Abstract
Human immunodeficiency virus (HIV)–specific CD4+ T cell cytokine secretion is characteristically weak during HIV infection, in part because HIV-specific CD4+ T cells undergo massive apoptotic deletion. Glucocorticoid-induced tumor necrosis factor (TNF) receptor family–related (GITR) protein triggering enhances murine antigen-specific T cell cytokine secretion by protecting T cells from apoptosis. Therefore, we investigated the impact of GITR triggering on HIV-specific CD4+ T cell cytokine secretion and on apoptosis of HIV-specific CD4+ T cells. In HIV-infected subjects, CD4+ T cell surface expression of GITR was greater than that in uninfected control subjects, and phytohemagglutinin induction of additional GITR expression was impaired. However, antibody triggering of GITR significantly increased HIV-specific CD4+ T cell expression of TNF-α and interferon (IFN)–γ. The percentage increase in HIV-specific CD4+ T cell expression of TNF-α correlated directly with the absolute peripheral CD4+ T cell count. Furthermore, GITR triggering reduced the expression of intracellular activated caspase-3 in HIV-specific CD4+ T cells. Taken together, these data suggest that, despite abnormal GITR expression during HIV infection, GITR triggering enhances HIV-specific CD4+ T cell cytokine expression and protects HIV-specific CD4+ T cells from apoptosis.
Apoptosis of CD4+ T cells is central to the pathogenesis of HIV disease. HIV-specific CD4+ T cells are preferentially infected by HIV [1], and there is massive apoptosis of CD4+ T cells starting early during HIV infection [2, 3]. The progressive apoptotic deletion of CD4+ T cells contributes to weakened HIV-specific cellular immune responses and to the development of AIDS [4–9].
Preventing CD4+ T cell apoptosis has the potential to preserve HIV-specific cellular immune responses and even forestall the development of AIDS. Interventions that are known to reduce apoptosis of CD4+ T cells during HIV infection include antiretroviral therapy [4, 10, 11], inhibition of the caspase cascade [4], interleukin (IL)–15 [12], protein kinase inhibition [13], inhibition of a cysteine protease [14], and programmed death (PD)–1 ligation [15].
Glucocorticoid-induced tumor necrosis factor (TNF) receptor family–related (GITR) protein is a member of the TNF receptor family of molecules that is expressed on activated and anitgen-specific lymphocytes. Triggering GITR with its natural ligand, GITR ligand, or with agonistic antibodies enhances antigen-specific effector T cell responses, in part by making T cells resistant to apoptosis [16–23].
Although triggering other members of the TNF receptor family has been explored as a means of heightening immune responses to HIV [24–27], the role played by GITR triggering in enhancing cellular immune responses to HIV or in protecting HIV-specific effector T cells from apoptosis has not been explored. However, GITR triggering has been shown to reverse effector T cell impairment during murine retroviral infection [28] and to intensify murine responses a retroviral vaccine when administered in conjunction with soluble CD40 ligand [29].
Accordingly, we hypothesized that GITR triggering would enhance HIV-specific CD4+ T cell responses by protecting HIV-specific CD4+ T cells from apoptosis. To test this hypothesis, we characterized the impact of HIV infection on GITR expression on CD4+ T cells and examined the impact of GITR triggering with a monoclonal antibody on HIV-specific CD4+ T cell cytokine expression and on apoptosis of HIV-specific CD4+ T cells.
METHODS
Subjects and cell isolation
HIV-infected adults and uninfected control subjects gave informed consent to donate whole blood in a research protocol approved by the Dartmouth College Committee for the Protection of Human Subjects. Peripheral blood mononuclear cells (PBMCs) were isolated by ficoll density gradient centrifugation and were cultured in RPMI 1640 supplemented with penicillin, streptomycin, HEPES buffer, L-glutamine, and 10% fetal calf serum.
