Abstract
Individuals with mechanical heart valve implants are plagued by flow-induced thromboembolic complications, which are undoubtedly caused by platelet activation. Flow fields in or around the affected regions involve brief exposure to pathologically high-shear stresses on the order of 100 to 1000 dyne/cm2. Although high shear is known to activate platelets directly, their subsequent behavior is not known. We hypothesize that the post-high-shear activation behavior of platelets is particularly relevant in understanding the increased thrombotic risk associated with blood-recirculating prosthetic cardiovascular devices. Purified platelets were exposed to brief (5–40 s) periods of high-shear stress, and then exposed to longer periods (15–60 min) of low shear. Their activation state was measured using a prothrombinase-based assay. Platelets briefly exposed to an initial high-shear stress (e.g., 60 dyne/cm2 for 40 s) activate a little, but this study shows that they are now sensitized, and when exposed to subsequent low shear stress, they activate at least 20-fold faster than platelets not initially exposed to high shear. The results show that platelets in vitro exposed beyond a threshold of high-shear stress are primed for subsequent activation under normal cardiovascular circulation conditions, and they do not recover from the initial high-shear insult.
Keywords: Thrombosis, Hemodynamics, Shear-induced platelet activation
INTRODUCTION
Platelet activation plays a central role in thrombus formation in mechanical heart valves (MHV).14,38 Flow-induced mechanisms of platelet activation under these pathological conditions are well established9,20 and involve disturbed hemodynamic conditions which briefly exert high-shear stresses (100–8000 dyne/cm2) on platelets.1,21,33 Brief exposures lasting a few milliseconds are known to cause platelet activation,34 but it is not known how platelet activation progresses after these brief exposures. This information is critical to understanding why patients with implanted prosthetic heart valves are at such high risk of thromboembolism, even with anticoagulation therapy.10,11 We propose that the cause of this behavior is platelet sensitization, in which, after the initial high-shear insult, platelets are much more susceptible to activation under low, non-pathological shear conditions.
Upon passage through constrictions such as heart valves and stenoses in the circulation, platelets are exposed to very brief durations of high-shear stress, followed by relatively long periods of normal shear exposure. Previous work in vitro on the exposure of platelets to brief high-shear stress followed by physiological shear stress has shown that, after activation during the high-shear phase, enhanced platelet aggregation in response to exogenous agonists is seen during the subsequent low-shear phase.40 Here, we extend those observations to a longer exposure to low shear, and to measurement of the intrinsic activation state of the platelets themselves.
Platelet aggregation in response to various exogenous chemical agonists and shear stress is the main method presently used to assess platelet function.8 However, a consensus on what level of shear stress is necessary to activate platelets and how platelets respond once activated is lacking. One particular question is whether platelets cross a critical shear threshold beyond which they are permanently primed or sensitized to further shear insult. While we have shown that platelets activated by high-shear stress can retain prior memory when exposed to subsequent pulses of high-shear stress,24 their response to extended subsequent periods of normal, non-pathological shear is not known.
Normal vascular flow is characterized by low wall and fluid shear stresses, ranging from 1 to 10 dyne/cm2 in venous flow to 50 dyne/cm2 in arterial flow, peaking at approximately 60 dyne/cm2 in the arterioles.15,17,33 Stresses in pathological regions exceed 50 dyne/cm2 and the fluid may be marked by flow separation, where the streamlines do not follow the contours of the vessel wall, or by vortices, which promote recirculation and increase the residence time of blood components.15,17 In the past, testing for device thrombogenicity has focused on hemolysis of red blood cells, which is estimated to occur at shear stress levels above approximately 6000 dyne/cm2.16,30 This is an order of magnitude higher than that required for platelet activation,27 and devices tested with red cells in this manner are not optimized to minimize flow-induced thrombogenicity.5,6
When starting this study, we knew from the work of many investigators and our own work that platelets exposed to high-shear stress, even for short durations, become partly activated. In this article, we address the question of what happens considerably later, when platelets return to normal low-shear conditions. Initially, we hypothesized that they might recover and revert to a low activation state, rather as platelets treated in an aggregometer with low concentrations of exogenous agonists can reverse their initial shape change and revert to a quiescent state.3 However, we see here that when shear is the activating principle, there is no recovery. Indeed, brief exposure to high shear, while it directly causes little platelet activation, sensitizes them so that they are substantially more susceptible to non-pathological shear stress.
MATERIALS AND METHODS
Platelet Preparation
Informed consent for blood donation, approved by the Stony Brook University IRB, was obtained from healthy adult volunteers of either sex who had not taken ibuprofen or aspirin for 2 weeks. Whole blood, 30 mL, was drawn by venipuncture, collected into a 3-mL acid-citrate dextrose (ACD-A), and centrifuged at 650 × g for 6 min to obtain platelet-rich plasma. Gel-filtered platelets (GFP) were prepared as described previously.24,28,37 Platelets were counted using a Z1 Particle Counter (Coulter Corporation, Miami, FL, USA) and diluted to 20000/µL final count in platelet buffer, a Hepes-modified Ca2+-free Tyrodes buffer with 0.1% bovine serum albumin, with 3 mM CaCl2 added 10 min prior to experiments. The viscosity of this mixture was measured as 1 cP at 37 °C.
Platelet Activation State
Platelet activation state (PAS) was quantified using a chemically modified prothrombinase-based assay that uses acetylated prothrombin to measure the rate of thrombin generation.18,19 As we have shown under other conditions that cause platelet activation, the results of this assay correlate well with intensity of P-selectin expression, as quantified with flow cytometry. 15 All prothrombinase assays were performed at a count of 5000 platelets/µL in the actual assay, and PAS values are thus independent of platelet count. PAS values were normalized against the activity of fully activated platelets, obtained by sonication (10 W for 10 s, Branson Sonifier 150 with microprobe, Branson, MO, USA). PAS values obtained are therefore expressed as a fraction of the maximum thrombin-generating capacity, with a maximum of 1.0.
Platelet activation rates (Tables 1 and 2) are the rates of change of PAS with time of incubation, with units of reciprocal time.
TABLE 1.
Platelet activation rates after pre-exposure to high-shear stress.
| Pre-exposure shear stress (dyne/cm2) |
Duration (s) | Integral shear stress (dyne·s/cm2) |
Platelet activation rate (ΔPAS/min) (×10−4 min−1) |
p vs. control |
|---|---|---|---|---|
| (a) Variable peak shear | ||||
| 1 (control) | 40 | 40 | 1.6 ± 0.6 | – |
| 20 | 40 | 800 | −0.2 ± 1.8 | >0.5 |
| 30 | 40 | 1200 | 1.3 ± 1.2 | >0.5 |
| 40 | 40 | 1600 | 5.2 ± 3.4 | >0.5 |
| 50 | 40 | 2000 | 12.3 ± 3.9 | >0.5 |
| 60 | 40 | 2400 | 24.4 ± 8.7 | <0.05 |
| 70 | 40 | 2800 | 25.6 ± 9.5 | <0.05 |
| (b) Variable peak duration | ||||
| 1 (control) | 40 | 40 | −1.3 ± 0.6 | – |
| 70 | 5 | 350 | −0.8 ± 0.8 | >0.5 |
| 70 | 10 | 700 | −0.3 ± 1.1 | >0.5 |
| 70 | 20 | 1400 | 2.3 ± 1.6 | >0.5 |
| 70 | 30 | 2100 | 4.8 ± 2.0 | >0.5 |
| 70 | 40 | 2800 | 14.9 ± 6.8 | <0.005 |
TABLE 2.
