Abstract
This study examined associations between the tendency to ruminate and two polymorphisms: the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) gene and 5-HTTLPR polymorphism in the serotonin transporter gene (SLC6A4). Participants were a homogenous group of healthy, unmedicated, never depressed individuals with few current symptoms of depression (N = 71). Results indicated that met heterozygotes of the BDNF allele were significantly more likely to ruminate than individuals homozygous for the val BDNF allele. There was no association between rumination and the 5-HTTLPR polymorphism. Further, the interaction between the 5-HTTLPR and BDNF polymorphisms did not predict rumination. Results suggest that variation in the BDNF gene may contribute to the tendency to ruminate. Since this association exists in healthy adults, it may represent a susceptibility factor for affective disorders.
Keywords: genetic association, rumination, depression, BDNF, 5-HTTLPR
The tendency to ruminate—a repetitive focus on one’s negative thoughts and feelings—has been posited to play an important role in the maintenance and etiology of depression (Nolen-Hoeksema, 2000). Indeed, several studies have now documented that rumination is associated with a more severe episode of depression, precedes the onset of depressive episodes, and contributes to more persistent depressive episodes (e.g., Beevers, Rohde, Stice, & Nolen-Hoeksema, 2007; Nolen-Hoeksema, 2000; Spasojevic & Alloy, 2001). Despite its well-documented role in depression, the etiology of rumination has received relatively little attention. The goal of this article is to examine the genetic etiology of rumination.
A single nucleotide polymorphism in the brain-derived neurotropic factor (BDNF) gene has been implicated in depression vulnerability (Nestler et al., 2002). BDNF is a protein involved in the growth and differentiation of new and existing neurons and synapses. Lower levels of BDNF have been associated with depressive states in animals and central administration of BDNF can reverse these states (Shirayama, Chen, Nakagawa, Russell, & Duman, 2002).
An amino acid substitution (valine to methionine) at codon 66 (Val66Met) of the BDNF gene results in two alleles: val and met. Slightly more than one third of Caucasians inherit at least one copy of the met allele, producing allele frequencies of .67 for val/val, .28 for val/met, and .05 for met/met (Egan et al., 2003). Importantly, the met allele is associated with decreased synaptic activity and plasticity in the hippocampus, abnormal hippocampal activation, lower BDNF secretion, and poorer episodic memory (Egan et al., 2003). Met allele carriers have been shown to have smaller hippocampal volumes (Frodl et al., 2007), reduced grey-matter volume in prefrontal cortex (Nemoto et al., 2006), and poorer executive functioning (Rybakowski et al., 2006) than val allele homozygotes. The BDNF genotype may therefore confer vulnerability to depression via its effect on cognitive functioning.
Consistent with this possibility, recent research has found an association between the BDNF gene Val66Met polymorphism and rumination (Hilt, Sander, Nolen-Hoeksema, & Simen, 2007). Among mothers who had experienced adult-onset depression, the val/met BDNF genotype was associated with greater rumination and depression than the val/val genotype. Further, analyses suggested that rumination mediated the relationship between the BDNF polymorphism and depressive symptoms. Rumination may therefore be an important intermediate phenotype that contributes to the expression of depression.
The serotonin transporter (SLC6A4) is a second gene that has been posited to be associated with rumination (Canli et al., 2006). The serotonin transporter (5-HTT) gene regulates the reuptake of serotonin to the presynaptic neuron for recycling or degradation after serotonin has been released. Importantly, the efficiency with which the transporter returns serotonin to the presynaptic neuron appears to be influenced by a polymorphism in the serotonin transporter gene-linked polymorphic region (5-HTTLPR).
A common polymorphism in the promoter region of the 5-HTT gene results in 2 variants: a short allele and a long allele. The presence of one or two short alleles, rather than two copies of the long allele, is associated with reduced transcriptional efficiency that putatively results in significant decreases (approximately 50%) in serotonin reuptake (Lesch et al., 1996). Short 5-HTTLPR allele carriers thus have increased levels of extracellular serotonin compared to long allele homozygotes. Further, it has recently been determined that the long 5-HTTLPR allele has two variants (i.e., LA and LG). In the first of two extra 20 to 23 bp repeats in the L allele, a common single nucleotide polymorphism occurs at the sixth nucleotide (adenine to guanine; A to G) (Wendland, Martin, Kruse, Lesch, & Murphy, 2006). The LG variant and the S allele appear to be very similar in terms of transcriptional activity; therefore, only the LA variant is high expressing with regard to transcriptional activity (Hu et al., 2005).