Antibodies and cell subsets
PBMCs were stained with fluorochrome-conjugated monoclonal antibodies against CD3 and CD4 or CD8 (BD Biosciences). T cells were defined as CD3+ cells within the lymphocyte cloud on a forward scatter–side scatter plot. All analyses were conducted on T cells expressing CD4 or CD8.
Inducible GITR expression on CD4+ T cells
PBMCs were incubated for 2 h at 37°C in 5% CO2 in either medium alone or medium plus phytohemagglutinin (PHA; Sigma), and the percentage of CD4+ T cells expressing surface GITR was characterized using a fluorochrome-conjugated monoclonal antibody (R&D Systems). Specificity of staining was confirmed using an isotype control.
Antigens
In assays of T cell cytokine responses and CD4+ T cell apoptosis, PBMCs were stimulated with the HIV protein p55 (National Institutes of Health [NIH] AIDS Research and Reference Reagent Program/BioMolecular Technologies). Responses to pooled peptides of cytomegalovirus, Epstein-Barr virus, and influenza virus (CEF; NIH AIDS Research and Reference Reagent Program) were assessed in parallel. Staphylococcal enterotoxin B (SEB; Sigma) was used as a positive control in assays of intracellular cytokine expression.
GITR triggering
Before intracellular cytokine staining, cells were incubated overnight in culture medium either with monoclonal antibodies against GITR at 10 μg/mL or with nonspecific antibodies. To exclude the possibility that GITR triggering would enhance responses independent of antigenic stimulation, all experiments included a control condition in which cells were incubated with monoclonal antibody against GITR without antigenic stimulation.
Intracellular cytokine staining
Intracellular expression of TNF-α and interferon (IFN)–γ was assessed as described elsewhere [30]. Briefly, after antigen stimulation for 2 h aided by antibodies against CD28 and CD49d, brefeldin A was added for 5 h to trap expressed cytokines in cells, and then a standard fixation and permeabilization protocol was used to stain cells after overnight refrigeration (Caltag).
Intracellular caspase-3 staining
Freshly isolated PBMCs from HIV-infected subjects not receiving antiretroviral therapy were stimulated for 2 h with experimental and control antigens, incubated for 5 h with brefeldin A, and then refrigerated overnight before cells were stained for surface expression of CD3 and CD4 and for intracellular expression of TNF-α and activated caspase-3 (BD Biosciences) using a standard fixation, permeabilization, and staining protocol (Caltag). SEB (Sigma) was used as a positive control for intracellular TNF-α staining, and monoclonal antibodies against CD95 (Upstate) were used as a positive control for the detection of apoptosis. An isotype control antibody was used to confirm the GITR specificity of the effects of anti-GITR antibodies.
Statistical analysis
CD4+ T cell expression of GITR was compared between conditions using a Mann-Whitney U test, as was the percentage of CD4+ T cells expressing intracellular activated caspase-3. The percentage of CD4+ or CD8+ T cells expressing intracellular cytokines in multiple subjects was compared in pooled samples using the Wilcoxon test. The correlation between the magnitude of the impact of GITR triggering and CD4+ T cell counts and serum HIV loads as well as antiretroviral treatment status was measured using a Pearson χ2 test. All analyses were conducted using Prism software (version 4; GraphPad).
