Abstract
Objective
To investigate mechanisms underlying gender differences in serum lipoprotein concentrations, the kinetic behavior of apoB-100 was assessed.
Methods and Results
Twenty subjects (<50 years; 12 men and 8 premenopausal women) were provided a Western diet for 4 to 6 weeks, after which the kinetics of apoB-100 in triglyceride-rich, intermediate-density, and low-density lipoprotein (TRL, IDL, and LDL) were determined in the fed state. Nonfasting plasma TC, LDL-C, and triglyceride concentrations were 23%, 34%, and 57% lower, respectively, in the women compared with men. Plasma TRL and LDL apoB 100 pool sizes were lower by 40% and 30%, respectively. These differences were accounted for by higher TRL and LDL apoB 100 fractional catabolic rates (FCR), rather than differences in production rates (PR). Plasma TRL-C and LDL-C were positively correlated with TRL and LDL apoB 100 concentrations and pool size, and negatively correlated with TRL and LDL apoB 100 FCR (women: r=−0.59, P<0.01 and r=−0.54, P<0.04, and men: r=−0.43, P<0.05 and r=−0.44, P<0.05). No significant associations were observed between plasma TRL-C and LDL-C and PR.
Conclusions
These data suggest the mechanism for lower TRL-C and LDL-C concentrations in women was determined predominantly by higher TRL and LDL FCR rather than lower PR. This could explain, in part, the lower CVD risk in premenopausal women relative to men.
Keywords: apolipoproteins, gender, metabolism, stable isotopes, CVD
Cardiovascular disease (CVD) incidence differs between men and women. Among men aged 20 to 44 years the prevalence of CVD is one-third higher than women of the same age (34% versus 24%, respectively). Between the ages of 45 to 54 years, the prevalence is similar, but above the age of 55 years, it is higher in women.1 Among women, CVD death rates after menopause are 2 to 3 times higher than women of the same age before menopause.2–4
Lipid abnormalities, hypertension, smoking, diabetes, atherogenic diet, and sedentary lifestyle are well established risk factors for CVD.1,5 Among the lipid risk factors, elevated LDL-C and triglycerides, and decreased HDL-C concentrations have been independently linked to the progression of CVD. Gender, age and menopausal differences in plasma concentrations of both LDL-C and HDL-C have been documented.6–11 Men tend to have higher plasma LDL-C and lower HDL-C concentrations than comparably aged premenopausal women, and some studies have also reported higher VLDL-C, triglycerides, and apoB concentrations.9,10,12–17 Data from cross sectional9,10,17–24 and longitudinal studies23,25–27 have shown that post- compared to premenopausal women have higher plasma total, LDL-C, VLDL-C, and triglyceride concentrations. It has been hypothesized that these differences might partly explain the lower CVD risk in premenopausal women and the higher but comparable CVD risk between postmenopausal women and men. However, limited information is available on the mechanism(s) underlying the gender related differences on circulating lipid and lipoprotein concentrations, especially in the younger age categories.
ApoB-100 is the major protein component associated with very low–density or triglyceride-rich, intermediate-density, and low-density lipoproteins (VLDL or TRL in the case of nonfasted plasma, IDL and LDL). These particles are linked via a delipidation and transfer cascade. Assessing the kinetic behavior of apoB-100 provides a unique opportunity to define mechanisms underlying the gender differences in lipoprotein profiles. In the present study, we investigated apoB-100 metabolism in premenopausal women and men (<50 years) using stable isotope methodology and multicompartmental modeling.
Methods
Subjects
Twenty subjects (n=8 women and n=12 men) aged <50 years participated in this study. All women were premenopausal (plasma estradiol 30±18 pg/mL and follicle-stimulating hormone [FSH] 5±1 mIU/mL) and were not taking oral contraceptives. Exclusion criteria were chronic illness, smoking, consumption of ≥2 alcoholic drinks per day, current use of medications known to affect lipid metabolism (lipid lowering drugs, fish-oil capsules, β blockers, or diuretics), LDL-C >3.36 mmol/L, women who had undergone a hysterectomy or oopherectomy, perimenopausal women with irregular menses, and postmenopausal women. The protocol was approved by the Human Investigation Review Committee of the New England Medical Center and Tufts University. All subjects gave written informed consents. Part of these data has been published previously to address a different question-the effect of menopausal status28 and age29 on apolipoprotein kinetics.
Experimental Design and Diet
Subjects were maintained for 4 to 6 weeks on a standardized Western diet providing 49% energy (%E) carbohydrate, 15%E protein, 35%E fat (14% saturated fatty acids; 15% monounsaturated fatty acids and 7% polyunsaturated fatty acids), and 180 mg cholesterol/1000 kcal. All food and drink were provided to the subjects who reported to the Metabolic Research Unit of Tufts University four times per week. Initial energy intakes were calculated using the Harris–Benedict equation, and adjustments made when necessary to maintain body weight throughout the study period.