A number of studies have reported that the 5-HTTLPR is associated with cognitive vulnerability to depression. For instance, carriers of the low expressing 5-HTTLPR allele report a more negative attributional style (Sheikh et al., 2008), endorse more negative thoughts following a mood induction (Beevers, Scott, McGeary, & McGeary, in press), display biased attention for emotional stimuli (Beevers, Gibb, McGeary, & Miller, 2007), and recall more negative self-referent words (Hayden et al., 2008) than long allele homozygotes. More relevant to the current study, Canli et al. (2006) found that low expressing 5-HTTLPR allele carriers who had recently experienced life stress reported higher levels of rumination than long allele homozygotes. No other studies have examined the association between the 5-HTTLPR and rumination.
The studies by Hilt and colleagues (2007) and Canli et al. (2006) provide important initial evidence regarding genetic associations with rumination. However, additional study of these associations is warranted for a number of reasons. First, initial behavioral genetics findings are often not replicated in subsequent studies (Ioannidis, Ntzani, Trikalinos, & Contopoulos-Ioannidis, 2001), so replication is critical to determine whether the initial findings are reliable. Second, no studies have examined the association between the BDNF Val66Met polymorphism and rumination in non-depressed samples. Depressive symptoms and rumination are typically strongly correlated; therefore, in samples that include depressed individuals, it is difficult to determine whether the candidate gene is associated with rumination per se or if depression severity accounts for the association between the candidate gene and rumination. This can be disentangled to a degree by examining the relationship between genetic variants and rumination among individuals with low levels of depression. Finally, no study has examined associations between rumination and more than one gene within the same sample. Studying multiple candidate genes within the same sample facilitates comparisons across genetic groups and also allows for the study of gene-by-gene interactions.
The current study builds upon this relatively small body of research and examines whether polymorphisms of the BDNF and 5-HTTLPR, both alone and in combination, are associated with self-reported rumination in a healthy, unmedicated, never depressed sample of college aged adults. Based on previous work, we expected met heterozygotes of the BDNF allele to report more rumination than val allele homozygotes. The 5-HTTLPR has been associated with rumination in the context of life stress in one small study (N = 21); however, the direct effect of the polymorphism on rumination was relatively small (Canli et al., 2006). Nevertheless, given the associations between 5-HTTLPR and other forms of cognitive vulnerability, we thought it was plausible that the 5-HTTLPR might also be associated with rumination.
Method
Participants
Participants were seventy-one undergraduate students recruited from introductory classes from a large, southwestern university (see Table 1 for demographic information). Participants received credit towards satisfying requirements of their introductory psychology class. The average laboratory session was approximately one hour. Participants completed self-report inventories that measured rumination, current depressive symptoms, the presence of past episodes of depression, current medication use, demographic information, and other assessments not included in this report. They also provided buccal cells used to extract DNA for genotyping. Inclusion criteria included minimal current depressive symptoms (BDI-II < 9) at the time of the testing, no history of major depressive disorder, and not currently taking any psychiatric medication.
Table 1.
Demographic data presented as a function of Val66Met and 5-HTTLPR allele status.
| BDNF | 5-HTTLPR | ||||
|---|---|---|---|---|---|
| Val/Val (n = 49) | Val/Met (n = 22) | S′S (n = 15) | S′L′ (n = 37) | L′L′ (n = 19) | |
| Age (years) | 19.4 (1.65) | 18.8 (1.05) | 19.5 (2.07) | 18.9 (1.05) | 18.7 (0.96) |
| % Gender (F/M) | 65/35 | 46/54 | 42/58 | 62/38 | 67/33 |
| % Hispanic (Yes/No) | 18/82 | 12/88 | 5/95 | 14/86 | 28/71 |
| % Caucasian/Other | 71/29 | 84/16 | 80/20 | 84/16 | 72/28 |
| Depressive symptoms | 3.09 (2.91) | 4.00 (3.37) | 3.68 (2.31) | 2.57 (2.54) | 2.87 (2.77) |
Note: within each genetic group, there were no statistically significant allele group differences on any of these variables.
