Abstract
Despite great progress in curing childhood acute lymphoblastic leukemia, survival after relapse remains poor. We analyzed survival following relapse among 9,585 pediatric patients enrolled on Children's Oncology Group clinical trials between 1988-2002. A total of 1961 patients (20.5%) experienced relapse at any site. The primary endpoint was survival. Patients were subcategorized by site of relapse and timing of relapse from initial diagnosis. Time to relapse remains the strongest predictor of survival. Patients experiencing early relapse less than 18 months from initial diagnosis had a particularly poor outcome with a 5-year survival estimate of 21.0±1.8%. Standard risk patients who relapsed had improved survival compared to their higher risk counterparts; differences in survival for the two risk groups was most pronounced for patients relapsing after 18 months. Adjusting for both time and relapse site, multivariate analysis showed that age (10+ yrs) and presence of CNS disease at diagnosis, male gender, and T-cell disease were significant predictors of inferior post-relapse survival. Of note, there was no difference in survival rates for relapsed patients in earlier versus later era trials. New therapeutic strategies are urgently needed for children with relapsed ALL and efforts should focus on discovering the biological pathways that mediate drug resistance.
Keywords: relapsed acute lymphoblastic leukemia, Children's Oncology Group, pediatric
Introduction
Overall cure rates for newly diagnosed children with acute lymphoblastic leukemia (ALL) approach 80% (1, 2) with currently available therapies. However, even with risk-stratified and more intensive frontline therapy, 20-25% of children with ALL still relapse, accounting for a large number of pediatric cancer patients(3-6). The treatment of patients with relapsed ALL remains unsatisfactory, with suboptimal re-induction remission rates and poor long-term overall survival rates ranging from 15-50% (7-11).
Previous studies have demonstrated that successful treatment of relapsed ALL has been closely correlated to the length of the first clinical remission (CR1) and the site of relapse (7, 12). Survival after a bone marrow (BM) relapse is worse than after an extramedullary (EM) event, and the worst outcomes are significantly associated with earlier relapse while on therapy. Most papers define early relapses occurring in the first 18 months from diagnosis, intermediate relapses occurring between 18-36 months from diagnosis, and late relapses occurring after 36 months. Survival for patients experiencing early BM relapse range from 0%-15% (13, 14), while those with intermediate medullary relapses have survival rates from 10-40% (12, 14). For patients with late BM relapses, survival rates range from 14-50% (15). Relapses involving sites other than bone marrow have better prognoses: the survival rates for CNS relapses approach 51% (16) and those for isolated testicular relapses range from 53-84% (17, 18).
Treatment for re-induction of relapsed ALL primarily involves many of the same traditional chemotherapy agents initially used as well as stem cell transplantation (SCT). The benefits of SCT versus aggressive chemotherapy for different patient groups remain unclear but the overall poor outcomes and long-term sequelae from both strategies renders neither of them ideal treatments (15, 19-22). Efforts must focus on improving our understanding of the biological factors contributing to relapse and identifying new agents that will increase the chances of a sustained second remission. We conducted this retrospective study in order to determine if patterns of survival after relapse have changed with more intensive frontline therapy on contemporary clinical trials. We describe the diagnostic characteristics and outcomes after relapse for a large number of patients relapsing on the generation of COG trials opened from 1988-2002, in order to determine if newer treatment strategies have altered the survival of ALL following relapse.
Materials and Methods
Clinical trials
Data used in this study were collected as part of Children's Cancer Group (CCG) protocols that enrolled patients between December 1988 and 2002. All patients and guardians participated on these trials with informed consent. A total of 9,585 children with newly diagnosed B-precursor or T-cell ALL were enrolled in CCG-1881, CCG-1882, CCG-1883, CCG-1891, CCG-1901, CCG-1922, CCG-1952, CCG-1953, CCG-1961, and CCG-1962 trials. For all protocols, any patient with a diagnosis of mature B-cell leukemia or AML was ineligible for enrollment. A description of these trials, accrual dates, total enrollment, and overall 5- year event-free survival (EFS) rates are listed in Table 1. Table 2 compares the clinical characteristics of the patients who relapsed with those who remained in CR1. The primary endpoint was overall survival after first relapse (information on subsequent adverse events was not collected). Following relapse on initial trials, the only data collected was survival. Thus, we cannot comment on event-free survival or analyze outcome based on the treatment administered post relapse.
Table 1. Clinical trials sponsored by CCG during 1988-2002.
| Trial | Accrual dates | Risk group | Study Question | N | 5- year EFS±SE1 | # of Relapses (% per protocol) | Median (range) length of CR12 | Median follow-up time3 | Median follow-up time4 |
|---|---|---|---|---|---|---|---|---|---|
| 1881 | 12/88-12/92 | Low | Randomization of DI5 vs. standard therapy. | 778 | 79.9±1.5% | 152 (19.5%) | 36.1 (2.6, 120.9) | 91.9 | 49.0 |
| 1882 | 5/89-6/95 | Higher | Efficacy of post-induction intensification for higher risk patients with a slow response to initial therapy. | 1525 | 68.3±1.3% | 388 (25.4%) | 23.1 (0.1, 113.4) | 35.7 | 8.6 |
| 1883 | 12/88-8/93 | Infant | Treatment of infants less than 12 months of age. | 135 | 37.6±4.3% | 75 (55.6%) | 5.5 (0.6, 69.3) | 16.6 | 6.6 |
| 1891 | 1/90-7/93 | Intermediate | Efficacy of double DI vs. single DI | 1204 | 79.6±1.2% | 223 (18.5%) | 28.7 (0.6, 124.8) | 73.7 | 33.7 |
| 1901 | 12/90-9/94 | High | Treatment of newly diagnosed children with multiple unfavorable presenting features | 418 | 73.0±2.3% | 79 (18.9%) | 16.1 (1.7, 67.8) | 28.9 | 8.7 |
| 1922 | 3/93-8/95 | Standard | Randomized comparison IV vs. oral 6-MP and dexamethasone vs. prednisone | 1061 | 82.3±1.3% | 179 (16.9%) | 30.5 (2.1, 86.7) | 67.7 | 25.0 |
| 1952 | 5/96-2/00 | NCI Standard | Randomized comparison of oral 6-MP vs. 6-TG and intrathecal MTX vs ITT. | 2174 | 81.7±0.1% | 371 (17.1%) | 31.5 (0.5, 110.6) | 69.0 | 26.6 |
| 1953 | 7/96-8/00 | Infant | Treatment of infants with intensive early systemic and intrathecal therapy. | 115 | 43.1±4.9% | 24 (20.9%) | 8.9 (1.1, 69.2) | 14.1 | 4.0 |
| 1961 | 9/96-5/02 | NCI High | Randomized comparison of standard versus augmented BFM and single versus double DI. | 2057 | 71.5±1.3% | 443 (21.5%) | 21.1 (0.2, 112.2) | 37.2 | 9.9 |
| 1962 | 3/97-11/98 | NCI Standard | Randomized comparison of PEG-asparaginase with native E.coli asparaginase on the standard arm of the CCG-1952 trials. | 118 | 78.8±4.3% | 27 (22.9%) | 30.2 (6.7, 93.1) | 68.6 | 24.1 |
| Total | 9585 | 1961 | 26.0 (0.1, 124.8) | 51.6 | 15.7 |
Standard error
Only among relapsed ALL patients (time in months)
Median follow-up time (time in months) for relapsed patients from initial diagnosis
Median follow-up time (time in months) for relapsed patients from relapse diagnosis
DI=Delayed Intensification
Table 2. Characteristics of 9585 patients enrolled on CCG trials between 1988-2002.