Measurement of Lipoprotein Kinetics
At the end of the controlled dietary period, a primed-constant infusion of deuterated-leucine was administered to the subjects in the fed state to determine the kinetic behavior of nonfasting TRL, IDL, and LDL apoB-100. After a 12-hour fast, subjects were fed the experimental diet as described hourly for 20 hours starting at 6 AM. Each identical meal consisted of 1/20 their daily caloric intake as previously described.30 Five hours after the first meal, subjects received an intravenous bolus dose (10 μmol/kg) followed by a constant infusion (10 μmol/kg/h) of [5,5,5-2H3]-L-leucine over a 15-hour period. Blood samples (20 mL) were collected via a second intravenous line at 0, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 10, 12, and 15 hours.
The protocol for plasma lipid and lipoprotein characterization, quantification, and isolation of the apolipoproteins, isotopic enrichment determinations and kinetic analysis were performed as described in detail previously.31–38 The fasting lipid and lipoprotein values reported are averages of 3 measurements taken at the end of the controlled feeding period. The nonfasting lipid and lipoprotein values are averages of 5 measurements corresponding to time points 1, 4, 8, 12, and 15 hours, during the infusion protocol.
Kinetic Analysis
The kinetic parameters of apoB-100 in TRL, IDL, and LDL fractions were determined by fitting the multicompartmental model to the tracer/tracee ratio (TTR) data using the SAAM II program (University of Washington, Seattle) as previously described.28,39 TRL, IDL, and LDL apoB pool sizes (PS) were determined by multiplying the measured apoB concentrations by estimated plasma volume. After fitting the respective compartment models to the observed data, fractional catabolic rates (FCR in pools/d) of apoB-100 were derived.37,39 Production rates (PR in mg/kg/d) were calculated as FCR (pools/d)×apo concentration (mg/L)×plasma volume (L)/body wt (kg).
It has been recently reported that use of TTR values for modeling can result in an underestimation of fractional secretion rate.40 For primed constant infusions the bias error percentage is approximately equal to the plateau enrichment. Recalculation of the present data revealed an underestimation of FCR by 7% in the women and 6% in the men. Because these differences fall within expected error limits, and to facilitate comparison with previously published studies, we have reported results calculated using TTR data.
Statistical Analyses
Before statistical testing, data were checked for normality. Variables that were log transformed included nonfasting plasma TRL-C and triglycerides, TRL apoB-100, TRL PS, and IDL PR. A square root transformation was performed for IDL apoB-100. Untransformed data are presented in text and tables as means±SEM. Unpaired Student t test (SAS version 8, SAS Institute Inc) was used to assess mean differences between groups. Spearman correlation coefficients were calculated to test for association between plasma lipoproteins and apoB-100 kinetic parameters.
Results
Baseline Characteristics and Lipoprotein Data
Mean age, body weight, and body mass index were not significantly different between women and men (Table 1). Nonfasting plasma TC, LDL-C, and triglyceride concentrations were significantly lower by 23%, 34%, and 57%, respectively, in women compared with men. Plasma TRL-C concentrations also tended to be lower (17%) in the women, but this difference did not attain statistical significance. The mean HDL-C concentrations of the women and men recruited for this study was not significantly different. The TC/HDL-C and LDL-C/HDL-C ratios were significantly more favorable in the women than men. The fasting plasma TC and LDL-C concentrations were 12% and 18% lower in the women compared to the men. No significant differences were observed between genders in the other fasting lipid parameters.
Table 1.
Baseline Characteristics and Plasma and Lipid and Lipoprotein Profile
| Variables | Women (n=8) | Men (n=12) | P Value* |
|---|---|---|---|
| Age, y | 25.6±0.8 | 36.8±2.8 | 0.09 |
| Weight, kg | 65.4±2.5 | 76.0±2.4 | 0.08 |
| BMI, kg/m2 | 23.0±0.7 | 24.9±0.9 | 0.14 |
| Nonfasting values, mmol/L† | |||
| Total cholesterol† | 3.6±0.2 | 4.7±0.3 | 0.01 |
| LDL-C | 1.9±0.2 | 2.9±0.3 | 0.02 |
| HDL-C | 1.1±0.1 | 1.0±0.1 | 0.28 |
| TRL-C‡ | 0.5±0.1 | 0.6±0.1 | 0.55 |
| Triglycerides†‡ | 1.0±0.4 | 2.3±0.3 | 0.001 |
| Total C/HDL-C | 3.5±0.4 | 5.0±0.5 | 0.04 |
| LDL-C/HDL-C | 1.9±0.4 | 3.2±0.4 | 0.04 |
| Fasting values, mmol/L|| | |||
| Total cholesterol | 4.5±0.7 | 5.1±1.3 | 0.03 |
| LDL-C | 2.8±0.8 | 3.4±1.1 | 0.04 |
| HDL-C | 1.3±0.3 | 1.2±0.3 | 0.48 |
| VLDL-C‡ | 0.4±0.2 | 0.5±0.3 | 0.81 |
| Triglycerides‡ | 0.8±0.4 | 1.0±0.4 | 0.16 |
| Total C/HDL-C | 3.5±1.0 | 4.5±1.5 | 0.10 |
| LDL-C/HDL-C | 2.2±0.9 | 3.0±1.2 | 0.12 |
Values are mean±SEM of untransformed data. To convert values for cholesterol and triglycerides to milligrams per deciliter, multiply by 38.67 and 88.54, respectively.
