Abstract
Pit1 null (Snell dwarf) and Proph1 null (Ames dwarf) mutant mice lack GH, PRL and TSH. Snell and Ames dwarf mice also exhibit reduced IGF-I, resistance to cancer and a longer lifespan than control mice. Endogenous glucose production during fasting is reduced in Snell dwarf mice compared to fasting control mice. In view of cancer cell dependence on glucose for energy, low endogenous glucose production may provide Snell dwarf mice with resistance to cancer. We investigated whether endogenous glucose production is lower in Snell dwarf mice during feeding. Inhibition of endogenous glucose production by glucose injection was enhanced in 12 to 14 month-old female Snell dwarf mice. Thus, we hypothesize that lower endogenous glucose production during feeding and fasting reduces cancer cell glucose utilization providing Snell dwarf mice with resistance to cancer. The elevation of circulating adiponectin, a hormone produced by adipose tissue, may contribute to the suppression of endogenous glucose production in 12 to 14 month-old Snell dwarf mice. We compared the incidence of cancer at time of death between old Snell dwarf and control mice. Only 18% of old Snell dwarf mice had malignant lesions at the time of death compared to 82% of control mice. The median ages at death for old Snell dwarf and control mice were 33 and 26 months, respectively. By contrast, previous studies showed a high incidence of cancer in old Ames dwarf mice at the time of death. Hence, resistance to cancer in old Snell dwarf mice may be mediated by neuroendocrine factors that reduce glucose utilization besides elevated adiponectin, reduced IGF-I and a lack of GH, PRL and TSH, seen in both Snell and Ames dwarf mice. Proteomics analysis of pituitary secretions from Snell dwarf mice confirmed the absence of GH and PRL, the secretion of ACTH and elevated secretion of Chromogranin B and Secretogranin II. Radioimmune assays confirmed that circulating Chromogranin B and Secretogranin II were elevated in 12 to 14 month-old Snell dwarf mice. In summary, our results in Snell dwarf mice suggest that the pituitary gland and adipose tissue are part of a neuroendocrine loop that lowers the risk of cancer during aging by reducing the availability of glucose.
Keywords: Glucose, Cancer, Adiponectin, Growth hormone, Dwarf mice, Pituitary, Chromogranins
1. Introduction
Snell dwarf mice are valuable laboratory models for investigating the mechanisms of cancer and aging. Snell dwarf mice are homozygous for the Pit1dw allele, which eliminates function of the transcription factor Pit1 (Li et al., 1990). Snell dwarf mice are deficient in growth hormone (GH), prolactin (PRL) and thyroid stimulating hormone (TSH) due to hypoplasia of the cells that produce these hormones in the anterior pituitary. Snell dwarf mice are resistant to chemically induced cancers and live longer than control mice which are either homozygous or heterozygous for the wild-type Pit1 allele (Flurkey et al., 2001; Rennels et al., 1965; Bielschowsky and Bielschowsky, 1959).
During fasting, endogenous glucose production and whole-body glucose utilization are lower in Snell dwarf mice than in control mice (Brooks et al., 2007). The reduction of endogenous glucose production may deprive cancer cells of energy and provide resistance to cancer. Rather than oxidative phosphorylation, cancer cells depend on glycolysis for ATP production and therefore rely on high levels of glucose for energy (Matoba et al., 2006; Brown, 1999; Gullino et al., 1967). Glucose deprivation causes cancer cells in culture to undergo cell death more readily than healthy cells (Funes et al., 2007; Elstrom et al., 2004; Shim et al., 1998). Elevated circulating glucose increases the risk of cancer and reduces the effectiveness of medical treatment in cancer patients (Caudle et al., in press; Stattin et al., 2007; Malin et al., 2005).
Low endogenous glucose production was previously reported in Snell dwarf mice during fasting (Brooks et al., 2007). Therefore, the first goal of this study was to determine whether increased inhibition of endogenous glucose production in Snell dwarf mice extends to the fed state. Glucose metabolism differs dramatically between the fed and fasted states. In the fed state (a) circulating levels of glucose and insulin are elevated, (b) the fraction of glucose in the circulation from the gastrointestinal tract is elevated, (c) circulating glucose is stored and used for energy and (d) glucose production from gluconeogenesis and glycogenolysis is not needed to replenish the disappearance of circulating glucose.
Hypophysectomy, surgical removal of the pituitary gland, was used as endocrine therapy to reduce hormone action before highly specific chemicals and monoclonal antibodies were available for the treatment of cancer (Luft et al., 1955). A lack of GH and low levels of insulin like growth factor I (IGF-I), a hormone secreted mainly by GH stimulation, play a key role in resistance to cancer. GH deficient rats are highly resistant to chemical induction of cancer unless GH is replaced (Shen et al., 2007). The suppression of IGF-I could provide resistance to cancer by reducing glucose utilization (Rossetti et al., 1991).
Resistance to cancer in Snell dwarf mice can also be mediated by the elevation of circulating adiponectin (Brooks et al., 2007; Combs et al., 2003). Adiponectin, a hormone produced exclusively by adipocytes, inhibits endogenous glucose production (Brooks et al., 2007; Combs et al., 2004; Combs et al., 2001). Adiponectin inhibits endogenous glucose production by the liver through the suppression G6Pase and PEPCK, rate limiting enzymes in hepatic glucose production (Combs et al., 2001). Numerous clinical studies suggest that breast cancer risk is decreased by elevated circulating adiponectin (Chen et al., 2006; Korner et al., 2007; Mantzoros et al., 2004; Miyoshi et al., 2003; Tworoger et al., 2007).
Elevated adiponectin has been reported in several GH deficient and long-lived mouse models (Berryman et al., 2004; Combs et al., 2003; Wang et al., 2006). Adiponectin inhibits endogenous glucose production and stimulates fatty acid utilization through its intracellular target, AMP activated protein kinase (AMPK) (Brooks et al., 2007; Nawrocki et al., 2006; Tomas et al., 2002; Yamauchi et al., 2002). AMPK is also implicated in the enhancement of fatty acid metabolism through exercise training (Winder, 2001). Gene expression array data in GH deficient mice, such as the Snell dwarf, Ames dwarf, GH release hormone receptor-null (little) and GH receptor-null mice, support the conclusion that fatty acid oxidation is elevated in these long-lived and cancer resistant mouse models (Stauber et al., 2005).
Ames dwarf mice are homozygous for the Prop1df allele which leads to the loss of the transcription factor Prop1 (Sornson et al., 1996). Prop1 regulates the expression of Pit1 and, as a result, Ames dwarf mice are deficient in GH, PRL and TSH similar to Snell dwarf mice. Ames dwarf mice live longer than control mice which are either homozygous or heterozygous for the wild-type Prop1 allele (Brown-Borg et al., 1996). The median age of death for Ames dwarf and control mice is 36 and 26 months, respectively; however, despite having a similar endocrine profile as Snell dwarf mice, old Ames dwarf mice exhibit a high incidence of cancer at the time of death (Ikeno et al., 2003). Necropsy at the time of death revealed malignant lesions in 72% of Ames dwarf mice, in comparison to 95% of control mice. Although young Snell dwarf mice exhibit resistance to chemically induced tumors, data on naturally occurring cancers in old Snell dwarf mice is currently not available. Thus, our second goal was to determine whether old Snell dwarf mice also have a high incidence of cancer at the time of death similar to Ames dwarf mice.