RESULTS
Increase in baseline CD4+ T cell expression of GITR and impairment of PHA-induced GITR expression during HIV infection
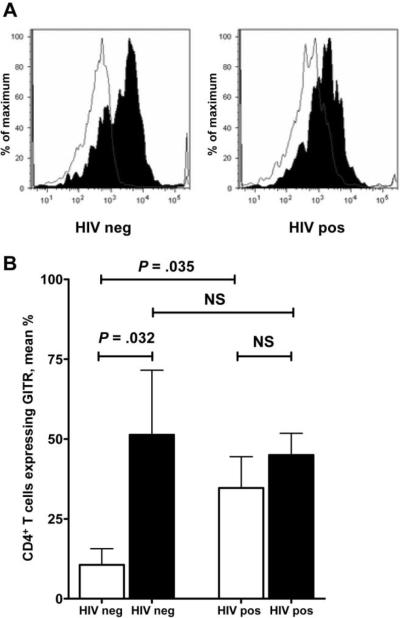
To characterize the impact of HIV infection on GITR expression, CD4+ T cells were stained for GITR before and after PHA stimulation of PBMCs from 8 HIV-infected subjects and 6 HIV-uninfected control subjects (average age, 42.5 and 45.2 years, respectively; P = .647). The percentage of CD4+ T cells expressing surface GITR was greater at baseline in the HIV-infected subjects than in the control subjects (mean, 34.70% vs. 10.59%; P = .035) (figure 1A). PHA stimulation increased surface expression of GITR in the control subjects (mean, 10.59% vs. 51.30%; P = .032) but not in the HIV-infected subjects (mean, 34.70% vs. 45.03%; P = .402) (figure 1B). PHA-stimulated GITR expression was not different between the HIV-infected subjects and the control subjects (mean, 45.03% vs. 51.30%; P = 1.000).
Figure 1.
Increase in CD4+ T cell surface expression of glucocorticoid-induced tumor necrosis factor receptor family–related (GITR) protein at baseline and impairment of phytohemagglutinin (PHA) induction of additional GITR expression in HIV-infected subjects. Panel A shows a representative plot in which the surface expression of GITR after PHA simulation is shaded. Panel B shows results for 6 HIV-negative and 8 HIV-positive subjects; the percentage of CD4+ T cells expressing GITR after PHA stimulation is shaded. Groups were compared using the Mann-Whitney U test. Error bars show SDs. NS, not significant.
Enhancement of HIV p55–specific CD4+ T cell cytokine se-cretion by GITR triggering
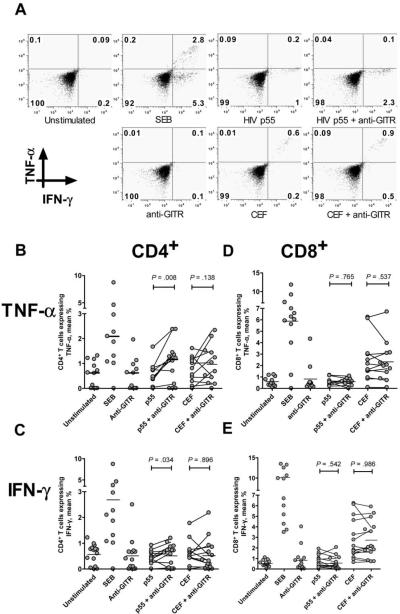
We tested the hypothesis that antibody triggering of GITR would enhance HIV-specific cytokine secretion by comparing the percentage of CD4+ and CD8+ T cells expressing intracellular TNF-α and IFN-γ to HIV p55 and other antigens via intracellular cytokine staining. HIV p55–specific CD4+ T cell expression of both TNF-α and IFN-γ was enhanced by GITR triggering, whereas GITR triggering alone or with pooled CEF peptides induced little additional expression of TNF-α and IFN-γ (figure 2A). We then studied the impact of GITR triggering on HIV-specific cytokine secretion in 12 subjects with chronic HIV infection. The mean age of these subjects was 46 years, 10 were male, and 9 were receiving antiretroviral therapy; the mean CD4+ T cell count was 356 cells/μL, and the mean HIV load was 51,208 copies/mL. GITR triggering significantly increased the percentage of CD4+ T cells expressing HIV p55–specific TNF-α (mean, 0.51% vs. 1.00%; P = .008) (figure 2B) and IFN-γ expression (mean, 0.37% vs. 0.51%; P = .034) (figure 2C). Antibody triggering of GITR did not alter HIV p55–specific CD8+ T cell expression of TNF-α (mean, 0.79% vs. 0.59%; P = .765) (figure 2D) or IFN-γ (mean, 0.63% vs. 0.56%; P = .542) (figure 2E). CEF-specific T cell responses were unaffected by GITR triggering (figure 2B–2E). GITR triggering did not change the percentage of HIV-specific T cells exhibiting dual cytokine secretion (data not shown).
