Abstract
Neural contributions to the age-related reduction in spatial vision are incontrovertible. Whether there are differential age-related changes in the magnocellular (M) and parvocellular (P) pathways across the life span has not been tested extensively. We studied psychophysically the contrast gain signature of the M and P pathways for 13 younger and 13 older observers. Two separate paradigms thought to separate the M and P pathways based on their contrast gain signature were used. A four-square array was presented as an increment or decrement on a background of 115 Td for 35 ms, with one test square presented at a slightly higher or lower retinal illumination. Using a four-alternative forced-choice procedure, the observer's task was to choose the unique square. The two paradigms differed only in the pretrial adaptation and inter-stimulus array. Data were fitted with models of contrast discrimination derived from the unique contrast gain signatures. The fitted models indicate a change in the discrimination functions with age for both the M and P pathways, revealing a shift in the contrast gain slope. Results indicate that both M and P pathways undergo age-related changes, but functional losses appear greater for the P pathway under the conditions tested.
Keywords: age-related changes, contrast gain, M and P pathways
Introduction
Senescent losses in spatial vision have been demonstrated extensively through measures of contrast sensitivity (Elliott, Whitaker, & MacVeigh, 1990; Elliott, 1987; Owsley, Sekuler, & Siemsen, 1983; Tulunay-Keesey, Ver Hoeve, & Terkla-McGrane, 1988). Optical factors are known to contribute (Burton, Owsley, & Sloane, 1993; Elliott et al., 2009; Owsley et al., 1983), but the extent of the age-related spatial sensitivity decline exceeds what is predicted by optics alone (Elliott et al., 1990; Elliott, 1987; Elliott et al., 2009; Morrison & McGrath, 1985). At the retinal (Curcio & Drucker, 1993; Curcio, Millican, Allen, & Kalina, 1993; Gao & Hollyfield, 1992; Marshall, 1978) and post-retinal (Hua et al., 2006; Karas & McKendrick, 2009; Schefrin, Bieber, McLean, & Werner, 1998; Schmolesky, Wang, Pu, & Leventhal, 2000) levels, age-related structural and functional changes have been observed, which may account for some neural sensitivity decline, but whether age-related functional changes in spatial vision are specific to the magnocellular (M) or parvocellular (P) pathways has received surprisingly little attention.
There is support for an age-related functional decline in the P pathway, but thus far, losses have only been demonstrated with chromatically varying stimuli. Specifically, measures of contrast sensitivity for (L–M) varying patterns reveal an average 0.25 log unit loss in sensitivity from ~20-to-70 years, becoming more pronounced with an increase in spatial frequency (Hardy, Delahunt, Okajima, & Werner, 2005). In addition, visual-evoked potentials show an age-related increase in latency and reduction in amplitude of the waveforms to both chromatic onset and pattern reversal stimuli varying along an isoluminant (L–M) axis (Crognale, 2002; Fiorentini, Porciatti, Morrone, & Burr, 1996). These age-related sensitivity losses cannot be attributed to optical factors, and the functional loss may be attributed to the P pathway due to its superior sensitivity to isoluminant chromatic stimuli (Kaplan, 2004).
In the spatial domain with luminance modulation, there are few studies evaluating age-related functional losses in the P pathway, although some reports have implicated the P pathway in the decline of contrast sensitivity (Crassini, Brown, & Bowman, 1988; Elliott et al., 1990). These reports argue that the P pathway's superior sensitivity to high spatial frequencies may account for the increase in sensitivity loss at higher spatial frequencies (Owsley et al., 1983; Tulunay-Keesey et al., 1988). However, there is still debate about the functional roles of the M and P pathways in contrast sensitivity (e.g., Plainis & Murray, 2005; Watson, 1992), so a functional decline that increases with spatial frequency may not unequivocally implicate the P pathway (Kaplan, 2004; Kaplan & Shapley, 1986).
There is also clear evidence that the M pathway undergoes functional changes with age, especially when probed with stimuli thought to target the superior contrast gain and temporal sensitivity of the M pathway (Kaplan, 2004). Schefrin, Tregear, Harvey, and Werner (1999) measured spatial contrast sensitivity using a retinal illumination of –0.85 log trolands (Td), an illumination where the contrast gain response is absent in physiological recordings of P cells (Purpura, Kaplan, & Shapley, 1988) and the high spatial frequency cut-off approximates the reduced sampling efficiency of M ganglion cells (Lennie & Fairchild, 1994). Older observers exhibited sensitivity losses at all tested spatial frequencies, but the loss measured at frequencies below 1.2 c/deg was significantly larger. Luminance impulse response functions (IRF) also point to age-related losses in the M pathway, where the two primary phases of the impulse response function are significantly reduced for older observers (Shinomori & Werner, 2003). This loss was attributed to the M pathway because many of the observers exhibited trimodal IRFs, a response pattern found only in physiological recordings of M cells at higher contrasts (Lee, Pokorny, Smith, & Kremers, 1994). Suprathreshold estimates of brightness magnitude also point to a functional age-related loss in the M pathway (Sturr, Van Orden, & Taub, 1987). At low levels of illumination (near threshold), older adults consistently rated a reference light as being much dimmer when the presentation times were very short (10 ms).
While previous results indicate that both the M and P pathways undergo age-related functional changes, it is difficult to determine from these findings whether one pathway is responsible for spatial sensitivity loss with age. Thus far, there are few studies evaluating the two pathways simultaneously and/or along the same stimulus dimension (e.g., using the same spatial configuration, temporal frequency, chromaticity, and/or background illumination to measure performance in the two pathways). One recent study that did evaluate the two pathways simultaneously reports an overall reduction in contrast sensitivity for older observers compared to younger observers, irrespective of pathway (McKendrick, Sampson, Wallard, & Babcock, 2007). However, McKendrick et al. (2007) focused on measuring sensitivity changes in glaucoma patients, so no controls were used for the optical differences between the age-matched healthy older and younger observers, and the stimuli used to assess the M and P pathways varied along different stimulus dimensions [a fast onset stimulus was thought to target the M pathway, while a large contrast change was used to target the contrast gain signature of the P pathway (Lenova, Pokorny, & Smith, 2003; Pokorny & Smith, 1997)]. Evaluating the functional role of the M and P pathways in the age-related spatial vision decline requires an experimental design that measures psychophysical performance along similar stimulus dimensions.
It is difficult to find a spatial stimulus that will generate a response from one pathway in isolation, as physiological measures show a large overlap in sensitivity in both the spatial and temporal domains (Derrington & Lennie, 1984). However, large physiological differences in the M and P pathways exist in their unique luminance contrast gain signatures. Because contrast sensitivity is thought to be proportional to the contrast gain of the underlying mechanism (Kaplan & Shapley, 1986; Shapley, Kaplan, & Purpura, 1993), contrast gain provides a unique signature to evaluate the mechanisms underlying spatial vision for individual observers. In the retina (Lee, Pokorny, Smith, Martin, & Valberg, 1990) and LGN (Kaplan & Shapley, 1986), the contrast gain signatures of M and P cells can be described by a Michaelis–Menten saturation function:
| (1) |
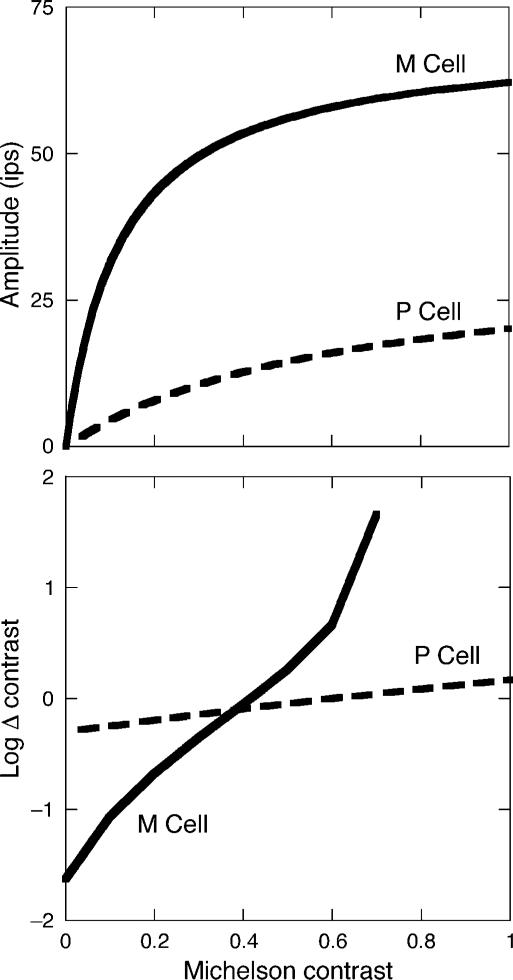
where R0 is the resting response level of the cell, Rmax is the maximum response amplitude in impulses per second (ips), C is the Michelson contrast of the stimulus, and Csaturation is the semisaturation constant (the contrast at which the response amplitude is half Rmax). The linear slope of this function is determined by the cell's percent contrast gain, (Rmax/Csaturation)/100, or the initial linear rise from zero contrast (Kaplan & Shapley, 1986; Pokorny & Smith, 1997). The contrast gain in M cells is 10 times higher than P cells, exhibiting a slope greater than unity. P cells have a shallow slope, with values typically less than 0.5. Examples of the unique contrast gain signatures measured physiologically in the LGN are shown in Figure 1 (top).
