Abstract
Although patients with human immunodeficiency virus (HIV) infection who live in the rural United States receive less expert care and less antiretroviral treatment, the impact of living in rural areas on mortality from HIV infection is unstudied. We compared mortality rates in 327 rural and 317 urban patients with HIV infection in a retrospective cohort study using a multivariate logistic regression model. Rural patients with HIV infection were older at the end of follow-up (43.4 vs. 41.4 years, p = 0.002), and more likely white (93.0% vs. 77.9%, p < 0.001), and a greater proportion were men who have sex with men (55.5% vs. 36.1%, p < 0.001). While the mean year of diagnosis was 1994 in rural patients and 1995 in urban patients (p < 0.001), the mean CD4+ T cell count at presentation was similar in the two groups: 376 vs. 351 cells/μl (p = 0.298). Rural patients in our cohort were more likely to receive antiretroviral medications at any CD4 count (73.7 vs. 62.1%, p = 0.0016), and received PCP prophylaxis at comparable rates (23.5% vs. 25.6%, p = 0.555). Mortality was higher in rural patients (10.4% vs. 6.0%, p = 0.028). The risk of mortality remained higher in rural patients when adjusting for age, sex, race, HIV risk factors, year of diagnosis, travel time, lack of insurance, and receipt of antiretroviral treatment or PCP prophylaxis in a logistic regression model (OR 2.11, 1.064 to 4.218, p = 0.047). Patients with HIV who live in rural areas have higher mortality rates than urban patients with HIV.
INTRODUCTION
The prevalence of human immunodeficiency virus (HIV) infection in rural areas is increasing,1–3 yet the impact of living in rural areas on clinical outcomes from HIV remains unknown.
Patients with HIV who live in rural areas receive substandard HIV care as compared to patients with HIV in urban areas. HIV-infected patients from rural areas are less likely to receive antiretroviral treatment or prophylaxis against Pneumocystis carinii pneumonia (PCP), and are more likely to see an inexperienced HIV provider. Rural patients with HIV are also more likely to experience significant inconvenience obtaining HIV care.4–6 However, the clinical impact of these differences in quality of HIV care between rural and urban areas has not been studied.
We investigated the impact of living in rural areas on mortality in patients with HIV in a retrospective cohort study of 644 patients with HIV in New England. Importantly, all patients in our cohort were seen by the same multidisciplinary team of HIV-expert providers, but mortality was higher in the rural patients with HIV.
MATERIALS AND METHODS
Subjects
We conducted a retrospective cohort study in all 644 adult HIV-infected patients seen in the four New England clinics of the Dartmouth-Hitchcock HIV Program from 1995 to 2005 who were seen for more than one clinic visit. Subjects were excluded if there were inadequate records on place of residency, CD4 count, or clinical outcomes. Subjects were seen in a multidisciplinary regional HIV program staffed by board-certified infectious disease physicians as well as nurses, psychologists, and support staff who specialize in HIV care.
Endpoint ascertainment
Clinical and demographic data were collected through a medical records review in a research protocol approved by the Dart-mouth Medical School Committee for the Protection of Human Subjects. Outcome variables included age, sex, race, country of birth, home town, location of HIV clinic, CD4 count and viral load history, receipt of antiretroviral and prophylactic medications, insurance status, and death. Subjects were characterized as receiving highly active antiretroviral therapy (HAART) if they received at least three antiretroviral medications from at least two drug classes. We derived the travel time between the patient(s zip code and the zip code of their clinic location using MapQuest.com. Cause of death was assessed by a retrospective medical record review and a vital records search.
Definitions
Patients were considered to live in rural areas if they lived outside of the major New England city and town areas defined by the Office of Management and Budget in 2004. Antiretroviral therapy in this cohort of patients followed from 1995 to 2005 included monotherapy, dual therapy, and HAART. PCP prophylaxis constituted receipt of trimethoprim/sulfamethoxazole, dapsone, atovaquone, or aerosolized pentamadine at the appropriate doses.
Statistical analysis
We conducted univariate comparisons between patients living in rural areas and urban areas using t tests. Variables that were different between rural and urban areas, or variables selected a priori to have a potential impact on HIV-related mortality, were incorporated into a multivariate logistic regression analysis in aggregate. This model included age as a continuous variable, sex, race, categorized risk behaviors, country of birth, and population of home town as a continuous variable, as well as quality of care markers such as insurance status, minutes traveled to clinic, and receipt of antiretroviral or prophylactic medications. We used this model to analyze the impact of living in rural areas on the primary endpoint, which was death from all causes. All statistical analyses were performed using STATA 8.0 (College Station, TX).