Unpaired Student t test.
Average of 5 measurements corresponding to time points 1, 4, 8, 12 and 15 hours, during the infusion protocol.
Log-transformed before statistical analysis.
Average of 3 measurements taken at the end of the dietary period.
ApoB-100 Kinetics
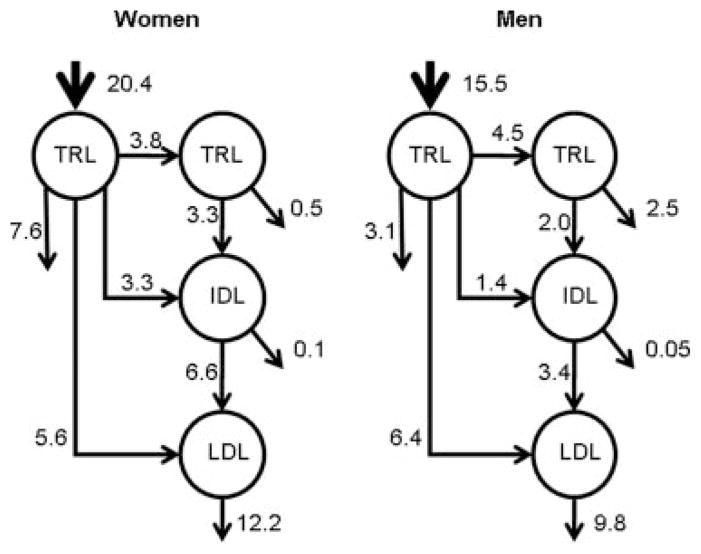
Plasma TRL apoB-100 pool size was significantly lower (40%) in women compared with men (Table 2). This difference was predominantly attributable to an 82% higher TRL FCR with no significant difference in PR. The IDL kinetic parameters tended to be higher in the women compared to the men, but only the differences in IDL PR reached statistical significance. This could be a consequence of the higher TRL to IDL apoB-100 transfer or conversion rate observed in the women (Table 2 and Figure 1). Both LDL apoB-100 FCR and PR were higher (44% and 24% [P<0.05], respectively) in the women relative to the men, and this resulted in net 30% lower LDL apoB-100 pool size (P<0.05) and 17% lower LDL apoB-100 concentration. The proportion of TRL apoB-100 converted to LDL via IDL was significantly higher in the women (37.1%) compared to the men (21.8%). There was no difference in the proportion of TRL apoB-100 converted directly to LDL, or in the conversion rate of apoB-100 from IDL to LDL (Table 2). However, the fraction of LDL apoB-100 derived from the direct conversion of TRL (Figure 2) was 46% (5.6/12.2*100) in women compared to 65% (6.4/9.8*100) in men (P=0.03). By difference, the fraction of LDL apoB-100 derived via the conventional cascade was 54% in the women compared to 35% in then men (P=0.04). This suggests that the transport of apoB-100 through TRL, IDL, and LDL fractions tended to be higher in women compared with men, and that this was compensated for by increased rates of catabolism resulting in lower apoB-100 concentrations. Inclusion of age and BMI as covariates in the analysis did not significantly influence any of the results.
Table 2.