After discovering greater inhibition of endogenous glucose production by intravenous injection of glucose (aim 1) and resistance to cancer at the time of death in old Snell dwarf mice (aim 2), we applied proteomics analysis to determine whether the pituitary of Snell dwarf mice overproduces or lacks other hormones besides GH, PRL and TSH that may play a role in the resistance to cancer (aim 3). Therefore, our third and final goal was to compare the proteins secreted from the pituitaries of Snell dwarf and control mice. Previous proteomics analyses of whole anterior pituitaries from outbred mice verified production of all known pituitary hormones except for THS (Blake et al., 2005). The effect of the Snell dwarf mutation on pituitary secretions has not been previously examined by proteomics analysis.
2. Materials and methods
2.1. Mice
Unless otherwise indicated, 12 to 14 month-old female mice in the F1 (DW/J × C57Bl/6J) background were used. Mice were housed in ventilated isolator cage systems in a pathogen-free isolator barrier facility at 23 °C, 55% humidity on a 12-h light/12-h dark cycle. Mice received a standard chow diet consisting of 73% carbohydrate, 18% protein, 4% fat and 5% ash (Purina). Mice were sacrificed by cervical dislocation for experiments requiring dissection of tissues. Experimental procedures were approved by the Institutional Animal Care and Use Committees at the University of North Carolina.
2.2. Intravenous glucose tolerance test
Mice were fasted after 7 am and studied between 1 and 3 pm. In the afternoon, mice received a single intravenous injection of glucose (0.8 mg/g body weight) mixed with tritium labeled glucose (d-[3-3H]glucose; 3H on carbon 3 of glucose) from Amersham Biosciences (Beard et al., 1986). The mixture was injected directly into the circulation through the ophthalmic plexus of conscious free-moving mice. The tail was nicked 2–4 mm from the tip, always below the end of the vertebrae. Blood samples (10–20 µL) were collected from the tail tip at 2.5, 5, 10, 20 and 30 min after injection for measurement of glucose and radioactivity as previously described (Brooks et al., 2007). The fraction of circulating glucose representing the injected glucose was calculated from the specific activity of the injected [3H]glucose/glucose solution (3 × 105 cpm/mg glucose). HPLC analysis was performed to verify that the radioactivity measured in dried plasma by scintillation counter represented [3H]glucose. An Agilent 1100 HPLC system connected to a beta-RAM Model 2B Detector (IN/US Systems) was used. An Aminex HPX-87C polystyrene divinylbenzene resin column (Bio-Rad) was used at 80 °C using a 0.6 mL/min flow of water. Mutant and control mice were always paired for the intravenous glucose tolerance test.
2.3. Cancer evaluation at necropsy
Gross pathologic examination was performed while gathering life span data at the Jackson Laboratories. Necropsy was performed on female Snell dwarf mice and control mice from a cohort of F1 (DW × C3He) mice. Cages were checked daily and necropsy was performed immediately when a dead mouse was found. The median age at time of death was 33 months for Snell dwarf mice and 26 months for control mice. A total of 11 Snell dwarf and 38 control mice underwent necropsy. Histology was used to determine whether neoplastic lesions were benign or malignant.
2.4. Proteomics analysis of pituitary secretions
Anterior pituitary glands from 12 to 14 month-old Snell dwarf and age-matched control mice were placed in serum-free modified Eagle’s medium immediately after dissection and incubated at 37 °C for 1 h. The media was transferred to new tubes and centrifuged at 45,000 rpm for 1 h. Proteomics analyses were performed at the UNC-Duke Proteomics Center. The supernatant was digested with trypsin at 37 °C for 1 h and analyzed by nano LC/MS/MS on a ThermoFisher Orbitrap hybrid FT-MS, equipped with an Eksigent nanoLC system, New Objective nanospray source and a nanobore PicoFrit column (ProteoPep™ II C18, 50 lm id × 10.0 cm, 10 µm tip size, New Objective). The Orbitrap data were processed using ThermoFisher’s Bioworks 3.3.1 software, the Sequest search engine and the FASTA Protein Database. A comprehensive report of the protein functions was prepared using BLAST on the NCBI database. Protein expression ratios were determined with GE Healthcare’s Decyder MS software (Johansson et al., 2006).
2.5. PCR reactions
Genotyping of Pit1dw allele carriers for breeding. The Pit1dw allele was detected from tail DNA to identify heterozygous breeding pairs by PCR using AGCTGCTAAGGATGCTCTGG (forward primer), CTCTGCCTTCGGTTGCAGAAA (reverse primer) and PlatTAQ (Invitrogen) under standard PCR conditions. The reverse primer was designed to detect the point mutation of Pit1dw allele (Li et al., 1990). Reverse Transcriptase PCR. Mice were sacrificed by cervical dislocation between 1 and 3 pm. Tissues were frozen in liquid nitrogen immediately after dissection and stored at 4 °C in RNA-Later (Ambion). RNA was isolated using a kit (Qiagen) according to the manufacturer’s protocol. Yield and purity were determined by spectrophotometric absorption analysis at 260/280 nm and gel electrophoresis. cDNA was prepared with reverse transcriptase (Invitrogen) and random hexamers. The PCR primer pair sequences were AAGAGGCCTGAGTGCCCAAC and ACGCTCCTCCTCCTCTTCC for Chromogranin A, GACCAGGACCAGAGCCAG and GACCAGGACCA GAGCCAG for Chromogranin B and GCAGTGGGAGGTCACAGAG and ATACCCACCCTTGGAGAGC for Secretogranin II.
2.6. Northern blot analysis
Total RNA (5 µg) was electrophoresed on 1.2% agarose/6% formaldehyde gel and transferred to Hybond-XL membrane (Amersham Biosciences). RNA was cross-linked to the membrane by ultraviolet irradiation and incubated overnight at 65 °C in hybridization buffer (Amersham Biosciences) mixed with radiolabeled DNA probe. Membranes were washed with 3× SSC, exposed to a PhosphorImager (Molecular Dynamics) screen for 24 h, and analyzed on a PhosphorImager using ImageQuant Software. Probe Synthesis. Radiolabeled probes were prepared by PCR using 2.5 mM[32P]dCTP (Perkin Elmer). The Secretogranin II probe was prepared using TGCTGAAACGGCCCGAGC and CAGGCGTGTCCACTGGGAA. The Chromogranin B probe was prepared using TGCTGAAACGGCCCG AGC and CAGGCGTGTCCACTGGAA. An oligonucleotide CTTCC TCTAGATAGTCAAGTTCGACCGTCT specific for 18S RNA was end labeled with [32P]dATP using T4 polynucleotide kinase (Invitrogen) and used to confirm equal RNA loading.
2.7. Hormone measurements
Adiponectin was measured in plasma from control and Snell dwarf mice by Western blot analysis as previously described (Brooks et al., 2007). Adiponectin was measured using rabbit anti-sera to murine adiponectin amino acid sequence 18–32 (mAC-RP30: 18–32) (Scherer et al., 1995). Chromogranin A, Chromogranin B and Secretogranin II were measured by radioimmune assay. Chromogranin A was measured using an antibody to human Chromogranin A amino acid sequence 324–337 (hCgA:324–337), which detects the WE-14 region of Chromogranin A (Stridsberg et al., 2004). Chromogranin B was measured using an antibody to human Chromogranin B amino acid sequence 312–331 (hCgB:312–331) (Stridsberg et al., 2005). Secretogranin II was measured using an antibody against the human Secretogranin II amino acid sequence 154–165 (hSgII:154–165) (Stridsberg et al., in press). The antibodies used for the measurements of granins have been tested for inter-species cross reactivity; all measure rodent granins with 100% cross reactivity. The detection limits were 10 fmol and the total assay variation was 10% for all assays.