Role of crosstalk in platelet sensitization.
| Condition | Integral shear stress (dyne·s/cm2) |
Platelet activation rate (ΔPAS/min) (×10−4 min−1) |
p-Values |
|---|---|---|---|
| (a) Platelet count | |||
| 20000/µL | 2800 | 45.1 ± 17.2 | – |
| 150000/µL | 2800 | 36.8 ± 8.9 | >0.5 |
| (b) Effect of quiescent platelets | |||
| Quiescent platelets only | 40 | 3.5 ± 5.3 | <0.1 |
| Sensitized platelets only | 2400 | 50.8 ± 30.1 | – |
| Sensitized + quiescent platelets | 2400 | 51.8 ± 15.0 | >0.8 |
| (c) Apyrase (1 U/mL) | |||
| Untreated | 2800 | 114.6 ± 6.4 | – |
| Apyrase-treated | 2800 | 76.9 ± 15.2 | <0.05 |
Platelet activation rates are means ± SEM for the post-exposure, low-shear phase (see “Results” section). (a) Effect of platelet count. (b) Effect of sensitized platelets on quiescent platelets. (c) Effect of apyrase addition.
Exposure of Platelets to Shear Stress in Hemodynamic Shearing Device
Platelets were exposed to shear stress in a computer-controlled cone-plate-Couette viscometer, or hemodynamic shearing device (HSD).24 Before experiments, all platelet-contacting surfaces were siliconized with Sigmacote (Sigma–Aldrich, St. Louis, MO, USA) and warmed to 37 °C. Platelets were exposed to a short varying period of varying high-shear stress, followed by an extended period of low-shear stress, 1 dyne/cm2, for a total duration of 15 min. The initial period of high-shear stress, here described as pre-exposure, involved exposure for up to 40 s, and shear stresses up to 70 dyne/cm2. The acceleration and deceleration times for this phase were 0.5 s. Samples for PAS measurement were taken from the annular region of the HSD at the start of the experiment, at 40 s, and every 3 min until 15 min. Platelet counts were taken at the start, at 40 s, and at the end, to measure any platelet loss. For both the variable peak shear stress and variable peak duration groups, data following the pre-exposure were fitted to a straight line, to determine the platelet activation rate (see above). In all cases, the validity of a linear fit was verified using a chi-square goodness-of-fit test.
Platelet Lysis
Platelet lysis was measured by the release of cytoplasmic lactate dehydrogenase (LDH), using a Cytotoxicity Detection Kit (Roche Applied Science, Indianapolis, IN, USA). LDH values were normalized against fully lysed platelets treated with the provided lysis reagent. The platelet lysis rate was determined by calculating the slope of a best-fit line for the LDH data in the post-exposure (low shear) period.
BAPTA Loading of Platelets
Platelets for experiments involving intracellular Ca2+ depletion were prepared by gel filtration in the absence of bovine serum albumin, which interferes with the Ca2+ inhibitor treatment. The volume of GFP obtained was split into two samples of equal volume in which one was preloaded with 20 µM BAPTA-AM (Sigma–Aldrich) and the other was defined as the control. Both samples were incubated at 37 °C for 30 min and then gel-filtered separately through two columns (to insure identical secondary filtration), to remove solution-phase BAPTA.
Platelet-Derived Microparticles (PMPs)
Pilot centrifugations were done with 500 µL samples of GFP to determine conditions under which 85–90% of normal platelets are pelleted. In our particular centrifuge, this corresponded to 3000 rpm for 90 s. The supernatant, containing any microparticles, was assayed for prothrombinase activity as described for platelets earlier. Samples were taken at the start of the experiment, immediately after 40 s exposure to 70 dyne/cm2, and at the conclusion of the 15 min low-shear exposure.
Statistics
To compare post-exposure activation rates, one-way ANOVA with Tukey’s post hoc test was performed for the varying conditions of pre-exposure to high shear. Exposure to a constant 1 dyne/cm2 shear stress for 15 min was considered as the control in each case. A threshold was defined as that integral shear stress, the product of the pre-exposure shear stress and duration, at which there was a statistically significant difference between pre-exposed and the non-exposed control (p < 0.05). For all other experiments, the Student’s t-test was used to establish significance (p < 0.05). Results are reported as mean ± standard error of the mean. All statistical analyses were done with SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
It should be noted that the fundamental question in this study is what happens after platelets have been briefly exposed to shear stress and return to normal circulation conditions for extended durations. Although we cannot experimentally mimic the extremely short high-shear exposure that might be seen in passage through, for instance, a heart valve, we emphasize that the time-integrated shear stresses generated are well within the pathological range.
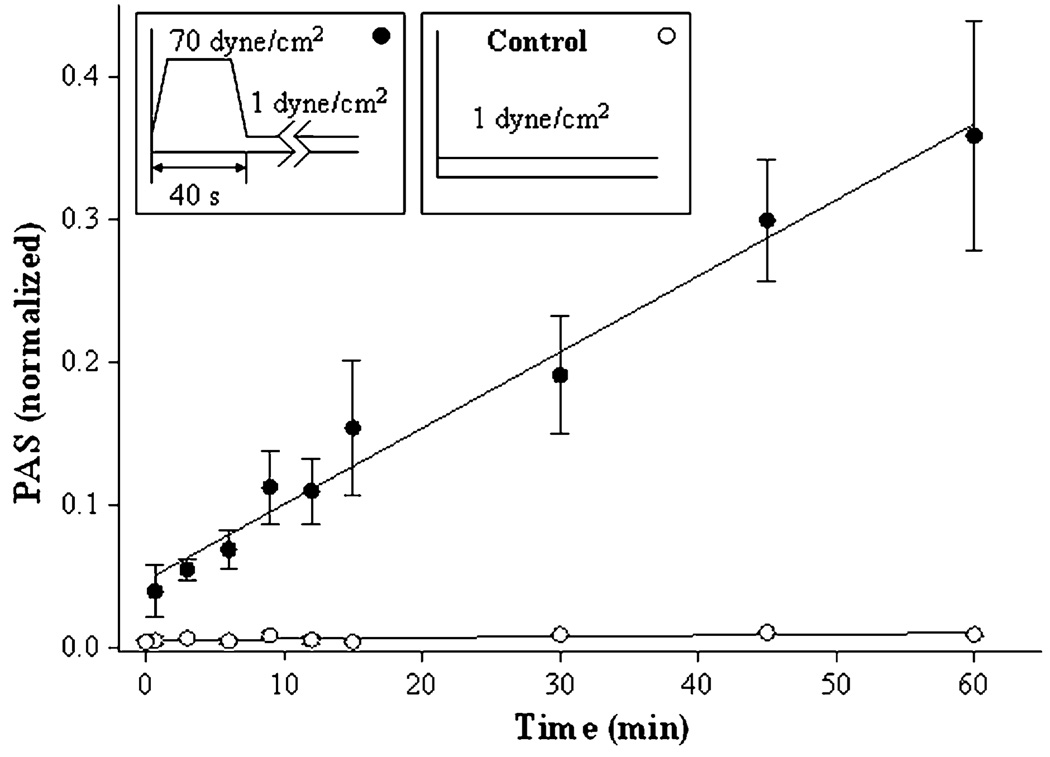
Figure 1 shows the basis of this study and the time course of platelet activation after a 40-s exposure to a shear stress of 70 dyne/cm2, and then to a shear stress of 1 dyne/cm2 for 60 min. Control experiments lacked the initial high-shear exposure. Measurement of PAS allowed two conclusions. First, the initial high-shear exposure causes measurable platelet activation, as expected, but the immediate extent of activation is small (<5%). Second, platelets do not recover to their initial pre-exposure activation state when subsequently exposed to low shear, and in fact they activate much faster under low-shear conditions than do platelets not pre-exposed to high shear. In other words, we see that high shear—an integral shear stress of 2800 dyne·s/cm2 in this case—sensitizes the platelets to subsequent low-shear conditions. To study this observation in more detail, we varied the conditions of the initial high-shear exposure, varying both shear stress and duration of exposure.