Figure 2.
Enhancement of CD4+ T cell expression of tumor necrosis factor (TNF)-α and interferon (IFN)-γ to HIV p55 but not to other viral antigens after glucocorticoid-induced tumor necrosis factor receptor family–related (GITR) protein triggering. A representative plot is shown in panel A. CD4+ T cell expression of TNF-α and IFN-γ to HIV p55 and to pooled peptides of cytomegalovirus, Epstein-Barr virus, and influenza virus (CEF) for 12 subjects with HIV infection is shown in panels B and C, respectively. CD8+ T cell expression of TNF-α and IFN-γ to HIV p55 and to CEF is shown in panels D and E, respectively. Pooled results were compared using the Wilcoxon test. SEB, staphylococcal enterotoxin B.
Correlation between the increase in HIV-specific CD4+ T cell expression of TNF-α after GITR triggering and peripheral CD4+ T cell count
To determine whether clinical progression of HIV infection impacts CD4+ T cell responsiveness to GITR triggering, we assessed the correlation between the peripheral CD4+ T cell count and the magnitude of the increase in the percentage of CD4+ T cells expressing TNF-α after GITR triggering in PBMCs from 12 HIV-infected subjects. The increase in the percentage of HIV p55−specific CD4+ T cells expressing intracellular TNF-α after GITR triggering correlated directly with peripheral absolute CD4+ T cell count (P = .009; Pearson R = 0.714) (figure 3) but not with plasma viral load (P = .13; Pearson R = −0.463) or antiretroviral treatment status (P = .13; Pearson R = −0.466).
Figure 3.
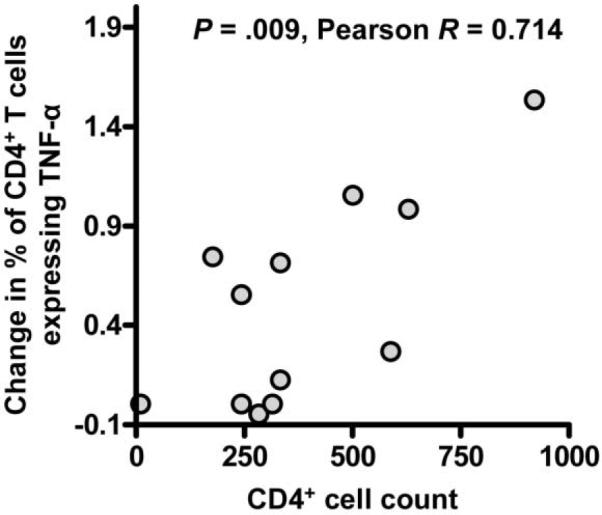
Correlation between the magnitude of the enhancement of CD4+ T cell expression of tumor necrosis factor (TNF)-α after glucocorticoid-induced tumor necrosis factor receptor family–related protein triggering and absolute CD4+ T cell counts in HIV-infected subjects.