| Features at initial diagnosis | Total number of patients | Number of patients in CCR (% of overall group) | # of relapsed patients (% in relapsed group) | 5-year EFS (SE) | |
|---|---|---|---|---|---|
| Gender | Male | 5374 | 4195 (55.0) | 1179 (60.1)* | 74.0 (0.6)@ |
| Female | 4211 | 3429 (45.0) | 782 (39.9) | 77.6 (0.7) | |
| Age | <1 year | 250 | 151 (2.0) | 99 (5.1)* | 40.1 (3.2)@ |
| 1-9.99 years | 6940 | 5626 (73.8) | 1314 (67.0) | 79.2 (0.5) | |
| ≥ 10 years | 2395 | 1847 (24.2) | 548 (27.9) | 68.5 (0.1) | |
| Race | White | 6830 | 5514 (72.3) | 1316 (67.1)* | 77.1 (0.5)@ |
| Black | 507 | 373 (4.9) | 134 (6.8) | 67.6 (2.4) | |
| Hispanic | 1636 | 1262 (16.6) | 374 (19.1) | 72.5 (1.2) | |
| Other | 612 | 475 (6.2) | 137 (7.0) | 73.6 (2.0) | |
| WBC | <50K | 7344 | 5974 (78.4) | 1370 (69.9)* | 78.6 (0.5) |
| 50-100K | 1009 | 776 (10.2) | 233 (11.9) | 70.4 (1.6) | |
| >100K | 1227 | 870 (11.4) | 357 (18.2) | 61.9 (1.6) | |
| Unknown | 5 | 4 | 1 | ||
| CNS Status | CNS 1 | 8359 | 6743 (90.3) | 1616 (84.3)* | 77.1 (0.5) |
| CNS 2 | 706 | 514 (6.9) | 192 (10.0) | 66.3 (1.9) | |
| CNS 3 | 317 | 208 (2.8) | 109 (5.7) | 58.1 (3.0) | |
| Unknown | 203 | 159 | 44 | ||
| Testicular disease | Normal | 5173 | 4040 (98.2) | 1133 (97.8) | 74.2 (0.7) |
| Enlarged | 101 | 76 (1.8) | 25 (2.2) | 65.0 (5.1) | |
| Unknown/NA | 4311 | 3508 | 803 | ||
| Immunophenotype | |||||
| B-lineage | 5754 | 4470(85.6) | 1284 (86.5) | 75.2 (0.6) | |
| T-lineage | 953 | 752 (14.4) | 201 (13.5) | 73.0 (1.6) | |
| Other/unknown | 2878 | 2402 | 476 | ||
p-value < 0.001 (Significant difference in clinical characteristic between relapsed vs. did not relapse)
p-value < 0.001 (Significant differences in EFS between categories for the clinical characteristic)
Risk stratification
The risk stratification algorithms used for patient eligibility are summarized below. For studies open prior to 1995: Low risk: 2 to 9 years of age at diagnosis with an initial WBC count less than 10,000/μL (23). Intermediate risk: ages 2 to 10 years with a WBC 10,000 to 50,000/mm3, no bulky extramedullary disease, or, in boys with platelet counts less than 100,000/mm3, a WBC less than 10,000/mm3, or in children 1 to 2 years of age, a WBC less than 50,000/mm3(24). High risk: 1 to 9 years old with WBC ≥ 50 × 109/L or ≥10 years old irrespective of WBC, excluding patients with lymphomatous features(21).
For studies including CCG 1922, 1952, 1953, 1961, and 1962, risk stratification followed the NCI-Rome risk criteria (25): Standard risk: Age 1 through 9.99 years and diagnostic WBC <50,000 μL. High risk: all others up to the age of 21 years.
Immunophenotypic classification was determined as follows: B lineage ALL: >30% CD19 positivity, +/- >30% CD10 or CD 20 positivity; T lineage ALL: >30% CD7 positivity. Diagnostic karyotyping of leukemic cells was performed by local institutions prior to the initiation of therapy; however results were not uniformly submitted for central review.
Statistical analyses
Differences in proportions of clinical characteristics at diagnosis between patients who relapsed and those who did not, were assessed using chi-square tests. Outcome analyses used life table methods and associated statistics. The primary endpoint examined was overall survival post relapse. Life table estimates were calculated by the Kaplan-Meier (KM) procedure(26) and the standard error (SE) of the life table estimate was obtained by the method of Peto(27). The log rank test was used to compare outcome between groups. Cox proportional hazards regression models were used to evaluate the significance of differences in survival between groups. When the proportional hazards assumption seemed unsatisfactory for a predictor in the Cox model, the effects of other variables were adjusted by stratifying the predictor in question. Five-year post-relapse survival rates are presented here for various subgroups.
