Abstract
Metabolic myopathies comprise a clinically and etiologically diverse group of disorders caused by defects in cellular energy metabolism, including the breakdown of carbohydrates and fatty acids to generate adenosine triphosphate, predominantly through mitochondrial oxidative phosphorylation. Accordingly, the three main categories of metabolic myopathies are glycogen storage diseases, fatty acid oxidation defects, and mitochondrial disorders due to respiratory chain impairment. The wide clinical spectrum of metabolic myopathies ranges from severe infantile-onset multisystemic diseases to adult-onset isolated myopathies with exertional cramps. Diagnosing these diverse disorders often is challenging because clinical features such as recurrent myoglobinuria and exercise intolerance are common to all three types of metabolic myopathy. Nevertheless, distinct clinical manifestations are important to recognize as they can guide diagnostic testing and lead to the correct diagnosis. This article briefly reviews general clinical aspects of metabolic myopathies and highlights approaches to diagnosing the relatively more frequent subtypes (Fig. 1).
Keywords: Metabolic myopathies, Glycogenosis, Fatty acid, Mitochondrial diseases
Introduction
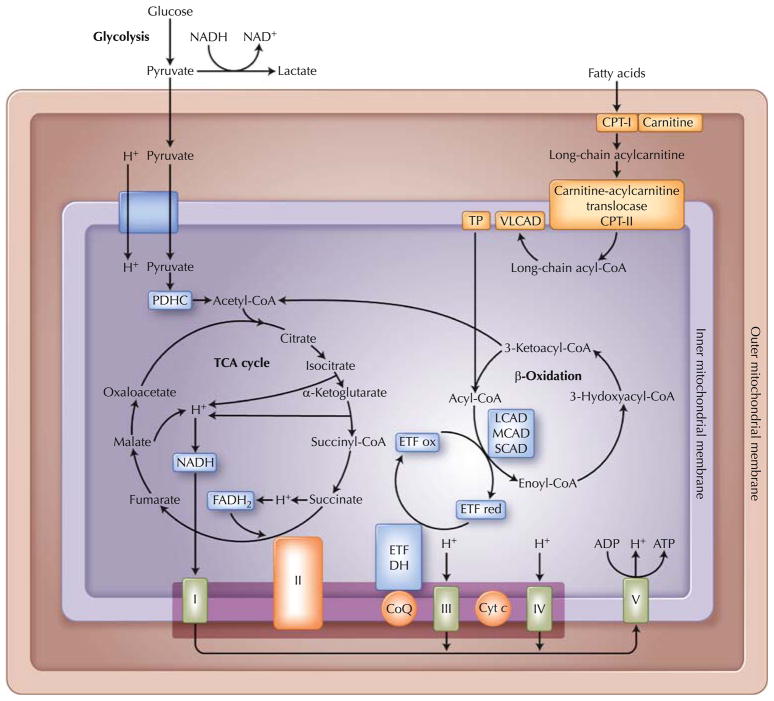
The main sources of adenosine triphosphate (ATP) in cells are glycogen, glucose, and free fatty acids. Glycogen is metabolized in the cytoplasm to pyruvate, which enters mitochondria (Fig. 2). Short-and medium-chain fatty acids cross freely into the mitochondria, whereas long-chain fatty acids require binding to carnitine for transport across the mitochondrial membrane, a process mediated by acylcarnitine translocase and carnitine palmitoyl transferases (CPTs) I and II (Fig. 2). Once in the mitochondria, all these substrates are turned into acetyl coenzyme A (CoA), which feeds into the Krebs cycle. In this critical cycle, reducing equivalents (electrons) combined with protons are bound to intermediate molecules, NADH and FADH2, which deliver the electrons to the mitochondrial respiratory chain to produce ATP and H2O.
Fig. 2.
Mitochondrial metabolism. Roman numerals refer to mitochondrial respiratory chain enzymes. ADP—adenosine diphosphate; ATP—adenosine triphosphate; CoA—coenzyme A; CoQ—coenzyme Q; CPT—carnitine palmitoyl transferase; Cyt c—cytochrome c; DH—dehydrogenase; ETF—electron transfer flavoprotein; LCAD—long-chain acyl-CoA dehydrogenase; MCAD—medium-chain acyl-CoA dehydrogenase; PDHC—pyruvate dehydrogenase complex; SCAD—short-chain acyl-CoA dehydrogenase; TCA—tricarboxylic acid; TP, trifunctional enzyme; VLCAD, very long-chain acyl-CoA dehydrogenase. (Adapted from Hirano [53], with permission.)
Defects in any of these pathways—glycogen catabolism (glycogenolysis and glycolysis), fatty acid oxidation, Krebs cycle, or mitochondrial respiratory chain and oxidative phosphorylation—may cause human disorders that often predominantly affect muscle because of its high energy requirements, particularly during exercise. Because most of the enzyme defects are partial, many of these diseases manifest in adulthood with isolated muscle symptoms and similar clinical features [1, 2•]. Nevertheless, there are salient clinical features that can guide clinicians to the exact diagnosis with the aid of complementary diagnostic tests.