Figure 1.
(Top) Contrast response functions of prototypical M (solid curve) and P (dashed curve) cells plotted as a function of Michelson contrast. The data (only the fitted Michealis–Menten curves shown) are from Kaplan and Shapley (1986) and represent average responses from 36 ganglion cell (impulses per second, ips) recordings in a rhesus monkey LGN. The stimuli were optimal spatial frequencies for each cell and were modulated at 4 Hz. Rmax was 65 and 45 ips, and Csaturation was 0.13 and 1.74 for the M and P functions, respectively. (Bottom) Predicted physiological contrast detection and discrimination for the M (solid curve) and P (dashed curve) pathways, plotted as a function of Michelson contrast. Predictions are based on Equation 2 using data reported in Kaplan and Shapley (1986) and a criterion of 10 ips. After Pokorny and Smith (1997), with permission.
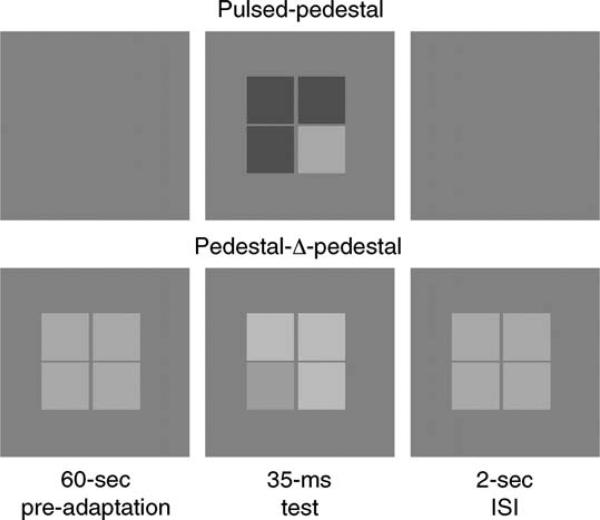
Recently, Pokorny and Smith (1997) developed a psychophysical probe to measure the contrast gain signatures of the M and P pathways in spatial vision. Specifically, two testing paradigms measure contrast discrimination for a series of luminance contrast steps (Weber contrast, ΔI/IB). The paradigms differ only in the pretrial adaptation and inter-stimulus array. The first paradigm is known as the pulsed pedestal, where observers first adapt to a uniform background. The trial period contains a brief presentation of a four-square array at a suprathreshold contrast as an increment or decrement to the background, with one test square presented at a slightly higher or lower retinal illumination compared to the other three. In between trials, observers maintain adaptation to the uniform background. In the second paradigm, the pedestal–Δ-pedestal, observers first adapt to a four-square array that is presented continuously. During the brief trial period, the illuminance of the trial foursquare array is incremented or decremented from the pretrial adaptation pedestal by a small amount, with the test square incremented or decremented by a slightly different amount. The brief trial period is followed by a period of readaptation to the continuous pedestal foursquare array. The observer's task in each paradigm is to discriminate the square that differed from the other three.
These unique paradigms are interpreted to target the separate physiological contrast gain signatures of the M and P pathways (Kaplan & Shapley, 1986; Pokorny & Smith, 1997). The spatiotemporal contrast step in the pulsed pedestal is intended to saturate the M pathway response, revealing discrimination thresholds characteristic of the P pathway, while the pedestal–Δ-pedestal condition is designed to target the M pathway by using minute steps in luminance contrast (<0.15 log units from the luminance used for adaptation). Using a modified version of the Michaelis–Menten saturation function (Equation 1), Pokorny and Smith (1997) developed a model to compare the psychophysical discrimination thresholds of inferred M and P pathways to the physiological contrast gain signatures measured in the LGN (Kaplan & Shapley, 1986). The physiological predictions, as derived from Equation 1, were calculated as follows:
| (2) |
where δ is a criterion difference in two contrast responses. The predicted discrimination thresholds for the M and P pathways using a criterion of 10 ips are illustrated in Figure 1 (bottom). This model proposes that discrimination thresholds will form a V-shaped function centered on the luminance used for adaptation (similar to a Michelson contrast of 0 in Figure 1, bottom), with a shallow V-shape for the P pathway and a steep V-shape for the M pathway.
The separate paradigms have been used in a series of studies to evaluate differential psychophysical characteristics of the M and P pathways, and robust differences related to the M and P pathways are consistently reported (Kachinsky Smith, & Pokorny, 2003; Pokorny, Sun, & Smith, 2003; Smith, Sun, & Pokorny, 2001). The paradigms have also been used to study the functional losses associated with the M and P pathways in disease. For instance, a study of patients with retinitis pigmentosa reported a greater functional loss in the M pathway (Alexander, Pokorny, Smith, Fishman, & Barnes, 2001), while others have evaluated losses in glaucoma patients (Battista, Badcock, & McKendrick, 2009; McKendrick et al., 2007).
In this study, we applied the technique of Pokorny and Smith (1997) to evaluate age-related functional changes in contrast gain related to the M and P pathways. The pedestal–Δ-pedestal and pulsed pedestal paradigms were used to evaluate the contrast gain signature separately in the putative M and P pathways to determine if the age-related decline in spatial vision is the result of a selective neural deficit in one pathway.
Methods
Observers
Thirteen younger (mean of 21.5 years, range 19–25, 8 males) and 13 older (mean of 73.4 years, range 64–82, 7 males) phakic observers participated in this experiment. Observers were tested monocularly using their preferred eye (i.e., the eye with superior visual acuity and health, or by individual preference). Refractive errors did not exceed ±5 diopter (D) sphere or ±2 D cylinder for any observer. Corrected Snellen acuity was equal to or better than 20/25 in the tested eye. The presence of abnormal ocular media and retinal disease was ruled out for each observer by conventional eye exam, including a slit lamp examination and ophthalmoscopy. All participants had no more small drusen than is considered normal for their age as assessed in fundus photos by a retina specialist and no abnormal vascular, retinal, choriodal, or optic nerve findings. All observers had intraocular pressure of <22 mm Hg, and all had normal color vision as measured with the Farnsworth D15 Color Vision Test. Written informed consent was obtained following the Tenets of Helsinki and with approval of the Institutional Review Board of the University of California, Davis, School of Medicine.
Apparatus
Stimuli were presented on a 17-inch EIZO FlexScan T566 CRT, driven by a Macintosh G4 computer with 8-bit color resolution. The refresh rate of the CRT was set to 85 Hz. To control for differences in pupil size between the two age groups, the monitor was viewed through a 2.16× magnification Keplerian telescope with a 2.5-mm exit pupil in a plane conjugate to the observer's pupil. The monitor was calibrated using a Minolta colorimeter (CS-100 Chroma Meter) following the procedures set forth by Brainard, Pelli, and Robson (2002). The experimental software was written in MATLAB 5.2.1 using the Psychophysics Toolbox extension (Brainard, 1997; Pelli, 1997). The observer's head was stabilized with the use of a bite bar on an x–y–z mount, and the observer's pupil was viewed with an auxiliary optical channel to maintain alignment with the exit pupil of the optical system. Trial lenses were mounted in a holder positioned in front of the eye to correct any refractive error.
Controlling for ocular media density
To minimize individual differences in prereceptoral transmission, each observer (young and old) equated the green phosphor to a constant luminance of the red phosphor (115 Td) by heterochromatic flicker photometry. This assumes that age-related changes in ocular media at long wavelengths (red phosphor) are negligible (<0.1 log unit; Werner, 1982). Observers first dark adapted for 5 min. An annular stimulus of 0.05°–2.05° was presented on a black background in square-wave counterphase between the red and green phosphors at an individually determined frequency. Due to differences in flicker sensitivity with age (Kim & Mayer, 1994), the flicker frequency was varied until the observer was able to find a flicker null. The mean flicker rate was therefore 18.7 Hz for young observers and 12.8 Hz for older observers. Each observer was instructed to adjust the luminance of the green phosphor using the method of adjustment until the flicker was minimized (i.e., the stimulus was isoluminant). Three-to-five flicker settings were averaged for each observer.