RESULTS
The mean age of subjects at the end of follow-up in our cohort was 42.4 years; 85.6% were white, 46.0% were men who have sex with men, and 13.5% were HIV infected through injection drug use. The mean age at presentation was 32 years, CD4 count at presentation was 364 cells/μl, and the mean year of diagnosis was 1995. Fifty percent of subjects in the cohort met criteria for the acquired immunodeficiency syndrome; 8% died during the period of follow-up (Table 1).
Table 1.
Characteristics of Rural and Urban Patients in Dartmouth HIV Program, 1995–2005
| Rural (n = 327) | Urban (n = 317) | p value by t test | |
|---|---|---|---|
| Age at end of follow-up, years | 43.4 | 41.4 | 0.002 |
| Male, % | 72.9 | 70.4 | |
| Race, % | |||
| White | 93.0 | 77.9 | <0.001 |
| Black | 6.1 | 20.8 | <0.001 |
| Asian | 0.3 | 1.3 | 0.168 |
| Risk behavior, % | |||
| Man who has sex with men (MSM) | 55.5 | 36.1 | <0.001 |
| Man who has sex with women and men | 25.9 | 32.2 | 0.082 |
| Intravenous drug use (IDU) | 12.2 | 14.8 | 0.336 |
| Woman who has sex with men (WSM) | 12.8 | 24.0 | <0.001 |
| MSM/IDU | 6.1 | 4.5 | 0.345 |
| WSM/IDU | 1.8 | 4.1 | 0.091 |
| Transfusion | <1 | <1 | 0.632 |
| Population of town, mean | 9,738 | 78,721 | <0.001 |
| Born in United States, % | 82.4 | 82.3 | 0.978 |
| Year of diagnosis, mean | 1994 | 1995 | 0.001 |
| CD4+ T cell count at diagnosis, cells/μl | 376 | 351 | 0.298 |
| Diagnosed at a CD4+ T cell count <200, % | 27.8 | 28.1 | 0.945 |
Individuals with HIV from rural areas were older (43.4 vs. 41.4 years, p = 0.002), were more likely to be white (93.0% vs. 77.9%, p < 0.001), and were more likely to be men who have sex with men (55.5% vs. 36.2%, p < 0.001) than subjects from urban areas. Rates of intravenous drug use, foreign birth, and male sex were similar between the two groups. The CD4 count at first presentation to our clinics was not different in rural areas (376 vs. 351 cells/μl, p = 0.298), nor was the percentage of people presenting with a CD4 count below 200 (27.8% vs. 28.1%, p = 0.945).
All patients received care by the same team of clinicians. Similar numbers of rural patients were uninsured (9.8% vs. 11.7%, p = 0.440). However, in rural areas private health insurance was more common than in urban areas (41.9 vs. 28.4, p < 0.001), while government-sponsored health insurance was less common (39.1 vs. 51.7, p < 0.001). Rural patients had longer travel time to HIV care than urban patients (56.0 vs. 34.4 min, p < 0.001).
Rural patients with HIV infection received antiretroviral medications more often at any CD4 count (73.7 vs. 62.1%, p = 0.002) and at all CD4 count strata. Rural patients with HIV infection were similarly likely to have received non-HAART antiretroviral therapy at some point during their treatment history (44.3 vs. 43.8%, p = 0.756). The odds of receiving antiretroviral treatment remained significantly higher for HIV-infected patients from rural areas when controlling for age, sex, black race, HIV acquisition risk factor, lack of health insurance, travel time, and CD4 count at presentation to our clinics in a logistic regression model [odds ratio (OR) 2.016, 1.36–2.99, p < 0.001).
There was no significant difference in the frequency of receipt of PCP prophylaxis between rural and urban patients with HIV in univariate analyses (23.5% vs. 25.6%, p = 0.555) or in multivariate logistic regression analyses incorporating the above demographic variables (OR 0.90, 0.627–1.29, p = 0.555).