From a clinical point of view, metabolic myopathies can be categorized into two different groups: 1) those that show symptoms and signs related to exercise (exercise intolerance, cramps, myalgias, myoglobinuria) with normal interictal examination and 2) those with fixed symptoms, such as muscle weakness, often associated with systemic involvement (eg, endocrinopathies or encephalopathies). When evaluating a patient with exercise-related symptoms, we should ask two questions: 1) What type of exercise provokes symptoms? 2) Are there associated triggering factors? If short bursts of high-intensity exercise trigger muscle cramps or pigmenturia, the patient may have a defect of glycogen metabolism [3]. Examples of this type of activity include weight lifting and sprinting. In the United States and other countries where baseball is popular, the “home run” (Haller) sign—that is, the inability to sprint around the bases because of exercise-induced muscle spasms—is a typical complaint in young patients with glycogenoses, such as McArdle disease (Dr. Ronald Haller, personal communication). In contrast, if the patient reports that prolonged exercise (eg, hiking or playing soccer) triggers myalgias, fatigue, and pigmenturia without acute contractures, he or she likely has a defect of fatty acid oxidation. The symptoms often occur when the patient is fasting. A prototypical example is a young adult with CPT II deficiency who enlists in military service and has difficulty completing long marches because of fatigue and myalgias followed by pigmenturia. Some patients with glycolytic or lipid disorders may develop progressive myopathy and persistent weakness. Patients with mitochondrial diseases may show a wide range of manifestations that are exercise induced, fixed, or both, but exercise intolerance is particularly common and manifests as the inability to perform activities because of premature fatigue out of proportion to weakness. This article briefly goes through general clinical aspects of these metabolic myopathies, emphasizing the more common forms (Table 1), and provides an algorithm for diagnosing these diverse conditions (Fig. 1).
Table 1.
Metabolic myopathies: adult-onset forms
| Characteristic | Mitochondrial diseases | Glycogen storage diseases (type) | Fatty acid oxidation diseases |
|---|---|---|---|
| Described diseases | Isolated myopathy (cyt b mutations) | Pompe’s disease (II) | CPT type II |
| MERRF | Cori-Forbes disease (III) | PCD | |
| MELAS | Andersen disease (IV) PPL deficiency (McArdle disease, V) |
β-Oxidation mutations VLCAD deficiency |
|
| CPEO/Kearns-Sayre syndrome | PFK deficiency (Tarui disease, VII) Phosphorylase b kinase deficiency (VIII) PGK deficiency (IX) PGAM deficiency (X) LDH deficiency (XI) |
LCAD deficiency MCAD deficiency SCAD deficiency LCHAD MADD |
|
| General clinical features | Usually multisystemic disorders | Premature fatigue during endurance-type activity | Symptoms precipitated by prolonged exercise activity (hours); fasting or infection may trigger episodes |
| Exercise intolerance manifested during endurance-type activity | Respiratory insuffiency + proximal weakness + macroglossia (II) | No cramps during but muscle pain and weakness after exercise since childhood; no 2nd wind | |
| Myoglobinuria is uncommon except with cyt b, COX, and A3260G mutations | Muscle cramping, myalgias, “2nd wind,” intense exercise-induced myoglobinuria, late proximal weakness (V, VII) | Recurrent myoglobinuria (CPT II) | |
| Proximal weakness and ocular muscle involvement | Later, distal weakness may develop (III) | Isolated proximal weakness (PCD) | |
| Laboratory findings | Serum CK and lactate may be elevated | Consistent CK elevation at all times | Selective neck weakness (MADD) |
| VO2max low in exercise testing | Failure of lactate to increase Exaggerated ammonia elevation; during metabolic crisis, may exceed 10,000 U/L Serum uric acid may be elevated (II, III) Hemolytic anemia (VII, IX) VO2max low in exercise testing |
Blood test usually normal between events During crisis, hypoglycemia, hyperkalemia, and CK elevation may be present Acylcarnitine profile elevated during metabolic crisis With fasting, ratio of serum free fatty acids to ketones increases 2:1 (block in β-oxidation) |
|
| Muscle biopsy | Ragged red fibers, COX negative, subsarcolemmal SDH increase staining (ragged blue) Paracrystalline inclusions Enzymatic measurement may show respiratory chain complex deficiency |
Increased glycogen concentration by PAS staining and electronic microscopy (II) Normal biopsy finding (III, V) Immunohistochemistry for different enzyme deficiencies |
May show increase in neutral lipids by oil red O or Sudan black, or may be completely normal (CPT deficiency) |
CK—creatine kinase; CoA—coenzyme A; COX—cytochrome c oxidase; CPEO—chronic progressive external ophthalmoplegia; CPT—carnitine palmitoyl transferase; cyt b—cytochrome b; LCAD—long-chain acyl-CoA dehydrogenase; LCHAD—long-chain 3-hydroxy/acyl-CoA dehydrogenase deficiency; LDH—lactate dehydrogenase; MADD—multiple acyl-CoA dehydrogenase deficiency. MCAD—medium-chain acyl-CoA dehydrogenase; MELAS—mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes; MERRF—myoclonus epilepsy with ragged red fibers; PAS—periodic acid-Schiff; PCD—primary carnitine deficiency; PFK—phosphofructokinase; PGAM—phosphoglycerate mutase; PGK—phosphoglycerate kinase; PPL—myophosphorylase; SCAD—short-chain acyl-CoA dehydrogenase; SDH—succinate dehydrogenase; VLCAD—very long–chain acyl-CoA dehydrogenase; Vo2max—maximal oxygen uptake
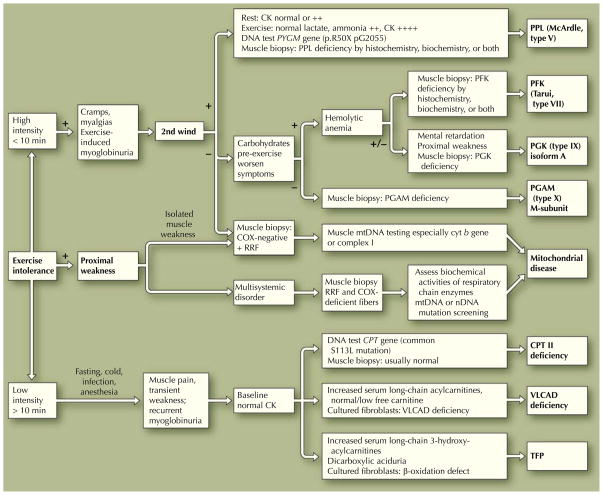
Fig. 1.