FIGURE 1.
Pre-exposure to high-shear stress, 40 s duration. Platelets were pre-exposed to shear stresses of 1 (control), and 70 dyne/cm2, as shown in the top bar, followed by exposure to 1 dyne/cm2 for 59 min. The means of four experiments are shown ±SEM.
Pre-Exposure to High Shear
Two variables were selected for the pre-exposure high-shear phase: variable shear stress for a fixed duration of 40 s (Fig. 2); and variable duration at a fixed shear stress of 70 dyne/cm2 (Fig. 3). The shear stress waveforms are shown. Figures 2 and 3 show only subsets of the range of shear conditions and exposures studied. The complete data are summarized in Fig. 4 and Table 1.
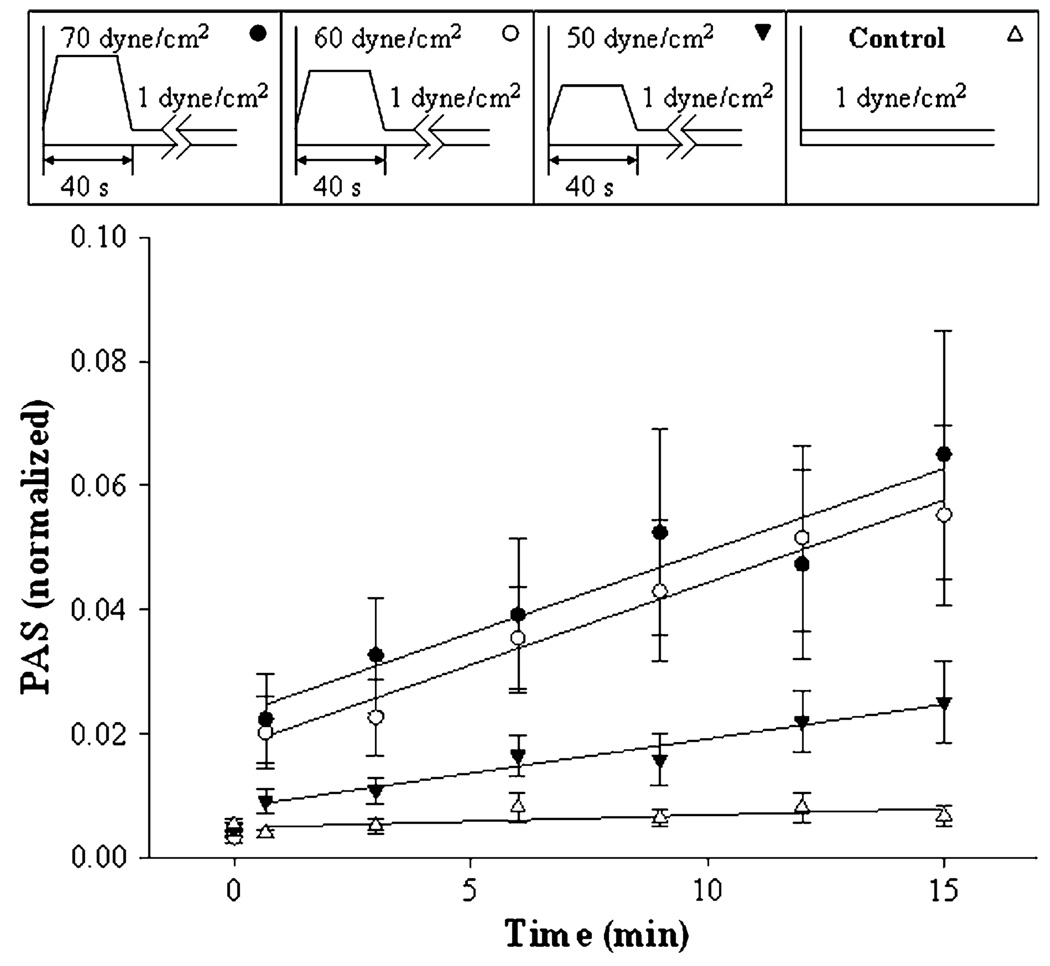
FIGURE 2.
Pre-exposure to varying shear stress, 40 s duration. Platelets were pre-exposed to shear stresses of 1 (control), 50, 60, and 70 dyne/cm2, as shown in the top bar, followed by exposure to 1 dyne/cm2 for 14 min. The means of nine experiments are shown ±SEM.