Stimuli
Two of the paradigms introduced by Pokorny and Smith (1997) that are thought to target the contrast gain signature of the M and P pathways were used. For both paradigms, the test stimulus was a 2.05° square array, with 4 squares each subtending 1° and separated by 3.25 arcmin. The stimulus configuration was chosen to be similar to that of Pokorny and Smith (1997) and to avoid threshold floor effects that occur with larger stimulus arrays (Smith et al., 2001). After equating the luminance for individual differences in ocular media density, the 22° × 18° surround was maintained at 115 Td using only the green CRT phosphor. The illuminance of the four-square array varied between 73 and 182 Td, providing contrast discrimination measures as both increments and decrements to the background. In the pulsed pedestal condition (thought to target the P pathway), the four-square array was presented only during the trial period, while observers maintained adaptation to the uniform background between trials. In the pedestal–Δ-pedestal condition (thought to target the M pathway), the four-square array was presented as a pedestal of higher retinal illuminance (145 Td) during the entire session, therefore maintaining adaptation to the background plus pedestal illuminance. During the trial period, the entire four-square array was incremented or decremented by no more than 0.08 log units compared to the pedestal retinal illuminance. The test square was chosen randomly and appeared of a slightly higher or lower retinal illuminance than the other three. The temporal profile in each paradigm was a square pulse of 35.29 ms (3 screen refreshes at 85 Hz), accompanied by an auditory cue (a short beep) and separated by a 2-s inter-stimulus interval (ISI). An example of the two testing paradigms is illustrated in Figure 2.
Figure 2.
Example of the testing sequence. (Top) The pulsed pedestal condition. Observers first adapted to a uniform field for 60 s. The test stimulus array was pulsed for 35.29 ms and included 3 squares at the pedestal luminance and one test square as an increment or decrement to the other three. (Bottom) Pedestal–Δ-pedestal condition. Observers adapted to the uniform field plus pedestal for 60 s, followed by the 35 ms test, where three squares were changed as an increment or decrement to the pedestal luminance. The test square increased or decreased by a slightly different amount.
Procedure
Observers were seated in a dark, windowless room and allowed to dark adapt for 5 min. Each test began with either 60-s adaptation to the uniform field (pulsed pedestal) or 60-s adaptation to the uniform field plus pedestal (pedestal–Δ-pedestal). The observer's task was to press 1 of 4 keys to identify the test square that differed from the other three, i.e., was of lower or higher retinal illuminance compared to the pedestal (or Δ-pedestal). A black fixation circle of 0.15° in the middle of the foursquare array provided a fixation and spatial location cue during the course of adaptation and for each trial presentation. Observers were instructed to maintain fixation throughout the experimental procedure. Contrast discrimination thresholds were obtained using a spatial 4-alternative forced-choice procedure with a double random alternating staircase. In one staircase, thresholds were measured in the increment direction (test square was a higher illumination than the pedestal), while the other measured thresholds in the decrement direction (test square was a lower illumination than the pedestal). Each staircase followed an asymmetric reversal rule, where the test stimulus decreased in contrast after 2 correct identifications but increased in contrast after 1 incorrect identification. At the beginning of the trial, the contrast of the test square was high so it was easily discriminated. After 2 correct identifications, the contrast between the test square and the pedestal was halved until a criterion step size of 0.009 log Td was reached (0.004 log units higher than the bit resolution of the video card). Each observer's final threshold was defined as the average of the last 8 of 10 contrast reversals at the criterion step size. Staircases thus required approximately 40 trials each to converge on the final threshold.
There were 18 different pedestal illuminance conditions, 9 for the pulsed pedestal with luminance steps of 0.05 log units centered on the background illuminance, and 9 for the pedestal–Δ-pedestal paradigm with luminance steps of 0.01 and 0.02 log units centered on the pedestal illuminance. Each condition was repeated twice for a total of 36 sessions. One 2-h session yielded approximately 14 contrast discrimination thresholds, so each observer required approximately 3 visits to the laboratory to complete all conditions.
Results
Separation of the putative M and P pathways
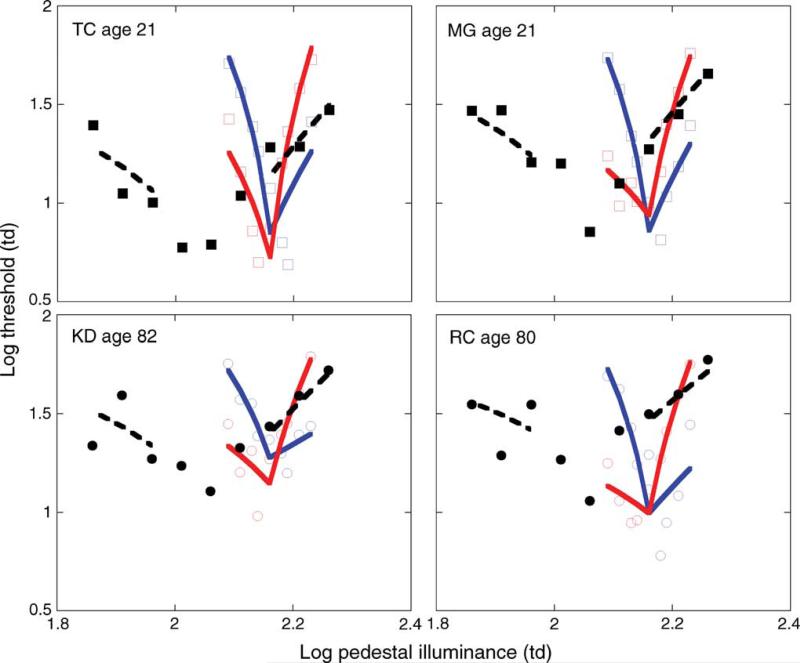
Data from four individual (two younger, and two older) observers are illustrated in Figure 3, where log thresholds are plotted as a function of the log retinal illuminance (in Td) of the pedestal. The discrimination functions obtained in the pulsed pedestal (solid symbols, dashed lines) and pedestal–Δ-pedestal (open symbols, solid lines) are consistent with discrimination thresholds mediated by two different pathways. With a large increment or decrement pulse in contrast, as in the pulsed pedestal, discrimination thresholds form a shallow V-shaped function. With a small increment or decrement pulse in contrast, as in the pedestal–Δ-pedestal, discrimination thresholds form a steep V-shaped function. These discrimination functions are consistent with the model of Pokorny and Smith (1997, model fits shown as dashed and solid lines, explained in detail below) and are thought to reveal the contrast gain signature of the P and M pathways, respectively (Pokorny & Smith, 1997).
Figure 3.
Data from (top) two younger and (bottom) two older observers. Solid symbols denote discrimination thresholds for the pulsed pedestal, open symbols denote discrimination thresholds for the pedestal–Δ-pedestal paradigm. Dashed and solid lines denote fits to the data generated from Equations 3 and 4, respectively. Error bars are not included for clarity.
Pulsed pedestal
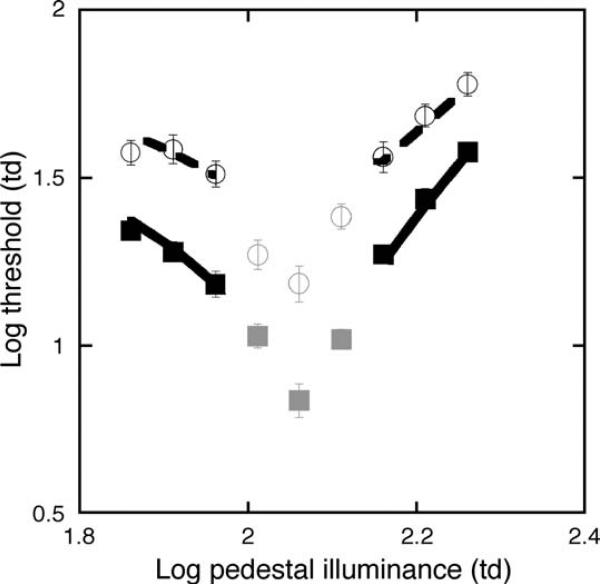
Figure 4 illustrates the average log discrimination thresholds (symbols) plotted as a function of the log retinal illuminance (in Td) in the pulsed pedestal condition for the 2 age groups. The data point for the highest pedestal condition (182 Td) represents the average of only 11 of the 13 older observers. One older observer was not able to return to complete the pulsed pedestal condition (1 full repeat of all pedestals and both repeats of the final data point), and one observer was removed due to a ceiling effect: the discrimination thresholds were at the maximum illumination of the CRT for the increment staircase.