Clinical algorithm for patients with exercise intolerance in whom a metabolic myopathy is suspected. CK—creatine kinase; COX—cytochrome c oxidase; CPT—carnitine palmitoyl transferase; cyt b—cytochrome b; mtDNA—mitochondrial DNA; nDNA—nuclear DNA; PFK—phosphofructokinase; PGAM—phosphoglycerate mutase; PGK—phosphoglycerate kinase; PPL—myophosphorylase; RRF—ragged red fibers; TFP—trifunctional protein deficiency; VLCAD—very long-chain acyl-coenzyme A dehydrogenase
Disorders of Glycogen Metabolism (Glycogenoses)
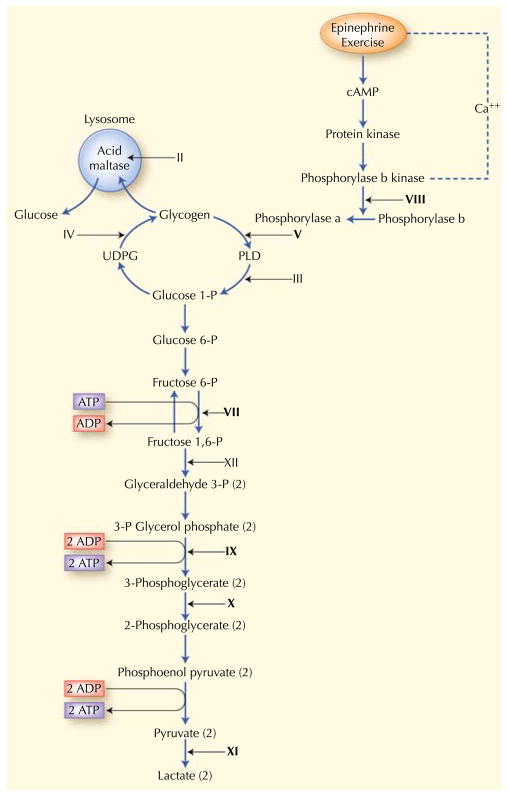
Abundant glucose is stored in liver and skeletal muscle in the form of a polysaccharide called glycogen. The glycogenoses include disorders characterized by genetic mutations in glycogen synthesis (glycogenogenesis), glycogen degradation (glycogenolysis), or glucose degradation (glycolysis). Clinical presentations of muscle glycogenoses are protean, ranging from profound multisystem disease in infancy [4] to exercise intolerance or isolated progressive muscle weakness in adulthood [5, 6]. To date, 14 glycogenoses have been identified (Fig. 3) [7]; most are autosomal recessive except for phosphoglycerate kinase (PGK) and phosphorylase b kinase deficiency, which are X-linked.
Fig. 3.
Glycogen metabolism and glycolysis. Roman numerals denote muscle glycogenoses due to defects in the following enzymes: II, acid maltase; III, debrancher; IV, brancher; V, myophosphorylase; VII, phosphofructokinase; VIII, phosphorylase b kinase; IX, phosphoglycerate kinase; X, phosphoglycerate mutase; XI, lactate dehydrogenase; and XII, aldolase. Bold numerals designate glycogenoses associated with exercise intolerance, cramps, and myoglobinuria. Non-bold numerals correspond to glycogenoses causing weakness. ADP—adenosine diphosphate; ATP—adenosine triphosphate; cAMP—cyclic adenosine monophosphate; PLD—phospholipase D; UDPG—uridine diphosphoglucose. (Adapted from Hirano [53], with permission.)
Myophosphorylase deficiency (McArdle disease, glycogenosis type V) is a prototypical glycolytic disorder with episodic muscle dysfunction and myoglobinuria. It is the most common disorder of skeletal muscle carbohydrate metabolism and one of the most frequent genetic myopathies [8]. Patients with McArdle disease typically exhibit intolerance to static or isometric muscle contractions and also to dynamic exercise that can trigger episodes of reversible “muscle crises.” Acute crises manifest mainly in the form of premature fatigue and contractures, frequently accompanied by muscle breakdown (rhabdomyolysis) with elevated serum creatine kinase and sometimes by myoglobinuria. Another important sign considered pathognomonic is the “second wind,” which is a marked improvement in exercise tolerance about 10 minutes into aerobic exercise involving large muscle masses (jogging or cycling) [9].