3. Results
3.1. Glucose metabolism in Snell dwarf mice
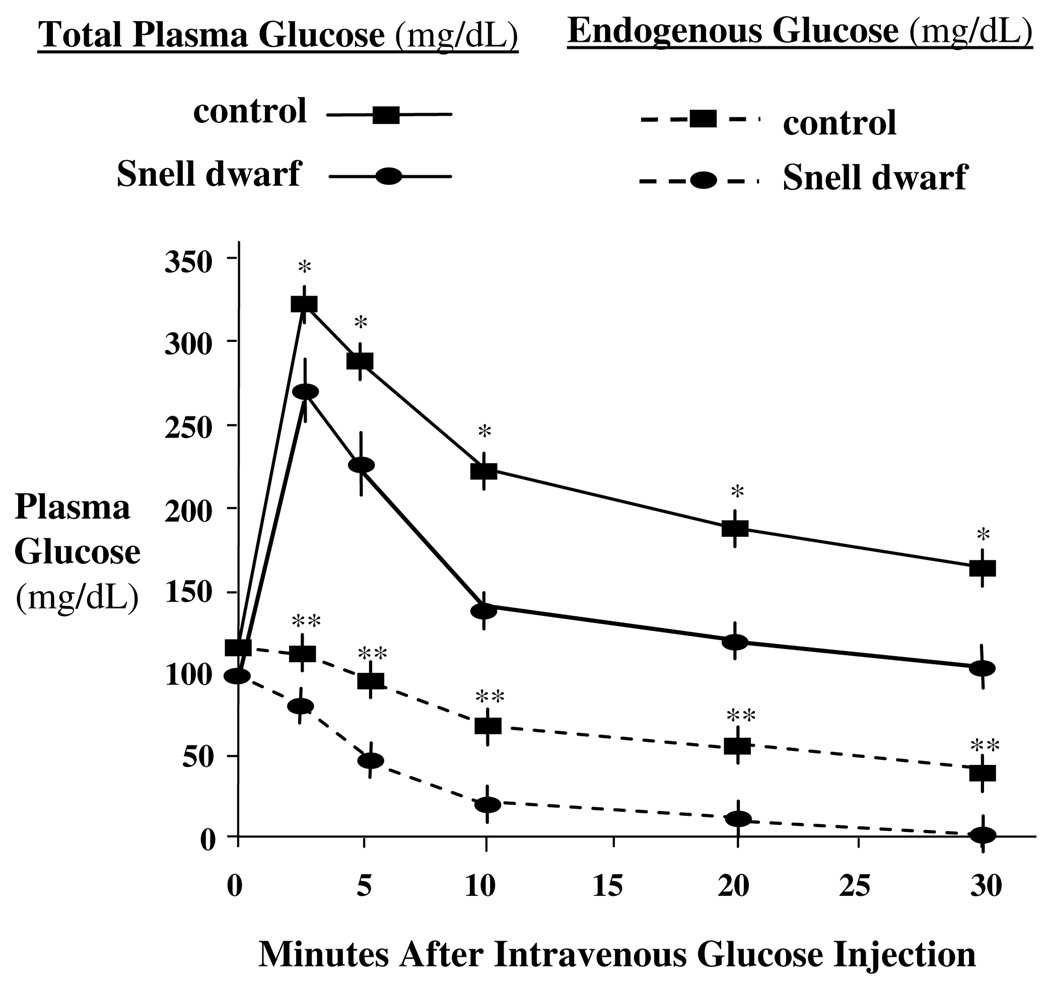
Low endogenous glucose production and whole-body glucose utilization were previously reported in Snell dwarf mice in the fasted (Brooks et al., 2007). Rather than the fasted state, the current data were obtained under conditions simulating the fed state (Fig. 1). Control and Snell dwarf mice received a single intravenous injection of glucose (0.8 mg/g body weight) mixed with trace amounts of [3H]glucose (3 × 105 cpm/mg glucose). Although plasma glucose levels were increased after glucose injection, they were lower in Snell dwarf than control mice at 2.5, 5, 10, 20 and 30 min. Endogenous glucose levels were suppressed after glucose injection, however they were reduced further in Snell dwarf than control mice at 2.5, 5, 10, 20 and 30 min. Thus, increased inhibition of glucose production in Snell dwarf mice in the fed state may restrict glucose utilization by cancer cells and thereby prevent cancer.
Fig. 1.
Endogenous glucose in 12 to 14 month-old female Snell dwarf mice after glucose injection. Control and Snell dwarf mice were injected with glucose (0.8 mg/g body weight) mixed with trace amounts of [3H]glucose (3 × 105 cpm/mg glucose). Compared to control mice, plasma glucose was reduced in Snell dwarf mice at 2.5 5, 10, 15, 20 and 30 min after injection, indicating elevated glucose tolerance. Endogenous glucose in plasma was also reduced in Snell dwarf mice at 2.5, 5, 10, 15, 20 and 30 min after injection, indicating greater inhibition of endogenous glucose production. Results are shown as means ± SEM. *, **Significantly different at indicated time-points by nonparametric Student t-test between Snell dwarf and control mice where p < 0.05 and N = 6 mice per group.
3.2. Incidence of cancer at time of death in old Snell dwarf mice
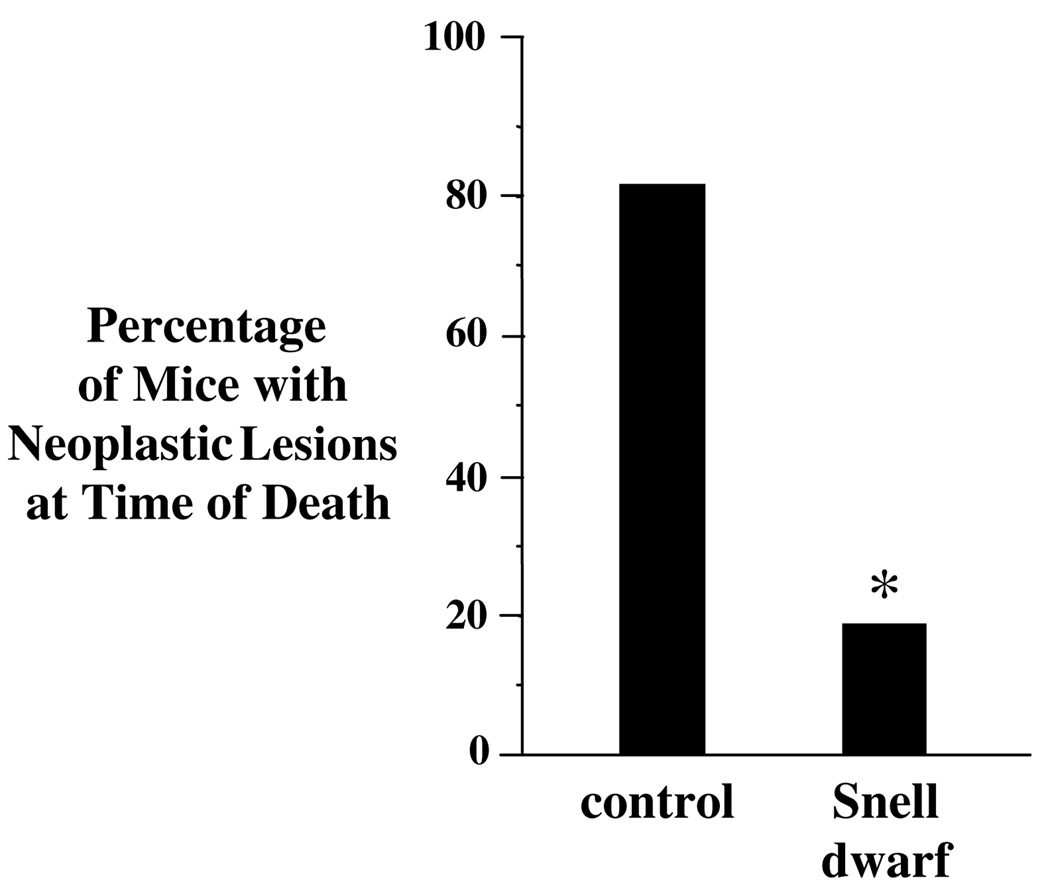
Snell dwarf mice are resistant to chemically induced cancers; however, the incidence of cancer at the time of death in old Snell dwarf mice has not been previously described (Rennels et al., 1965; Bielschowsky and Bielschowsky, 1959). We observed that significantly fewer old Snell dwarf mice had neoplastic lesions at necropsy. Only 18% of Snell dwarf mice had one or more malignant lesions at the time of death, compared to 82% of control mice (Fig. 2). These results are in contrast with previous evidence of high tumor incidence at time of death in old Ames dwarf mice (Ikeno et al., 2003).