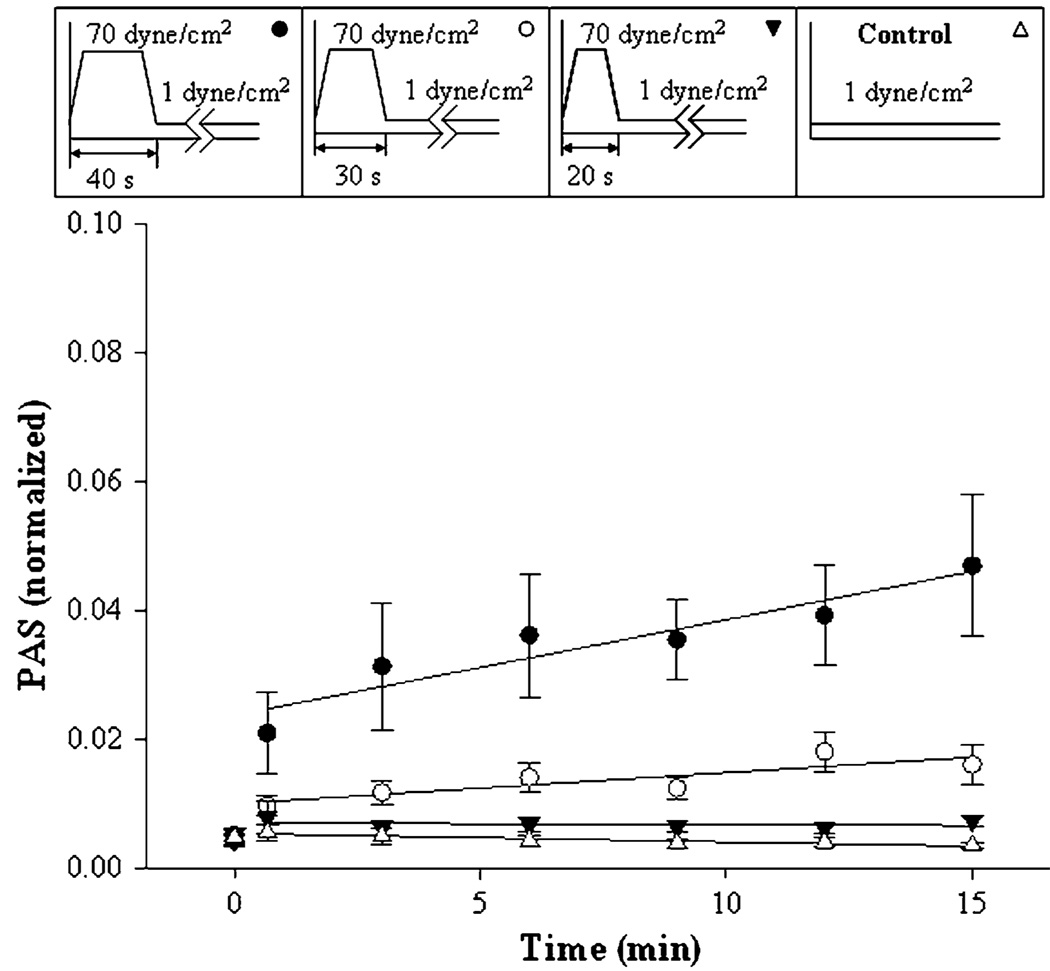
FIGURE 3.
Pre-exposure to fixed high-shear stress for varying duration. Platelets were pre-exposed to 70 dyne/cm2 shear stress for 20, 30, and 40 s, as shown in the top bar, followed by 1 dyne/cm2 for 14 min. Means of 11 experiments are shown ±SEM.
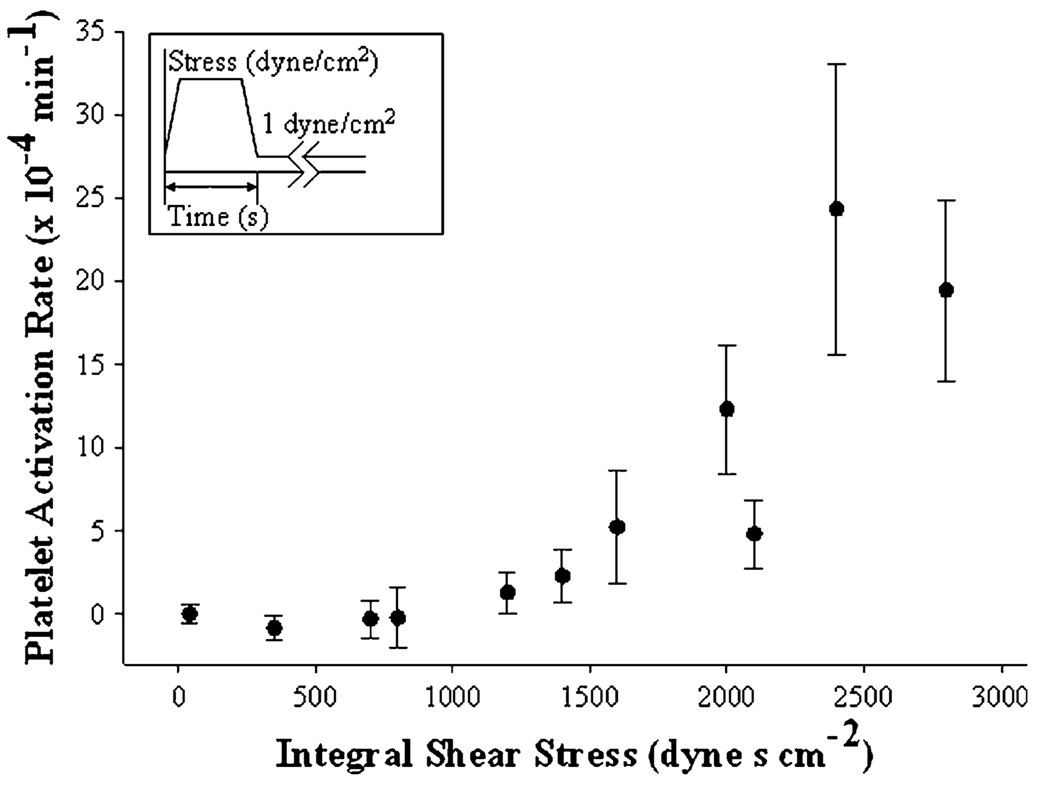
FIGURE 4.
Sensitization threshold. Platelet activation rates after high-shear pre-exposure (Figs. 1 and 2, and Table 1) are plotted against the time integral of the initial high-shear stress.
For the first case, seven peak shear stress values were chosen: 70, 60, 50, 40, 30, 20, and 1 dyne/cm2. Nine experiments were done for each of the conditions, with controls; and four of these are shown in Fig. 2, and the remainder in Table 1. During the initial 40 s phase, the higher-shear stress experiments caused a small but significant increase in PAS. After the brief exposure, the shear stress was lowered to 1 dyne/cm2 for the remaining 14 min. A linear increase in PAS was observed during this phase. However, only with pre-exposure to 60 and 70 dyne/cm2 were subsequent platelet activation rates significantly different from the 1 dyne/cm2 control (see Table 1 for a summary of activation rates and statistical analyses).
For the second case, six peak shear stress durations at 70 dyne/cm2 were chosen: 40, 30, 20, 10, 5 s, and again a 1 dyne/cm2 control. Four are shown in Fig. 3, and the remaining data in Table 1. Eleven experiments were done for each of the conditions (Table 1, Fig. 3). Only after high-shear exposure for 40 s was the platelet activation rate significantly different from the control (Table 1).
Threshold Determination
The varying pre-exposure regimens shown in Figs. 2 and 3 were integrated to provide an overall measure of the initial high-shear exposure, expressed in dyne·s/cm2. The subsequent rates of platelet activation were collected for each condition (n = 9 or 11) and plotted against the integral shear stress, defined as the product of the peak shear stress and duration (Fig. 4). Only pre-exposure to 70 dyne/cm2 for 40 s (integral = 2800 dyne·s/cm2) and 60 dyne/cm2 for 40 s (integral = 2400 dyne·s/cm2) resulted in statistically significant changes in platelet activation rate over the 1 dyne/cm2 control (integral = 40 dyne·s/cm2, Table 1).