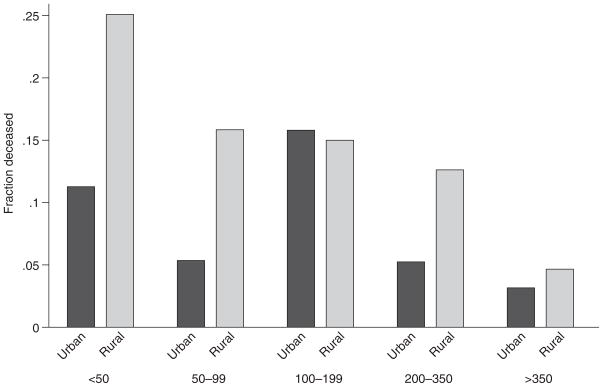
Rural patients with HIV had a higher mortality rate than urban patients with HIV in a univariate analysis (10.4% vs. 6.0%, p = 0.028; Fig. 1). The unadjusted odds of death was significantly higher in rural patients (OR 1.9, 1.1–3.5, p = 0.030). After controlling for age, sex, black race, and HIV acquisition risk in a logistic regression model, the odds of death remained higher in rural patients with HIV (OR 2.0, 1.1–3.7, p = 0.032). Incorporating markers of access to care including travel time, year of diagnosis, lack of health insurance, receipt of prophylaxis against PCP, or antiretroviral medications into this model, the odds of death remained higher in rural patients (OR 2.1, 1.1–4.2, p = 0.033) with adequate goodness of fit by the Pearson chi-squared test (p > 0.624) and an area under the receiver operating characteristic curve of 0.801.
FIG. 1.
Univariate analysis of rural versus urban patients with HIV by a CD4+ count at presentation. Rural patients had a higher mortality rate than urban patients.
When death rates were analyzed by demographic risk factors (Table 2), only injection drug users living in rural areas with HIV infection had significantly higher death rates than their urban peers in univariate analyses (17.5 vs. 4.2%, p = 0.044). No other demographic group showed a statistically significant increase in death in rural compared to urban areas. No risk group had significantly higher death rates in urban areas in multivariate analyses.
Table 2.
Death Rates in Rural and Urban Patients in the Dartmouth HIV Program, Listed by Demographic Group
| Risk factor | Univariate comparisons |
Multivariate comparisons |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality (%) |
Death in rural patients |
Death in rural patients |
|||||||
| Rural | Urban | p value | Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Female sex | 10.2 | 6.5 | 0.360 | 1.65 | 0.56–4.85 | 0.361 | 1.34 | 0.34–5.24 | 0.676 |
| Injection drug user | 17.5 | 4.2 | 0.044 | 4.78 | 0.93–24.47 | 0.061 | 3.56 | 0.62–20.56 | 0.156 |
| Man who has sex with men | 8.0 | 4.5 | 0.254 | 1.83 | 0.64–5.24 | 0.258 | 1.71 | 0.54–5.42 | 0.362 |
| Black | 5.0 | 6.1 | 0.861 | 0.82 | 0.09–7.75 | 0.859 | 0.37 | 0.03–5.54 | 0.474 |
Although patients who died in our cohort resided in non-significantly smaller towns than those who survived (mean town population 34,188 vs. 44,472 persons, p = 0.120), the population of the patient’s home town did not correlate with either receipt of antiretroviral medications or death in univariate or multivariate comparisons.
The demographic characteristics of rural and urban decedents with HIV in our cohort are summarized in Table 3. Rural decedents were more likely to be white (97.1 vs. 77.8%, p = 0.025) than their urban peers, but the racial distribution of patients was similar to that seen in rural versus urban patients in our cohort as a whole. Otherwise, there were no significant demographic differences between rural and urban patients, although rural decedents tended nonsignificantly to be men who have sex with men.
Table 3.
Characteristics of Rural and Urban Descendents in the Dartmouth HIV Program, 1995–2005
| Rural (n = 35) | Urban (n = 19) | p value by t test | |
|---|---|---|---|
| Age at end of follow-up, years | 45.3 | 42.1 | 0.204 |
| Male, % | 73.5 | 72.2 | 0.921 |
| Race, % | |||
| White | 97.1 | 77.8 | 0.025 |
| Black | 2.9 | 22.2 | 0.025 |
| Risk behavior, % | |||
| Man who has sex with men | 39.4 | 22.2 | 0.222 |
| Intravenous drug use | 17.6 | 11.1 | 0.544 |
| Woman who has sex with men | 14.7 | 22.2 | 0.505 |
| Born in the United States | 81.5 | 72.7 | 0.561 |
| Year of diagnosis, mean | 1994 | 1993 | 0.553 |
| Mean last CD4 count (cells/μl) | 194 | 178 | 0.779 |
| Received antiretroviral treatment, % | 79.4 | 72.2 | 0.567 |
The year of death was equivalent in rural decedents (2001 vs. 2000, p = 0.168), and the average time to death from the year of diagnosis was similar in rural and urban decedents (7.1 vs. 6.9 years, p = 0.890). The most common causes of death in our cohort were opportunistic infection (30.8%), liver disease (19.2%), and cancer (13.5%). The distribution of the causes of death was not different between rural and urban decedents with HIV infection (Table 4).