Results
Overview of clinical trials
Between 1988 and 2002, a total of 9,585 patients with de novo ALL were enrolled on CCG clinical trials and 1961 children experienced a relapse at any site. The distribution of relapse sites was as follows: Isolated marrow 57.3%, Concurrent marrow 13.5%, Isolated CNS 20.9%, Isolated testicular 5.3%, Other extramedullary +/- CNS 3.1%. Overall enrollment and 5-year event-free survival (EFS) rates by protocol are given in Table 1. Event-free survival rates ranged from 37.6% to 82.3%. Among all relapsed patients, the median follow-up time from initial diagnosis was 51.6 months. After experiencing relapse, the median follow-up time was 15.7 months. Duration of CR1 for patients who relapsed varied accordingly with NCI risk group at diagnosis, with shorter duration of remission coinciding largely with higher risk features at diagnosis (Table 1 and Table 2). Of 1961 total patients who relapsed, 837 were alive at their last follow-up. Three patients died on the day of relapse. In addition, follow-up data after relapse was not available for 49 patients. For era of trial enrollment, we defined early trials to include 1881, 1882, 1883, 1891, 1901, and 1922. Later trials included 1952, 1953, 1961, and 1962. Of note, at the time of this analysis, results from CCG 1991 for standard risk ALL patients were still blinded and not available.
A comparison of diagnostic features of patients who relapsed with those who did not is given in Table 2. The relapsed cohort had a higher percent of patients who fell in the age range < 1 or ≥10 years (p=0.0001). Similarly, there was a higher proportion of males in the relapsed group (p=0.0002). Nearly 20% of the patients who relapsed presented with an initial WBC >100,000/μL. In comparison to Caucasians, more children of African American or Hispanic ethnicity experienced relapse (p=0.0001). There was no significant difference in the distribution of immunophenotype (B-precursor or T cell) between the patients who relapsed versus those who did not. It was not possible to determine the distribution of other diagnostic variables such as cytogenetics, because of insufficient data.
Measurements of early response to therapy were captured by day 7 and/or day 14 marrow morphology status, though not all studies uniformly captured both time points. Of the 4,064 patients achieving an M1 marrow by day 7, 662 (16.2%) relapsed, while 537 of 2,547 (21.1%) patients with a slower response to initial therapy (e.g. achieving an M1 marrow by day 14) experienced relapse (p=0.0001). Of the 630 patients who were M2 or M3 on day 14, 263 (41.7%) relapsed (p=0.0001). Taken together, this data supports the previous observation that slow early response is associated with inferior EFS.
Features associated with survival after relapse
Relapse events were defined by time from initial diagnosis (early: <18 months; intermediate: 18-36 months; late: ≥ 36 months) and site (isolated marrow, concurrent marrow, isolated CNS, isolated testicular, other extramedullary +/- CNS). Table 3 gives five-year survival rates following relapse by site and timing of relapse.
Table 3. Overall five-year survival (OS) rates after relapse, by site and time to relapse.
| 5-year Survival Post Relapse | Relapse Site | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated Marrow Relapse | Concurrent Marrow Relapse | Isolated CNS Relapse | Isolated Testicular Relapse | Other extramedullary +/-CNS Relapse | |||||||||||
| Time to relapse | n | Survival (%) | SE | n | Survival (%) | SE | n | Survival (%) | SE | n | Survival (%) | SE | n | Survival (%) | SE |
| Early1 | 412 | 11.49 | 1.91 | 86 | 11.64 | 4.89 | 175 | 43.51 | 4.45 | 15 | 13.64 | 16.56 | 23 | 10.38 | 11.32 |
| Intermediate1 | 324 | 18.42 | 3.09 | 54 | 39.79 | 9.31 | 180 | 67.99 | 4.53 | 35 | 52.20 | 11.41 | 17 | 50.47 | 25.12 |
| Late1 | 387 | 43.46 | 5.23 | 124 | 60.34 | 8.29 | 54 | 78.25 | 8.85 | 54 | 59.96 | 15.49 | 21 | 85.15 | 14.67 |
| Overall2 | 1123 | 24.1 | 2.1 | 264 | 39.4 | 5.0 | 409 | 58.7 | 3.2 | 104 | 58.0 | 8.2 | 61 | 55.9 | 9.9 |
p-value < 0.0001 compares survival rates by sites of relapse within each time period.
“Overall survival” refers to an unadjusted comparison of sites of relapse.
Overall post-relapse survival rates were higher (p <0.0001) for patients with isolated CNS relapse (58.7±3.2%) than for patients with either isolated (24.1±2.1%) or concurrent bone marrow (39.4±5.0%) relapses. Survival rates after early relapse were higher for patients with isolated CNS relapse than for patients with either isolated or combined bone marrow relapse (5 year survival rates – isolated CNS: 43.5±4.5% vs isolated marrow: 11.5±1.9% vs concurrent marrow: 11.6±4.9%, p<0.0001). This was also true for intermediate and late relapsing patients. Survival rates were also significantly higher for patients with concurrent marrow relapses compared to those with isolated marrow relapses – overall (p<0.0001), intermediate (p=0.002), late (p=0.02). Survival rates were identical for the early relapse patients with any marrow involvement. Survival curves post relapse by timing of relapse are given in figures 1A, 1B, and 1C for isolated marrow, concurrent marrow and isolated CNS relapses, respectively. Estimates of 5-year survival rates for isolated marrow relapse in early (n=412), intermediate (n=324), and late (n=387) relapsing patients were 11.5±1.9%, 18.4±3.1%, and 43.5±5.2%, respectively (Table 3, Figure 1A, p<0.0001). Overall, patients with early isolated marrow relapse had a relative risk of death about 3.7-fold higher than patients with a late relapse and it was 2.3-fold higher for patients with an intermediate isolated marrow relapse patients compared to those with late relapses. Similarly, the rates for concurrent marrow relapse were 11.6±4.5% (n=86), 39.8±9.3% (n=54), and 60.3±8.3% (N=124), respectively (Table 3, Figure 1B, p<0.0001). Relative risk of death was 4.9-fold and 2.0-fold higher for early and intermediate relapse patients respectively, compared to patients with late concurrent marrow relapse. Five-year survival rates post isolated CNS relapse were: Early (N=175): 43.5±4.5%, Intermediate (N=180): 68.0±4.6%, Late (N=54): 78.2±8.8% (Table 3, Figure 1C, p<0.0001). The relative risk of death for patients with early and intermediate CNS relapses were 3.4-fold and 1.5-fold respectively, compared to that for patients experiencing late CNS relapses. Overall survival rates after relapse, by timing of relapse were similar for 104 patients experiencing an isolated testicular relapse; their 5-year post-relapse survival rate was 58±8.2% (data not shown). There was no significant difference in survival rates for patients experiencing early versus intermediate versus late testicular events.