Figure 4.
Mean log discrimination thresholds (symbols) and the model fits (lines) from Equation 3 for the pulsed pedestal paradigm for younger (squares, solid line) and older (circles, dashed line) observers. The gray symbols denote data removed from the least-squares fits and data analyses, as they may represent M pathway discrimination thresholds. Error bars are ±1 SEM.
Thresholds obtained with the increment and decrement staircases were not significantly different for the younger (p = 0.14, paired two-tailed t-test) or older age group (p = 0.14, paired two-tailed t-test) and were therefore averaged for each age group. The minimum threshold occurred when the pedestal was equal to the surround illuminance, and the data form a V-shaped function. The illuminance of the central 3 pedestal conditions differed by <0.1 log unit from the surround and may not saturate the M pathway sufficiently to isolate the P pathway response (Kachinsky et al., 2003; Pokorny & Smith, 1997). Therefore, these 3 pedestal conditions (denoted by gray symbols) were removed from the pulsed pedestal analysis. Overall, it is clear that log discrimination thresholds are elevated for older individuals, irrespective of pedestal illuminance (main effect of age, F(1,142) = 174.50, p < 0.0001). The average increase in discrimination thresholds was 0.27 log units compared to the young observers.
Data were fitted with the saturation equation for the contrast response of the P pathway as revised by Smith et al. (2001):
| (3) |
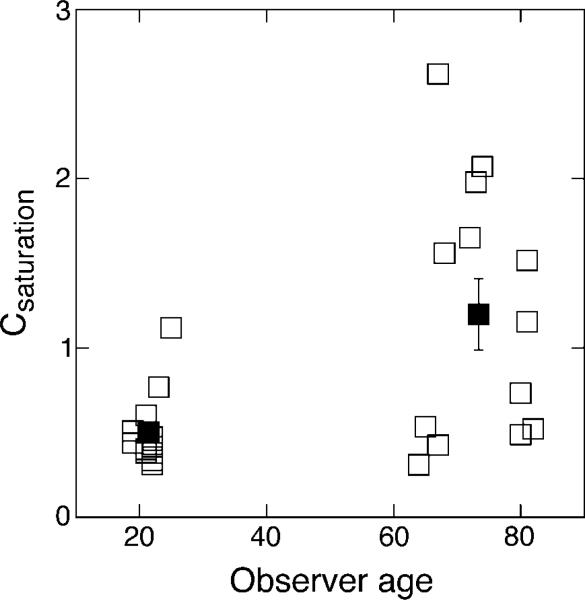
where |C| represents the absolute Weber contrast of the pulsed pedestal (ΔI/Is), Kc is the criterion increment firing rate (comparable to δ/Rmax in Pokorny & Smith, 1997), and K represents the overall scaling constant to account for threshold sensitivity. Kc was set at 0.001 to reflect the distinct saturation signature of the P pathway (i.e., it does not approach saturation; Kachinsky et al., 2003), and Csaturation and K were allowed to vary. The fits were good, with an average of 0.04 ± 0.01 and 0.06 ± 0.01 log units of absolute error between the fitted function and individual data points for younger and older observers, respectively. The scaling parameter, K, was similar for all observers within each age group but was higher for the older age group overall (mean of 0.14 ± 0.01 and 0.19 ± 0.02 for young and old, respectively; F(1,24) = 6.98, p = 0.01), reflecting the vertical shift in discrimination thresholds. The Csaturation values, a reflection of the contrast gain signature of the underlying neural pathway, are consistent with previous reports and reflect discrimination thresholds in the P pathway (Kachinsky et al., 2003; Pokorny & Smith, 1997; Smith et al., 2001). The mean Csaturation values were 0.50 ± 0.06 for younger and 1.19 ± 0.21 for older observers, a 2.38 factor increase for the older age group. This difference is statistically significant (main effect of age, F(1,24) = 10.15, p < 0.01) and is illustrated in Figure 5, where the individual Csaturation values are plotted as a function of the observer's age. The Csaturation value determines the shape of the discrimination function, with a higher value reflecting a more shallow function (comparable to the physiological semi-saturation constant in a Michaelis–Menten saturation function, Equation 1). This means that older observers required more contrast at lower pedestal contrasts to discriminate the contrast change than they did at higher pedestal contrasts, consistent with a more shallow contrast gain slope.
Figure 5.
Csaturation values obtained from the fitted Equation 3. Open symbols denote individual observers plotted as a function of age; closed symbols denote age group means ±1 SEM.
Pedestal–Δ-pedestal
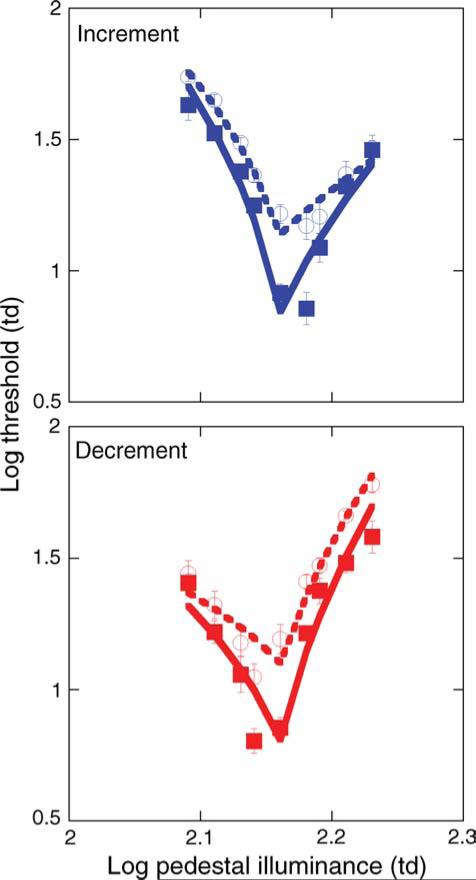
Discrimination thresholds obtained in the pedestal–Δ-pedestal condition are illustrated in Figure 6 (symbols). The increment (Figure 6, top) and decrement (Figure 6, bottom) staircases revealed differential discrimination functions (i.e., asymmetry around the steady pedestal used for adaptation) and were therefore separated in the analyses. Specifically, increment thresholds are lower than decrement thresholds when the test stimulus is presented on an increment Δ-pedestal, and decrement thresholds are lower than increment thresholds on a decrement Δ-pedestal. This difference suggests that contrast discrimination thresholds were mediated by the putative ON and OFF pathways. For one young observer, the two lowest (in the increment staircase) and the two highest (in the decrement staircase) Δ-pedestal conditions revealed extremely low discrimination thresholds. Compared to the other young observers, these 4 data points significantly biased this observer's data, and in turn notably shifted the mean for the young age group. Therefore, these four data points were removed for this single observer. Otherwise, the data are consistent across observers and replicate previous findings (Kachinsky et al., 2003; Pokorny et al., 2003).
Figure 6.
Open and solid symbols denote contrast discrimination thresholds, while dashed and solid lines denote fits to the data based on Equation 4 for older and younger observers, respectively. The top panel shows the increment data and the bottom panel shows the decrement data. Error bars are ±1 SEM.
Data for the increment and decrement staircases were fitted (lines) separately using a modification of Equation 3:
| (4) |
where Kc was set at 0.01 to reflect the contrast saturation signature of the M pathway, and an additional variable, κ, was added to account for the reduction in effective contrast for discrimination thresholds obtained with decrement tests on a decrement Δ-pedestal and increment tests on an increment Δ-pedestal (Kachinsky et al., 2003). The values of K, Csaturation, and . were allowed to vary in the least-squares calculation.
The fits were good for both increment and decrement data across observers. The average error was 0.10 ± 0.01 and 0.07 ± 0.01 log units for younger and older observers, respectively, for the increment data. The average error in the decrement data was 0.11 ± 0.02 and 0.08 ± 0.01 log units for younger and older observers, respectively. The largest amount of error between the data and the fits were at Δ-pedestal contrasts near the pretrial adaptation pedestal (<0.03 log units), where sub-threshold summation is likely to occur (Kachinsky et al., 2003). The scaling parameter, K, was higher overall for older observers for both the increment (0.53 ± 0.03 vs. 0.64 ± 0.03, F(1,24) = 7.59, p = 0.01) and decrement data (0.45 ± 0.03 vs. 0.62 ± 0.03, F(1,24) = 19.15, p < 0.01), which reflected the overall shift in thresholds for both the increment (F(1,210) = 54.43, p < 0.0001) and decrement staircase (F(1,210) = 65.89, p < 0.0001). The RMANOVA revealed no difference in the age-related threshold elevation between the two staircases (F(1,210) = 0.13, p = 0.25).