Assessments
Beck Depression Inventory – II (BDI-II; Beck, Brown, & Steer, 1996)
The BDI-II is a widely used self-report questionnaire to assess depression severity. The BDI-II consists of 21 items and measures the presence and severity of cognitive, motivational, affective, and somatic symptoms of depression. Past reports indicate test-retest reliability is adequate and the BDI-II has been found to be valid among college student samples (Beck et al., 1996). Internal consistency reliability in the current study was good (α = .81). Participants were required to have BDI-II scores of less than 9 at the time of testing to be included in this study.
The Inventory for Diagnosing Depression-Lifetime Version (IDD-L; Zimmerman & Coryell, 1987)
The IDD-L is a 24-item self-report questionnaire used to diagnose lifetime presence of major depressive disorder. It has been shown to have similar sensitivity and specificity as the Diagnostic Interview Schedule, and good construct validity and test-retest reliability (Zimmerman & Coryell, 1987). People who endorsed the presence of five of nine symptoms for a two week period or greater were classified as having a history of depression. Participants were required not to have a past history of depression to be included in this study.
Ruminative Responses Scale (RRS; Treynor, Gonzalez, & Nolen Hoeksema, 2003)
The RRS is a 10-item self-report scale that measures rumination, typically defined as a repetitive and passive focus on one’s negative emotional state. Each question on the RRS has a four-item response option ranging from 1 (almost never) to 4 (almost always). The RRS provides an assessment of rumination that is not confounded with depressive symptoms. This scale has shown good internal reliability and predictive validity (Treynor et al., 2003). The RRS also contains two related subscales, reflection and brooding. These subscales were moderately correlated in the present study (r = .50). Total score and subscale scores were used in this study.
Genotyping
Genomic DNA were isolated from buccal cells using a modification of published methods (e.g., Freeman et al., 1997). The cheeks and gums are rubbed for 20 s with three sterile, cotton-tipped wooden swabs. The swabs are placed in a 50-ml capped polypropylene tube containing lysis buffer (500 μl of 1 M Tris-HCl; 200 mM disodium ethylene diaminetetracetic acid (EDTA), pH 8.0; 500 μl of 10% sodium docecyl sulfate; and 100 μl of 5 M sodium chloride). The subjects then rinse out the mouth vigorously with 10 ml of distilled water for 20 sec and this was added to the 50-ml tube. The tubes were stored at 4°C until the DNA was extracted.
Brain derived neurotropic factor (BDNF)
The Val66Met polymorphism (rs6265) was genotyped using Taqman assay C___11592758_10 (Applied Biosystems) using an ABI 7300 Real time PCR system. The frequency of the BDNF genotypes (val/val, n = 49; val/met, n = 22; met/met, n = 4) did not differ from the Hardy-Weinberg equilibrium (χ2 = 0.08, p = 0.77). Because the rare met/met allele group only contained 4 participants, these individuals were not included in this study due to low statistical power.
Serotonin transporter promoter region polymorphism (5-HTTLPR)
The 5-HTTLPR gene, which maps to 17q11.1–17q12, contains a 44 bp insertion/deletion in the 5′ regulatory region of the gene (Heils et al., 1996). This polymorphism in the promoter appears to be associated with variations in transcriptional activity: the long variant (528 bp) has approximately three times the basal activity of the shorter promoter (484 bp) with the deletion (Lesch et al., 1996). The assay is a modification of the method of Lesch and colleagues (Lesch et al., 1996). The primer sequences are: forward, 5′-GGCGTTGCCGCTCTGAATGC-3′ (fluorescently labeled), and reverse, 5′-GAGGGACTGAGCTGGACAACCAC-3′. These primer sequences yield products of 484 or 528 bp. Allele sizes are scored by two investigators independently and inconsistencies were reviewed and rerun when necessary.