Figure 1.
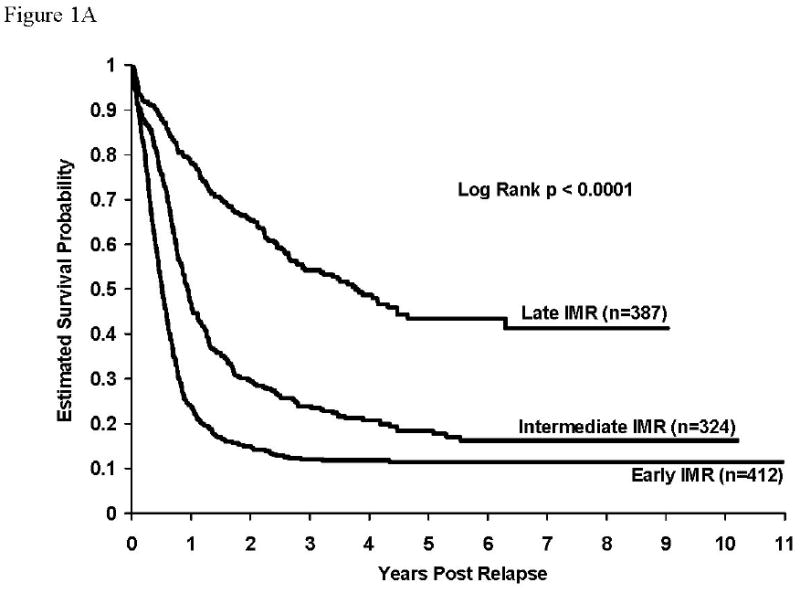
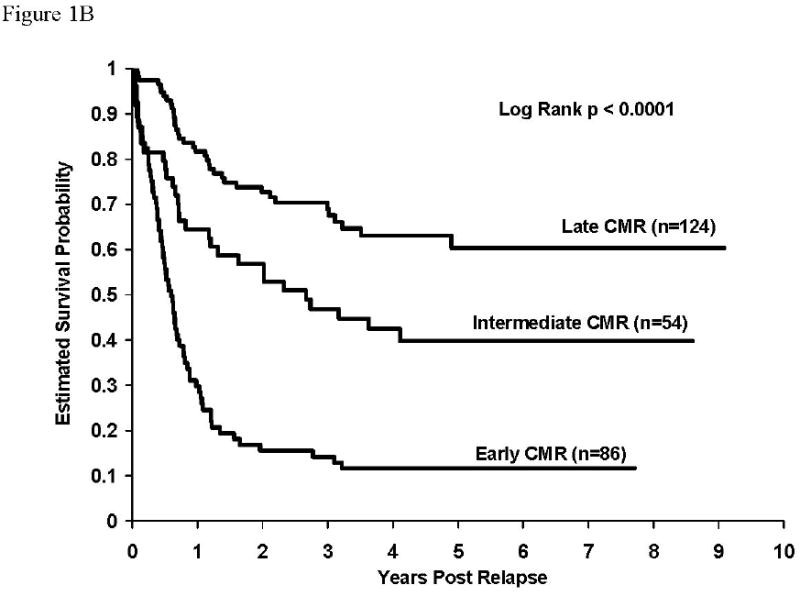
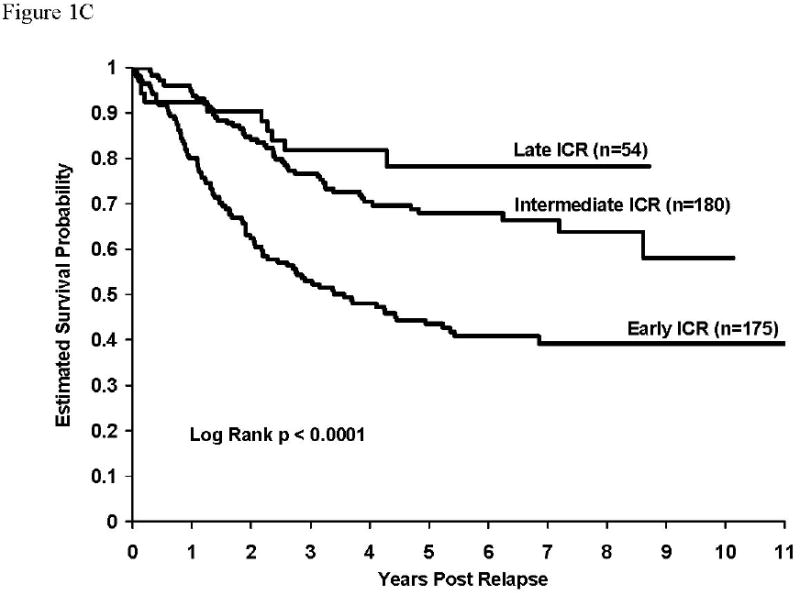
Figure 1A: Survival after relapse for patients experiencing isolated marrow relapse. Survival rates at 5 years after relapse for patients with isolated marrow relapse by timing of relapse (early: 11.5±1.9%, intermediate: 18.4±3.1%, late: 43.5±5.2%), p<0.0001. RHR: early vs late = 3.7, intermediate vs late = 2.3.