Fig. 2.
Percentage of old female Snell dwarf mice with neoplastic lesions at time of death. The percentage of mice with one or more neoplastic lesion at time of death was lower for Snell dwarf than control mice. The median age at time of death was 26 months for control and 33 months for Snell dwarf mice. Neoplastic lesions identified by histology included histiocytic sarcoma, lymphoma, mammary adenocarcinoma, fibrosarcoma, hepatocellular carcinoma, leiomyosarcoma, myelogenous leukemia and hemangiosarcoma. Bar graphs show results as means ± SEM. *Significantly different by χ2 analysis where p < 0.05 and N = 33 control mice and N = 11 Snell dwarf mice.
3.3. Adiponectin levels in Snell dwarf mice
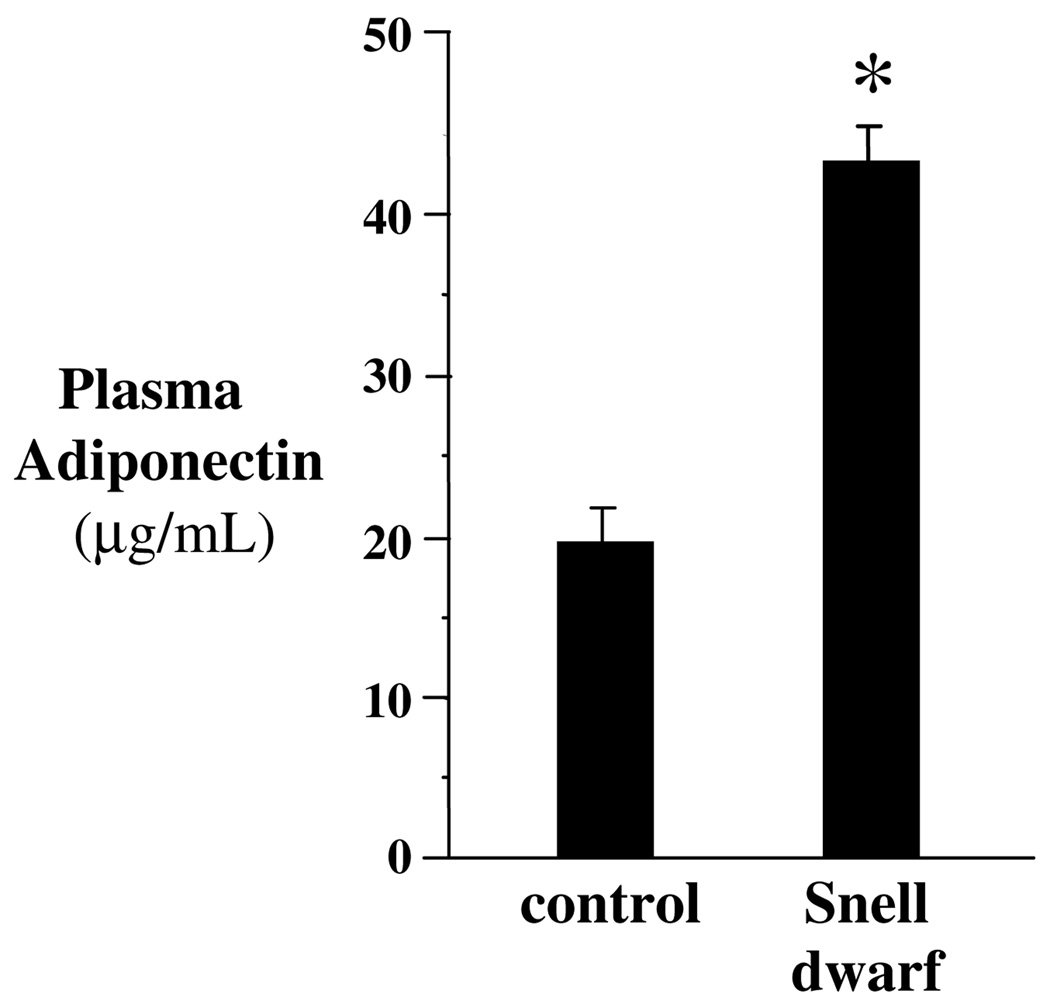
Adiponectin is a hormone that inhibits endogenous glucose production (Brooks et al., 2007; Combs et al., 2004, 2001). If elevated adiponectin protects Snell dwarf mice from cancer by reducing glucose production, adiponectin must remain elevated in older mice. Elevation of plasma adiponectin was previously reported in 3 to 6-month old Snell dwarf and Ames dwarf mice (Brooks et al., 2007; Wang et al., 2006; Combs et al., 2003). We found that plasma adiponectin was also elevated in 12 to 14 month-old Snell dwarf mice indicating a possible role for adiponectin in cancer resistance (Fig. 3). Of course, if elevated adiponectin causes the difference in resistance to cancer between Snell and Ames dwarf mice, we predict that adiponectin will be higher over the lifespan of Snell dwarf than Ames dwarf mice.
Fig. 3.
Circulating adiponectin in 12 to 14 month-old female Snell dwarf mice. Plasma adiponectin was elevated in Snell dwarf mice by Western blot analysis. Bar graphs show results as means ± SEM. *Significantly different by nonparametric Student t-test where p < 0.05 and N = 6 mice per group.
3.4. Proteomics analysis of Snell dwarf pituitary secretions
Proteomics analysis was applied to determine whether there are any additional endocrine differences between Snell dwarf and control mice. Pituitary secretions were collected from Snell dwarf and control mice ex vivo. Proteomics analysis confirmed the pituitary gland of the Snell dwarf mouse produces ACTH but not GH or PRL. In addition, the analysis revealed the Snell dwarf pituitary produces Chromogranin A, Chromogranin B and Secretogranin II (Table 1). The analysis identified a total of 107 proteins from control mouse pituitaries and 52 proteins from Snell dwarf pituitaries including nuclear, metabolic, protein structure, cell structure, signaling and secreted proteins. Detection of cellular proteins among pituitary secretions was indicative of ex vivo pituitary cell lysis. Decyder MS software analysis indicated that Chromogranin A, Chromogranin B and Secretogranin II were elevated based on matching peptide masses and retention time.