The second wind, as manifested by a marked decrease in early exertional tachycardia (eg, a decrease from ~140–150 bpm to ~120 bpm) starting after around 7 minutes of exercise, does not occur in patients with other disorders associated with exercise intolerance, such as glycogenoses types VII (phosphofructokinase [PFK] deficiency, Tarui disease), VIII (phosphorylase b kinase deficiency), and X (phosphoglycerate mutase deficiency); mitochondrial myopathies; and disorders of lipid metabolism (deficiencies of CPT II) or very long-chain acyl-CoA dehydrogenase (VLCAD) [10–13].
When a glycogenosis is suspected, other clinical features, in addition to the second wind, may offer diagnostic inclues. Glucose or sucrose intake before exercise exacerbates muscle symptoms (out-of-wind phenomenon) in PFK deficiency [14], in which the metabolic block occurs below the entry of glucose into glycolysis, whereas in McArdle disease, sugar intake ameliorates symptoms because the metabolic block is upstream of glucose catabolism [15]. In other cases, such as phosphoglycerate mutase deficiency, this intervention does not produce changes in exercise performance [16].
Hemolytic anemia (elevated indirect bilirubin and reticulocytes) is seen in glycogenosis due to defects in genes partially expressed in erythrocytes, such as PFK, PGK (type IX), and aldolase A (type XII) deficiency [17–19]. Mental retardation or dementia often is associated with the adult polyglucosan body disease form of branching enzyme deficiency (type IV), PGK deficiency, and aldolase A deficiency [20–22].
In the late-onset form of acid maltase deficiency (type II, Pompe’s disease), patients have fixed proximal muscle weakness and early respiratory insufficiency rather than exercise-induced symptoms. In other cases, such as debrancher deficiency (type III, Cori-Forbes disease), distal muscle weakness may be combined with cardiomyopathy and peripheral neuropathy in patients who in infancy had shown hepatomegaly, hypoglycemia, and failure to thrive, all of which usually improve around puberty [23].
Disorders of Lipid Metabolism
Fatty acids are the primary energy source for muscle at rest and during periods of prolonged low-intensity exercise. Short-and medium-chain fatty acids cross freely into the mitochondria, whereas long-chain fatty acids require binding to carnitine for transport across the inner mitochondrial membrane, a process mediated by acylcarnitine translocase and CPT I and II (Fig. 2). Fatty acids are catabolized by the β-oxidation enzymes, which cleave two-carbon fragments with each cycle. Thus, lipidoses arise as a result of the failure of fatty acids to transport into mitochondria secondary to carnitine or CPT I or II deficiencies or because of defects in intramitochondrial β-oxidation [24, 25•]. These disorders are inherited as autosomal recessive traits. The more severe variants present in infancy or childhood with primary involvement of liver or brain, whereas the milder adult forms are predominantly myopathic and include the myopathic variant of CPT II deficiency, trifunctional protein deficiency, and VLCAD.
Since DiMauro and Melis-DiMauro [26] described the first patients with CPT II deficiency, this disease has been the most frequently diagnosed disorder of lipid metabolism. The first symptoms occur most often between 6 and 20 years of age, but age at onset may be later than 50 years or as early as 4 years. The symptomatology usually consists of recurrent attacks of myalgias and muscle stiffness or weakness, often associated with myoglobinuria. The attacks usually are acute, and the consequences may be prolonged up to several weeks. The patients usually are asymptomatic between attacks. The frequency of these attacks is highly variable. Occasionally, the rhabdomyolysis may be complicated by two types of life-threatening events: more commonly, acute renal failure secondary to myoglobinuria and, much less frequently, respiratory insufficiency secondary to respiratory muscle involvement. Symptoms usually are prompted by prolonged exercise and less commonly by prolonged fasting, high fat intake, exposure to cold, mild infection (especially in children), fever, emotional stress, general anesthesia, or drugs such as diazepam and ibuprofen. In all cases, the clinical symptomatology is restricted to skeletal muscle, without liver or heart involvement.
Defects of most β-oxidation enzymes typically manifest in infancy with severe multisystem disease; however, the clinical presentation of VLCAD deficiency may be indistinguishable from CPT II deficiency [27].
Multiple acyl-CoA dehydrogenation deficiency (MADD), also known as glutaric aciduria type II, is an autosomal recessive disorder caused by mutations in genes encoding either one of the two subunits of the electron transfer flavoprotein (ETFA and ETFB) or by mutations in the gene encoding the electron transfer flavoprotein dehydrogenase (ETFDH) and resulting in abnormal fatty acid, amino acid, and choline metabolism. Less severely affected patients might present with progressive muscle weakness and lipid storage myopathies with secondary muscle coenzyme Q10 (CoQ10) deficiency, mainly in adulthood, and sometimes respond to riboflavin treatment (riboflavin-responsive MADD) [28] or CoQ10 supplementation [29].