Figure 1B: Survival after relapse for patients experiencing concurrent marrow relapse. Survival rates at 5 years after relapse for patients with concurrent marrow relapse by timing of relapse (early: 11.6±4.5%, intermediate: 39.8±9.3%, late: 60.3±8.3%), p<0.0001. RHR: early vs late = 4.9, intermediate vs late = 2.0.
Figure 1C: Survival after relapse for patients experiencing isolated CNS relapse. Survival rates at 5 years after relapse for patients with isolated CNS relapse by timing of relapse (early: 43.5±4.5%, intermediate: 68.0±4.6%, late: 78.2±8.8%), p<0.0001. RHR: early vs late = 3.4, intermediate vs late = 1.5.
Overall, patients who were NCI SR at diagnosis had better outcomes than NCI HR patients (RHR=2.4, p<0.0001; 5-year survival rates- NCI SR: 50.4±2.4% versus NCI HR: 22.6±2.1%). There were significant differences in outcome for those SR versus HR patients experiencing either an early (33.1±3.6% vs 14.9±2.1%, RHR=2.0, p<0.0001), intermediate (52.2±3.7% vs 22.0±3.9%, RHR=2.4, p<0.0001) or late relapse (59.6±4.6% vs 39.5±7.2%, RHR=2.0, p< 0.0001)(Figure 2).
Figure 2. Kaplan-Meier estimates of survival after relapse for patients stratified by NCI risk group at diagnosis and timing of relapse.
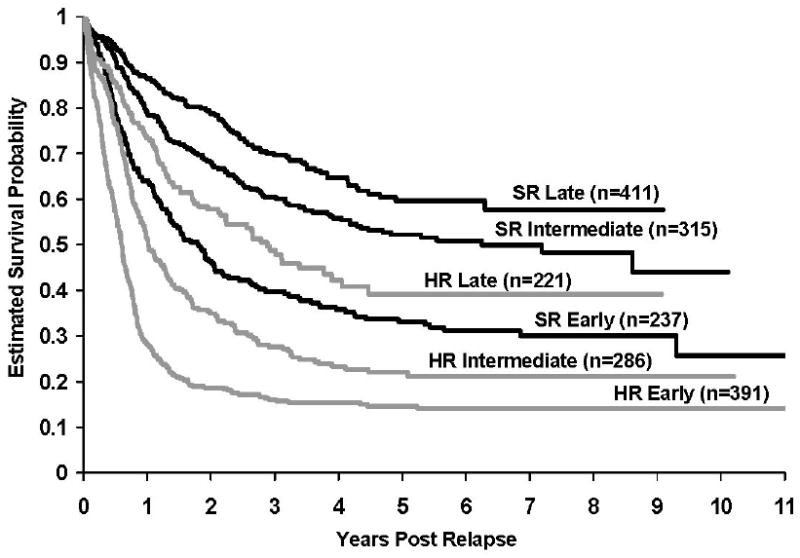
There were significant differences in outcome for those SR versus HR patients experiencing either an early (33.1±3.6% vs 14.9±2.1%, p<0.0001), intermediate (52.2±3.7% vs 22.0±3.9%, p<0.0001) or late relapse (59.6±4.6% vs 39.5±7.2%, p< 0.0001)).
In general, frontline therapy for ALL has intensified over time in order to improve event-free survival. It has been speculated that patients treated intensively at initial diagnosis might be more refractory to salvage therapy. In order to examine this further, we assessed survival after relapse for patients with any marrow relapse stratified by treatment era (early vs late trials). We defined early treatment protocols to include CCG 1881, 1882, 1883, 1891, 1901, and 1922, and later era trials to include CCG1952, 1953, 1961, and 1962. A total of 1961 children were included in this analysis (Figure 3). The results demonstrated that patients who relapsed early had dismal survival regardless of treatment era (5-year post-relapse survival: 19.3±2.1% versus 23.4±3.4%, p=0.10). Similarly, there were no statistically significant differences in survival between those treated on early vs late trials, for patients experiencing either an intermediate (39.3±3.3% versus 36.0±4.8%, p=0.49) or late relapse (53.3±4.3% versus 54.4±9.2%, p=0.66).
Figure 3. Kaplan-Meier estimates of survival after relapse for patients stratified by treatment era and timing of relapse.
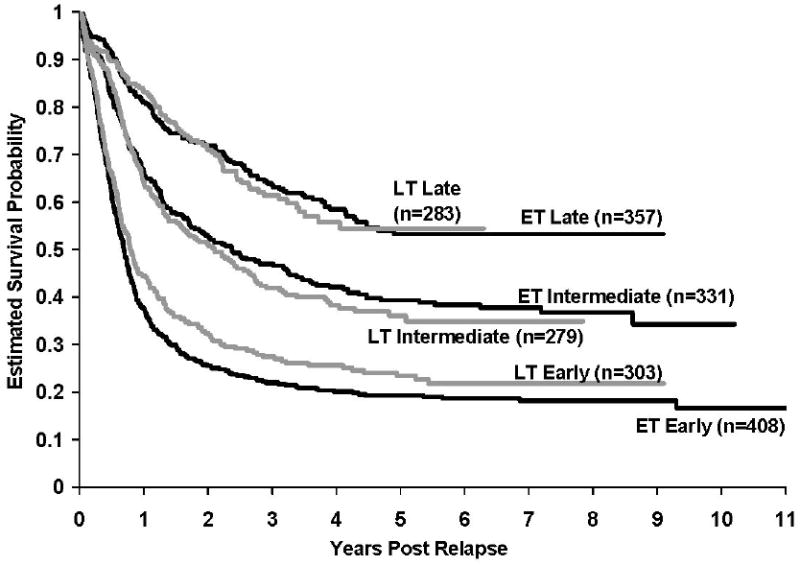
There was no difference in survival between Early and Late trials among patients who experienced either an early relapse (19.3±2.1% vs 23.4±3.4%, p=0.10), or intermediate relapse (39.3±3.3% vs 36.0±4.8%, p=0.49), or late relapses (53.3±4.3% vs 54.4±9.2%, p=0.66).