Table 1.
Proteomics analysis of secreted proteins from the pituitary of Snell dwarf and control mice
| Secreted pituitary proteins by proteomics analysis | Control | Snell dwarf |
|---|---|---|
| Growth hormone | + | ND |
| Prolactin | + | ND |
| ACTH | + | + |
| Chromogranin A | + | ++ |
| Chromogranin A | + | ++ |
| Secretogranin II | + | ++ |
Proteomics analysis confirmed that the pituitary of the Snell dwarf does not secrete GH or PRL and verified the secretion of ACTH, Chromogranin A, Chromogranin B and Secretogranin II secretion. LH, FSH and TSH secretion was not detected from Snell dwarf or control and pituitaries. The plus sign (+) indicates detected, two plus signs (++) indicate a 2-fold difference and ND indicates not detected. Pituitary secretion levels were compared using quantitative proteomics analysis software (Decyder MS).
3.5. Chromogranin A, Chromogranin B and Secretogranin II
The pituitary gland expresses Chromogranin A, Chromogranin B and Secretogranin II mRNA (Grino et al., 1989; Nicol et al., 2002; Wei et al., 1995). PCR reactions were performed for Chromogranin A, Chromogranin B and Secretogranin II mRNA expression using pituitary cDNA from control and Snell dwarf mice. PCR results showed that Chromogranin A, Chromogranin B and Secretogranin II mRNA were expressed in Snell dwarf and control pituitaries (data not shown). Primer pair sequences are provided in Materials and Methods.
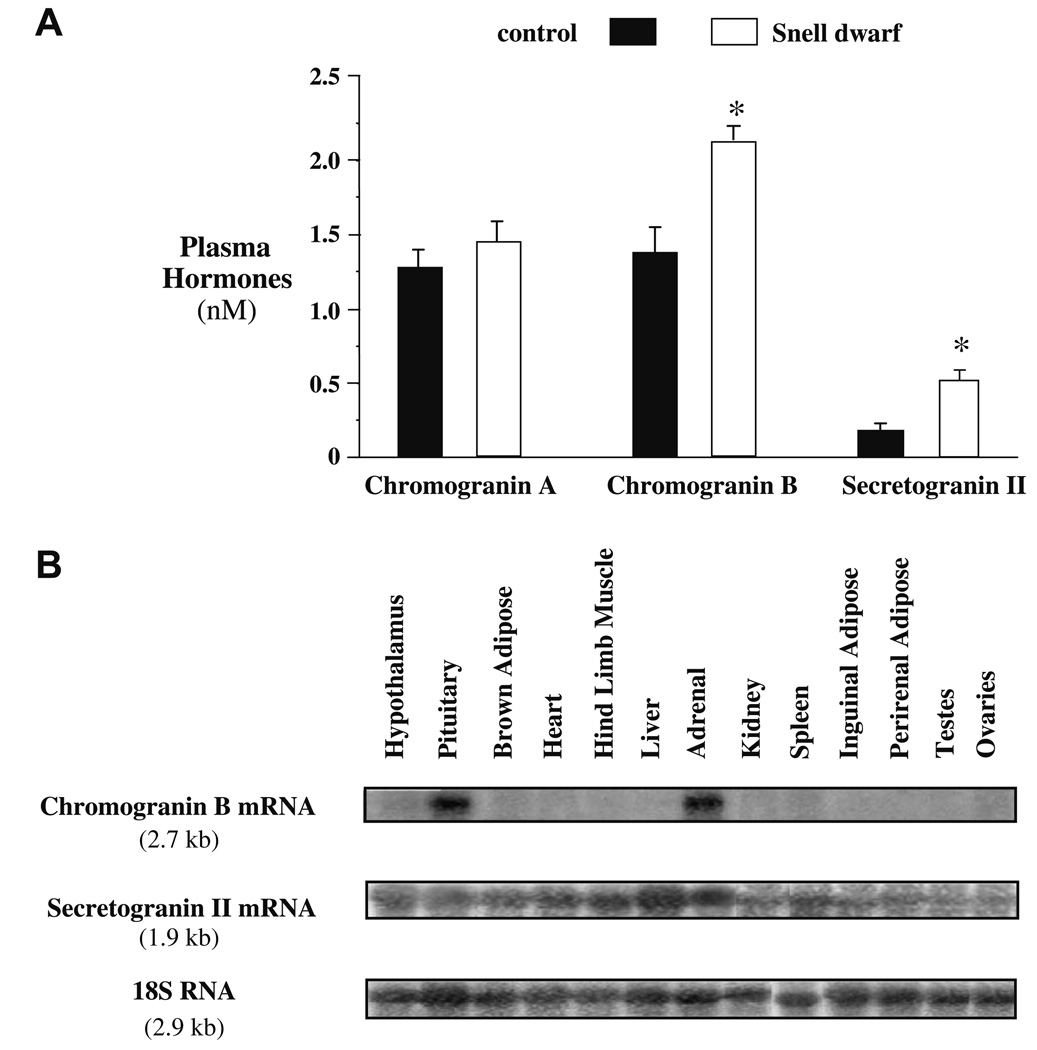
Circulating levels of Chromogranin A, Chromogranin B and Secretogranin II were measured in control and Snell dwarf mice by radioimmune assay (Stridsberg et al., 2004, 2005, in press). Significantly elevated circulating Chromogranin B and Secretogranin II levels were detected in Snell dwarf mice at p < 0.05 by nonparametric Student t-test (Fig. 4A).
Fig. 4.
(A) Circulating Chromogranin A, Chromogranin B and Secretogranin II in 12 to 14 month-old female Snell dwarf mice. Plasma Chromogranin B and Secretogranin II were elevated in Snell dwarf mice by radioimmune assay. Bar graphs show results as means ± SEM. *Significantly different by nonparametric Student t-test where p < 0.05 and N = 15 mice per group for Chromogranin A and N = 5 mice per group for Chromogranin B and Secretogranin II. (B) Tissue Chromogranin B and Secretogranin II mRNA expression by Northern blot analysis in wild-type mice. Chromogranin B mRNA expression was detected in the pituitary and the adrenal glands while Secretogranin II mRNA expression was detected ubiquitously.
The elevation of circulating Chromogranin B and Secretogranin II led us to investigate whether the pituitary is a major determinant of these circulating proteins (Fig. 4B). Northern blot analysis for mRNA expression suggested that the pituitary and the adrenal glands are the major determinants of circulating Chromogranin B while Secretogranin II was expressed ubiquitously.
4. Discussion
Our main finding was that endogenous glucose production is inhibited further after glucose injection in Snell dwarf mice than age-matched controls. Inhibition of endogenous glucose production by injection of glucose is simulating the fed state. These results complement the previous data showing suppressed endogenous glucose production during fasting in Snell dwarf mice (Brooks et al., 2007). The decrease in glucose production could restrict glucose utilization by cancer cells and inhibit the development of malignant lesions that appear with high frequency with aging. Diabetes is often associated with elevated glucose production (Basu et al., 2005; Natali and Ferrannini, 2006; Radziuk and Pye, 2002; Stefan et al., 2003; Wajngot et al., 2001) and in a recent clinical study, not a single diabetic colorectal cancer patient showed a complete pathologic response to treatment (Caudle et al., in press).