Platelet Lysis and Activation
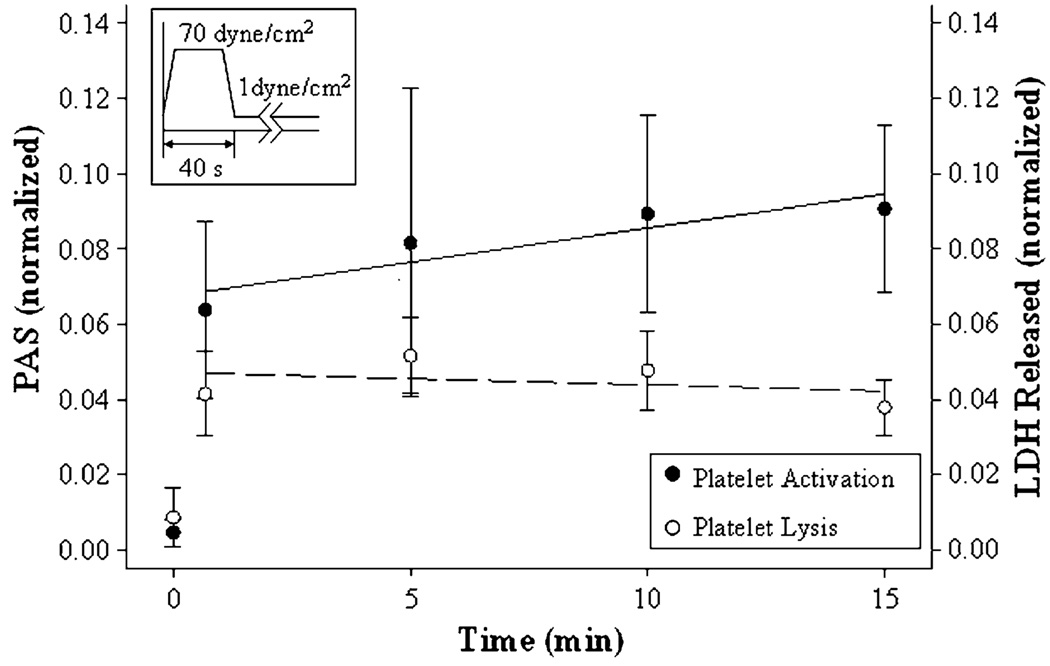
LDH release was measured (see “Materials and Methods” section) to determine whether the high pre-exposure shear was simply causing overt platelet lysis rather than causing sub-lytic activation of intact platelets, and compared with the PAS measured by the prothrombinase assay (n =5). Platelets were exposed to 70 dyne/cm2 for 40 s, followed by exposure to 1 dyne/cm2 for 14 min (Fig. 5). The initial exposure to high shear causes small but significant platelet activation and lysis, but the subsequent behavior under low shear is very different; while platelet activation continues, there is no further LDH release. Thus, while lysis might be involved in the initial small extent of activation under high-shear stress, subsequent activation of the platelets does not involve further lysis (Pearson’s coefficient r = 0.065, p > 0.5).
FIGURE 5.
Comparison of PAS and platelet lysis. Platelets were pre-exposed to 70 dyne/cm2 shear stress for 40 s, followed by 1 dyne/cm2 for 14 min. Samples were assessed for PAS, and for platelet lysis by LDH release.
Intracellular Ca2+ and Sensitization
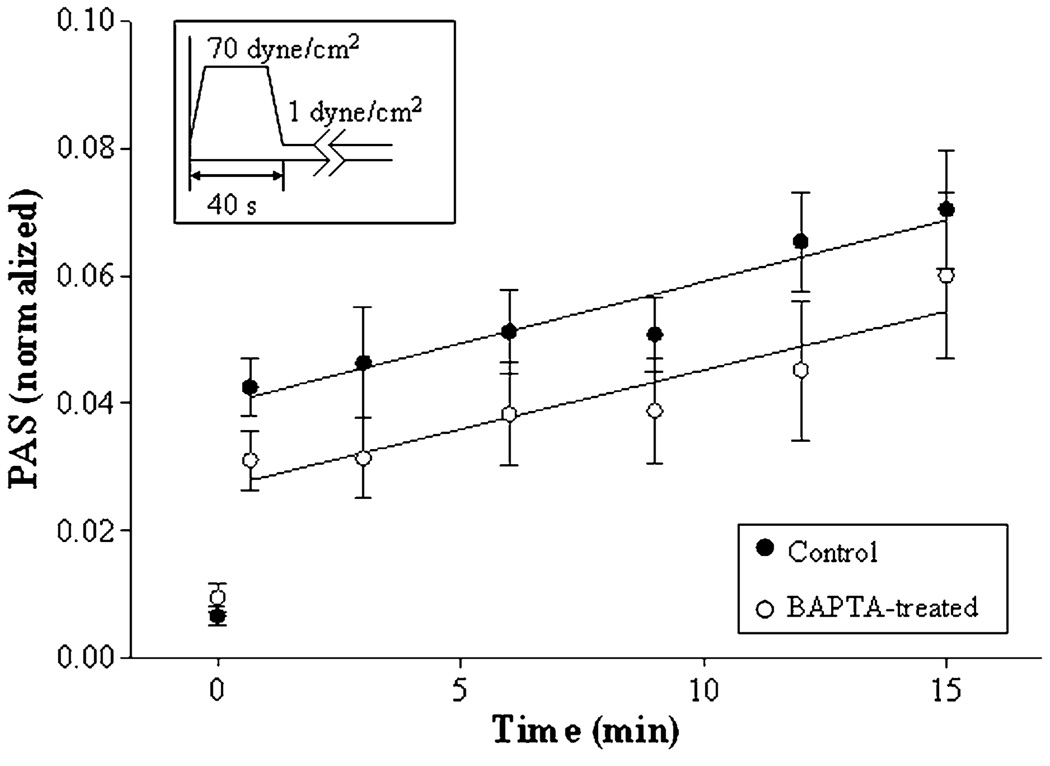
To block the effects of intracellular Ca2+ release, platelets were loaded with the Ca2+-binding inhibitor BAPTA-AM under standard conditions, and gel-filtered again to remove solution-phase inhibitor. Five experiments were performed for each condition (20 µM BAPTA-treated and untreated platelets) with PAS determined as described earlier (Fig. 6). While BAPTA loading causes a small reduction in the extent of initial activation under high-shear stress, it is barely significant (p ~ 0.09). Furthermore, there is no difference in post-exposure platelet activation rates for the BAPTA-treated and untreated platelets [20.9 ± 5.3 vs. 19.3 ± 5.7 (×10−4) min−1, p > 0.5]. While the mechanism of sensitization is unknown, these results show that subsequent activation of platelets under low-shear conditions does not apparently involve the release of intracellular platelet Ca2+.
FIGURE 6.
Intracellular Ca2+ depletion and platelet sensitization. Platelets treated with 20 µM BAPTA and untreated platelets were pre-exposed to 70 dyne/cm2 shear stress for 40 s, followed by 1 dyne/cm2 for 14 min.