Kinetic Parameters of Nonfasting TRL, IDL, and LDL ApoB-100
| Variables | Women (n=8) | Men (n=12) | P Value* |
|---|---|---|---|
| TRL apo B100†, mg/dL | 3.6±0.4 | 5.2±0.7 | 0.07 |
| TRL Pool Size†, mg | 107.6±16.1 | 179.4±24.9 | 0.03 |
| TRL FCR†, pools/day | 14.2±2.8 | 7.8±1.2 | 0.03 |
| TRL PR, mg/kg/day | 20.4±2.9 | 15.5±1.1 | 0.14 |
| IDL apo B100†, mg/dL | 1.6±0.2 | 1.1±0.2 | 0.06 |
| IDL Pool Size, mg | 46.5±5.5 | 37.9±5.7 | 0.31 |
| IDL FCR, pools/day | 10.3±1.7 | 7.0±1.0 | 0.08 |
| IDL PR‡, mg/kg/day | 6.6±0.7 | 3.4±0.6 | 0.01 |
| LDL apo B100, mg/dL | 71.6±4.9 | 87.3±8.9 | 0.14 |
| LDL Pool Size, mg | 2114.2±175.7 | 3035.3±339.4 | 0.03 |
| LDL FCR, pools/day | 0.39±0.03 | 0.27±0.04 | 0.04 |
| LDL PR†, mg/kg/day | 12.2±0.6 | 9.8±1.1 | 0.05 |
| TRL to IDL conversion | 37.9±6.7 | 22.1±3.5 | 0.04 |
| IDL to LDL conversion | 97.5±2.2 | 98.5±0.9 | 0.65 |
| TRL to LDL conversion via IDL | 37.1±6.8 | 21.8±3.5 | 0.04 |
| TRL to LDL conversion (direct) | 31.2±5.1 | 42.4±5.5 | 0.18 |
| TRL to LDL conversion (total) | 68.3±9.5 | 64.2±5.9 | 0.71 |
Values are mean±SEM of untransformed data.
Unpaired Student t test.
Log-transformed before statistical analysis.
Square root transformed before statistical analysis.
Figure 1.
Mean production (mg/kg/d) and conversion rates of apoB-100 in TRL, IDL, and LDL in women and men.
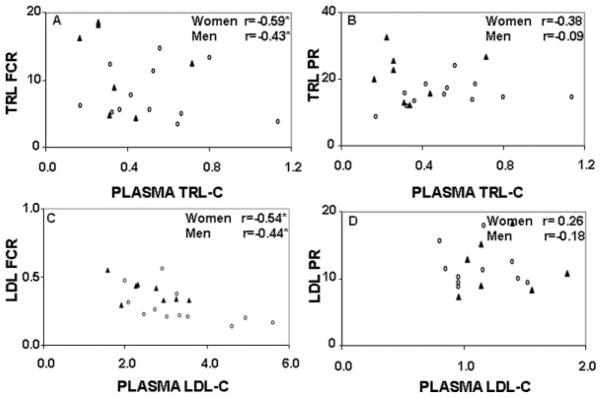
Figure 2.
Correlation between plasma TRL-C concentrations (mmol/L) and TRL apoB-100 FCR (pools/d; A) and TRL apoB-100 PR (mg/kg/d; B); plasma LDL-C concentrations (mmol/L) and LDL apoB-100 FCR (pools/d; C) and LDL apoB-100 PR (mg/kg/d; D). The (▲) represents the premenopausal women, and the (○) represents the men. *P<0.05.
To determine whether the differences observed in plasma TRL-C and LDL-C concentrations were associated with differences in apoB-100 kinetic parameters, correlations coefficients were calculated. Plasma TRL-C concentrations were positively correlated with TRL apoB-100 (r=0.80, P<0.05 and r=0.59, P<0.05) and pool size (r=0.71, P<0.05 and r=0.68, P<0.01) in both women and men, respectively. In contrast, plasma TRL-C concentrations were correlated negatively with TRL apoB-100 FCR in both women and men (Figure 2A). No significant association was observed with TRL PR (Figure 2B). Plasma LDL-C concentrations were correlated negatively with LDL apoB-100 FCR (Figure 2C) and positively with LDL apoB-100 (r=0.85, P<0.01 and r=0.91, P<0.0001) and pool size (r=0.80; P<0.01 and r=0.93; P<0.0001), in both women and men, respectively. Plasma LDL-C concentrations and the PR of LDL apoB-100 were not significantly correlated (Figure 2D).
Discussion
Elevated LDL-C and triglyceride and low HDL-C concentrations are well established risk factors for CVD in women and men. Although women tend to have a more favorable lipid profile than men from age 20 to 50 years, CVD risk increases in women after the onset of menopause, as well as with age in both women and men.1,41 In the present study we assessed the mechanisms for the gender difference in lipids between premenopausal women and men by studying the kinetic behavior of the major apolipoprotein associated with TRL, IDL, and LDL particles in the fed state. Our data demonstrate that the more favorable plasma lipoprotein profile in premenopausal women relative to men is primarily attributable to differences in TRL and LDL apoB-100 FCR rather than PR.