Univariate analyses (n=1961) were conducted to study the association of presenting clinical and laboratory features at initial diagnosis, time to relapse, and site of relapse with survival post relapse (Table 4). Significant associations were found with site and timing of relapse, age at diagnosis, WBC at diagnosis, lineage (B vs T), CNS status at diagnosis, race, and NCI risk; exceptions were trial era and sex. Multivariate analyses stratified by timing of relapse and site of relapse were conducted on the subset of patients (n=1391) with complete data for all the variables (Table 5). Infants < 1 year of age at diagnosis were excluded from the multivariate analyses, since they cannot be classified by NCI risk group. Age at diagnosis, CNS disease, sex, lineage, and NCI risk group were significant predictors of survival post relapse. Trial era, WBC at diagnosis, and race were not significant in predicting survival in the multivariate analysis. Multivariate analyses stratified for time to relapse, site of relapse, and age at diagnosis resulted in the same significant predictors as before. Trial era, WBC at diagnosis, and race were not significant factors.
Table 4. Univariate analyses of risk factors associated with survival after relapse.
| Univariate Analyses of Survival Post Relapse risk Factors (N=1961) | 5-year Survival rates post relapse ± SE (%) | p-value | |
|---|---|---|---|
| Time to relapse | Early | 21.0±1.8 | <0.0001 |
| Intermediate | 37.9±2.7 | ||
| Late | 53.1±3.9 | ||
| Relapse Site | Isolated marrow | 24.1±2.1 | <0.0001 |
| Concurrent marrow | 39.4±5.0 | ||
| Isolated CNS | 58.7±3.2 | ||
| Isolated testicular | 58.0±8.2 | ||
| Other extramed ± CNS | 55.9±9.9 | ||
| Age Group | <1 Yr | 19.8±4.4 | <0.0001 |
| 1-9 Yrs | 45.0±2.1 | ||
| 10+ Yrs | 18.2±2.8 | ||
| Trial Era | Early | 36.2±1.9 | 0.66 |
| Late | 36.6±3.1 | ||
| Sex | M | 35.4±2.2 | 0.51 |
| F | 38.0±2.5 | ||
| Lineage | B | 37.2±2.1 | <0.0001 |
| T | 23.0±4.0 | ||
| CNS Status at Diagnosis | CNS-3 | 14.5±4.5 | <0.0001 |
| CNS-2 & CNS-1 | 37.7±1.7 | ||
| WBC | <50K | 41.6±2.0 | <0.0001 |
| >=50K | 24.3±2.6 | ||
| Race | White | 38.5±2.0 | 0.02 |
| Hispanic | 30.5±3.8 | ||
| Black | 30.1±5.9 | ||
| Other | 38.1±6.9 | ||
| NCI Risk | Standard | 50.4±2.4 | <0.0001 |
| High | 22.6±2.1 | ||
Table 5. Multivariate analyses of risk factors associated with survival after relapse: Stratified by Time to and Site of relapse.
| Multivariate Analysis of Survival Post Relapse Risk Factors (Stratified by Time to Relapse and Site) (N=1391) | RHR | 95% CI | |
|---|---|---|---|
| Age Group | 1-9 Yrs | --- | --- |
| 10+ Yrs | 1.373 | (1.065, 1.771) | |
| Trial Era | Early | 1.056 | (0.909, 1.226) |
| Late | --- | --- | |
| Sex | M | 1.280 | (1.095, 1.497) |
| F | --- | --- | |
| Lineage | B | --- | --- |
| T | 1.355 | (1.101, 1.664) | |
| CNS Status at Diagnosis | CNS-3 | 1.501 | (1.083, 2.083) |
| CNS-2 & CNS-1 | --- | --- | |
| WBC | <50K | --- | --- |
| >=50K | 1.151 | (0.889, 1.488) | |
| Race | White | 0.853 | (0.707, 1.030) |
| Hispanic | --- | --- | |
| Black | 0.870 | (0.640, 1.182) | |
| Other | 0.774 | (0.564, 1.062) | |
| NCI Risk | Standard | --- | --- |
| High | 1.593 | (1.163, 2.184) | |
Discussion
Despite improved treatments and increasing dose intensity in primary therapy for newly diagnosed patients with ALL, patients with relapsed disease continue to account for a large number of pediatric cancer patients (28). The current study analyzed survival following relapse for almost 10,000 children diagnosed with ALL between 1988-2002, of whom 1,961 (20.5%) relapsed. Not surprisingly, survival after relapse remains most dismal for those patients experiencing a bone marrow relapse within 18 months of initial diagnosis. High risk patients have higher mortality than their standard risk counterparts irrespective of the time to relapse. Male sex, age < 1 year or ≥10 years at diagnosis, T-cell lineage, and CNS disease at diagnosis persist as predictors of increased risk of death after relapse, even when controlled for by site and time to relapse. New studies clearly need to address how to effectively treat relapsed patients and maintain durable remissions.
Our analysis failed to demonstrate any substantial differences in survival after relapse for patients treated on a recent generation COG sponsored ALL trial versus 762 children enrolled on earlier CCG trials between 1983-1989 (12). While we have witnessed improved EFS for newly diagnosed patients by exposing them to more intense treatment and potentially greater morbidity, we remain unable to salvage the majority of children who fail initial therapy. The data presented here support our ongoing efforts to optimize risk stratification to identify patients at diagnosis who require more intense or novel therapies to achieve cure. In particular, this report provides compelling data that NCI HR patients do exceptionally poorly after relapsing at any time, thus warranting new approaches to optimizing therapy at diagnosis.
It is curious to note that while no differences in survival following relapse were noted for patients relapsing off older era trials versus more contemporary trials, patients who are SR at diagnosis have improved survival compared to their HR counterparts irrespective of time to relapse. Certainly, the distribution of patients experiencing early, intermediate, and late relapses were highly skewed towards NCI HR patients in the former group and NCI SR in the latter group (Figure 2b). While NCI SR patients receive less intense therapy, the data suggests that intrinsic differences in the biology of the leukemic blasts is correlated with different mechanisms and timing of relapse. For instance, NCI SR patients have a much higher incidence of favorable genotypes like hyperdiploidy and TEL-AML1 while older patients are more likely to be BCR-ABL positive.