Reduction in HIV-specific CD4+ T cell apoptosis induced by GITR triggering
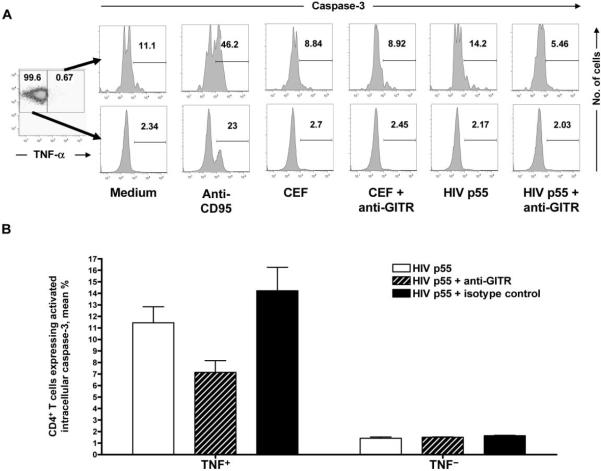
Because GITR triggering augmented CD4+ T cell responses to HIV p55 but not to CEF and because GITR triggering has been shown to protect T cells from apoptosis in murine models [20–23], we tested the hypothesis that GITR triggering enhances HIV-specific CD4+ T cell cytokine secretion by protecting HIV-specific CD4+ T cells from apoptosis. To avoid confounding, we studied the impact of GITR triggering on apoptosis in HIV-specific CD4+ T cells from patients not receiving antiretroviral therapy. Simultaneous intracellular expression of TNF-α and activated caspase-3 by CD4+ T cells was assessed in unfractionated PBMCs from HIV-infected subjects with viremia after stimulation with control medium, HIV p55, or pooled CEF peptides with and without GITR triggering. Intracellular expression of activated caspase-3 was greater in TNF-α−expressing CD4+ T cells after stimulation with HIV p55, compared with that in unstimulated cells (mean, 12.15% vs. 7.41%; P < .001) (figure 4A). Intracellular expression of activated caspase-3 was not, however, increased in CD4+ T cells responding to CEF (mean, 10.22% vs. 7.41%; P = .200). Furthermore, activated caspase-3 expression was significantly reduced after GITR triggering in CD4+ T cells expressing TNF-α to HIV p55 (mean, 12.15% vs. 10.45%; P < .001) (figure 4B) but not in CEF-specific CD4+ T cells (mean, 10.22% vs. 9.76%; P = 1.000). By contrast, GITR triggering had no impact on the intracellular expression of caspase-3 in CD4+ T cells not expressing TNF-α. Furthermore, isotype control antibody had no effect on intracellular caspase-3 expression in any experimental condition.
Figure 4.
Decrease in apoptosis of HIV-specific CD4+ T cells after glucocorticoid-induced tumor necrosis factor receptor family–related (GITR) protein triggering. In panel A, the percentage of CD4+ T cells expressing in tracellular activated caspase-3 is shown for cells expressing tumor necrosis factor (TNF)-α to the indicated antigen in the top row and for cells not expressing TNF-α to the indicated antigen in the bottom row. Depicted results are representative of 3 independent experiments. Panel B shows that the percentage of HIV p55–specific CD4+ T cells expressing intracellular activated caspase-3 is significantly decreased. Groups were compared using the Mann-Whitney U test. Error bars show SDs. CEF, pooled peptides of cytomegalovirus, Epstein-Barr virus, and influenza virus.
DISCUSSION
HIV-specific, HIV-infected, and bystander CD4+ T cells undergo dramatic and progressive apoptotic deletion during the course of HIV infection [1, 4, 5, 7–9, 31, 32]. Our data show that, despite abnormal GITR expression on CD4+ T cells during HIV infection, GITR triggering protects HIV-specific CD4+ T cells from apoptosis and enhances HIV-specific CD4+ T cell cytokine secretion.
GITR, a member of the TNF receptor family, is expressed transiently on antigen-specific and activated lymphocytes [16, 17, 23], and, in the healthy host, GITR triggering potentiates effector T cell responses [19, 21, 22]. Other members of the TNF receptor family of cell surface molecules, such as OX40, 4-1BB, and herpesvirus entry mediator (HVEM), also are involved in modulating and sustaining antigen-specific T cell responses [24], and OX40 and 4-1BB triggering has been shown to modulate HIV-specific T cell responses [25–27]. Our data extend prior findings that GITR triggering can enhance murine immune responses to retroviruses or retroviral vaccines [28, 29] by showing that GITR triggering enhances HIV-specific CD4+ T cell cytokine secretion in humans. GITR is therefore a member of the TNF receptor family that will be important to further explore in novel approaches to HIV immunotherapy.
