research, is an adviser to Lilly, owns stock in Corcept, and has received honoraria for unrestricted educational activity from Novartis and Pfizer. Dr. Gildengers receives research support for an investigator-initiated trial for GlaxoSmithKline. Dr. Whyte has received research support for investigator-initiated trials for Pfizer, Forrest, Ortho-McNeil, and Lilly.
Author Contributions: Study concept and design: Martire, Schulz, Reynolds. Acquisition of data: Martire, Reynolds, Karp, Gildengers, and Whyte. Analysis and interpretation of data: Martire, Schulz, Reynolds, Karp, Gildengers, and Whyte. Preparation of manuscript: Martire, Schulz, Reynolds, Karp, Gildengers, and Whyte.
Sponsor's Role: The funding organizations had no roles in the design and conduct of the study; analysis and interpretation of the data; or preparation, review, and approval of the manuscript.
References
- 1.Bruce ML, Ten Have TR, Reynolds CF, III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: A randomized controlled trial. JAMA. 2004;291:1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds CF, III, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354:1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- 3.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 4.Unutzer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manage Care. 2008;14:95–100. [PMC free article] [PubMed] [Google Scholar]
- 5.Lenze EJ, Dew MA, Mazumdar S, et al. Combined pharmacotherapy and psychotherapy as maintenance treatment for late-life depression: Effects on social adjustment. Am J Psychiatry. 2002;159:466–468. doi: 10.1176/appi.ajp.159.3.466. [DOI] [PubMed] [Google Scholar]
- 6.Katon WJ, Schoenbaum M, Fan MY, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry. 2005;62:1313–1320. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- 7.Langa KM, Valenstein MA, Fendrick AM, et al. Extent and cost of informal caregiving for older Americans with symptoms of depression. Am J Psychiatry. 2004;161:857–863. doi: 10.1176/appi.ajp.161.5.857. [DOI] [PubMed] [Google Scholar]
- 8.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 9.Vitaliano PP, Zhang J, Scanlan J. Is caregiving hazardous to one's physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 10.Andreescu C, Mulsant BH, Houck PR, et al. Empirically derived decision trees for the treatment of late-life depression. Am J Psychiatry. 2008;165:855–862. doi: 10.1176/appi.ajp.2008.07081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;6:113–120. doi: 10.1097/01.jcp.0000204471.07214.94. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds CF, Dew MA, Martire LM, et al. Treating major depression to remission in older adults: A controlled evaluation of combined interpersonal psychotherapy and escitalopram. Int J Geriatr Psychiatry. doi: 10.1002/gps.2443. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lingler JH, Martire LM, Schulz R. Caregiver-specific outcomes in antidementia clinical drug trials: A systematic review and meta-analysis. J Am Geriatr Soc. 2005;53:983–990. doi: 10.1111/j.1532-5415.2005.53313.x. [DOI] [PubMed] [Google Scholar]
- 14.Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer's disease: Efficacy and safety of sertraline therapy, and the benefits of depression reduction: The DIADS. Arch Gen Psychiatry. 2003;60:737–746. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- 15.Hinrichsen GA, Hernandez NA. Factors associated with recovery from and relapse into major depressive disorder in the elderly. Am J Psychiatry. 1993;150:1820–1825. doi: 10.1176/ajp.150.12.1820. [DOI] [PubMed] [Google Scholar]
- 16.Teri L, Truax P, Logsdon R, et al. Assessment of behavioral problems in dementia: The Revised Memory and Behavior Problems Checklist. Psychol Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 17.Zarit SH, Zarit JM. The Memory and Behaviour Problems Checklist 1987R and the Burden Interview (Technical report) State College, PA: Pennsylvania State University; 1987. [Google Scholar]