Only one study42 has directly compared the gender-related differences in TRL, IDL, and LDL apoB-100 metabolism in the fed state. No significant differences were found in any kinetic parameter between postmenopausal women and men. This is not unexpected because the apoB-100 concentrations were similar between groups. Additionally, the study population included older individuals (>50 years) which could have obscured any potential gender difference at younger ages because increases in apoB-100 concentrations with advancing years have been documented.17,43 The kinetic basis for the age effect has been attributed to both an increase in production or a decrease in catabolism.28,29,44 We have previously reported28 that the shift toward a more atherogentic lipoprotein profile with menopause was related to lower TRL and LDL catabolism, which could also account for the comparable results observed between the postmenopausal women and men.
In the present study TRL apoB-100 FCR was approximately 1.8 times higher in premenopausal women than in men, and was the primary determinant of plasma TRL-C concentrations. These results are concordant with the findings of a meta-analysis of 21 kinetic studies of VLDL (fasted state) and TRL (fed state) apoB-100.45 Pooled analysis showed that the FCR was 50% higher in women than in men (8.38±0.55 versus 12.59±1.6 pools/d; P=0.007). No significant difference in PR was reported. A recent study46 of VLDL apoB-100 kinetics in the fasted state reported that the lower VLDL apoB concentrations in women relative to men was attributable to lower VLDL apoB-100 PR. Interestingly, women also had shorter VLDL apoB-100 residence times (which is indicative of a faster FCR). Although the FCR data are similar to that observed in the present study, the lack of a difference in PR is likely attributable to the differences in prandial state.
With regard to LDL apoB-100 kinetics, FCR and PR were approximately 1.4 and 1.2 times higher, respectively, in the women relative to the men. This translated into a net reduction of 30% in LDL apoB-100 pool size, and 17% in LDL apoB-100 concentrations. These data suggest that the higher production rate did not compensate for the higher clearance rate observed. No comparable studies on the determinants of LDL apoB-100 kinetics in premenopausal women relative to men have been reported.
The mechanism(s) responsible for the higher TRL and LDL apoB-100 catabolic rate in premenopausal women is not fully understood. One possible explanation is the stimulatory effect of estrogen and the inhibitory effects of androgen on hepatic LDL and remnant receptors.47 In support of this concept, Walsh and colleagues48 reported that oral estradiol significantly reduced LDL-C concentrations by 10%, and this was accounted for by a 36% increase in apoB-100 FCR which offset a 21% increase in PR. In a subsequent analysis, Campos et al49 showed that estrogen predominantly affects the metabolism of large LDL apoB-100, with a 63% increase in FCR and a 42% increase in PR. The apoB-100 concentration of dense LDL was not affected. In this study the lower plasma LDL-C concentrations (34%) in the premenopausal women relative to men was primarily attributable to a 44% higher LDL apoB-100 FCR, despite a 24% higher PR. These data are consistent with the hormonal dependent effect on lipoprotein metabolism.
In the present study, significantly less LDL apoB-100 was derived from TRL via the direct pathway in women compared with men. This suggests that apoB-100 transport through TRL, IDL, and LDL fractions was greater in women, and this was compensated for by increased rates of catabolism resulting in lower apoB-100 concentration. Given that the development of CVD in women is delayed relative to men, it would appear that the increased apoB-100 transport rates coupled with the increased FCRs resulting in a shorter residence time of apoB-100 in the TRL, IDL, and LDL fractions accounts, in part, for the reduced risk. Another potential explanation for the protective advantage in premenopausal women may be attributable to the higher clearance or lower production rates of apoB-48 associated with postprandial chylomicrons. We could not determine apoB-48 kinetic behavior because the apoB-48 enrichment values in the study subjects were below detection limits.
Differences in age and body composition could have also contributed to the observed gender differences. Although there was no statistically significant difference in age and BMI between the men and women who participated in this study, the men tended to be older, taller, and weigh more. Consequently, we cannot fully exclude the impact of these variables on the gender differences observed, nor can we extrapolate these results to the obese population. Disparities in fat distribution between women and men could have also contributed to the differences in kinetic parameters observed. Men tend to have higher visceral fat mass,50 and it has been suggested that enhanced free fatty acid release from these stores can increase production of triglycerides by hepatocytes and result in higher TRL apoB-100 production rates. This would favor a decreased conversion rate of IDL to LDL and delayed FCR of LDL, via downregulation of hepatic LDL receptor activity. Additionally, higher hepatic lipase activity and lower lipoprotein lipase activity have also been reported in men than in women51 which could result in greater lipolysis of triglyceride enriched IDL and LDL and impaired removal of TRL, respectively. This hypothesis is partially supported by the slower TRL to IDL transfer rates and lower TRL and LDL apoB-100 FCR observed in the men compared with women.
In conclusion, the more favorable lipoprotein profile and consequently lower CVD risk in premenopausal women relative to similarly aged men is attributable, in part, to the faster clearance rates of atherogenic apoB-100 containing lipoproteins rather than lower production rates.