Maximal baseline GITR expression in HIV-infected individuals may signify the induction of a protective host response against HIV infection, perhaps in response to the chronic immunological activation of HIV infection. This maximal baseline induction of GITR expression in HIV-infected subjects likely explains why additional induction of GITR expression with even a potent immunological stimulus was impaired. Analogous abnormalities of the expression of other costimulatory molecules, such as CD40 ligand and cytotoxic T lymphocyte antigen 4, have been reported in HIV infection [33–35].
Highly active antiretroviral therapy reduces apoptosis of HIV-specific T cells, as does PD-1 ligation, stimulation with IL-15, and inhibition of proapoptotic intracellular pathways [4, 10–15]. However, GITR triggering represents a novel means of preventing apoptosis of CD4+ T cells, one that appears to have a preferential impact on HIV-specific CD4+ T cells. It will be interesting to explore whether GITR triggering and antiretroviral therapy have synergistic effects on CD4+ T cell apoptosis.
Our data do not demonstrate a restoration of HIV-specific CD8+ T cell cytokine secretion after antibody triggering of GITR. It is possible that CD4+ T cells were at greater risk for apoptotic deletion during HIV infection and, thus, GITR triggering produced a preferential augmentation in cytokine secretion and protection from apoptosis in this cell population. Furthermore, restoration of CD4+ T cell help for CD8+ T cells may restore CD8+ T cell responses over a longer period of time than was assayed in the brief in vitro assays used in this study [36, 37]. Another possibility is that CD4+ and CD8+ T cell responses are impacted differentially by GITR triggering, a pattern that has been observed in other models [38].
CD4+CD25+ regulatory T (Treg) cells express GITR, as do activated T cells [17]. In murine models, triggering GITR abrogated Treg cell suppression of effector T cell responses [19, 21, 39]. However, in humans, GITR triggering may not relieve Treg cell suppression of effector T cell responses [40]. Given that CD4+CD25+ T cells are infected by HIV [41–43], modulate HIV-specific cellular immune responses [44, 45], are depleted by HIV infection [46, 47], and are dysfunctional during HIV infection [46, 48], it will be interesting to explore whether GITR triggering impacts Treg cell modulation of HIV-specific cellular immune responses or HIV infection of CD4+CD25+ T cells.
GITR triggering is a novel and modifiable host protective mechanism against CD4+ T cell deletion during HIV infection. Thee present data support additional investigations into the role played by GITR triggering in the modulation of HIV-specific cellular immune responses and in the protection of HIV-specific CD4+ T cells from apoptosis.
Acknowledgments
We thank Eric Rosenberg, Charles Wira, and C. Fordham von Reyn, for mentorship; Galit Alter and Daniel Kaufmann, for hands-on teaching; Kim Wood and Betsy Eccles, for clinical support; and our study subjects, for inspiration and generosity.