Also, significantly fewer old Snell dwarf mice had neoplastic lesions at necropsy. Our study showed that only 18% had one or more malignant lesions, compared to 82% of control mice at time of death. The low incidence of cancer in Snell dwarf mice is in contrast to the high incidence of cancer at time of death in old Ames dwarf mice (Ikeno et al., 2003). Necropsy revealed that 72% of Ames dwarf mice showed at least one fatal neoplastic lesion at the time of death, compared to 95% of control mice. The difference in genetic background between Snell mice used in the present study and Ames dwarf mice is probably not the reason for the difference in cancer resistance as controls for both models show similar susceptibility to cancer.
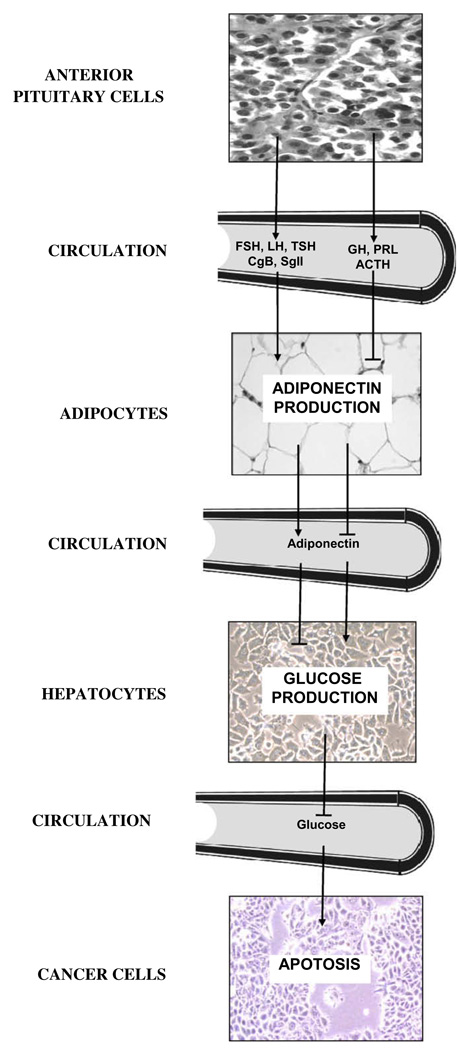
Catecholamines, glucagon and glucocorticoids are hormones that stimulate endogenous glucose production (Kraus-Friedmann, 1984). Adiponectin, a hormone produced by adipose tissue, inhibits glucose production (Brooks et al., 2007; Combs et al., 2004, 2001; Berg et al., 2001). Adiponectin can lower cancer cell glucose utilization by inhibiting liver production of glucose (Fig. 5). The elevation of circulating adiponectin in Snell dwarf mice is an endocrine signal that suppresses endogenous glucose production and elevates fatty acid oxidation. Elevated utilization of fatty acids for energy, fat burning, may be associated with a higher level of fitness compared to elevated utilization of carbohydrates (Brooks et al., 2007).
Fig. 5.
Neuroendocrine inhibition of endogenous glucose production and resistance to cancer. Snell dwarf mice show elevated adiponectin, Chromogranin B and Secretogranin II and low endogenous glucose production. Furthermore, Snell dwarf mice are highly resistant to cancer. Adiponectin, Chromogranin B and Secretogranin II are shown as part of a complex neuroendocrine axis that can ultimately provide resistance to cancer.
In the present study, Snell dwarf and control mice received the same dose of glucose per unit body weight for the intravenous glucose tolerance test. Striking differences in nutrient metabolism are observed in Ames dwarf and control mice despite similar daily food intake per body weight (Argentino et al., 2005; Dominici et al., 2003; Hauck et al., 2001; Mattison et al., 2000; Borg et al., 1995). Elevated glucose utilization for energy can increase oxidative stress (Nishikawa et al., 2000). Carbohydrate conversion to fatty acids and the oxidation of fatty acids for ATP may increase longevity in Snell dwarf mice by reducing oxidative stress (Brooks et al., 2007). Furthermore, the switch in whole-body substrate utilization may impair cancer cell growth and proliferation.
Glucose utilization can also be reduced in Snell dwarf mice by other mechanisms in addition to greater inhibition of endogenous glucose production. IGF-I and thyroid hormone deficiency in Snell dwarfs could lower tissue demand for glucose (Itoh et al., 2001; Rossetti et al., 1991). Snell dwarf mice also show elevated insulin sensitivity as indicated by circulating glucose and insulin levels and by glucose and insulin tolerance tests (Brooks et al., 2007; Combs et al., 2003; Mirand and Osborn, 1953, 1952). The elevation of insulin sensitivity lowers circulating insulin levels which can reduce GLUT4 mediated transport by insulin responsive tissues, mainly muscle and adipose (DeFronzo et al., 1981; Rossetti et al., 1997).
The neuroendocrine basis for resistance to cancer in old Snell dwarf mice is currently unknown but is obviously linked the loss of function of the Pit1 gene. Pit1 overexpression is associated with transformation, proliferation and tumor growth. Pituitary adenomas show greater expression of Pit1 than healthy pituitaries (Asa et al., 1993; Delhase et al., 1993; Sanno et al., 1996). Pit1 overexpression in human breast adenocarcinoma stimulates GH production, cell growth and cell proliferation (Gil-Puig et al., 2005). Pit1 antisense oligonucleotides reduce GH mRNA expression and [3H]thymidine incorporation in pituitary somatotrophs and lactotrophs, suggesting that Pit1 stimulates DNA replication and cell proliferation (Castrillo et al., 1991). By contrast, the potential role of Pit1 as a tumor suppressor may be limited to somatotrophs (Canibano et al., 2007).
As a first step towards determining the neuroendocrine basis for the difference in the incidence of cancer between old Snell and Ames dwarf mice, we tested whether there are any other pituitary hormone differences in Snell dwarf mice besides a lack of GH, PRL and TSH. Proteomics analysis and revealed that pituitary secretion of Chromogranin A, Chromogranin B and Secretogranin II were elevated in Snell dwarf mice. Chromogranin A, Chromogranin B and Secretogranin II constitute the main members of a unique family of secretory proteins (Helle, 2004). The effects of Chromogranin A, Chromogranin B and Secretogranin II on endogenous glucose production are unknown however other effects on glucose metabolism have been reported. Chromogranin A and Chromogranin B inhibit insulin secretion therefore they can lower glucose utilization by insulin responsive tissues (Schmid et al., 2007; Karlsson et al., 2000; Rossetti et al., 1997). GLUT4 colocalizes with Secretogranin II in large dense core vesicles (Hudson et al., 1993). Our plasma measurements revealed that circulating levels of Chromogranin B and Secretogranin II are elevated in Snell dwarfs. Of course, if the elevation of Chromogranin B and Secretogranin II is responsible for the differences in cancer incidence between Snell dwarf and Ames dwarf mice, then these hormones should not be elevated in the Ames dwarf.
It is of great interest to determine the mechanisms that reduce cancer in old Snell dwarf mice, as they may suggest clinical interventions. Thus, future studies will determine whether adiponectin, Chromogranin B and Secretogranin II play a role in the inhibition of endogenous glucose production and resistance to cancer with old age. Fig. 5 illustrates an endocrine link between the pituitary gland, adipose tissue and the liver that can provide resistance to cancer by restricting endogenous glucose production. Although it is not indicated in Fig. 5, the anterior pituitary gland is under the influence of the hypothalamus; the liver’s ability to produce glucose is also directly inhibited by the hypothalamus through the hepatic branch of the vagus nerve (Pocai et al., 2005). The low incidence of cancer in Snell dwarf mice should be investigated further considering the mechanism is related to neuroendocrine factors other than an absence of GH and IGF-I.