Acknowledgments
We are indebted to the Metabolic Research Unit staff for their expert care of the study subjects, to Margaret Diffenderfer for technical assistance, and to the study subjects without whom this investigation would not have been possible.
Sources of Funding
This work was supported by grants HL 54727 (to N.R.M. and A.H.L.), HL 39236 from the National Institute of Health (to E.J.S.), and NIBIB P41 EB-001975 (to P.H.R.B.). P.H.R.B. is a fellow of the National Health and Medical Research Council of Australia.
Footnotes
Disclosures
None.
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://atvb.ahajournals.org/cgi/content/full/28/10/1838
References
- 1.American Heart Association Writing Group. Heart Disease and Stroke Statistics-2006 Update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006:1–67. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 3.Hsia J, Barad D, Margolis K, Rodabough R, McGovern PG, Limacher MC, Oberman A, Smoller S. Usefulness of prior hysterectomy as an independent predictor of Framingham risk score (The Women’s Health Initiative) Am J Cardiol. 2003;92:264–269. doi: 10.1016/s0002-9149(03)00621-0. [DOI] [PubMed] [Google Scholar]
- 4.Hurst W. The Heart, Arteries and Veins. New York, NY: McGraw-Hill; 2002. [Google Scholar]
- 5.National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 6.Ferrara A, Barrett-Connor E, Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984–1994. Circulation. 1997;96:37–43. doi: 10.1161/01.cir.96.1.37. [DOI] [PubMed] [Google Scholar]
- 7.Godsland IF, Wynn V, Crook D, Miller NE. Sex, plasma lipoproteins, and atherosclerosis: prevailing assumptions and outstanding questions. Am Heart J. 1987;114:1467–1503. doi: 10.1016/0002-8703(87)90552-7. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB. The Framingham Study: historical insight on the impact of cardiovascular risk factors in men versus women. J Gender Spec Med. 2002;5:27–37. [PubMed] [Google Scholar]
- 9.Schaefer EA, Lamon-Fava S, Cohn SD, Schaefer MM, Ordovas JM, Castelli WP, FWPW Effects of age, gender, and menopausal status on plasma low density lipoprotein cholesterol and apoliporpotein B levels in the Framingham Offspring Study. J Lipid Res. 1994;35:779–792. [PubMed] [Google Scholar]
- 10.Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98:83–90. doi: 10.1016/0021-9150(93)90225-j. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PW, Anderson KM, Harris T, Kannel WB, Castelli WP. Determinants of change in total cholesterol and HDL-C with age: the Framingham Study. J Gerontol. 1994;49:M252–M257. doi: 10.1093/geronj/49.6.m252. [DOI] [PubMed] [Google Scholar]
- 12.Bello N, Mosca L. Epidemiology of coronary heart disease in women. Prog Cardiovasc Dis. 2004;46:287–285. doi: 10.1016/j.pcad.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Costanza MC, Cayanis E, Ross BM, Flaherty MS, Alvin GB, Das K, Morabia A. Relative contributions of genes, environment, and interactions to blood lipd concentrations in a general adult population. Am J Epidemiol. 2004;161:714–724. doi: 10.1093/aje/kwi103. [DOI] [PubMed] [Google Scholar]
- 14.Despres JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women. The health, risk factors, exercise training, and genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20:1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 15.Gardner CD, Tribble DL, Young DR, Ahn D, Fortmann SP. Population frequency distributions of HDL, HDL2, and HDL3 cholesterol and apolipoproteins A-I and B in healthy men and women and associations with age, gender, hormonal status and sex hormone use: The Stanford Five City Project. Prev Med. 2000;31:335–345. doi: 10.1006/pmed.2000.0715. [DOI] [PubMed] [Google Scholar]
- 16.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease. A prospective follow-up study of 14,786 middle-aged men and women in Finland. Circulation. 1999;99:1165–1172. doi: 10.1161/01.cir.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, McNamara JR, Fruchart JC, Luc G, Bard JM, Ordovas JM, Wilson PWF, Schaefer EJ. Effects of gender and menopausal status on plasma lipoprotein subspecies and particle sizes. J Lipid Res. 1996;37:1886–1896. [PubMed] [Google Scholar]