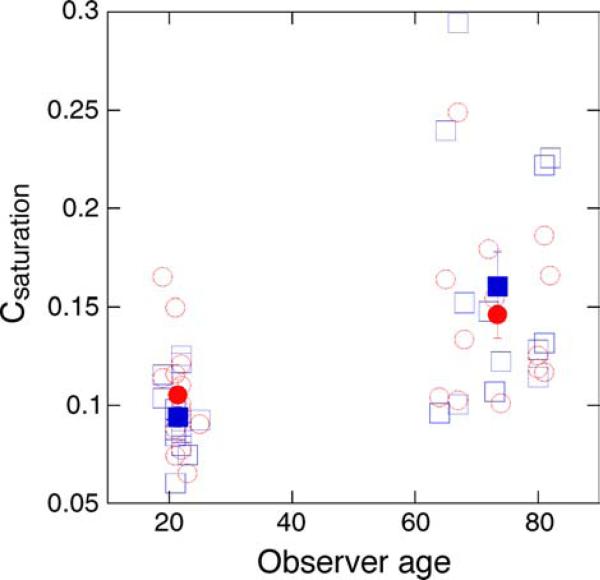
The discrimination functions showed a significant change with age, becoming shallower, as revealed in the Csaturation values for the two age groups. The increase in Csaturation as a function of age is illustrated in Figure 7. For the increment data (blue symbols), Csaturation values were 0.09 ± 0.01 and 0.16 ± 0.02 for younger and older observers, respectively (main effect of age, F(1,24) = 12.95, p < 0.01). For the decrement data (red symbols), Csaturation values were 0.11 ± 0.01 for younger and 0.15 ± 0.01 for older observers (main effect of age, F(1,24) = 8.19, p < 0.01). The age-related increase in Csaturation was significantly larger for the increment staircase compared to the decrement staircase, as revealed in the interaction effect of the RMANOVA (F(1,24) = 4.46, p < 0.05). In general, Csaturation values for the younger observers are in agreement with previous reports (Kachinsky et al., 2003; Pokorny et al., 2003). They are much lower than values obtained in the pulsed pedestal and are consistent with the physiological signature of the M pathway (i.e., a steep contrast gain slope and early saturation; Kaplan & Shapley, 1986; Pokorny & Smith, 1997).
Figure 7.
Open symbols denote Csaturation values obtained with the pedestal–Δ-pedestal paradigm, plotted as a function of the observer's age. Blue and red symbols are values obtained with the increment and decrement staircases, respectively. Solid symbols denote age group means ±1 SEM.
Differential decline in the putative M and P pathways
To determine whether M and P pathways differ in the rate of age-related functional decline, the discrimination thresholds and Csaturation values for the three conditions (pulsed pedestal, and the two staircases of the pedestal–Δ-pedestal) were compared between age groups using separate within-subjects analyses. Two RMANOVAs revealed significant interactions between age group and discrimination thresholds (F(1,146) = 47.52, p < 0.001, RMANOVA 1) and between age group and the Csaturation values (F(1,24) = 12.11, p < 0.002, RMANOVA 2). We used contrast decompositions in both analyses to determine whether thresholds and Csaturation values in the pulsed pedestal condition increased or decreased significantly with age compared to the pedestal–Δ-pedestal condition, and all comparisons proved significant. Thresholds increased significantly more with age in the pulsed pedestal condition (0.27 log units) compared to the increment (increasing by 0.14 log units, F(1,146) = 13.33, p < 0.001, contrast 1) and decrement (increasing by 0.17 log units, F(1,146) = 14.28, p < 0.001, contrast 2) staircases of the pedestal–Δ-pedestal condition. The age-related change in Csaturation was also significantly larger for the pulsed pedestal (a 2.38 factor increase) compared to both the increment (a 1.71 factor increase, F(1,24) = 8.63, p < 0.01, contrast 1) and decrement (a 1.39 factor increase, F(1,24) = 9.45, p < 0.01, contrast 2) staircases of the pedestal–Δ-pedestal condition.
Discussion
The goal of the current study was to examine whether age-related changes in spatial vision are specific to the M or P pathways. Previous reports provide strong support for the idea that differing signatures of the M and P pathways can be revealed through the pedestal–Δ-pedestal and pulsed pedestal paradigms, respectively (Kachinsky et al., 2003; Pokorny & Smith, 1997; Pokorny et al., 2003; Smith et al., 2001). Our results are consistent with this view as data obtained with the young observers replicate previous findings.
Discrimination thresholds measured with the two pedestal conditions revealed age-related differences in both the inferred M and P pathways. We interpret the losses in sensitivity to be due to neural rather than optical factors. Equating the retinal illuminance for individual observers controlled for age-related changes in ocular media (Weale, 1988; Werner, 1982) and pupil size (Winn, Whitaker, Elliott, & Phillips, 1994). Any increase in high-order aberrations for our older population should not have a significant impact on discrimination thresholds due to the small exit pupil of the optical system (Applegate, Donnelly, Marsack, & Koenig, 2007; Calver, Cox, & Elliott, 1999). There is the potential that an increase in intraocular scatter (Hennelly, Barbur, Edgar, & Woodward, 1998; Westheimer &Liang, 1995) may have a significant effect on the contrast discrimination functions. However, previous studies indicate that optical factors including intraocular scatter do not fully account for differences between older and younger observers on increment thresholds and grating detection tasks (Bennett, Sekuler, & Ozin, 1999; Werner, Schelble, & Bieber, 2000; Whitaker & Elliott, 1992). Therefore, it is likely that the differences in discrimination functions measured here are not due to differences in the optical quality of the eye and are, in fact, neural in origin.
Differences between M and P pathways
For the younger observers, the shape of the discrimination functions and the Csaturation values from the fitted model compared well with those reported previously for both pedestal conditions (Kachinsky et al., 2003; Pokorny et al., 2003; Smith et al., 2001). However, older observers exhibited an elevation in discrimination thresholds and a shift in the discrimination functions compared to young observers. Thresholds in the pulsed pedestal increased by an average of 0.27 log units, while thresholds in the pedestal–Δ-pedestal condition increased by an average of 0.14 and 0.17 log units for the increment and decrement staircases, respectively. In general, the threshold elevations were larger at lower pedestal contrasts, as revealed from the Csaturation value. The Csaturation values increased for older observers by a factor of 2.38 in the pulsed pedestal condition and by 1.71 and 1.39 for the increment and decrement staircase, respectively, in the pedestal–Δ-pedestal condition. This means that older observers required more contrast to discriminate contrast changes at low pedestal contrasts compared to higher pedestal contrasts, indicative of a shallower contrast gain slope.
The differential degree of threshold elevation and change in the discrimination functions for the two pedestal conditions suggests that while age-related neural changes occur in both pathways, the functional decline is larger for the P pathway. Previous reports have inferred age-related functional losses in the M (Schefrin et al., 1999; Shinomori & Werner, 2003; Sturr et al., 1987) and P (Crassini et al., 1988; Crognale, 2002; Elliott et al., 1990; Fiorentini et al., 1996; Hardy et al., 2005) pathways, and our results agree with such findings. For instance, the age-related threshold elevation measured in the pedestal–Δ-pedestal condition is consistent with a mean threshold increase of 0.14 log units reported by Schefrin et al. (1999) for older observers between 61–88 years using stimuli thought to target the M pathway. Furthermore, Hardy et al. (2005) and McKendrick et al. (2007) show an age-related increase in thresholds for stimuli thought to target the P pathway of 0.25 and 0.22 log units for older observers between 65–77 and 52–87 years, respectively, similar to the 0.27 log unit increase in discrimination thresholds measured here. However, previous studies revealed functional losses along separate stimulus dimensions, making it difficult to conclude whether one pathway undergoes larger functional changes that may underlie age-related sensitivity losses in spatial vision. Using methods differing only in the pretrial adaptation and inter-stimulus array to target the individual pathways, we found a significant difference in the functional decline of the two pathways. Although both the M and P pathways likely play a role in the age-related spatial vision decline, the larger change in the P pathway may provide one explanation for the pattern of age-related contrast sensitivity loss [e.g., greater loss at higher spatiotemporal frequencies (Crassini et al., 1988; Elliott et al., 1990)].