To distinguish between the S, LA, and LG fragments, the PCR fragment was digested with MspI according to the methods found in Wigg et al. (2006). The resulting polymorphic fragments were separated using an ABI 377 DNA sequencer (S: 297, 127, 62 bp, LA: 340, 127, and 62 bp, LG: 174, 166, 127, and 62 bp). Using this approach, allele frequencies were S: n = 63 (44.4%), LA: n = 75 (52.8%), LG: n = 4 (2.8%). Consistent with previous research, the low expressing S and LG alleles were designated S′ and the higher expressing LA allele was designated L′. We therefore formed three groups: (a) S′S′ (i.e., SS: n = 15 (21.1%), SLG: n = 0 (0.0%), LGLG: n = 0 (0%)), (b) S′L′ (i.e., SLA: n = 33 (46.5%), LGLA: n = 4 (5.6%)), and (c) L′L′ (i.e., LALA: n = 19 (26.8%)). Distributions for the triallelic genotypes were in Hardy-Weinberg equilibrium using the exact test for multiple alleles (p = 0.25).
Procedure
Participants were initially recruited based on their scores on the short form of the Beck Depression Inventory completing during mass pre-testing sessions at the beginning of the academic semester. Individuals with low scores (< 4) were contacted and invited to participate in the current study. Upon arrival to the laboratory, participants were oriented to the laboratory, provided informed consent, completed the self-report questionnaires (including a second assessment of depression severity), and provided buccal cells via a cheek swab for genotyping. Upon completion of study procedures, participants were debriefed and were assigned course credit for their participation. The internal review board approved all study procedures.
Results
Sample Characteristics
Descriptive statistics for the sample are presented in Table 1 stratified by BDNF and 5-HTTLPR allele groups. There were no significant differences for any of the participant variables as a function of allele grouping for the BDNF and 5-HTTLPR (see Table 1).
BDNF
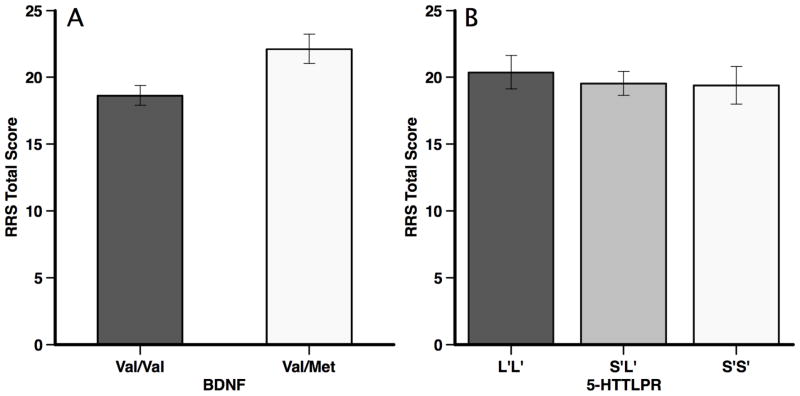
A one-way analysis of variance (ANOVA) with BDNF group (allele: val/val, val/met) as a between subjects factor with total score on the RRS was conducted. Results indicated a significant main effect for BDNF allele group, F(1, 69) = 6.93, p < .05, η2 = .09; the val/met group reported significantly more rumination than the val/val group (d = 0.66; see Figure 1). Identical analyses were conducted for the reflection and brooding subscales of the RRS. The val/met group reported significantly more reflective rumination, F(1, 69) = 7.00, p < .05, η2 = .09, than the val/met group (M = 6.50, SD = 3.58 and M = 4.28, SD = 3.11, respectively; d = .66). The val/met group also tended to endorse more brooding rumination, F(1, 69) = 3.22, p < .10, η2 = .05, than the val/val group (M = 4.37, SD = 2.73 and M = 5.63, SD = 2.80, respectively; d = .45), although this effect fell short of statistical significance.
Figure 1.
Mean and standard errors for total rumination scores, measured with the Ruminative Responses Scale, presented as a function of (a) BDNF and (b) 5-HTTLPR allele groups.