The myopathic form of CoQ10 deficiency is characterized by proximal myopathy with premature fatigue, weakness, and increased serum levels of creatine kinase and lactate. Muscle biopsies show excessive numbers of lipid droplets, predominantly in type 1 fibers; combined deficiency of respiratory chain complexes I and III; and CoQ10 levels below 50% of normal. Mutations in ETFDH leading to a decrement in complex electron transfer flavoprotein–ubiquinone oxidoreductase activity have been identified in adult-onset MADD and some cases of myopathy with CoQ10 deficiency, indicating they may be allelic diseases [30•].
Mitochondrial Diseases
The mitochondrion is a complex organelle that performs numerous essential functions not only for cellular metabolism, including lipid metabolism, the Krebs cycle, amino acid metabolism, and energy production, but also for mediation of the apoptotic cascade [1]. The respiratory chain is composed of four multi-subunit enzymatic complexes (I, II, III, and IV), which generate a proton gradient across the inner mitochondrial membrane that, in turn, drives ATP synthesis by complex V. In addition, CoQ10 and cytochrome c are critical components of the mitochondrial respiratory chain, serving as “electron shuttles” between the complexes [31, 32].
Mitochondria are unique human organelles by virtue of being the products of two genomes, nuclear DNA and mitochondrial DNA (mtDNA). mtDNA is a 16,569-bp, double-stranded, circular molecule containing 37 genes, 24 of which participate in mitochondrial protein synthesis (two ribosomal RNAs and 22 transfer RNAs [tRNAs]); the other 13 genes encode subunits of respiratory chain enzyme complexes [33•]. In addition, 1000 to 2000 nuclear DNA genes are required for mitochondrial functions [34–36]. The mitochondrial genome is maternally inherited, so mothers pass on their mtDNA (and mtDNA mutations) to all their children, but only the daughters will pass it on to the next generation, whereas mutations in nuclear genes segregate according to mendelian rules. Thus, mitochondrial diseases may be inherited maternally or via autosomal dominant, autosomal recessive, or X-linked patterns. As a result of this genetic complexity, pathogenetic mechanisms of mitochondrial diseases are heterogeneous and include primary mtDNA mutations, autosomal recessive disorders of respiratory chain components, intergenomic communication due to nuclear gene defects causing secondary instability of mtDNA (mainly depletion and multiple deletions of mtDNA), mitochondrial lipid membranes (Barth syndrome), and mitochondrial protein importation. Disorders of mitochondrial dynamics (fusion, fission, and movement) comprise an expanding group of diseases.
One of the most common symptoms affecting patients with mitochondrial diseases is exercise intolerance due to premature fatigue with activities as mild as walking up a single flight of stairs. After a short rest, patients usually can resume their activity, but symptoms recur. Patients with mitochondrial disease often report subjective heaviness or burning of muscles with exertion but, in contrast to patients with glycogenoses, typically do not manifest stiffness, cramps, or second wind phenomenon. The exercise intolerance usually is disproportionately severe relative to muscle weakness. This symptom may be isolated or associated with muscle weakness and multisystemic involvement. Exercise testing is particularly helpful as an evaluation and screening tool in mitochondrial myopathies. Elevated lactate levels at rest and exaggerated lactate response after even trivial exercise are useful clues to the diagnosis of mitochondrial disease. One of the hallmarks of mitochondrial myopathies is a reduction in maximal whole-body oxygen consumption (Vo2max) demonstrated by a characteristic deficit in peripheral oxygen extraction (arteriovenous O2 difference) and an enhanced oxygen delivery (hyperkinetic circulation) [10]. Alternatively, in specialized referral centers, 31P nuclear magnetic resonance spectroscopy may reveal decreased basal levels of high-energy phosphate compounds (eg, ATP and phosphocreatine) at rest or prolonged recovery of ATP and phosphocreatine after exercise in patients with mitochondrial myopathies.
Several multisystemic mitochondrial syndromes can be recognized by their characteristic combinations of clinical features. For example, the typically sporadic disorder Kearns-Sayre syndrome (KSS) is defined by the obligate triad of onset before age 20, extraocular muscle weakness (ptosis and ophthalmoparesis), and pigmentary retinopathy, plus one of the following: ataxia, cerebrospinal fluid protein level greater than 100 mg/dL, or cardiac conduction block. The vast majority (>90%) of KSS patients harbor a pathogenic single large-scale mtDNA deletion that often is undetectable in blood. In addition to the deletion, there may be a related single duplication mutation (ie, mitochondrial genomes composed of full-length plus the nondeleted segment of mtDNA) that may be detectable in blood buffy coat or urine sediment. Nevertheless, muscle biopsy is the gold standard for diagnosing KSS because histology shows abnormal mitochondrial proliferation as ragged red fibers (RRFs) [37] on modified Gomori trichrome stain, as well as cytochrome c oxidase-deficient fibers, while Southern blot analysis of muscle DNA detects the single mtDNA deletion [38]. Succinate dehydrogenase histochemical staining is even more sensitive than Gomori trichrome for detecting excessive mitochondrial proliferation, also called “ragged blue fibers.”
Mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS) syndrome is characterized clinically by strokes that are atypical in that they usually occur in people less than 40 years old and generally do not conform to large vessel distribution [39]. Although MELAS is a maternally inherited condition, the proband often is the only family member with the full MELAS syndrome, whereas oligosymptomatic matrilineal relatives may manifest milder signs (eg, diabetes, deafness, migraine-like headaches) and some mutation carriers are asymptomatic. The diagnosis of MELAS can be confirmed by detection of the pathogenic mtDNA point mutation in blood. About 80% of MELAS patients harbor the m.3243A>G mutation in the tRNALeu(UUR) gene [40, 41], but at least 20 additional mtDNA mutations have been reported to cause this disorder. For unclear reasons, the MELAS mutation often is present at higher levels of heteroplasmy in urine sediment than in blood; therefore, it may be preferable to test urine rather than blood samples [42–44].
Another maternally inherited disorder, myoclonus epilepsy with ragged red fibers (MERRF), manifests with the clinical triad of myoclonus, epilepsy, and ataxia, plus numerous RRFs in the muscle biopsy. Although matrilineal relatives may be oligosymptomatic or asymptomatic, often multiple family members manifest the MERRF syndrome. About 80% of MERRF patients have the m.8344A>G tRNALys gene mutation [45], and other patients harbor rarer mtDNA mutations that can be detected in blood [46].
An unusual group of mitochondrial disease patients present with sporadic isolated myopathies, with exercise intolerance variably accompanied by proximal muscle weakness and myoglobinuria. It is important for clinicians to be aware of this condition, which in several cases has been misdiagnosed as chronic fatigue syndrome or fibromyalgia. Resting venous lactic acid is elevated in most patients with this condition; therefore, blood lactate is a useful noninvasive screening test for this syndrome. In most cases, muscle biopsies reveal RRFs that are cytochrome c oxidase positive because the gene defect often is a mutation in the cytochrome b gene, which encodes a subunit of complex III [47], or in an ND gene, encoding a subunit of complex I. Other mutations in mtDNA have been associated with exercise intolerance, including tRNA mutations [48, 49] and mutations in protein-coding genes for subunits of respiratory chain complexes I [50, 51] and IV [52]. Because the mutation is not detectable in blood in most of these patients, muscle biopsy generally is required to make the diagnosis.
Conclusions
Metabolic myopathies should be considered in the differential diagnosis of exercise intolerance, and a detailed clinical approach will help determine which of the three main disorders (glycogenoses, lipid-related disorders, or mitochondrial diseases) is the underlying cause. It is important to determine whether the patient has fixed or exercise-induced manifestations and, in the latter case, if they are related to short and high-intensity exercise or to prolonged, mild exercise. Second wind phenomenon and other related events, such as fasting, fever episodes, and pre-exercise carbohydrate intake, may provide additional clues to restrict the differential diagnosis.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Darras BT, Friedman NR. Metabolic myopathies: a clinical approach: part I. Pediatr Neurol. 2000;22:87–97. doi: 10.1016/s0887-8994(99)00133-2. [DOI] [PubMed] [Google Scholar]
- 2•.van Adel BA, Tarnopolsky MA. Metabolic myopathies: update 2009. J Clin Neuromuscul Dis. 2009;10:97–121. doi: 10.1097/CND.0b013e3181903126. This article reviews the key clinical features, diagnostic tests, and treatment recommendations for the more common metabolic myopathies. [DOI] [PubMed] [Google Scholar]
- 3.DiMauro S, Lamperti C. Muscle glycogenoses. Muscle Nerve. 2001;24:984–999. doi: 10.1002/mus.1103. [DOI] [PubMed] [Google Scholar]
- 4.Tein I. Neonatal metabolic myopathies. Semin Perinatol. 1999;23:125–151. doi: 10.1016/s0146-0005(99)80046-9. [DOI] [PubMed] [Google Scholar]
- 5.Eymard B, Laforêt P. Metabolic myopathies in adulthood. Features and clues for diagnosis [in French] Rev Med Interne. 2001;22:328–337. [PubMed] [Google Scholar]
- 6.Spiegel R, Gomez EA, Akman HO, et al. Myopathic form of phosphoglycerate kinase (PGK) deficiency: a new case and pathogenic considerations. Neuromuscul Disord. 2009;19:207–211. doi: 10.1016/j.nmd.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Stojkovic T, Vissing J, Petit F, et al. Muscle glycogenosis due to phosphoglucomutase 1 deficiency. N Engl J Med. 2009;23:425–427. doi: 10.1056/NEJMc0901158. [DOI] [PubMed] [Google Scholar]
- 8.Haller RG. Treatment of McArdle disease. Arch Neurol. 2000;57:923–924. doi: 10.1001/archneur.57.7.923. [DOI] [PubMed] [Google Scholar]