Financial support: National Institutes of Health (grant 1K08AI069915-01 to T.L.); Hitchcock Foundation (Henry Heyl Award to T.L.). This work was done while T.L. was enrolled in the Harvard Medical School Scholars in Clinical Sciences Program (K30#HL004095).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 2.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yue FY, Kovacs CM, Dimayuga RC, et al. Preferential apoptosis of HIV-1-specific CD4+ T cells. J Immunol. 2005;174:2196–204. doi: 10.4049/jimmunol.174.4.2196. [DOI] [PubMed] [Google Scholar]
- 5.Seth N, Kaufmann D, Lahey T, Rosenberg ES, Wucherpfennig KW. Expansion and contraction of HIV-specific CD4 T cells with short bursts of viremia, but physical loss of the majority of these cells with sustained viral replication. J Immunol. 2005;175:6948–58. doi: 10.4049/jimmunol.175.10.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gougeon ML. Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol. 2003;3:392–404. doi: 10.1038/nri1087. [DOI] [PubMed] [Google Scholar]
- 7.Cottrez F, Manca F, Dalgleish AG, Arenzana-Seisdedos F, Capron A, Groux H. Priming of human CD4+ antigen-specific T cells to undergo apoptosis by HIV-infected monocytes: a two-step mechanism involving the gp120 molecule. J Clin Invest. 1997;99:257–66. doi: 10.1172/JCI119154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottrez F, Capron A, Groux H. Selective CD4+ T cell deletion after specific activation in HIV-infected individuals; protection by anti-CD28 monoclonal antibodies. Clin Exp Immunol. 1996;105:31–8. doi: 10.1046/j.1365-2249.1996.d01-716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–6. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg H, Blumenthal R. HIV gp41-induced apoptosis is mediated by caspase-3-dependent mitochondrial depolarization, which is inhibited by HIV protease inhibitor nelfinavir. J Leukoc Biol. 2006;79:351–62. doi: 10.1189/jlb.0805430. [DOI] [PubMed] [Google Scholar]
- 11.Ensoli F, Fiorelli V, Alario C, et al. Decreased T cell apoptosis and T cell recovery during highly active antiretroviral therapy (HAART) Clin Immunol. 2000;97:9–20. doi: 10.1006/clim.2000.4915. [DOI] [PubMed] [Google Scholar]
- 12.Chang KH, Kim JM, Kim HY, et al. Spontaneous programmed cell death of peripheral blood mononuclear cells from HIV-infected persons is decreased with interleukin-15. Yonsei Med J. 2000;41:112–8. doi: 10.3349/ymj.2000.41.1.112. [DOI] [PubMed] [Google Scholar]
- 13.Okada H, Takei R, Tashiro M. Inhibition of HIV-1 Nef-induced apoptosis of uninfected human blood cells by serine/threonine protein kinase inhibitors, fasudil hydrochloride and M3. FEBS Lett. 1998;422:363–7. doi: 10.1016/s0014-5793(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Liu ZH, Ware CF, Ashwell JD. A cysteine protease inhibitor prevents activation-induced T-cell apoptosis and death of peripheral blood cells from human immunodeficiency virus-infected individuals by inhibiting upregulation of Fas ligand. Blood. 1997;89:550–7. [PubMed] [Google Scholar]
- 15.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suvas S, Kim B, Sarangi PP, Tone M, Waldmann H, Rouse BT. In vivo kinetics of GITR and GITR ligand expression and their functional significance in regulating viral immunopathology. J Virol. 2005;79:11935–42. doi: 10.1128/JVI.79.18.11935-11942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005;35:1016–22. doi: 10.1002/eji.200425818. [DOI] [PubMed] [Google Scholar]
- 18.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–6. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 19.Kanamaru F, Youngnak P, Hashiguchi M, et al. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–14. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 20.Zhan Y, Funda DP, Every AL, et al. TCR-mediated activation promotes GITR upregulation in T cells and resistance to glucocorticoid-induced death. Int Immunol. 2004;16:1315–21. doi: 10.1093/intimm/dxh134. [DOI] [PubMed] [Google Scholar]
- 21.Ronchetti S, Zollo O, Bruscoli S, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte sub-populations. Eur J Immunol. 2004;34:613–22. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 22.Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. Role of GITR in activation response of T lymphocytes. Blood. 2002;100:350–2. doi: 10.1182/blood-2001-12-0276. [DOI] [PubMed] [Google Scholar]