Platelet Crosstalk
In an earlier study, we established that the extent of platelet activation under the conditions of average shear (9 dyne/cm2) was significantly dependent on platelet count, being both more non-linear and much more potent at 200000 than at 20000 platelets/µL.28 That study, however, only addressed shear-dependent activation, not behavior under subsequent low-shear exposure. In this study, we used the same approach of varying platelet count. Platelet activation rates (Table 2) show no evidence of count dependence (20000 vs. 150000/µL, n = 4 vs. 4; p > 0.5), suggesting a lack of any major crosstalk.
Although this indirect evidence suggested that crosstalk is not involved, we additionally asked whether shear-sensitized platelets can cause the activation of quiescent (non-exposed) platelets. To examine this, we added shear-exposed platelets (60 dyne/cm2 for 40 s) to non-exposed platelets, doubling the count to 40000/µL, and then exposed the mixture to low shear for 15 min as before. PAS was measured at the standard fixed platelet count (5000/µL in the assay: see “Materials and Methods” section) from 3 to 15 min, so that activation rates were determined on a per-platelet basis. Adding quiescent platelets did not change the net activation rate (Table 2), showing that shear-sensitized platelets may activate added quiescent platelets.
To examine further whether ADP might be involved in subsequent low-shear activation, we exposed platelets, in the presence of the ADP scavenger apyrase (1 U/mL, Sigma–Aldrich), to 70 dyne/cm2 for 40 s, followed by 1 dyne/cm2 for 15 min. Apyrase caused a 33% reduction in activation rate during the low-shear phase, which was statistically significant (Table 2; n = 4 vs. 4; p < 0.05). ADP may thus account for some of the increase in the activation of sensitized platelets during the low-shear phase.
Role of Microparticles
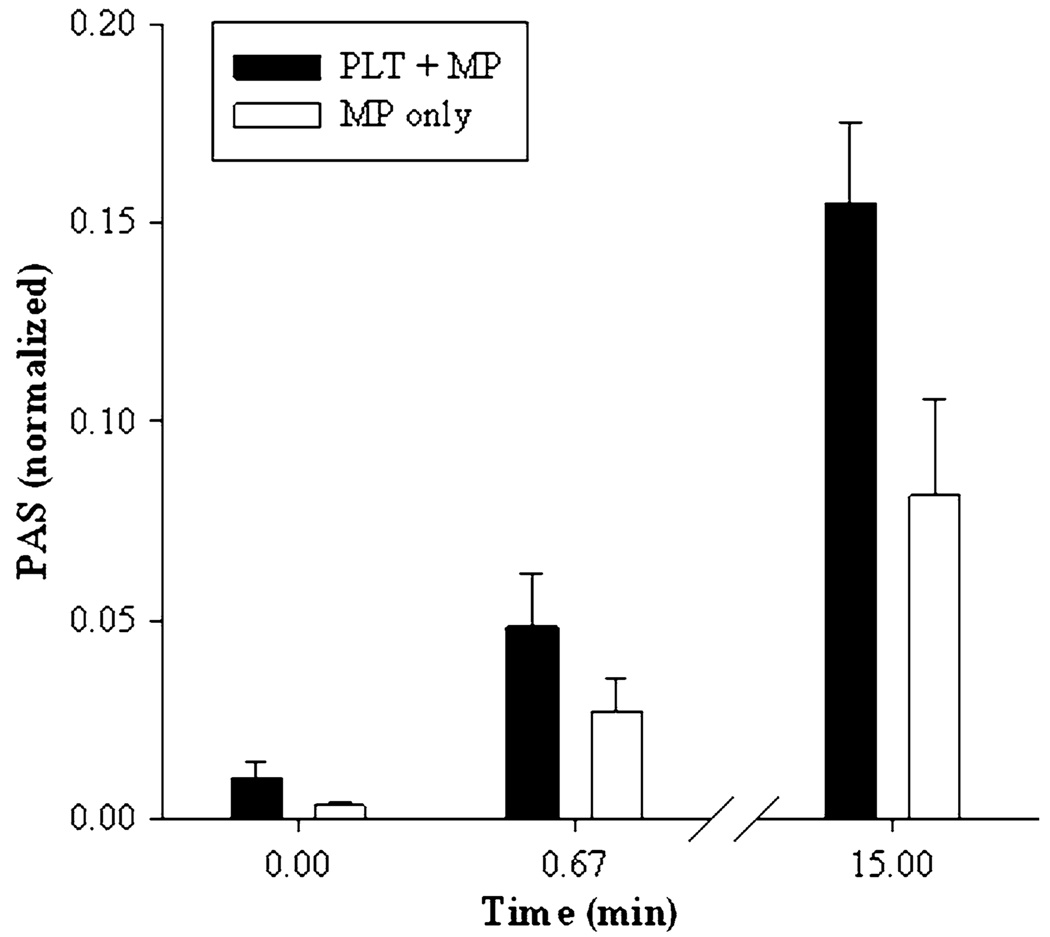
The prothrombinase activity of PMP was measured using the centrifugation supernatant (see “Materials and Methods” section) of platelet samples at 0 min, after 40 s exposure to high shear, and after 15 min to low shear (Fig. 7). It is clear that a substantial proportion of the total prothrombinase activity is due to microparticle formation, both immediately after high-shear exposure and after extended low-shear exposure. Significantly, the proportion of activity due to PMP remains approximately the same throughout, suggesting that microparticle formation continues throughout the low-shear activation phase.
FIGURE 7.
PMPs and sensitization. Comparison of total (platelet + microparticle, black) and microparticle (white) activity after single 40-s exposure to 70 dyne/cm2 and subsequent 15-min exposure to 1 dyne/cm2.
DISCUSSION
Shear-induced platelet activation is a well-documented phenomenon that has been studied extensively since the late 1970s. A multitude of activation markers have been used to examine the response of platelets to shear stress, including the measure of serotonin release,2,12 thromboglobulin release,32,34 anionic phospholipid exposure,29 P-selectin exposure, 22,39,40 and prothrombin activation (“platelet factor 3” activity).19,28
However, there is little information about what happens to platelets after exposure to pathological shear stress, such as will occur in vivo after passage through an MHV. Do the platelets recover; do they remain stably activated; or do they undergo further activation? Several studies have examined platelet hyperreactivity, defined as increased platelet function with regards to activation and aggregation.20 However, these studies have focused on platelet response to exogenous agonists such as epinephrine, ADP, collagen, and thrombin-receptor-activating peptide,35,36 rather than the intrinsic activity of the platelets themselves. In this study, we focus on the possibility that brief exposure to high shear should be considered as a natural physical agonist that sensitizes platelets to later, quite minor, insult.
The present results (Figs. 1–4) allow two key conclusions: (1) While platelets are activated a little by short periods of high shear, they do not recover their normal quiescent activity state under subsequent normal conditions of low shear. (2) Rather, if the initial exposure is sufficiently severe (≥2000 dyne·s/cm2 integral shear stress), they are rendered substantially more sensitive to subsequent low-shear stress. We further examined the mechanisms responsible for this change in platelet activation behavior.