Previously, Gaynon and colleagues reported survival after ALL relapse of children enrolled on Children's Cancer Group clinical trials between 1983-1989 (12). In that analysis, the strongest predictor of prolonged survival after relapse was an initial time to first relapse ≥ 36 months. Importantly, patients with an isolated BM relapse who failed initial therapy less than 18 months from diagnosis had a 4.5-fold higher relative risk for death than those relapsing after 36 months. In our updated analysis and a review of the literature, the two most significant predictors of survival after relapse remain the same—site and time. This observation continues to raise essential questions about the biology of relapsed ALL which will need to be addressed if we are going to be remotely successful in curing these patients.
Indeed, recent analyses have indicated that there is a unique and distinct pattern of gene expression in pairs of relapsed samples from patients who relapse early from those who relapse later (29). It has been shown that paired samples from patients experiencing early relapse are more similar in expression patterns than paired samples from those relapsing later. Early relapse is also characterized by increased expression of genes involved in proliferation and survival (29). It is likely that resistant leukemia cells in patients destined to relapse early are present in the initial diagnostic field of blasts. Late relapse may be due to the acquisition of further somatic and/or epigenetic changes. Moreover been shown by analyzing microsatellite markers that some very late relapses of TEL/AML1 positive leukemia likely represent a new event that occurs in a quiescent precursor leukemia cell harboring an otherwise silent fusion gene that has escaped eradication during initial therapy(30, 31).
Studying the biology of these diseases, both at diagnosis, in minimal residual disease states after selection by chemotherapy, and at relapse, would ideally improve an understanding of how to use current therapy as well as identify new targets. To date, several drug combinations have achieved substantial complete response 2 (CR2) rates varying from 68 to 94% depending on the timing of relapse; however, patients who fail to enter CR2 are not likely to be salvaged with a different combination suggesting intrinsic drug resistance and a defect in terminal cell death pathways. Moreover re-induction rates for patients with multiply relapsed ALL cluster around 40%, suggesting that we have yet to truly understand and circumvent the mechanisms by which resistant lymphoblasts escape cytotoxicity(8-11, 32, 33).
For newly diagnosed ALL patients, the challenge that remains is to accurately identify patients who will benefit the most from intensive primary therapy. However, this analysis demonstrates that survival for patients with relapsed ALL using conventional therapy remains woefully inadequate, particularly for those who relapse within 36 months of initial diagnosis. As a result of these and other analyses, recent efforts in the Children's Oncology Group have focused on offering uniform clinical trials for patients with relapsed ALL while gathering important biologic data, thus taking the first steps toward defining whether agents not typically used in treatment of ALL at initial diagnosis can improve outcome for relapse. The extremely poor survival following relapse underscores the need to focus on improving the outcome of primary therapy for these patients who are unlikely to be salvaged if they relapse. Promising new therapies should be rapidly integrated into trials for subsets of higher risk patients at initial diagnosis.
Acknowledgments
This work has been sponsored by the Doris Duke Charitable Foundation (KN), NIH K22 CA113557, The Leukemia Lymphoma Society (LLS 2157-08), and the Frank Campini Foundation (MLL), NIH U10 CA98543-01 (ML, ER, HS, WLC, NW, SH, PG, and MLL). Dr. Loh is a Clinical Scholar of the Leukemia Lymphoma Society
Footnotes
This paper was accepted for poster presentation at the American Society of Hematology 48th annual meeting on December 9-12, 2006 as abstract 1855.
References
- 1.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006 Jan 12;354(2):166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006 Mar-Apr;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Gaynon PS, Trigg ME, Heerema NA, Sensel MG, Sather HN, Hammond GD, et al. Children's Cancer Group trials in childhood acute lymphoblastic leukemia: 1983-1995. Leukemia. 2000 Dec;14(12):2223–33. doi: 10.1038/sj.leu.2401939. [DOI] [PubMed] [Google Scholar]
- 4.Henze G, Fengler R, Hartmann R, Kornhuber B, Janka-Schaub G, Niethammer D, et al. Six-year experience with a comprehensive approach to the treatment of recurrent childhood acute lymphoblastic leukemia (ALL-REZ BFM 85). A relapse study of the BFM group. Blood. 1991 Sep 1;78(5):1166–72. [PubMed] [Google Scholar]
- 5.Pui CH, Pei D, Sandlund JT, Campana D, Ribeiro RC, Razzouk BI, et al. Risk of adverse events after completion of therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005 Nov 1;23(31):7936–41. doi: 10.1200/JCO.2004.01.0033. [DOI] [PubMed] [Google Scholar]
- 6.Rivera GK, Zhou Y, Hancock ML, Gajjar A, Rubnitz J, Ribeiro RC, et al. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. 2005 Jan 15;103(2):368–76. doi: 10.1002/cncr.20743. [DOI] [PubMed] [Google Scholar]
- 7.Chessells JM, Veys P, Kempski H, Henley P, Leiper A, Webb D, et al. Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003 Nov;123(3):396–405. doi: 10.1046/j.1365-2141.2003.04584.x. [DOI] [PubMed] [Google Scholar]
- 8.Kolb EA, Steinherz PG. A new multidrug reinduction protocol with topotecan, vinorelbine, thiotepa, dexamethasone, and gemcitabine for relapsed or refractory acute leukemia. Leukemia. 2003 Oct;17(10):1967–72. doi: 10.1038/sj.leu.2403097. [DOI] [PubMed] [Google Scholar]