- 23.Gurney AL, Marsters SA, Huang RM, et al. Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr Biol. 1999;9:215–8. doi: 10.1016/s0960-9822(99)80093-1. [DOI] [PubMed] [Google Scholar]
- 24.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 25.Yu Q, Yue FY, Gu XX, Schwartz H, Kovacs CM, Ostrowski MA. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J Immunol. 2006;176:2486–95. doi: 10.4049/jimmunol.176.4.2486. [DOI] [PubMed] [Google Scholar]
- 26.Serghides L, Bukczynski J, Wen T, et al. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J Immunol. 2005;175:6368–77. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 27.Bukczynski J, Wen T, Wang C, et al. Enhancement of HIV-specific CD8 T cell responses by dual costimulation with CD80 and CD137L. J Immunol. 2005;175:6378–89. doi: 10.4049/jimmunol.175.10.6378. [DOI] [PubMed] [Google Scholar]
- 28.Dittmer U, He H, Messer RJ, et al. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 29.Stone GW, Barzee S, Snarsky V, et al. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J Virol. 2006;80:1762–72. doi: 10.1128/JVI.80.4.1762-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altfeld M, Addo MM, Eldridge RL, et al. Vpr is preferentially targeted by CTL during HIV-1 infection. J Immunol. 2001;167:2743–52. doi: 10.4049/jimmunol.167.5.2743. [DOI] [PubMed] [Google Scholar]
- 31.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 32.Gougeon ML, Montagnier L. Programmed cell death as a mechanism of CD4 and CD8 T cell deletion in AIDS: molecular control and effect of highly active anti-retroviral therapy. Ann NY Acad Sci. 1999;887:199–212. doi: 10.1111/j.1749-6632.1999.tb07934.x. [DOI] [PubMed] [Google Scholar]
- 33.Subauste CS, Wessendarp M, Portilllo JA, et al. Pathogen-specific induction of CD154 is impaired in CD4+ T cells from human immunodeficiency virus–infected patients. J Infect Dis. 2004;189:61–70. doi: 10.1086/380510. [DOI] [PubMed] [Google Scholar]
- 34.Subauste CS, Wessendarp M, Smulian AG, Frame PT. Role of CD40 ligand signaling in defective type 1 cytokine response in human immunodeficiency virus infection. J Infect Dis. 2001;183:1722–31. doi: 10.1086/320734. [DOI] [PubMed] [Google Scholar]
- 35.Steiner K, Waase I, Rau T, Dietrich M, Fleischer B, Broker BM. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin Exp Immunol. 1999;115:451–7. doi: 10.1046/j.1365-2249.1999.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 37.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muriglan SJ, Ramirez-Montagut T, Alpdogan O. GITR activation induces an opposite effect on alloreactive CD4+ and CD8+ T cells in graft-versus-host disease. J Exp Med. 2004;200:149–57. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tone M, Tone Y, Adams E, et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100:15059–64. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levings MK, Sangregorio R, Sartirana C, et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–46. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou CS, Ramilo O, Vitetta ES. Highly purified CD25− resting T cells cannot be infected de novo with HIV-1. Proc Natl Acad Sci USA. 1997;94:1361–5. doi: 10.1073/pnas.94.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramilo O, Bell KD, Uhr JW, Vitetta ES. Role of CD25+ and CD25− T cells in acute HIV infection in vitro. J Immunol. 1993;150:5202–8. [PubMed] [Google Scholar]
- 43.Oswald-Richter K, Grill SM, Shariat N, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–9. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinter AL, Hennessey M, Bell A, et al. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331–43. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eggena MP, Barugahare B, Jones N, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–14. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 47.Shet A, Mehandru S, Lopez P, et al. CD4+CD25+hi T regulatory-cell dynamics during acute and chronic HIV infection, and response to therapy (abstract 315). Program and abstracts of the 12th Conference on Retroviruses and Opportunistic Infections; Boston. 2005. [Google Scholar]
- 48.Apoil PA, Puissant B, Roubinet F, Abbal M, Massip P, Blancher A. FOXP3 mRNA levels are decreased in peripheral blood CD4+ lymphocytes from HIV-positive patients. J Acquir Immune Defic Syndr. 2005;39:381–5. doi: 10.1097/01.qai.0000169662.30783.2d. [DOI] [PubMed] [Google Scholar]