Elevated shear stress is known to produce PMPs, and they retain the procoagulant properties of platelets. 23,25 We found a significant increase in platelet microparticles on brief exposure to elevated shear stress (70 dyne/cm2 for 40 s). Microparticle generation continued over the next 15 min low-shear phase and approximately 50% of the increased prothrombinase activity was attributed to microparticles both after initial high-shear exposure and after 15 min exposure to low shear (Fig. 7). This finding may be relevant to the observations of increased microparticle formation numbers in cardiovascular disease patients.26
We also examined the role of ADP on post-sensitization behavior, and found that ADP depletion by the addition of apyrase partially reduces platelet activation rate in the low-shear phase (Table 2). Thus, ADP-induced platelet activation may play a role in the sensitization process, but addition of apyrase does not eliminate this behavior. Moreover, separate experiments showed evidence that quiescent platelets may be subject to activation by shear-exposed platelets (Table 2). However, with regard to possible platelet–platelet crosstalk, there is no evidence that platelet activation after high shear is affected by platelet count (Table 2).
Because lysis is a well-known result of high shear, we also examined whether lysis is correlated with subsequent activation. Compared with red blood cells, platelets are more prone to lysis, as they have less flexible membranes and experience a higher strain when exposed to shear stress.31 Wurzinger et al.34 showed that platelet activation and lysis (marked by LDH release) appear to go hand-in-hand, but the conditions studied involved much higher shear stress (2550 dyne/cm2 for 7 ms and 1700 dyne/cm2 for 113 ms). Under the conditions we have studied, lysis is minor in the high-shear phase (approximately 5% of maximal), and this correlates with a minor increase in PAS as measured by prothrombinase activity (approximately 6% maximal). However, in the latter low-shear phase, while the platelets undergo continuing activation, no further lysis occurs (Fig. 5).
We have shown that sensitization is not inhibited by an intracellular Ca2+ inhibitor, BAPTA-AM. Intracellular Ca2+ release plays a major role in platelet activation, and introduction of BAPTA-AM abolishes the depolarization and degranulation of platelets caused by thrombin, and reduces activation in a constant-shear flow loop.4,13 Our results indicate that while BAPTA causes a reduction in platelet activation over the initial 40 s exposure to 70 dyne/cm2, subsequent platelet activation at low shear stress was unaffected (Fig. 6). It therefore seems unlikely that intracellular Ca2+ release is involved in activation during the extended low-shear phase.
At this point, it is relevant to note that these studies were done in vitro with purified platelets, and in particular that they lacked other blood cells or the presence of endothelial cells. The latter are particularly potent regulators of platelet function, and it is thus feasible that the post-exposure activation that we observe here may be substantially modulated in vivo or in the presence of other relevant cell types. This awaits further study.
The sensitization behavior may partially explain the high risk of thromboembolic complications in patients with implanted MHVs. Wall shear stresses typically range from 100 to 8000 dyne/cm2 in MHVs.21,38 Although exposure to such high-shear stress typically lasts only about 10−3 to 10−1 s,7,38 the peak shear stress in these situations is substantially higher than we can yet emulate experimentally. Thus, while this study demonstrated sensitization at a lower (but still pathological) shear stress, it required a longer exposure time. Both shear stress and time obviously play a role, but we do not know if the integral shear stress is in fact the main controlling variable. It certainly appears that sensitization is not linearly related to the integral, and that a pathological threshold of exposure must be exceeded.
In summary, platelets exposed to high-shear stress for brief durations are substantially sensitized to subsequent, much lower levels of shear stress, but this does not occur unless the initial high-shear exposure exceeds a threshold. Under the conditions of this study (maximal shear stress 70 dyne/cm2), this threshold was in the region 2000–2400 dyne·s/cm2. Although we cannot yet mimic experimentally the extremely high and short exposures to shear that platelets can experience upon passage through such devices as MHVs, we propose that the observed sensitization is relevant in the known high risk of thrombotic complications in individuals whose circulation includes such zones of very high-shear stress. Future study will further address whether inter-platelet or platelet–endothelial–cell crosstalk affected by other natural agonists or inhibitors play a significant role.
ACKNOWLEDGMENTS
The authors would like to thank Philip Chiu, Mohammed Hoque, and Thomas Claiborne for their contributions to the experiments in this study. This study was supported by the American Heart Association (Award no. 0340143N, DB) and the National Institutes of Health (Award no. 1R01EB008004-01, DB).
REFERENCES
- 1.Akins CW. Results with mechanical cardiac valvular prostheses. Ann. Thorac. Surg. 1995;60:1836–1844. doi: 10.1016/0003-4975(95)00766-0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GH, Hellums JD, Moake J, Alfrey CP., Jr Platelet response to shear stress: changes in serotonin uptake, serotonin release, and ADP induced aggregation. Thromb. Res. 1978;13:1039–1047. doi: 10.1016/0049-3848(78)90232-3. [DOI] [PubMed] [Google Scholar]
- 3.Aursnes I, Sundal J, Nome T. Shear stress activation of platelets with subsequent refractoriness. Thromb. Res. 1987;45:29–37. doi: 10.1016/0049-3848(87)90254-4. [DOI] [PubMed] [Google Scholar]
- 4.Bahou WF, Scudder L, Rubenstein D, Jesty J. A shear-restricted pathway of platelet procoagulant activity is regulated by IQGAP1. J. Biol. Chem. 2004;279:22571–22577. doi: 10.1074/jbc.M402561200. [DOI] [PubMed] [Google Scholar]
- 5.Bluestein D. Towards optimization of the thrombogenic potential of blood recirculating cardiovascular devices using modeling approaches. Expert. Rev. Med. Devices. 2006;3:267–270. doi: 10.1586/17434440.3.3.267. [DOI] [PubMed] [Google Scholar]
- 6.Bluestein D, Yin W, Affeld K, Jesty J. Flow-induced platelet activation in mechanical heart valves. J. Heart Valve Dis. 2004;13:501–508. [PubMed] [Google Scholar]
- 7.Boreda R, Fatemi RS, Rittgers SE. Potential for platelet stimulation in critically stenosed carotid and coronary arteries. J. Vasc. Invest. 1995;1:26–37. [Google Scholar]