Age-related changes in contrast gain
The observed shift in the contrast gain function is consistent with previous measures of spatial vision loss with age. For instance, Sloane, Owsley, and Jackson (1988) measured contrast sensitivity for young and old observers with spatial frequencies ranging from 0.5 to 8 c/deg and luminance levels ranging from mesopic to photopic. At a low temporal frequency, the slope of the sensitivity function (inverse of the threshold function) spanning the range of luminance was steeper for older than younger observers at spatial frequencies below 4 c/deg. This means that older observers required more contrast at low luminance levels to detect a sine-wave grating than their younger counterparts. This pattern of contrast sensitivity loss is consistent with a reduction in the contrast gain slope of the underlying mechanism at low spatial frequencies. In addition, suprathreshold measures of spatial vision performance also support a reduction in contrast gain within one (or both) of the pathways. Losses in contrast sensitivity with age are diminished when the stimuli are above threshold, as measured with contrast matching functions where contrast discrimination losses are absent (Delahunt, Hardy, Okajima, & Werner, 2005; Tulunay-Keesey et al., 1988). This means that while older individuals require more contrast than young observers to detect specific spatial patterns, they are able to dependably match the contrast of different spatial frequencies to a standard when the contrast is above threshold. This finding suggests that the slope of the contrast gain function at lower levels of visual processing (i.e., within the M and P pathways) is steeper for young than older observers (Spear, 1993). Suprathreshold estimates of brightness magnitude, which reveal a dual-branched function thought to reflect the contrast gain of the M and P pathways, also point to a change in the contrast gain slope (Sturr et al., 1987). At low levels of illumination (near threshold), older adults show a reduction in the slope of their brightness function, especially for very short (10 ms) stimulus presentation times, but at higher levels of illumination, the slope does not differ from young observers. All of these results imply that the contrast gain signature becomes more shallow with age, consistent with the changes in discrimination functions measured here.
Possible neural mechanisms
Changes in contrast gain
One potential locus for the shift in contrast gain may be age-related changes within the photoreceptor layer. At the ganglion cell level, contrast gain depends on the photon flux over the receptive field (Enroth-Cugell & Shapley, 1973). With a loss in photoreceptor number or a change in efficiency, the photon flux at the receptive field may be reduced. In the peripheral retina, cone numbers can be reduced by up to 22% (Curcio et al., 1993; Gao & Hollyfield, 1992; Panda-Jonas, Jonas, & Jakobczyk-Zmija, 1995), but due to a small number of samples (Curcio et al., 1993) or the inability to accurately count cone numbers (Panda-Jonas et al., 1995), the extent of photo-receptor loss in the foveal region is not clear. In addition, with such a wide variation in cone numbers across observers, Gao and Hollyfield (1992) note that at least 20% of the total cone population would have to be lost in the fovea before a significant reduction would be measurable. Despite the lack of clear evidence on cone number, foveal cones do undergo age-related structural changes. Curcio et al. (1993) report the presence of refractive particles at the ellipsoid–myoid junction and a displacement of the photoreceptor nuclei to the inner segment. In the outer segments, convolutions appear to form within the disks (Marshall, 1978). While photopigment optical density is relatively stable across the life span (Elsner, Berk, Burns, & Rosenberg, 1988; Renner, Knau, Neitz, Neitz, & Werner, 2004), age-related changes do occur in outer retinal response dynamics (Birch, Hood, Locke, Hoffman, & Tzekov, 2002; Gerth, Sutter, & Werner, 2003). These retinal changes are accompanied by functional changes measured psychophysically. At low illumination levels or with small changes in cone excitation, losses in the sensitivity of cone mechanisms are reported (Schefrin, Shinomori, & Werner, 1995; Werner & Steele, 1988). Even a small loss in photo-receptor number or change in photoreceptor function could impact the strength of input signals at the ganglion cell level and thereby alter the contrast gain signature, especially in the foveal region where there are fewer photoreceptors per individual ganglion cell.
Another possible neural explanation for these data is a reduction in ganglion cell number, which may be reduced by up to 16% for older observers in the foveal region (Curcio & Drucker, 1993; Gao & Hollyfield, 1992). The change in contrast gain may reflect a reorganization of retinal inputs to the ganglion cell layer or a reorganization of LGN and/or cortical cells to compensate for the loss in ganglion cell number. A reorganization of cortical cells has been proposed as an explanation for the increase in scotopic spatial summation (Schefrin et al., 1998) that cannot be accounted for by an age-related enlargement in the ganglion cell receptive field size (Schefrin, Hauser, & Werner, 2004). This functional change is analogous to the physiological enlargements of cortical cell receptive fields following the loss of the afferent inputs from a retinal laser burn (Chino, Kass, Smith, Langston, & Cheng, 1992; Gilbert & Wiesel, 1992). A reorganization of ganglion cell inputs may produce a change in the contrast gain of post-retinal mechanisms.
Discrimination threshold elevation
It has been shown previously that discrimination thresholds obtained in the pulsed pedestal condition also reveal temporal (Pokorny & Smith, 1997) and spatial summation (Smith et al., 2001) characteristics of the P pathway. In both cases, a decrease in temporal presentation time or area of the 4-square array results in an overall increase in thresholds for young observers, but the shape of the discrimination function is not changed (i.e., Csaturation value). A similar pattern could likely be revealed for the M pathway with the pedestal–Δ-pedestal paradigm, but this has not been measured directly (although, see Pokorny et al., 2003). The higher thresholds in our older population may therefore reflect differences in spatial or temporal summation. Thus far, there is no evidence for a significant age-related change in foveal spatial summation under photopic conditions (Malania et al., 2009; Zele, O'Loughlin, Guymer, & Vingrys, 2005), but evidence against age-related changes in temporal summation is less clear.
For threshold detection tasks, age-related differences in temporal sensitivity typically appear when the stimulus is presented at higher spatial and/or temporal frequencies (Elliott et al., 1990; Kim & Mayer, 1994; Sloane et al., 1988; Tulunay-Keesey et al., 1988). The difference in contrast discrimination thresholds for the older observers measured here may therefore reflect a loss in temporal sensitivity. For instance, the increase in discrimination thresholds measured with the pedestal–Δ-pedestal paradigm agree well with Kim and Mayer (1994). Their data indicate an approximate temporal sensitivity reduction of 0.1 log units at 28 Hz (approximate frequency of our stimulus) for older observers compared to young observers at equivalent ages to our population. It is not clear if temporal sensitivity losses differ for the M and P pathways, and our results cannot provide further insight into this matter. Although the threshold elevations were larger in the inferred P pathway, this may be a consequence of the high temporal frequency of the stimulus and not necessarily a reflection of greater age-related temporal sensitivity loss specific to the P pathway.
Differences in putative ON and OFF pathways (M pathway)
In the pedestal–Δ-pedestal paradigm, increment and decrement staircases revealed distinct discrimination thresholds for both age groups, consistent with mediation by the ON and OFF pathways (Kachinsky et al., 2003; Pokorny et al., 2003). For the young observers, discrimination functions in the two staircases were reversed, but the contrast gain signatures were comparable, consistent with data in previous reports (Kachinsky et al., 2003; Pokorny et al., 2003). On the other hand, the increment staircase revealed a much larger change in Csaturation (a 1.71-fold increase vs. a 1.39-fold increase in the decrement staircase) and in the corresponding discrimination function for the older observers. This observation may reveal a larger age-related change in the ON compared to the OFF pathway. However, caution should be noted with this interpretation. A point-by-point comparison of the threshold elevations reveals that the age-related losses were similar (the average threshold elevation was only 0.03 log units different, a nonsignificant difference) for the two staircases. Therefore, it is possible that the methods used in this study do not accurately reveal differential neural losses in the M ON and OFF pathways.
It is not unreasonable to suspect a larger change in one pathway over the other, as clear physiological (Nelson & Kolb, 2004) and psychophysical differences exist between the ON and OFF pathways, such as their spatial tuning (Tyler, Chan, & Liu, 1992) and differences in adaptation mechanisms (Chichilnisky & Wandell, 1996). However currently, there is a lack of research on age-related changes associated with the separate ON and OFF pathways. The temporal dynamics of the M ON and OFF pathways do show differences for younger observers (Pokorny et al., 2003), and further testing with the pedestal–Δ-pedestal paradigm may reveal larger differences with age. The change in the putative M ON pathway discrimination functions for our older observers may mirror a change in the M ON pathway impulse response function with age (Shinomori & Werner, 2003), but there are no reports of an age-related change in the temporal dynamics of the M OFF pathway. Further research is needed to evaluate differential patterns of age-related sensitivity loss in the ON and OFF pathways.
Conclusion
Whether age-related changes in spatial vision are specific to the M or P pathway was evaluated with methods thought to target the distinct M and P contrast gain signatures; the pedestal–Δ-pedestal and pulsed pedestal paradigms, respectively. For both pedestal conditions, contrast discrimination thresholds were elevated for older observers, and a change in the discrimination function was revealed with a quantitative model of contrast saturation for the M and P pathways. The change in discrimination functions is consistent with a shift in the contrast gain slope, which becomes shallower with age. The M and P pathways each show significant sensitivity decline with age, an indication that both pathways may play a role in age-related spatial sensitivity loss. However, the methods used here reveal a larger functional decline in the P pathway.