5-HTTLPR
A one-way analysis of variance (ANOVA) with 5-HTTLPR group (allele: S′S, S′L′, L′L′) as a between subjects factor with total score on the RRS was conducted. Results indicated a non-significant main effect for 5-HTTLPR allele group, F(2, 68) = 0.18, ns, η2 = .00; there were no group differences among 5-HTTLPR allele groups (see Figure 1). Not surprisingly, there were no 5-HTTLPR allele effects for either the reflection, F(2, 68) = 0.43, ns, η2 = .01, or brooding, F(2, 68) = 0.11, ns, η2 = .00, subscales of the RRS.
BDNF-by-5-HTTLLPR interaction
The final analysis served two purposes: (a) to examine whether the BDNF Val66Met polymorphism continued to predict rumination after the 5-HTTLPR polymorphism was entered simultaneously into an ANOVA, and (b) whether the 5-HTTLPR moderated the effect of the Val66Met BDNF polymorphism on total score rumination. Consistent with the previous analyses, there was a main effect for the BDNF polymorphism, F(1, 65) = 6.93, p < .05, η2 = .10, but not the 5-HTTLPR polymorphism, F(2, 65) = 0.32, ns, η2 = .01. Further, the interaction between the 5-HTTLPR and the BDNF did not approach statistical significance, F(2, 65) = 0.46, ns, η2 = .01.
Discussion
The overall aim of the current study was to examine whether individual differences in the BDNF and 5-HTTLPR genes were associated with the tendency to ruminate in a healthy, non-medicated, non-depressed sample. Results suggest that val/met BDNF heterozygotes are significantly more likely to ruminate than individuals homozygous for the val allele. In contrast, there was no association between the 5-HTTLPR and rumination. Indeed, it was notable that the rumination scores were nearly identical across the 5-HTTLPR allele groups.
These findings provide additional support that individual differences in the BDNF gene contribute to the tendency to ruminate. This association has now been observed in adult women who varied in current and past depression (Hilt et al., 2007) and healthy adults with no current or history of depression. The fact that similar results were observed in studies that used different methodologies and samples suggests that this finding may be reliable. Nevertheless, additional replication in studies with large samples is still needed.
Additional work is also needed to determine why the met allele of the BDNF gene is associated with rumination. BDNF plays a critical role in cortical functioning by influencing the development and maintenance of cortical neurons and cortical synapses (Weickert et al., 2003). Genetically mediated alterations in the regulation of BDNF could therefore contribute to poorer executive functioning. Interestingly, depression is often characterized by poor prefrontal control, such as difficulty inhibiting negative thoughts or stimuli. For instance, Joormann (2004) reported that current dysphoria and lifetime depression were both associated with poor inhibitory control when processing negative word stimuli.
Inhibitory processes are thought to be responsible for updating working memory and for preventing irrelevant information from entering working memory. It follows that poor inhibitory control could lead to repeated processing of irrelevant information—a defining feature of rumination. Consistent with this possibility, Joormann (2006) has demonstrated that rumination is associated with poor inhibitory control, even after controlling for current depressive symptoms. More specifically, individuals high in rumination experienced more difficulty inhibiting irrelevant information than individuals low in rumination regardless of depressive symptoms. Similarly, Davis and Nolen-Hoeksema (2000) found that ruminators had a more inflexible cognitive style, as measured by the Wisconsin Card Sorting Task.
This raises the possibility that the BDNF Val66Met polymorphism contributes to rumination via its influence on inhibitory control. Met BDNF carriers may have poorer inhibitory control over irrelevant stimuli due to altered BDNF regulation in brain regions that are critical for inhibitory control, such as the prefrontal cortex. This poor inhibitory control, in turn, may contribute to the tendency to ruminate. Additional work is needed to test this possibility. Indeed, additional interdisciplinary work that comprehensively examines the etiology of rumination across levels of analysis (e.g., neural, cognitive, genetic) within the same sample may be critical for developing a better understanding of the origins of rumination.