- 18.Campos H, McNamara JR, Wilson PW, Ordovas JM, Schaefer EJ. Differences in low density lipoprotein subfractions and apolipoproteins in premenopausal and postmenopausal women. J Clin Endocrinol Metabol. 1988;67:30–35. doi: 10.1210/jcem-67-1-30. [DOI] [PubMed] [Google Scholar]
- 19.Dallongeville J, Marecaux N, Isorez D, Zylbergberg G, Fruchart JC, Amouyel P. Multiple coronary heart disease risk factors are associated with menopause and influenced by substitutive hormonal therapy in a cohort of French women. Atherosclerosis. 1995;118:123–133. doi: 10.1016/0021-9150(95)05599-r. [DOI] [PubMed] [Google Scholar]
- 20.Davis CE, Pajak A, Rywik S, Williams DH, Broda G, Pazucha T, Ephross S. Natural menopause and cardiovascular disease risk factors. The Poland and US Collaborative Study on Cardiovascular Disease Epidemiology. Ann Epidemiol. 1994;4:445–448. doi: 10.1016/1047-2797(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 21.Nerbrand C, Lidfeldt J, Nyberg P, Schersten B, Samsioe G. Serum lipids and lipoproteins in relation to endogenous and exogenous female sex steroids and age. The Womwn’s Health in the Lund Area (WHILA) study. Maturitas. 2004;48:161–169. doi: 10.1016/j.maturitas.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Peters JC, Lawson KD, Middleton SJ, Triebwasser KC. Assessment of the nutritional effects of olestra, a nonabsorbed fat replacement: summary. J Nutr. 1997;127:1719S–1728S. doi: 10.1093/jn/127.8.1719S. [DOI] [PubMed] [Google Scholar]
- 23.Poehlman ET, Toth MJ, Ades PA, Rosen CJ. Menopause-associated changes in plasma lipids, insulin-like growth factor I and blood pressure: a longitudinal study. Eur J Clin Invest. 1997;27:322–326. doi: 10.1046/j.1365-2362.1997.1160662.x. [DOI] [PubMed] [Google Scholar]
- 24.Staessen JA, Ginocchio G, Thijs L, Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. J Hum Hypertens. 1997;11:507–514. doi: 10.1038/sj.jhh.1000476. [DOI] [PubMed] [Google Scholar]
- 25.Burnette MM, Meilahn E, Wing RR, Kuller LH. Smoking cessation, weight gain, and changes in cardiovascular risk factors during menopause: the Healthy Women Study. Am J Public Health. 1998;88:93–96. doi: 10.2105/ajph.88.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casiglia E, d’Este D, Ginocchio G, Colangeli G, Onesto C, Tramontin P, Ambrosio GB, Pessina AC. Lack of influence of menopause on blood pressure and cardiovascular risk profile: a 16-year longitudinal study concerning a cohort of 568 women. J Hypertens. 1996;14:729–736. doi: 10.1097/00004872-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Hall G, Collins A, Csemiczky G, Landgren BM. Lipoproteins and BMI: a comparison between women during transition to menopause and regularly menstruating healthy women. Maturitas. 2002;41:177–185. doi: 10.1016/s0378-5122(01)00258-4. [DOI] [PubMed] [Google Scholar]
- 28.Matthan NR, Jalbert SM, Lamon-Fava S, Dolnikowski GG, Welty FK, Barrett HR, Schaefer EJ, Lichtenstein AH. TRL, IDL, and LDL apolipoprotein B-100 and HDL apolipoprotein A-I kinetics as a function of age and menopausal status. Arterioscler Thromb Vasc Biol. 2005;25:1691–1696. doi: 10.1161/01.ATV.0000172629.12846.b8. [DOI] [PubMed] [Google Scholar]
- 29.Millar JS, Lichtenstein AH, Cuchel M, Dolnikowski GG, Hachey DL, Cohn JS, Schaefer EJ. Impact of age on the metabolism of VLDL, IDL and LDL apolipoprotein B-100 in men. J Lipid Res. 1995;36:1155–1167. [PubMed] [Google Scholar]
- 30.Lichtenstein AH, Cohn JS, Hachey DL, Millar JS, Ordovas JM, Schaefer EJ. Comparison of deuterated leucine, valine, and lysine in the measurement of human apolipoprotein A-I and B-100 kinetics. J Lipid Res. 1990;31:1693–1701. [PubMed] [Google Scholar]
- 31.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 32.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clinica Chimica Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 34.Ordovas JM, Peterson JP, Santaniello P, Cohn JS, Wilson PW, Schaefer EJ. Enzyme-linked immunosorbent assay for human plasma apolipoprotein B. J Lipid Res. 1987;28:1216–1224. [PubMed] [Google Scholar]
- 35.Velez-Carrasco W, Lichtenstein AH, Barrett PH, Sun Z, Dolnikowski GG, Welty FK, Schaefer EJ. Human apolipoprotein A-I kinetics within triglyceride-rich lipoproteins and high density lipoproteins. J Lipid Res. 1999;40:1695–1700. [PubMed] [Google Scholar]