- 8.Bray PF. Platelet hyperreactivity: predictive and intrinsic properties. Hematol. Oncol. Clin. North Am. 2007;21:633–645. doi: 10.1016/j.hoc.2007.06.002. v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown CH, III, Lemuth RF, Hellums JD, Leverett LB, Alfrey CP. Response of human platelets to shear stress. Trans. Am. Soc. Artif. Intern. Organs. 1975;21:35–39. [PubMed] [Google Scholar]
- 10.Butchart EG, Ionescu A, Payne N, Giddings J, Grunkemeier GL, Fraser AG. A new scoring system to determine thromboembolic risk after heart valve replacement. Circulation. 2003;108 Suppl 1:II68–II74. doi: 10.1161/01.cir.0000087383.62522.1e. [DOI] [PubMed] [Google Scholar]
- 11.Butchart EG, Lewis PA, Grunkemeier GL, Kulatilake N, Breckenridge IM. Low risk of thrombosis and serious embolic events despite low-intensity anticoagulation. Experience with 1,004 Medtronic Hall valves. Circulation. 1988;78:I66–I77. [PubMed] [Google Scholar]
- 12.Colantuoni G, Hellums JD, Moake JL, Alfrey CP., Jr The response of human platelets to shear stress at short exposure times. Trans. Am. Soc. Artif. Intern. Organs. 1977;23:626–631. doi: 10.1097/00002480-197700230-00169. [DOI] [PubMed] [Google Scholar]
- 13.Davies TA, Drotts DL, Weil GJ, Simons ER. Cytoplasmic Ca2+ is necessary for thrombin-induced platelet activation. J. Biol. Chem. 1989;264:19600–19606. [PubMed] [Google Scholar]
- 14.Edmunds LH., Jr Thrombotic and bleeding complications of prosthetic heart valves. Ann. Thorac. Surg. 1987;44:430–445. doi: 10.1016/s0003-4975(10)63816-7. [DOI] [PubMed] [Google Scholar]
- 15.Goldsmith HL, Turitto VT. Rheological aspects of thrombosis and haemostasis: basic principles and applications. ICTH Report—Subcommittee on Rheology of the International Committee on Thrombosis and Haemostasis. Thromb. Haemost. 1986;55:415–435. [PubMed] [Google Scholar]
- 16.Grigioni M, Daniele C, D’Avenio G, Barbaro V. A discussion on the threshold limit for hemolysis related to Reynolds shear stress. J. Biomech. 1999;32:1107–1112. doi: 10.1016/s0021-9290(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 17.Hathcock JJ. Flow effects on coagulation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2006;26:1729–1737. doi: 10.1161/01.ATV.0000229658.76797.30. [DOI] [PubMed] [Google Scholar]
- 18.Jesty J, Bluestein D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal. Biochem. 1999;272:64–70. doi: 10.1006/abio.1999.4148. [DOI] [PubMed] [Google Scholar]
- 19.Jesty J, Yin W, Perrotta P, Bluestein D. Platelet activation in a circulating flow loop: combined effects of shear stress and exposure time. Platelets. 2003;14:143–149. doi: 10.1080/0953710031000092839. [DOI] [PubMed] [Google Scholar]
- 20.Kottke-Marchant K. Importance of platelets and platelet response in acute coronary syndromes. Cleve. Clin. J. Med. 2009;76 Suppl 1:S2–S7. doi: 10.3949/ccjm.76.s1.01. [DOI] [PubMed] [Google Scholar]
- 21.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88:1525–1541. [PubMed] [Google Scholar]
- 22.Leytin V, Allen DJ, Mykhaylov S, Mis L, Lyubimov EV, Garvey B, Freedman J. Pathologic high shear stress induces apoptosis events in human platelets. Biochem. Biophys. Res. Commun. 2004;320:303–310. doi: 10.1016/j.bbrc.2004.05.166. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88:3456–3464. [PubMed] [Google Scholar]
- 24.Nobili M, Sheriff J, Morbiducci U, Redaelli A, Bluestein D. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J. 2008;54:64–72. doi: 10.1097/MAT.0b013e31815d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nomura S. Function and clinical significance of platelet-derived microparticles. Int. J. Hematol. 2001;74:397–404. doi: 10.1007/BF02982082. [DOI] [PubMed] [Google Scholar]
- 26.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Ramstack JM, Zuckerman L, Mockros LF. Shear-induced activation of platelets. J. Biomech. 1979;12:113–125. doi: 10.1016/0021-9290(79)90150-7. [DOI] [PubMed] [Google Scholar]
- 28.Schulz-Heik K, Ramachandran J, Bluestein D, Jesty J. The extent of platelet activation under shear depends on platelet count: differential expression of anionic phospholipid and factor Va. Pathophysiol. Haemost. Thromb. 2005;34:255–262. doi: 10.1159/000093104. [DOI] [PubMed] [Google Scholar]
- 29.Shankaran H, Alexandridis P, Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood. 2003;101:2637–2645. doi: 10.1182/blood-2002-05-1550. [DOI] [PubMed] [Google Scholar]
- 30.Sutera SP, Mehrjardi MH. Deformation and fragmentation of human red blood cells in turbulent shear flow. Biophys. J. 1975;15:1–10. doi: 10.1016/S0006-3495(75)85787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travis BR, Marzec UM, Leo HL, Momin T, Sanders C, Hanson SR, Yoganathan AP. Bileaflet aortic valve prosthesis pivot geometry influences platelet secretion and anionic phospholipid exposure. Ann. Biomed. Eng. 2001;29:657–664. doi: 10.1114/1.1385808. [DOI] [PubMed] [Google Scholar]
- 32.Voisin P, Guimont C, Stoltz JF. Experimental investigation of the rheological activation of blood platelets. Biorheology. 1985;22:425–435. doi: 10.3233/bir-1985-22506. [DOI] [PubMed] [Google Scholar]
- 33.Wootton DM, Ku DN. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu. Rev. Biomed. Eng. 1999;1:299–329. doi: 10.1146/annurev.bioeng.1.1.299. [DOI] [PubMed] [Google Scholar]
- 34.Wurzinger LJ, Opitz R, Blasberg P, Schmid-Schonbein H. Platelet and coagulation parameters following millisecond exposure to laminar shear stress. Thromb. Haemost. 1985;54:381–386. [PubMed] [Google Scholar]
- 35.Yee DL, Bergeron AL, Sun CW, Dong JF, Bray PF. Platelet hyperreactivity generalizes to multiple forms of stimulation. J. Thromb. Haemost. 2006;4:2043–2050. doi: 10.1111/j.1538-7836.2006.02089.x. [DOI] [PubMed] [Google Scholar]
- 36.Yee DL, Sun CW, Bergeron AL, Dong JF, Bray PF. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood. 2005;106:2723–2729. doi: 10.1182/blood-2005-03-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin W, Gallocher S, Pinchuk L, Schoephoerster RT, Jesty J, Bluestein D. Flow-induced platelet activation in a St. Jude mechanical heart valve, a trileaflet polymeric heart valve, and a St. Jude tissue valve. Artif. Organs. 2005;29:826–831. doi: 10.1111/j.1525-1594.2005.29109.x. [DOI] [PubMed] [Google Scholar]
- 38.Yoganathan AP, He Z, Casey Jones S. Fluid mechanics of heart valves. Annu. Rev. Biomed. Eng. 2004;6:331–362. doi: 10.1146/annurev.bioeng.6.040803.140111. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JN, Bergeron AL, Yu Q, Sun C, McBride L, Bray PF, Dong JF. Duration of exposure to high fluid shear stress is critical in shear-induced platelet activation-aggregation. Thromb. Haemost. 2003;90:672–678. doi: 10.1160/TH03-03-0145. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JN, Bergeron AL, Yu Q, Sun C, McIntire LV, Lopez JA, Dong JF. Platelet aggregation and activation under complex patterns of shear stress. Thromb. Haemost. 2002;88:817–821. [PubMed] [Google Scholar]