- 18.First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis 1 Disorders, SCID 1: Clinical Version, Administration Booklet. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Hinrichsen GA. Adjustment of caregivers to depressed older adults. Psychol Aging. 1991;6:631–639. doi: 10.1037//0882-7974.6.4.631. [DOI] [PubMed] [Google Scholar]
- 22.Perlick DA, Rosenheck RR, Clarkin JF, et al. Impact of family burden and affective response on clinical outcome among patients with bipolar disorder. Psychiatr Serv. 2004;55:1029–1035. doi: 10.1176/appi.ps.55.9.1029. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Shelbourne CD. The MOS 36-item short-form health survey (SF-36): I: Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 27.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 28.Lenze EJ, Rogers JC, Martire LM, et al. The association of late-life depression and anxiety with physical disability: A review of the literature and prospectus for future research. Am J Geriatr Psychiatry. 2001;9:113–135. [PubMed] [Google Scholar]
- 29.Hebert R, Bravo G, Preville M. Reliability, validity and reference values of the Zarit Burden Interview for assessing informal caregivers of community-dwelling older persons with dementia. Can J Aging. 2000;19:494–507. [Google Scholar]
- 30.Schreiner AS, Morimoto T, Arai Y, et al. Assessing family caregiver's mental health using a statistically derived cut-off score for the Zarit Burden Interview. Aging Mental Health. 2006;10:107–111. doi: 10.1080/13607860500312142. [DOI] [PubMed] [Google Scholar]
- 31.Wisniewski S, Belle SH, Coon DW, et al. The Resources for Enhancing Alzheimer's Caregiver Health (REACH): Project design and baseline characteristics. Psychol Aging. 2003;18:375–384. doi: 10.1037/0882-7974.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulz R, Martire LM, Klinger JN. Evidence-based caregiver interventions in geriatric psychiatry. Psychiatr Clin North Am. 2005;28:1007–1038. doi: 10.1016/j.psc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Martire LM, Schulz R. Involving family in psychosocial interventions for chronic illness. Curr Direct Psychol Sci. 2007;16:90–94. [Google Scholar]
- 34.Miklowitz DJ, Simoneau TL, George EL, et al. Family-focused treatment of bipolar disorder: 1-year effects of a psychoeducational program in conjunction with pharmacotherapy. Biol Psychiatry. 2000;48:582–592. doi: 10.1016/s0006-3223(00)00931-8. [DOI] [PubMed] [Google Scholar]
- 35.Charney DS, Reynolds CF, III, Lewis L, et al. Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry. 2003;60:664–672. doi: 10.1001/archpsyc.60.7.664. [DOI] [PubMed] [Google Scholar]
- 36.Sewitch MJ, McCusker J, Dendukuri N, et al. Depression in frail elders: Impact on family caregivers. Int J Geriatr Psychiatry. 2004;19:655–665. doi: 10.1002/gps.1135. [DOI] [PubMed] [Google Scholar]
- 37.Scazufca M, Menezes PR, Almeida OP. Caregiver burden in an elderly population with depression in São Paulo, Brazil. Soc Psychiatry Psychiatr Epidemiol. 2002;27:416–422. doi: 10.1007/s00127-002-0571-6. [DOI] [PubMed] [Google Scholar]
- 38.Monin JK, Schulz R. Interpersonal effects of suffering in older adult caregiving relationships. Psychol Aging. 2009;24:681–695. doi: 10.1037/a0016355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz R, Hebert RS, Dew MA, et al. Patient suffering and caregiver compassion: New opportunities for research, practice, and policy. Gerontologist. 2007;47:4–13. doi: 10.1093/geront/47.1.4. [DOI] [PubMed] [Google Scholar]
- 40.Ayalon L, Arean PA, Alvidrez J. Adherence to antidepressant medications in black and Latino elderly patients. Am J Geriatr Psychiatry. 2005;13:572–580. doi: 10.1176/appi.ajgp.13.7.572. [DOI] [PubMed] [Google Scholar]
- 41.Freimuth VS, Quinn SC, Thomas SB, et al. African American's views on research and the Tuskegee syphilis study. Soc Sci Med. 2001;52:797–808. doi: 10.1016/s0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]