- 9.Haller RG, Vissing J. Spontaneous ‘second wind’ and glucose-induced second ‘second wind’ in McArdle disease: oxidative mechanisms. Arch Neurol. 2002;59:1395–1402. doi: 10.1001/archneur.59.9.1395. [DOI] [PubMed] [Google Scholar]
- 10.Taivassalo T, Jensen TD, Kennaway N, et al. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain. 2003;126:413–423. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- 11.Ørngreen MC, Schelhaas HJ, Jeppesen TD, et al. Is muscle glycogenolysis impaired in X-linked phosphorylase b kinase deficiency? Neurology. 2008;70:1876–1882. doi: 10.1212/01.wnl.0000289190.66955.67. [DOI] [PubMed] [Google Scholar]
- 12.Deschauer M, Wieser T, Zierz S. Muscle carnitine palmitoyltransferase II deficiency: clinical and molecular genetic features and diagnostic aspects. Arch Neurol. 2005;62:37–41. doi: 10.1001/archneur.62.1.37. [DOI] [PubMed] [Google Scholar]
- 13.Ørngreen MC, Dunø M, Ejstrup R, et al. Fuel utilization in subjects with carnitine palmitoyl-transferase 2 gene mutations. Ann Neurol. 2005;57:60–66. doi: 10.1002/ana.20320. [DOI] [PubMed] [Google Scholar]
- 14.Haller RG, Vissing J. No spontaneous second wind in muscle phosphofructokinase deficiency. Neurology. 2004;62:82–86. doi: 10.1212/wnl.62.1.82. [DOI] [PubMed] [Google Scholar]
- 15.Vissing J, Haller RG. The effect of oral sucrose on exercise tolerance in patients with McArdle’s disease. N Engl J Med. 2003;349:2503–2509. doi: 10.1056/NEJMoa031836. [DOI] [PubMed] [Google Scholar]
- 16.Vissing J, Quistorff B, Haller RG. Effect of fuels on exercise capacity in muscle phosphoglycerate mutase deficiency. Arch Neurol. 2005;62:1440–1443. doi: 10.1001/archneur.62.9.1440. [DOI] [PubMed] [Google Scholar]
- 17.Toscano A, Musumeci O. Tarui disease and distal glycogenoses: clinical and genetic update. Acta Myol. 2007;26:105–107. [PMC free article] [PubMed] [Google Scholar]
- 18.Beutler E. PGK deficiency. Br J Haematol. 2007;136:3–11. doi: 10.1111/j.1365-2141.2006.06351.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsujino S, Nonaka I, DiMauro S. Glycogen storage myopathies. Neurol Clin. 2000;18:125–150. doi: 10.1016/s0733-8619(05)70181-x. [DOI] [PubMed] [Google Scholar]
- 20.Regelsberger G, Höftberger R, Pickl WF, et al. Danon disease: case report and detection of new mutation. J Inherit Metab Dis. 2009 Jul 7; doi: 10.1007/s10545-009-1097-9. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Klein CJ, Boes CJ, Chapin JE, et al. Adult polyglucosan body disease: case description of an expanding genetic and clinical syndrome. Muscle Nerve. 2004;29:323–328. doi: 10.1002/mus.10520. [DOI] [PubMed] [Google Scholar]
- 22.Noel N, Flanagan JM, Ramirez Bajo MJ, et al. Two new phosphoglycerate kinase mutations associated with chronic haemolytic anaemia and neurological dysfunction in two patients from Spain. J Haematol. 2006;132:523–529. doi: 10.1111/j.1365-2141.2005.05882.x. [DOI] [PubMed] [Google Scholar]
- 23.Shen J, Bao Y, Liu HM, et al. Mutations in exon 3 of the glycogen debranching enzyme gene are associated with glycogen storage disease type III that is differentially expressed in liver and muscle. J Clin Invest. 1996;98:352–357. doi: 10.1172/JCI118799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet. 2006;142:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Bruno C, DiMauro S. Lipid storage myopathies. Curr Opin Neurol. 2008;21:601–606. doi: 10.1097/WCO.0b013e32830dd5a6. This article provides an excellent update on lipid storage myopathies. [DOI] [PubMed] [Google Scholar]
- 26.DiMauro S, DiMauro PM. Muscle carnitine palmityltransferase deficiency and myoglobinuria. Science. 1973;182:929–931. doi: 10.1126/science.182.4115.929. [DOI] [PubMed] [Google Scholar]
- 27.Hamano H, Shinohara Y, Takizawa S, et al. Very-long-chain acyl-CoA dehydrogenase deficiency presenting with recurrent rhabdomyolysis in an adult. Rinsho Shinkeigaku. 2003;43:253–257. [PubMed] [Google Scholar]
- 28.Curcoy A, Olsen RK, Ribes A, et al. Late-onset form of beta-electron transfer flavoprotein deficiency. Mol Genet Metab. 2003;78:247–249. doi: 10.1016/s1096-7192(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 29.Horvath R, Schneiderat P, Schoser BG, et al. Coenzyme Q10 deficiency and isolated myopathy. Neurology. 2006;66:253–255. doi: 10.1212/01.wnl.0000194241.35115.7c. [DOI] [PubMed] [Google Scholar]
- 30•.Gempel K, Topaloglu H, Talim B, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130:2037–2044. doi: 10.1093/brain/awm054. This is the first documentation of ETF-dehydrogenase mutation riboflavin-responsive MADD, indicating that this disorder and the myopathic form of CoQ10 deficiency may be allelic diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elston T, Wang H, Oster G. Energy transduction in ATP synthase. Nature. 1998;391:510–513. doi: 10.1038/35185. [DOI] [PubMed] [Google Scholar]