Acknowledgements
We are grateful to Dr. Karen B. Helle from the Department of Biomedicine, University of Bergen (Norway) for helpful suggestions, Elizabeth Tornquist from the School of Nursing at University of North Carolina at Chapel Hill for editorial help, Patricia Harrison, Pam Krason and Elizabeth Adler from the Jackson Laboratories for reliable assistance. This research was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK075573 and DK056350), the National Institute on Aging (AG026074, AG025007 and AG025707) and the National Institute of Environmental Health Sciences (ES10126). The UNC-Duke Proteomics Center was partially funded by a gift from an anonymous donor in honor of Michael Hooker.
Abbreviations
- ACTH
adrenalcorticotropic hormone
- AMPK
AMP activated protein kinase
- FSH
follicle stimulating hormone
- GH
growth hormone
- GLUT4
glucose transporter 4
- IGF-I
insulin like growth factor I
- LH
luteinizing hormone
- PRL
prolactin
- SEM
standard error of the mean
- TSH
thyroid stimulating hormone
References
- Argentino DP, Dominici FP, Munoz MC, Al-Regaiey K, Bartke A, Turyn D. Effects of long-term caloric restriction on glucose homeostasis and on the first steps of the insulin signaling system in skeletal muscle of normal and Ames dwarf (Prop1df/Prop1df) mice. Exp. Gerontol. 2005;40:27–35. doi: 10.1016/j.exger.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Asa SL, Puy LA, Lew AM, Sundmark VC, Elsholtz HP. Cell type-specific expression of the pituitary transcription activator pit-1 in the human pituitary and pituitary adenomas. J. Clin. Endocrinol. Metab. 1993;77:1275–1280. doi: 10.1210/jcem.77.5.8077321. [DOI] [PubMed] [Google Scholar]
- Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes. 2005;54:1942–1948. doi: 10.2337/diabetes.54.7.1942. [DOI] [PubMed] [Google Scholar]
- Beard JC, Bergman RN, Ward WK, Porte D., Jr The insulin sensitivity index in nondiabetic man. Correlation between clamp-derived and IVGTT-derived values. Diabetes. 1986;35:362–369. doi: 10.2337/diab.35.3.362. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm. IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Bielschowsky F, Bielschowsky M. Carcinogenesis in the pituitary dwarf mouse. The response to methylcholanthrene injected subcutaneously. Br. J. Cancer. 1959;13:302–305. doi: 10.1038/bjc.1959.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake CA, Kakhniashvili DG, Goodman SR. Mouse anterior pituitary gland: analysis by ion trap mass spectrometry. Neuroendocrinology. 2005;81:229–243. doi: 10.1159/000087434. [DOI] [PubMed] [Google Scholar]
- Borg KE, Brown-Borg HM, Bartke A. Assessment of the primary adrenal cortical and pancreatic hormone basal levels in relation to plasma glucose and age in the unstressed Ames dwarf mouse. Proc. Soc. Exp. Biol. Med. 1995;210:126–133. doi: 10.3181/00379727-210-43931. [DOI] [PubMed] [Google Scholar]
- Brooks NL, Trent CM, Raetzsch CF, Flurkey K, Boysen G, Perfetti MT, Jeong YC, Klebanov S, Patel KB, Khodush VR, et al. Low utilization of circulating glucose after food withdrawal in Snell dwarf mice. J. Biol. Chem. 2007;282:35069–35077. doi: 10.1074/jbc.M700484200. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown JM. The hypoxic cell: a target for selective cancer therapy – eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- Canibano C, Rodriguez NL, Saez C, Tovar S, Garcia-Lavandeira M, Borrello MG, Vidal A, Costantini F, Japon M, Dieguez C, Alvarez CV. The dependence receptor Ret induces apoptosis in somatotrophs through a Pit-1/p53 pathway, preventing tumor growth. EMBO J. 2007;26:2015–2028. doi: 10.1038/sj.emboj.7601636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo JL, Theill LE, Karin M. Function of the homeodomain protein GHF1 in pituitary cell proliferation. Science. 1991;253:197–199. doi: 10.1126/science.1677216. [DOI] [PubMed] [Google Scholar]
- Caudle AS, Kim HJ, Tepper JE, O’Neil BH, Lange LA, Goldberg RM, Bernard SA, Calvo BF, Meyers MO. Diabetes mellitus affects response to neoadjuvant chemoradiotherapy in the management of rectal cancer. Ann. Surg. Oncol. doi: 10.1245/s10434-008-9873-6. in press, doi:10.1245/S10434-008-9873-6. PMID: 18418656. [DOI] [PubMed] [Google Scholar]
- Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, Chen HY, Hou MF, Yuan SS. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Delhase M, Vergani P, Malur A, Velkeniers B, Teugels E, Trouillas J, Hooghe-Peters EL. Pit-1/GHF-1 expression in pituitary adenomas: further analogy between human adenomas and rat SMtTW tumours. J. Mol. Endocrinol. 1993;11:129–139. doi: 10.1677/jme.0.0110129. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Argentino DP, Bartke A, Turyn D. The dwarf mutation decreases high dose insulin responses in skeletal muscle, the opposite of effects in liver. Mech. Ageing Dev. 2003;124:819–827. doi: 10.1016/s0047-6374(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. USA. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes JM, Quintero M, Henderson S, Martinez D, Qureshi U, Westwood C, Clements MO, Bourboulia D, Pedley RB, Moncada S, Boshoff C. Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc. Natl. Acad. Sci. USA. 2007;104:6223–6228. doi: 10.1073/pnas.0700690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Puig C, Seoane S, Blanco M, Macia M, Garcia-Caballero T, Segura C, Perez-Fernandez R. Pit-1 is expressed in normal and tumorous human breast and regulates GH secretion and cell proliferation. Eur. J. Endocrinol. 2005;153:335–344. doi: 10.1530/eje.1.01962. [DOI] [PubMed] [Google Scholar]
- Grino M, Wohlfarter T, Fischer-Colbrie R, Eiden LE. Chromogranin A messenger RNA expression in the rat anterior pituitary is permissively regulated by the adrenal gland. Neuroendocrinology. 1989;49:107–110. doi: 10.1159/000125098. [DOI] [PubMed] [Google Scholar]
- Gullino PM, Grantham FH, Courtney AH, Losonczy I. Relationship between oxygen and glucose consumption by transplanted tumors in vivo. Cancer Res. 1967;27:1041–1052. [PubMed] [Google Scholar]
- Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp. Biol. Med. (Maywood) 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- Helle KB. The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: comparative and functional aspects. Biol. Rev. Camb. Philos. Soc. 2004;79:769–794. doi: 10.1017/s146479310400644x. [DOI] [PubMed] [Google Scholar]
- Hudson AW, Fingar DC, Seidner GA, Griffiths G, Burke B, Birnbaum MJ. Targeting of the “insulin-responsive” glucose transporter (GLUT4) to the regulated secretory pathway in PC12 cells. J. Cell Biol. 1993;122:579–588. doi: 10.1083/jcb.122.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Kaneshige M, Suzuki H, Cook M, Sokoloff L, Cheng SY, Nunez J. Brain glucose utilization in mice with a targeted mutation in the thyroid hormone alpha or beta receptor gene. Proc. Natl. Acad. Sci. USA. 2001;98:9913–9918. doi: 10.1073/pnas.171319498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Samskog J, Sundstrom L, Wadensten H, Bjorkesten L, Flensburg J. Differential expression analysis of Escherichia coli proteins using a novel software for relative quantitation of LC-MS/MS data. Proteomics. 2006;6:4475–4485. doi: 10.1002/pmic.200500921. [DOI] [PubMed] [Google Scholar]