- 36.Velez-Carrasco W, Lichtenstein AH, Welty FK, Li Z, Lamon-Fava S, Dolnikowski GG, Schaefer EJ. Dietary restriction of saturated fat and cholesterol decreases HDL ApoA-I secretion. Arterioscler Thromb Vasc Biol. 1999;19:918–924. doi: 10.1161/01.atv.19.4.918. [DOI] [PubMed] [Google Scholar]
- 37.Matthan NR, Welty FK, Barrett PH, Harausz C, Dolnikowski GG, Parks JS, Eckel RH, Schaefer EJ, Lichtenstein AH. Dietary hydrogenated fat increases high-density lipoprotein apoA-I catabolism and decreases low-density lipoprotein apoB-100 catabolism in hypercholesterolemic women. Arterioscler Thromb Vasc Biol. 2004;24:1092–1097. doi: 10.1161/01.ATV.0000128410.23161.be. [DOI] [PubMed] [Google Scholar]
- 38.Welty FK, Lichtenstein AH, Barrett PH, Dolnikowski GG, Ordovas JM, Schaefer EJ. Decreased production and increased catabolism of apolipoprotein B-100 in apolipoprotein B-67/B-100 heterozygotes. Arterioscler Thromb Vasc Biol. 1997;17:881–888. doi: 10.1161/01.atv.17.5.881. [DOI] [PubMed] [Google Scholar]
- 39.Parhofer KG, Barrett PHR, Bier DM, Schonfeld G. Determination of kinetic parameters of apolipoprotein B metabolism using amino acids labeled with stable isotopes. J Lipid Res. 1991;32:1311–1323. [PubMed] [Google Scholar]
- 40.Ramakrishnan R. Studying apolipoprotein turnover with stable isotope tracers: correct analysis is by modeling enrichments. J Lipid Res. 2006;47:2738–2753. doi: 10.1194/jlr.M600302-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive summary of the third report of the National Cholesterol Education Program (NCEP) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 42.Welty FK, Lichtenstein AH, Barrett PH, Dolnikowski GG, Schaefer EJ. Human apolipoprotein (Apo) B-48 and ApoB-100 kinetics with stable isotopes. Arterioscler Thromb Vasc Biol. 1999;19:2966–2974. doi: 10.1161/01.atv.19.12.2966. [DOI] [PubMed] [Google Scholar]
- 43.Schaefer EJ, Foster DM, Zech LA, Lindgren FT, Brewer HB, Jr, Levy RI. The effects of estrogen administration on plasma lipoprotein metabolism in premenopausal females. J Clin Endocrinol Metabol. 1983;57:262–267. doi: 10.1210/jcem-57-2-262. [DOI] [PubMed] [Google Scholar]
- 44.Marsh JB, Welty FK, Lichtenstein AH, Lamon-Fava S, Schaefer EJ. Apolipoprotein B metabolism in humans: studies with stable isotope-labeled amino acid precursors. Atherosclerosis. 2002;162:227–244. doi: 10.1016/s0021-9150(01)00709-2. [DOI] [PubMed] [Google Scholar]
- 45.Watts GF, Moroz P, Barrett PHR. Kinetics of very-low density lipoprotein apolipoprotein B-100 in normolipidemic subjects: pooled analysis of stable isoptope studies. Metabolism. 2000;49:1204–1210. doi: 10.1053/meta.2000.8621. [DOI] [PubMed] [Google Scholar]
- 46.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low density lipoproteins than men. J Clin Endocrinol Metabol. 2006;92:1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 47.Knopp RH, Paramsothy P, Retzlaff BM, Fish B, Walden C, Dowdy A, Tsunehara C, Aikawa K, Cheung MC. Sex differences in lipoprotein metabolism and dietary response: basis in hormonal differences and implications for cardiovascular disease. Curr Cardiol Rep. 2006;8:452–459. doi: 10.1007/s11886-006-0104-0. [DOI] [PubMed] [Google Scholar]
- 48.Walsh BW, Li H, Sacks FM. Effects of postmenopausal hormone replacement with oral and transdermal estrogen on high density lipoprotein metabolism. J Lipid Res. 1994;35:2083–2093. [PubMed] [Google Scholar]
- 49.Campos H, Walsh BW, Judge H, Sacks FM. Effect of estrogen on very low density lipoprotein and low density lipoprotein subclass metabolism in postmenopausal women. J Clin Endocrinol Metabol. 1997;82:3955–3963. doi: 10.1210/jcem.82.12.4437. [DOI] [PubMed] [Google Scholar]
- 50.William CM. Lipid metabolism in women. Proc Nutr Soc. 2004;63:153–160. doi: 10.1079/PNS2003314. [DOI] [PubMed] [Google Scholar]
- 51.Deeb SS, Zambon A, Carr MC, Ayyobi AF, Brunzell JD. Hepatic lipase and dyslipidemia: interactions among genetic variants, obesity, gender, and diet. J Lipid Res. 2003;44:1279–1286. doi: 10.1194/jlr.R200017-JLR200. [DOI] [PubMed] [Google Scholar]