- 9.Crooks GM, Sato JK. Ifosfamide and etoposide in recurrent childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1995 Feb;17(1):34–8. doi: 10.1097/00043426-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein ML, Abshire TC, Pollock BH, Devine S, Toledano S, Steuber CP, et al. Idarubicin and cytosine arabinoside reinduction therapy for children with multiple recurrent or refractory acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Pediatr Hematol Oncol. 1997 Jan-Feb;19(1):68–72. doi: 10.1097/00043426-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Harris RE, Sather HN, Feig SA. High-dose cytosine arabinoside and L-asparaginase in refractory acute lymphoblastic leukemia: the Children's Cancer Group experience. Med Pediatr Oncol. 1998 Apr;30(4):233–9. doi: 10.1002/(sici)1096-911x(199804)30:4<233::aid-mpo5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Gaynon PS, Qu RP, Chappell RJ, Willoughby ML, Tubergen DG, Steinherz PG, et al. Survival after relapse in childhood acute lymphoblastic leukemia: impact of site and time to first relapse--the Children's Cancer Group Experience. Cancer. 1998 Apr 1;82(7):1387–95. doi: 10.1002/(sici)1097-0142(19980401)82:7<1387::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Bleyer WA, Sather H, Hammond GD. Prognosis and treatment after relapse of acute lymphoblastic leukemia and non-Hodgkin's lymphoma: 1985. A report from the Childrens Cancer Study Group. Cancer. 1986 Jul 15;58(2 Suppl):590–4. doi: 10.1002/1097-0142(19860715)58:2+<590::aid-cncr2820581330>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler K, Richards S, Bailey C, Chessells J. Comparison of bone marrow transplant and chemotherapy for relapsed childhood acute lymphoblastic leukaemia: the MRC UKALL X experience. Medical Research Council Working Party on Childhood Leukaemia. Br J Haematol. 1998 Apr;101(1):94–103. doi: 10.1046/j.1365-2141.1998.00676.x. [DOI] [PubMed] [Google Scholar]
- 15.Chessells JM. Relapsed lymphoblastic leukaemia in children: a continuing challenge. Br J Haematol. 1998 Jul;102(2):423–38. doi: 10.1046/j.1365-2141.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 16.Barredo JC, Devidas M, Lauer SJ, Billett A, Marymont M, Pullen J, et al. Isolated CNS relapse of acute lymphoblastic leukemia treated with intensive systemic chemotherapy and delayed CNS radiation: a pediatric oncology group study. J Clin Oncol. 2006 Jul 1;24(19):3142–9. doi: 10.1200/JCO.2005.03.3373. [DOI] [PubMed] [Google Scholar]
- 17.Grundy RG, Leiper AD, Stanhope R, Chessells JM. Survival and endocrine outcome after testicular relapse in acute lymphoblastic leukaemia. Arch Dis Child. 1997 Mar;76(3):190–6. doi: 10.1136/adc.76.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wofford MM, Smith SD, Shuster JJ, Johnson W, Buchanan GR, Wharam MD, et al. Treatment of occult or late overt testicular relapse in children with acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Clin Oncol. 1992 Apr;10(4):624–30. doi: 10.1200/JCO.1992.10.4.624. [DOI] [PubMed] [Google Scholar]
- 19.Borgmann A, von Stackelberg A, Hartmann R, Ebell W, Klingebiel T, Peters C, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003 May 15;101(10):3835–9. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- 20.Kersey JH. Fifty years of studies of the biology and therapy of childhood leukemia. Blood. 1997 Dec 1;90(11):4243–51. [PubMed] [Google Scholar]
- 21.Mattano LA, Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children's Cancer Group. J Clin Oncol. 2000 Sep 15;18(18):3262–72. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: report from the Late Effects Study Group. J Clin Oncol. 2003 Dec 1;21(23):4386–94. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 23.Hutchinson RJ, Gaynon PS, Sather H, Bertolone SJ, Cooper HA, Tannous R, et al. Intensification of therapy for children with lower-risk acute lymphoblastic leukemia: long-term follow-up of patients treated on Children's Cancer Group Trial 1881. J Clin Oncol. 2003 May 1;21(9):1790–7. doi: 10.1200/JCO.2003.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Rackoff WR, Ge J, Sather HN, Cooper HA, Hutchinson RJ, Lange BJ. Central venous catheter use and the risk of infection in children with acute lymphoblastic leukemia: a report from the Children's Cancer Group. J Pediatr Hematol Oncol. 1999 Jul-Aug;21(4):260–7. doi: 10.1097/00043426-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996 Jan;14(1):18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Association. 1958;53:457–81. [Google Scholar]
- 27.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grovas A, Fremgen A, Rauck A, Ruymann FB, Hutchinson CL, Winchester DP, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997 Dec 15;80(12):2321–32. doi: 10.1002/(sici)1097-0142(19971215)80:12<2321::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Bhojwani D, Kang H, Moskowitz NP, Min DJ, Lee H, Potter JW, et al. Biologic pathways associated with relapse in childhood acute lymphoblastic leukemia: a Children's Oncology Group study. Blood. 2006 Jul 15;108(2):711–7. doi: 10.1182/blood-2006-02-002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford AM, Fasching K, Panzer-Grumayer ER, Koenig M, Haas OA, Greaves MF. Origins of “late” relapse in childhood acute lymphoblastic leukemia with TEL-AML1 fusion genes. Blood. 2001 Aug 1;98(3):558–64. doi: 10.1182/blood.v98.3.558. [DOI] [PubMed] [Google Scholar]
- 31.Zuna J, Ford AM, Peham M, Patel N, Saha V, Eckert C, et al. TEL deletion analysis supports a novel view of relapse in childhood acute lymphoblastic leukemia. Clin Cancer Res. 2004 Aug 15;10(16):5355–60. doi: 10.1158/1078-0432.CCR-04-0584. [DOI] [PubMed] [Google Scholar]
- 32.Kurtzberg J, Asselin B, Pollack B, Berstein M, Buchanan G. PEG-L-asparaginase (PEGasp) vs native E. coli asparaginase (asp) for reinduction of relapsed acute lymphoblastic leukemia (ALL): Pediatric Oncology Group (POG) 8866 Phase II trial. Proceedings of the American Society of Clinical Oncology. 1993;1993(12):325. [Google Scholar]
- 33.Wells RJ, Feusner J, Devney R, Woods WG, Provisor AJ, Cairo MS, et al. Sequential high-dose cytosine arabinoside-asparaginase treatment in advanced childhood leukemia. J Clin Oncol. 1985 Jul;3(7):998–1004. doi: 10.1200/JCO.1985.3.7.998. [DOI] [PubMed] [Google Scholar]