Table 4.
Cause of Death in Rural and Urban Descendents in the Dartmouth HIV Program, 1995–2005
| Rural % (n = 35) | Urban % (n = 19) | p value by t test | |
|---|---|---|---|
| Any infection | 41.2 | 33.4 | 0.350 |
| Opportunistic infection | 32.4 | 27.8 | 0.740 |
| Other infection | 8.8 | 5.6 | 0.681 |
| Liver disease | 23.5 | 11.1 | 0.2888 |
| Cancer | 14.7 | 11.1 | 0.724 |
| Medical, not infectious | 8.8 | 27.8 | 0.074 |
| Trauma and suicide | 8.8 | 11.1 | 0.795 |
| Unknown | 3.0 | 5.5 | 0.649 |
DISCUSSION
Rural patients with HIV infection were shown in previous studies to see less expert providers, and were less likely to receive PCP prophylaxis and antiretroviral therapy.4–6 In our cohort, rural patients with HIV infection had higher mortality rates than their urban peers, even though provider expertise was equivalent between groups, and rural patients received at least as aggressive HIV care. The source of the increased mortality in rural patients with HIV infection is not clear, and is likely multifactorial. However, our data help segregate likely from unlikely contributors.
Demographic factors such as age, sex, race, and HIV acquisition risk can impact mortality in HIV infection,7–11 yet are an unlikely source for the increased mortality observed in rural patients in our cohort. Not only did our multivariate analysis control for these factors, but the rural population in which we observed increased mortality was more likely to be comprised of white men with better predicted health outcomes. Furthermore, poorer outcomes were seen in rural patients within disparate demographic groups such as injection drug users and men who have sex with men, suggesting that living in rural areas with HIV infection exerts an impact on mortality in a fashion that is independent of such demographic characteristics.
We did note in univariate analyses that injection drug users who live in rural areas had significantly higher death rates than their urban peers. However, this relationship did not persist in the multivariate analysis. While it is conceivable that injection drug users are particularly vulnerable to the effects of inadequate access to health care on clinical outcomes, and a major source for the difference between rural and urban outcomes, we believe the finding of worse clinical outcomes in rural patients in multiple demographic groups and in the multivariate model signals that living in rural areas has a greater impact on mortality in patients with HIV infection than individual demographic characteristics.
In previous studies, rural patients with HIV infection had differentially restricted access to clinicians with HIV expertise.5 This was not a factor in this study, however, since the same multidisciplinary team of expert providers saw all of the study patients. Furthermore, rural patients were no less likely to receive HAART than their urban peers. It is possible, however, that rural patients with HIV infection were referred to our team later than urban patients, although the equivalent CD4 counts at presentation to our clinic in the two groups argue against this as a major contributor to our findings.
Mortality in rural patients with HIV may be higher because living in a rural or remote area could restrict patient access to quality health care. Restricted access to care could result from unmeasured but potentially important clinical variables like appointment frequency, adherence to medications, and degree of local HIV-specific social support. In addition, other socioeconomic determinants of health outcomes could affect mortality in rural patients with HIV infection, such as patient income. Lastly, migration patterns have previously been suggested to impact comparisons of clinical outcome between patients with HIV infection in rural and urban areas.12,13 We are currently assessing these possibilities in order to better understand the increased mortality in rural patients with HIV infection.
Our study has important potential shortcomings that should be considered. On average, rural patients in our cohort were diagnosed one calendar year earlier than urban patients. Although differential timing of HIV diagnosis could conceivably impact mortality rates, rural patients were diagnosed at a similar CD4 count at presentation to our clinic and had a similar time between diagnosis and death as their urban counterparts in our cohort. Furthermore, when year of diagnosis was incorporated into our multivariate mortality model, the results remained the same. Similarly, right-censoring the last year of rural mortality data did not alter our findings. Regardless, differential follow-up time is a potential source for bias in any retrospective cohort study that lacks the longitudinal follow-up required for a survival analysis.
Selection bias is another potential source for bias in any retrospective cohort study such as this. For instance, patients were excluded from our study if there was inadequate information on their home town or inadequate assessment of clinical outcome. It is conceivable that patients excluded due to inadequate data were differentially susceptible to poor clinical outcomes. However, there is no reason to suspect that this phenomenon would occur preferentially in rural or urban areas in a way that would impact the results of this study.