- 32.Noji H, Yoshida M. The rotary machine of the cell, ATP synthase. J Biol Chem. 2001;276:1665–1668. doi: 10.1074/jbc.R000021200. [DOI] [PubMed] [Google Scholar]
- 33•.DiMauro S, Schon E. Mitochondrial disorders in the nervous system. Ann Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. This is an excellent, comprehensive review of mitochondrial diseases. [DOI] [PubMed] [Google Scholar]
- 34.Suomalainen A, Kaukonen J. Diseases caused by nuclear genes affecting mtDNA stability. Am J Med Genet. 2001;106:53–61. doi: 10.1002/ajmg.1379. [DOI] [PubMed] [Google Scholar]
- 35.Spinazzola A, Zeviani M. Disorders of nuclear-mitochondrial intergenomic signaling. Biosci Rep. 2007;27:39–51. doi: 10.1007/s10540-007-9036-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Peng X, Guan MX, et al. Pathogenic mutations of nuclear genes associated with mitochondrial disorders. Acta Biochim Biophys Sin (Shanghai) 2009;41:179–187. doi: 10.1093/abbs/gmn021. [DOI] [PubMed] [Google Scholar]
- 37.Rowland LP, Blake DM, Hirano M, et al. Clinical syndromes associated with ragged red fibers. Rev Neurol. 1991;147:467–473. [PubMed] [Google Scholar]
- 38.Zeviani M, Moraes CT, DiMauro S, et al. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1998;51:1525–1533. doi: 10.1212/wnl.51.6.1525-a. [DOI] [PubMed] [Google Scholar]
- 39.Sproule DM, Kaufmann P. Mitochondrial encephalopathy, lactic acidosis, and strokelike episodes: basic concepts, clinical phenotype, and therapeutic management of MELAS syndrome. Ann N Y Acad Sci. 2008;1142:133–158. doi: 10.1196/annals.1444.011. [DOI] [PubMed] [Google Scholar]
- 40.Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 41.Mehrazin M, Shanske S, Kaufmann P, et al. Longitudinal changes of mtDNA A3243G mutation load and level of functioning in MELAS. Am J Med Genet A. 2009;149:584–587. doi: 10.1002/ajmg.a.32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonnell MT, Schaefer AM, Blakely EL. Noninvasive diagnosis of the 3243A>G mitochondrial DNA mutation using urinary epithelial cells. Eur J Hum Genet. 2004;12:778–781. doi: 10.1038/sj.ejhg.5201216. [DOI] [PubMed] [Google Scholar]
- 43.Shanske S. Varying loads of the mitochondrial DNA A3243G mutation in different tissues: implications for diagnosis. Am J Med Genet. 2004;130:134–137. doi: 10.1002/ajmg.a.30220. [DOI] [PubMed] [Google Scholar]
- 44.Marotta R, Reardon K, McKelvie PA, et al. Association of the MELAS m.3243A>G mutation with myositis and the superiority of urine over muscle, blood and hair for mutation detection. J Clin Neurosci. 2009;16:1223–1225. doi: 10.1016/j.jocn.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Wiedemann FR, Bartels C, Kirches E, et al. Unusual presentations of patients with the mitochondrial MERRF mutation A8344G. Clin Neurol Neurosurg. 2008;110:859–863. doi: 10.1016/j.clineuro.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Melone MA, Tessa A, Petrini S, et al. Revelation of a new mitochondrial DNA mutation (G12147A) in a MELAS/MERFF phenotype. Arch Neurol. 2004;61:269–272. doi: 10.1001/archneur.61.2.269. [DOI] [PubMed] [Google Scholar]
- 47.Andreu AL, Hanna MG, Reichmann H, et al. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N Engl J Med. 1999;341:1037–1044. doi: 10.1056/NEJM199909303411404. [DOI] [PubMed] [Google Scholar]
- 48.Bortot B, Barbi E, Biffi S, et al. Two novel cosegregating mutations in tRNAMet and COX III, in a patient with exercise intolerance and autoimmune polyendocrinopathy. Mitochondrion. 2009;9:123–129. doi: 10.1016/j.mito.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Pulkes T, Liolitsa D, Wills AJ, et al. Nonsense mutations in mitochondrial DNA associated with myalgia and exercise intolerance. Neurology. 2005;64:1091–1092. doi: 10.1212/01.WNL.0000154471.33156.55. [DOI] [PubMed] [Google Scholar]
- 50.Andreu AL, Tanji K, Bruno C, et al. Exercise intolerance due to a nonsense mutation in the mtDNA ND4 gene. Ann Neurol. 1999;45:820–823. doi: 10.1002/1531-8249(199906)45:6<820::aid-ana22>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 51.Downham E, Winterthun S, Nakkestad HL, et al. A novel mitochondrial ND5 (MTND5) gene mutation giving isolated exercise intolerance. Neuromusc Disord. 2008;18:310–314. doi: 10.1016/j.nmd.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 52.McFarland R, Taylor RW, Chinnery PF, et al. A novel sporadic mutation in cytochrome c oxidase subunit II as a cause of rhabdomyolysis. Neuromusc Disord. 2004;14:162–166. doi: 10.1016/j.nmd.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Hirano M. Metabolic myopathies. In: Gilman S, editor. Neurobiology of Disease. San Diego, CA: Elsevier Academic Press; 2007. pp. 947–956. [Google Scholar]