Acknowledgments
This work was supported by funds from the National Institute on Aging (Grant AG04058) and a Research to Prevent Blindness Senior Scientist Award to J. S. Werner. We thank Joel Pokorny for helpful comments on the methodology, and Susan Garcia for assistance with subject recruitment and screening.
Footnotes
Commercial relationships: none.
References
- Alexander KR, Pokorny J, Smith VC, Fishman GA, Barnes C. Contrast discrimination deficits in retinitis pigmentosa are greater for stimuli that favor the magnocellular pathway. Vision Research. 2001;41:671–683. doi: 10.1016/s0042-6989(00)00286-8. [PubMed] [DOI] [PubMed] [Google Scholar]
- Applegate RA, Donnelly WJ, Marsack JD, Koenig DE. Three-dimensional relationship between high-order root-mean-square wavefront error, pupil diameter, and aging. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2007;24:578–587. doi: 10.1364/josaa.24.000578. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista J, Badcock DR, McKendrick AM. Spatial summation properties for magnocellular and parvocellular pathways in glaucoma. Investigative Ophthalmology & Visual Science. 2009;50:1221–1226. doi: 10.1167/iovs.08-2517. [PubMed] [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Sekuler AB, Ozin L. Effects of aging on calculation efficiency and equivalent noise. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1999;16:654–668. doi: 10.1364/josaa.16.000654. [PubMed] [DOI] [PubMed] [Google Scholar]
- Birch DG, Hood DC, Locke KG, Hoffman DR, Tzekov RT. Quantitative electroretinogram measures of phototransduction in cone and rod photoreceptors: Normal aging, progression with disease, and test–retest variability. Archives of Ophthalmology. 2002;120:1045–1051. doi: 10.1001/archopht.120.8.1045. [PubMed] [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–446. [PubMed] [PubMed] [Google Scholar]
- Brainard DH, Pelli DG, Robson T. Display characterization. In: Hornak J, editor. The encyclopedia of imaging science and technology. John Wiley and Sons; New York: 2002. pp. 172–188. [Google Scholar]
- Burton KB, Owsley C, Sloane ME. Aging and neural spatial contrast sensitivity: Photopic vision. Vision Research. 1993;33:939–946. doi: 10.1016/0042-6989(93)90077-a. [PubMed] [DOI] [PubMed] [Google Scholar]
- Calver RI, Cox MJ, Elliott DB. Effect of aging on the monochromatic aberrations of the human eye. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1999;16:2069–2078. doi: 10.1364/josaa.16.002069. [PubMed] [DOI] [PubMed] [Google Scholar]
- Chichilnisky EJ, Wandell BA. Seeing gray through the ON and OFF pathways. Visual Neuroscience. 1996;13:591–596. doi: 10.1017/s0952523800008270. [PubMed] [DOI] [PubMed] [Google Scholar]
- Chino YM, Kass JH, Smith EL, Langston AL, Cheng H. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vision Research. 1992;32:789–796. doi: 10.1016/0042-6989(92)90021-a. [PubMed] [DOI] [PubMed] [Google Scholar]
- Crassini B, Brown B, Bowman K. Age-related changes in contrast sensitivity in central and peripheral retina. Perception. 1988;17:315–332. doi: 10.1068/p170315. [PubMed] [DOI] [PubMed] [Google Scholar]
- Crognale MA. Development, maturation, and aging of chromatic visual pathways: VEP results. Journal of Vision. 2002;2(6):2, 438–450. doi: 10.1167/2.6.2. http://journalofvision.org/2/6/2/, doi:10.1167/2.6.2. [PubMed][Article] [DOI] [PubMed]
- Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer's disease and aging. Annals of Neurology. 1993;33:248–257. doi: 10.1002/ana.410330305. [PubMed] [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investigative Ophthalmology & Visual Science. 1993;34:3278–3296. [PubMed][Article] [PubMed] [Google Scholar]
- Delahunt PB, Hardy JL, Okajima K, Werner JS. Senescence of spatial chromatic contrast sensitivity. II. Matching under natural viewing conditions. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2005;22:60–67. doi: 10.1364/josaa.22.000060. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurons in the lateral geniculate nucleus of macaque. The Journal of Physiology. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D, Whitaker D, MacVeigh D. Neural contribution to spatiotemporal contrast sensitivity decline in healthy ageing eyes. Vision Science. 1990;30:541–547. doi: 10.1016/0042-6989(90)90066-t. [PubMed] [DOI] [PubMed] [Google Scholar]
- Elliott DB. Contrast sensitivity decline with ageing: A neural or optical phenomenon? Ophthalmic & Physiological Optics. 1987;7:415–419. [PubMed] [PubMed] [Google Scholar]
- Elliott SL, Choi SS, Doble N, Hardy JL, Evans JW, Werner JS. Role of high-order aberrations in senescent changes in spatial vision. Journal of Vision. 2009;9(2):24, 1–16. doi: 10.1167/9.2.24. http://journalofvision.org/9/2/24/, doi:10.1167/9.2.24. [PubMed][Article] [DOI] [PMC free article] [PubMed]
- Elsner AE, Berk L, Burns SA, Rosenberg PR. Aging and human cone photopigments. Journal of the Optical Society of America A, Optics and Image Science. 1988;5:2106–2112. doi: 10.1364/josaa.5.002106. [PubMed] [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Shapley RM. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. The Journal of Physiology. 1973;233:311–326. doi: 10.1113/jphysiol.1973.sp010309. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A, Porciatti V, Morrone MC, Burr DC. Visual ageing: Unspecific decline of the responses to luminance and colour. Vision Research. 1996;36:3557–3566. doi: 10.1016/0042-6989(96)00032-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Aging of the human retina. Investigative Ophthalmology & Visual Science. 1992;33:1–17. [PubMed][Article] [PubMed] [Google Scholar]
- Gerth C, Sutter EE, Werner JS. mfERG response dynamics of the aging retina. Investigative Ophthalmology & Visual Science. 2003;44:4443–4450. doi: 10.1167/iovs.02-1056. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- Hardy JL, Delahunt PB, Okajima K, Werner JS. Senescence of spatial chromatic contrast sensitivity. I. Detection under conditions controlling for optical factors. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2005;22:49–59. doi: 10.1364/josaa.22.000049. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennelly ML, Barbur JL, Edgar DF, Woodward EG. The effect of age on the light scattering characteristics of the eye. Ophthalmic & Physiological Optics. 1998;18:197–203. [PubMed] [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiology of Aging. 2006;27:155–162. doi: 10.1016/j.neurobiolaging.2004.11.012. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kachinsky ES, Smith VC, Pokorny J. Discrimination and identification of luminance contrast stimuli. Journal of Vision. 2003;3(10):2, 599–609. doi: 10.1167/3.10.2. http://journalofvision.org/3/10/2/, doi:10.1167/3.10.2. [PubMed][Article] [DOI] [PubMed]
- Kaplan E. The M, P, and K pathways of the primate visual system. In: Chalupa LE, Werner JS, editors. The visual neurosciences. Vol. 1. MIT Press; Cambridge, MA: 2004. pp. 481–493. [Google Scholar]
- Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proceedings of the National Academy of Sciences. 1986;83:2755–2757. doi: 10.1073/pnas.83.8.2755. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas R, McKendrick AM. Aging alters surround modulation of perceived contrast. Journal of Vision. 2009;9(5):11, 1–9. doi: 10.1167/9.5.11. http://journalofvision.org/9/5/11/, doi:10.1167/9.5.11. [PubMed][Article] [DOI] [PubMed]
- Kim CBY, Mayer MJ. Foveal flicker sensitivity in healthy aging eyes: II. Cross-sectional aging trends from 18 through 77 years of age. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1994;11:1958–1969. doi: 10.1364/josaa.11.001958. [PubMed] [DOI] [PubMed] [Google Scholar]
- Lee BB, Pokorny J, Smith VC, Kremers J. Responses to pulses and sinusoids in macaque ganglion cells. Vision Research. 1994;34:3081–3096. doi: 10.1016/0042-6989(94)90074-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- Lee BB, Pokorny J, Smith VC, Martin PR, Valberg A. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. Journal of the Optical Society of America A, Optics and Image Science. 1990;7:2223–2236. doi: 10.1364/josaa.7.002223. [PubMed] [DOI] [PubMed] [Google Scholar]