- Karlsson E, Stridsberg M, Sandler S. Chromogranin-B regulation of IAPP and insulin secretion. Regul. Pept. 2000;87:33–39. doi: 10.1016/s0167-0115(99)00105-6. [DOI] [PubMed] [Google Scholar]
- Korner A, Pazaitou-Panayiotou K, Kelesidis T, Kelesidis I, Williams CJ, Kaprara A, Bullen J, Neuwirth A, Tseleni S, Mitsiades N, et al. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J. Clin. Endocrinol. Metab. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- Kraus-Friedmann N. Hormonal regulation of hepatic gluconeogenesis. Physiol. Rev. 1984;64:170–259. doi: 10.1152/physrev.1984.64.1.170. [DOI] [PubMed] [Google Scholar]
- Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Luft R, Olivecrona H, Ikkos D, Kornerup T, Ljunggren H. Hypophysectomy in man; further experiences in severe diabetes mellitus. Br. Med. J. 1955;2:752–756. doi: 10.1136/bmj.2.4942.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin A, Matthews CE, Shu XO, Cai H, Dai Q, Jin F, Gao YT, Zheng W. Energy balance and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 2005;14:1496–1501. doi: 10.1158/1055-9965.EPI-04-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, Papadiamantis Y, Markopoulos C, Spanos E, Chrousos G, Trichopoulos D. Adiponectin and breast cancer risk. J. Clin. Endocrinol. Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Wright C, Bronson RT, Roth GS, Ingram DK, Bartke A. Studies of aging in Ames dwarf mice: effects of caloric restriction. J. Am. Aging Assoc. 2000;23:9–16. doi: 10.1007/s11357-000-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirand EA, Osborn CM. Effect of fasting on blood sugars in hereditary hypopituitary dwarf mice. Proc. Soc. Exp. Biol. Med. 1952;81:706–708. doi: 10.3181/00379727-81-19995. [DOI] [PubMed] [Google Scholar]
- Mirand EA, Osborn CM. Insulin sensitivity in the hereditary hypopituitary dwarf mouse. Proc. Soc. Exp. Biol. Med. 1953;82:746–748. doi: 10.3181/00379727-82-20234. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin. Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49:434–441. doi: 10.1007/s00125-006-0141-7. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Nicol L, McNeilly JR, Stridsberg M, Crawford JL, McNeilly AS. Influence of steroids and GnRH on biosynthesis and secretion of secretogranin II and chromogranin A in relation to LH release in LbetaT2 gonadotroph cells. J. Endocrinol. 2002;174:473–483. doi: 10.1677/joe.0.1740473. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Radziuk J, Pye S. Quantitation of basal endogenous glucose production in Type II diabetes: importance of the volume of distribution. Diabetologia. 2002;45:1053–1084. doi: 10.1007/s00125-002-0841-6. [DOI] [PubMed] [Google Scholar]
- Rennels EG, Anigstein DM, Anigstein L. A cumulative study of the growth of sarcoma 180 in anterior pituitary dwarf mice. Tex. Rep. Biol. Med. 1965;23:776–781. [PubMed] [Google Scholar]
- Rossetti L, Frontoni S, Dimarchi R, DeFronzo RA, Giaccari A. Metabolic effects of IGF-I in diabetic rats. Diabetes. 1991;40:444–448. doi: 10.2337/diab.40.4.444. [DOI] [PubMed] [Google Scholar]
- Rossetti L, Stenbit AE, Chen W, Hu M, Barzilai N, Katz EB, Charron MJ. Peripheral but not hepatic insulin resistance in mice with one disrupted allele of the glucose transporter type 4 (GLUT4) gene. J. Clin. Invest. 1997;100:1831–1839. doi: 10.1172/JCI119711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno N, Teramoto A, Matsuno A, Osamura RY. Expression of human Pit-1 product in the human pituitary and pituitary adenomas. Immunohistochemical studies using an antibody against synthetic human Pit-1 product. Arch. Pathol. Lab. Med. 1996;120:73–77. [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Schmid GM, Meda P, Caille D, Wargent E, O’Dowd J, Hochstrasser DF, Cawthorne MA, Sanchez JC. Inhibition of insulin secretion by betagranin, an N-terminal chromogranin A fragment. J. Biol. Chem. 2007;282:12717–12724. doi: 10.1074/jbc.M700788200. [DOI] [PubMed] [Google Scholar]
- Shen Q, Lantvit DD, Lin Q, Li Y, Christov K, Wang Z, Unterman TG, Mehta RG, Swanson SM. Advanced rat mammary cancers are growth hormone dependent. Endocrinology. 2007;148:4536–4544. doi: 10.1210/en.2007-0513. [DOI] [PubMed] [Google Scholar]
- Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc. Natl. Acad. Sci. USA. 1998;95:1511–1516. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Stattin P, Bjor O, Ferrari P, Lukanova A, Lenner P, Lindahl B, Hallmans G, Kaaks R. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30:561–567. doi: 10.2337/dc06-0922. [DOI] [PubMed] [Google Scholar]
- Stauber AJ, Brown-Borg H, Liu J, Waalkes MP, Laughter A, Staben RA, Coley JC, Swanson C, Voss KA, Kopchick JJ, Corton JC. Constitutive expression of peroxisome proliferator-activated receptor alpha-regulated genes in dwarf mice. Mol. Pharmacol. 2005;67:681–694. doi: 10.1124/mol.104.007278. [DOI] [PubMed] [Google Scholar]
- Stefan N, Stumvoll M, Vozarova B, Weyer C, Funahashi T, Matsuzawa Y, Bogardus C, Tataranni PA. Plasma adiponectin and endogenous glucose production in humans. Diabetes Care. 2003;26:3315–3319. doi: 10.2337/diacare.26.12.3315. [DOI] [PubMed] [Google Scholar]
- Stridsberg M, Eriksson B, Öberg K, Janson ET. A panel of eleven region-specific radioimmunoassays against different parts of the human chromogranin A molecule. Regul. Pept. 2004;117:219–227. doi: 10.1016/j.regpep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Stridsberg M, Eriksson B, Öberg K, Janson ET. A panel of 13 region-specific radioimmunoassays for measurements of human chromogranin B. Regul. Pept. 2005;125:193–199. doi: 10.1016/j.regpep.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Stridsberg M, Eriksson B, Janson ET. Measurements of secretogranins II, III, V and proconvertases 1/3 and 2 in plasma from patients with neuroendocrine tumours. Regul. Pept. doi: 10.1016/j.regpep.2008.03.007. in press, doi:10.1016/j.regpep.2008.03.007: PMID: 18448176. [DOI] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CcC, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. USA. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, Hankinson SE. Plasma adiponectin concentrations and risk of incident breast cancer. J. Clin. Endocrinol. Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- Wajngot A, Chandramouli V, Schumann WC, Ekberg K, Jones PK, Efendic S, Landau BR. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metabolism. 2001;50:47–52. doi: 10.1053/meta.2001.19422. [DOI] [PubMed] [Google Scholar]
- Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- Wei N, Kakar SS, Neill JD. Measurement of secretogranin II release from individual adenohypophysial gonadotropes. Am. J. Physiol. 1995;268:E145–E152. doi: 10.1152/ajpendo.1995.268.1.E145. [DOI] [PubMed] [Google Scholar]
- Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J. Appl. Physiol. 2001;91:1017–1028. doi: 10.1152/jappl.2001.91.3.1017. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]