In contrast to the BDNF allele, there was virtually no association between the 5-HTTLPR polymorphism and rumination. Indeed, rumination was nearly identical across the 5-HTTLPR allele groups (see Figure 1). This is in contrast to other research, which has found that the 5-HTTLPR is associated with other forms of cognitive vulnerability to depression (for a review, see Beevers & Wells, in press). However, it should be noted that one study has found that the 5-HTTLPR combines with recent life stressors to predict higher levels of rumination (Canli et al., 2006). This suggests that the 5-HTTLPR gene may influence the tendency to ruminate following recent adversity. Thus, whereas the BDNF polymorphism may directly influence rumination, the 5-HTTLPR polymorphism may contribute to rumination following the occurrence of life stress.
Although previous work has found an interaction between BDNF and 5-HTTLPR for neural structures involved in emotion processing (Pezawas et al., 2008), we found no evidence of epistasis in the current study. Nevertheless, future work should continue exploring the contribution of gene-by-gene interactions to rumination. It is highly probable that multiple genes contribute to the expression of a complex phenotype such as rumination. Indeed, given that the BDNF gene putatively influences neural plasticity, it may be an excellent gene with which to study gene-by-gene interactions. Consistent with this possibility, Nagel et al. (2008), reported that BDNF met carriers performed significantly worse on executive functioning tasks if they were also Catechol-O-Methyltransferase (COMT) Val allele carriers. In other words, the BDNF gene moderated the effect of the COMT polymorphism on prefrontal cognitive performance. Given these genetic associations with executive function, this finding may have particular relevance for understanding rumination. It also suggests that additional research should examine associations between polymorphisms of the COMT gene and rumination.
Several limitations of this study should be noted. Small sample size is an important limitation, as effects observed in small samples are less likely to be replicated than effects initially observed in large samples. Further, small sample size may have also limited our ability to detect significant gene-by-gene interactions. Given the positive findings for the association between the BDNF polymorphism and rumination, larger scale studies involving participants with greater variability in depression and age seem warranted. Similar studies that also measure life stress are needed to further establish a link between 5-HTTLPR and rumination. As with any genetic association study, population stratification is a potential concern. This confound is unlikely as the vast majority of participants were Caucasian and ethnicity was unrelated to rumination or allele status. Third variable explanations, such as the possibility that the BDNF and 5-HTTLPR polymorphisms are in linkage disequilibrium with another functional genetic marker, should also be considered as alternative explanations for the observed effects.
Despite these limitations, we believe this study makes an important and interesting contribution to understanding rumination. Individuals who inherit the met variant of the BDNF gene may be more likely to ruminate. This proclivity to ruminate may contribute vulnerability to depression and therefore represent one psychological pathway that connects individual differences in the BDNF gene to the expression of depression. However, the expression of depression is obviously more complex than a single pathway. Additional work that tests complex etiological models of depression across levels of analyses (genetic, neural, cognitive, environmental) may allow for the development more comprehensive models, and further our understanding of this important and debilitating disorder.
Acknowledgments
Christopher G. Beevers and Tony T. Wells, Department of Psychology, University of Texas at Austin; John E. McGeary, Providence Veterans Affairs Medical Center and Center for Alcohol and Addiction Studies, Brown University. The authors thank the research assistants of the Mood Disorders Laboratory at the University of Texas for their help with data collection.
Preparation of this article was facilitated by a grant (R01MH076897) from the National Institute of Mental Health to Christopher Beevers and a shared equipment grant from the National Center for Research Resources (S10RR023457) to John McGeary.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/emo.