- Lennie P, Fairchild MD. Ganglion cell pathways for rod vision. Vision Research. 1994;34:477–482. doi: 10.1016/0042-6989(94)90161-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- Lenova A, Pokorny J, Smith VC. Spatial frequency processing in inferred P- and M-pathways. Vision Research. 2003;43:2133–2139. doi: 10.1016/s0042-6989(03)00333-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Malania M, Devinck F, Hardy JL, Delahunt PB, Knoblauch K, Werner JS. Test of senescent changes in photopic grating summation [Abstract]. Journal of Vision. 2009;9(8):1074, 1074a. doi: 10.1167/11.10.15. http://journalofvision.org/9/8/1074/, doi:10.1167/9.8.1074. [DOI] [PMC free article] [PubMed]
- Marshall J. Ageing changes in the human cones. In: Shimizu K, Oosterhuis JA, editors. XXIII Concilium Ophthalmologicum, Kyoto. Elsevier; North Holland, Amsterdam, The Netherlands: 1978. pp. 375–378. [Google Scholar]
- McKendrick AM, Sampson GP, Walland MJ, Babcock DR. Contrast sensitivity changes due to glaucoma and normal aging: Low-spatial frequency losses in both magnocellular and parvocellular pathways. Investigative Ophthalmology & Visual Science. 2007;48:2115–2122. doi: 10.1167/iovs.06-1208. [PubMed][Article] [DOI] [PubMed] [Google Scholar]
- Morrison JD, McGrath C. Assessment of the optical contributions to the age-related deterioration in vision. Quarterly Journal of Experimental Psychology. 1985;70:249–269. doi: 10.1113/expphysiol.1985.sp002907. [PubMed][Article] [DOI] [PubMed] [Google Scholar]
- Nelson R, Kolb H. ON and OFF pathways in the vertebrate retinal and visual system. In: Chalupa LE, Werner JS, editors. The visual neurosciences. Vol. 1. MIT Press; Cambridge, MA: 2004. pp. 260–278. [Google Scholar]
- Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Research. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Retinal photoreceptor density decreases with age. Ophthalmology. 1995;102:1853–1859. doi: 10.1016/s0161-6420(95)30784-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- Pelli DG. The videotoolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [PubMed] [Google Scholar]
- Plainis S, Murray IJ. Magnocellular channel subservers the human contrast-sensitivity function. Perception. 2005;34:933–940. doi: 10.1068/p5451. [PubMed] [DOI] [PubMed] [Google Scholar]
- Pokorny J, Smith VC. Psychophysical signatures associated with magnocellular and parvocellular pathway contrast gain. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1997;14:2477–2486. doi: 10.1364/josaa.14.002477. [PubMed] [DOI] [PubMed] [Google Scholar]
- Pokorny J, Sun VCW, Smith VC. Temporal dynamics of early light adaptation. Journal of Vision. 2003;3(6):3, 423–431. doi: 10.1167/3.6.3. http://journalofvision.org/3/6/3/, doi:10.1167/3.6.3. [PubMed][Article] [DOI] [PubMed]
- Purpura K, Kaplan E, Shapley RM. Background light and the contrast gain of primate P and M retinal ganglion cells. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4534–4537. doi: 10.1073/pnas.85.12.4534. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner AB, Knau H, Neitz M, Neitz J, Werner JS. Photopigment optical density of the human foveola and a paradoxical senescent increase outside the fovea. Visual Neuroscience. 2004;21:827–834. doi: 10.1017/S0952523804216030. [PuMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefrin BE, Bieber ML, McLean R, Werner JS. The area of complete scotopic spatial summation enlarges with age. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1998;15:340–348. doi: 10.1364/josaa.15.000340. [PubMed] [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Hauser M, Werner JS. Evidence against age-related enlargements of ganglion cell receptive field centers under scotopic conditions. Vision Research. 2004;44:423–428. doi: 10.1016/j.visres.2003.09.030. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefrin BE, Shinomori K, Werner JS. Contributions of neural pathways to age-related losses in chromatic discrimination. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1995;12:1233–1241. doi: 10.1364/josaa.12.001233. [PubMed] [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Tregear SJ, Harvey LO, Werner JS. Senescent changes in scotopic contrast sensitivity. Vision Research. 1999;39:3728–3736. doi: 10.1016/s0042-6989(99)00072-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nature Neuroscience. 2000;3:384–390. doi: 10.1038/73957. [PubMed] [DOI] [PubMed] [Google Scholar]
- Shapley R, Kaplan E, Purpura K. Contrast sensitivity and light adaptation in photoreceptors or in the retinal network. In: Shapley R, Lam DM, editors. Contrast sensitivity. MIT Press; Cambridge, MA: 1993. pp. 103–116. [Google Scholar]
- Shinomori K, Werner JS. Senescence of temporal impulse response to a luminance pulse. Vision Research. 2003;43:617–627. doi: 10.1016/S0042-6989(03)00009-9. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane ME, Owsley C, Jackson CA. Aging and luminance-adaptation effects on spatial contrast sensitivity. Journal of the Optical Society of America A, Optics and Image Science. 1988;5:2181–2190. doi: 10.1364/josaa.5.002181. [PubMed] [DOI] [PubMed] [Google Scholar]
- Smith VC, Sun VCW, Pokorny J. Pulse and steady-pedestal contrast discrimination: Effect of spatial parameters. Vision Research. 2001;41:2079–2088. doi: 10.1016/s0042-6989(01)00085-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- Spear PD. Neural bases of visual deficits during aging. Vision Research. 1993;33:2589–2609. doi: 10.1016/0042-6989(93)90218-l. [PubMed] [DOI] [PubMed] [Google Scholar]
- Sturr JF, Van Orden K, Taub HA. Selective attenuation of brightness for brief stimuli and at low intensities supports age-related transient channel losses. Experimental Aging Research. 1987;13:145–149. doi: 10.1080/03610738708259316. [PubMed] [DOI] [PubMed] [Google Scholar]
- Tulunay-Keesey U, Ver Hoeve JN, Terkla-McGrane C. Threshold and suprathreshold spatiotemporal response throughout adulthood. Journal of the Optical Society of America A, Optics and Image Science. 1988;5:2191–2200. doi: 10.1364/josaa.5.002191. [PubMed] [DOI] [PubMed] [Google Scholar]
- Tyler CW, Chan H, Liu L. Differential spatial tunings for ON and OFF pathway stimulation. Ophthalmic & Physiological Optics. 1992;12:233–240. doi: 10.1111/j.1475-1313.1992.tb00297.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Watson AB. Transfer of contrast sensitivity in linear visual networks. Visual Neuroscience. 1992;8:65–76. doi: 10.1017/s0952523800006507. [PubMed] [DOI] [PubMed] [Google Scholar]
- Weale R. Age and the transmittance of the human crystalline lens. The Journal of Physiology. 1988;395:577–587. doi: 10.1113/jphysiol.1988.sp016935. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JS. Development of scotopic sensitivity and the absorption spectrum of the human ocular media. Journal of the Optical Society of America. 1982;72:247–258. doi: 10.1364/josa.72.000247. [PubMed] [DOI] [PubMed] [Google Scholar]
- Werner JS, Schelble KA, Bieber ML. Age-related increases in photopic increment thresholds are not due to an elevation in intrinsic noise. Color Research & Application. 2000;26:S48–S52. doi: 10.1002/1520-6378(2001)26:1+<::AID-COL11>3.0.CO;2-P. [PubMed][Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JS, Steele VG. Sensitivity of human foveal color mechanisms throughout the life span. Journal of the Optical Society of America A, Optics and Image Science. 1988;5:2122–2130. doi: 10.1364/josaa.5.002122. [PubMed] [DOI] [PubMed] [Google Scholar]
- Westheimer G, Liang J. Influence of ocular light scatter on the eye's optical performance. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1995;12:1417–1424. doi: 10.1364/josaa.12.001417. [PubMed] [DOI] [PubMed] [Google Scholar]
- Whitaker D, Elliott DB. Simulating age-related optical changes in the human eye. Documenta Ophthalmologica. 1992;82:307–316. doi: 10.1007/BF00161018. [PubMed] [DOI] [PubMed] [Google Scholar]
- Winn B, Whitaker D, Elliott DB, Phillips NJ. Factors affecting light-adapted pupil size in normal human subjects. Investigative Ophthalmology & Visual Science. 1994;35:1132–1137. [PubMed][Article] [PubMed] [Google Scholar]
- Zele AJ, O'Loughlin RK, Guymer RH, Vingrys AJ. Disclosing disease mechanisms with a spatio-temporal summation paradigm. Graefe's Archives of Clinical Experimental Ophthalmology. 2005;244:425–432. doi: 10.1007/s00417-005-0121-5. [PubMed] [DOI] [PubMed] [Google Scholar]