These results are applicable to areas outside of New England. Our cohort does not include patients from a major metropolitan area, but the lack of a major metropolitan area in our study population would likely cause an underestimation of outcome differences between rural and urban domiciles. It is also important to recognize that the demographic characteristics of rural populations are different around the country. For instance, rural patients with HIV infection in the Southeast are more likely to be black and female than their urban counterparts.12,13 Such differences do not, however, limit the applicability of our findings, since the increased mortality in rural patients transcended racial and other demographic differences in a multivariate model, and plausibly stem from more universal obstacles to optimal health care access. Furthermore, understanding the different demographic characteristics of rural patients in New England will help contribute to quality HIV care in these patients.
It will be critical to identify the exact causes of increased mortality in rural patients with HIV infection in order to design interventions aimed at redressing this disparity in health outcomes.
Acknowledgments
Supported in part by a Dartmouth-Hitchcock Department of Medicine Junior Faculty Development Award. This work was undertaken while T.L. was enrolled in the Harvard Medical School Scholars in Clinical Science Program, K30#HL004095.
Footnotes
The authors have no financial conflicts of interest to declare.
References
- 1.Steinberg S, Fleming P. The geographic distribution of AIDS in the United States: Is there a rural epidemic? J Rural Health. 2000;16(1):11–19. doi: 10.1111/j.1748-0361.2000.tb00432.x. [DOI] [PubMed] [Google Scholar]
- 2.Young RA, Feldman S, Brackin BT, Thompson E. Sero-prevalence of human immunodeficiency virus among adolescent attendees of Mississippi sexually transmitted disease clinics: A rural epidemic. South Med J. 1992;85(5):460–463. doi: 10.1097/00007611-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Fordyce EJ, Thomas P, Shum R. Evidence of an increasing AIDS burden in rural America. Stat Bull Metrop Insur Co. 1997;78(2):2–9. [PubMed] [Google Scholar]
- 4.Samuels ME, Shi L, Stoskoph CH, Richter DL, Baker SL, Sy FS. Rural physicians: A survey analysis of HIV/AIDS patient management. AIDS Patient Care. 1995;9(6):281–289. doi: 10.1089/apc.1995.9.281. [DOI] [PubMed] [Google Scholar]
- 5.Cohn SE, Berk ML, Berry SH, et al. The care of HIV-infected adults in rural areas of the United States. J Acquir Immune Defic Syndr. 2001;28(4):385–392. doi: 10.1097/00126334-200112010-00013. [DOI] [PubMed] [Google Scholar]
- 6.Heckman TG, Somlai AM, Kalichman SC, Franzoi SL, Kelly JA. Psychosocial differences between urban and rural people living with HIV/AIDS. J Rural Health. 1998;14(2):138–145. doi: 10.1111/j.1748-0361.1998.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 7.Nash D, Katyal M, Shah S. Trends in predictors of death due to HIV-related causes among persons living with AIDS in New York City: 1993–2001. J Urban Health. 2005;82(4):584–600. doi: 10.1093/jurban/jti123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redelings MD, Frye DM, Sorvillo F. High incidence of HIV-associated mortality among black and Hispanic infants and women of childbearing age in the United States 1990–2001. J Acquir Immune Defic Syndr. 2005;39(4):496–498. doi: 10.1097/01.qai.0000153425.38453.ad. [DOI] [PubMed] [Google Scholar]
- 9.Lai D, Hardy RJ. An update on the impact of HIV/AIDS on life expectancy in the United States. AIDS. 2004;18(12):1732–1734. doi: 10.1097/01.aids.0000131383.15232.95. [DOI] [PubMed] [Google Scholar]
- 10.McGinnis KA, Fine MJ, Sharma RK, et al. Understanding racial disparities in HIV using data from the veterans aging cohort 3-site study and VA administrative data. Am J Public Health. 2003;93(10):1728–1733. doi: 10.2105/ajph.93.10.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muga R, Roca J, Egea JM, et al. Mortality of HIV-positive and HIV-negative heroin abusers as a function of duration of injecting drug use. J Acquir Immune Defic Syndr. 2000;23(4):332–338. doi: 10.1097/00126334-200004010-00009. [DOI] [PubMed] [Google Scholar]
- 12.Lansky A, Nakashima AK, Diaz T, et al. Human immunodeficiency virus infection in rural areas and small cities of the southeast: Contributions of migration and behavior. J Rural Health. 2000;16(1):20–30. doi: 10.1111/j.1748-0361.2000.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 13.Rumley RL, Shappley NC, Waivers LE, Esinhart JD. AIDS in rural eastern North Carolina—patient migration: A rural AIDS burden. AIDS. 1991;5(11):1373–1378. doi: 10.1097/00002030-199111000-00015. [DOI] [PubMed] [Google Scholar]