Contributor Information
Christopher G. Beevers, University of Texas at Austin
Tony T. Wells, University of Texas at Austin
John E. McGeary, Providence Veterans Affairs Medical Center and Center for Alcohol and Addiction Studies, Brown University
References
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin Transporter Genetic Variation and Biased Attention for Emotional Word Stimuli Among Psychiatric Inpatients. Journal of Abnormal Psychology. 2007;116(1):208. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Rohde P, Stice E, Nolen-Hoeksema S. Recovery from major depressive disorder among female adolescents: A prospective test of the scar hypothesis. Journal of Consulting and Clinical Psychology. 2007;75(6):888. doi: 10.1037/0022-006X.75.6.888. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Scott WD, McGeary C, McGeary JE. Negative cognitive response to a sad mood induction: Associations with polymorphisms of the serotonin transporter (5-HTTLPR) gene. Cognition & Emotion (in press) [Google Scholar]
- Beevers CG, Wells T. Genetic associations with cognitive vulnerablity to depression. In: Ingram RE, editor. The International Encyclopedia of Depression. New York: Springer; (in press) [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, et al. Neural correlates of epigenesis. PNAS. 2006;103(43):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RN, Nolen-Hoeksema S. Cognitive inflexibility among ruminators and nonruminators. Cognitive Therapy and Research. 2000;24(6):699–711. [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Graig I, Plomin R. DNA by mail: An inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavioral Genetics. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, et al. Association of the Brain-Derived Neurotrophic Factor Val66Met Polymorphism With Reduced Hippocampal Volumes in Major Depression. Arch Gen Psychiatry. 2007;64(4):410–416. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Dougherty LR, Maloney B, Olino TM, Sheikh H, Durbin CE, et al. Early-emerging cognitive vulnerability to depression and the serotonin transporter promoter region polymorphism. Journal of Affective Disorders. 2008;107(1–3):227–230. doi: 10.1016/j.jad.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of the human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Sander LC, Nolen-Hoeksema S, Simen AA. The BDNF Val66Met polymorphism predicts rumination and depression differently in young adolescent girls and their mothers. Neurosci Lett. 2007 doi: 10.1016/j.neulet.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Joormann J. Attentional bias in dysphoria: The role of inhibitory processes. Cognition & Emotion. 2004;18(1):125–147. [Google Scholar]
- Joormann J. Differential Effects of Rumination and Dysphoria on the Inhibition of Irrelevant Emotional Material: Evidence from a Negative Priming Task. Cognitive Therapy and Research. 2006;30(2):149. [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li S-C, von Oertzen T, Sander T, Villringer A, et al. Human aging magnifies genetic effects on executive functioning and working memory. Frontiers in Human Neuroscience. 2008;2 doi: 10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto K, Ohnishi T, Mori T, Moriguchi Y, Hashimoto R, Asada T, et al. The Val66Met polymorphism of the brain-derived neurotrophic factor gene affects age-related brain morphology. Neuroscience Letters. 2006;397(1–2):25–29. doi: 10.1016/j.neulet.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of Depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–511. [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, Kolachana BS, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Molecular Psychiatry. 2008;13(7):709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Skibinska M, Szczepankiewicz A, Kapelski P, Leszczynska-Rodziewicz A, et al. Prefrontal cognition in schizophrenia and bipolar illness in relation to Val66Met polymorphism of the brain-derived neurotrophic factor gene. Psychiatry and Clinical Neurosciences. 2006;60(1):70–76. doi: 10.1111/j.1440-1819.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Sheikh HI, Hayden EP, Singh SM, Dougherty LR, Olino TM, Durbin CE, et al. An examination of the association between the 5-HTT promoter region polymorphism and depressogenic attributional styles in childhood. Personality and Individual Differences. 2008;45(5):425–428. doi: 10.1016/j.paid.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen ACH, Nakagawa S, Russell DS, Duman RS. Brain-Derived Neurotrophic Factor Produces Antidepressant Effects in Behavioral Models of Depression. J Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojevic J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1(1):25–37. doi: 10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen Hoeksema S. Rumination Reconsidered: A Psychometric Analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8(6):592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11(3):224. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wigg KG, Takhar A, Ickowicz A, Tannock R, Kennedy JL, Pathare T, et al. Gene for the serotonin transporter and ADHD: No association with two functional polymorphisms. American journal of medical genetics Part B, Neuropsychiatric genetics. 2006;141(6):566–570. doi: 10.1002/ajmg.b.30247. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W. The inventory to diagnose depression, lifetime version. Acta Psychiatrica Scandinavica. 1987;75(5):495–499. doi: 10.1111/j.1600-0447.1987.tb02824.x. [DOI] [PubMed] [Google Scholar]