Abstract
Mechanisms underlying successful composite tissue transplantation must include an analysis of transplant chimerism, which is little studied, particularly in calcified tissue. We have developed a new method enabling determination of lineage of selected cells in our model of vascularized bone allotransplantation.
Vascularized femoral allotransplantation was performed from female Dark Agouti (DA) donor rats to male Piebald Virol Glaxo (PVG) recipients, representing a major histocompatibility mismatch. 4 groups differed in use of immunosuppression (+/- 2 weeks Tacrolimus) and surgical revascularization, by implantation of either a patent or a ligated saphenous arteriovenous (AV) bundle. Results were assessed at 18 weeks. Bone blood flow was measured by the hydrogen washout technique and transverse specimens were prepared for histology. Real-time PCR was performed on DNA from laser capture microdissected cortical bone regions to determine the extent of chimerism. To do so, we analyzed the relative expression ratio of the sex-determining region Y (Sry) gene, specific only for recipient male rat DNA, to the cyclophilin housekeeper gene.
Substantial transplant chimerism was seen in cortical bone of all groups (range 77-97%). Rats without immunosuppression and with a patent AV bundle revealed significantly higher chimerism than those with immunosuppression and a ligated AV bundle, which maintained transplant cell viability. We describe a new method to study the extent of chimerism in rat vascularized bone allotransplants, including a sex-mismatched transplantation model, laser capture microdissection of selected bone regions, and calculation of the relative expression ratio.
Keywords: microdissection, bone, allotransplant, real-time PCR, chimerism
Introduction
The reconstruction of massive bone and/or joint defects is frequently necessary following limb-sparing resection of primary and metastatic tumors, trauma, infection, or prosthetic implant failure. Currently available methods are problematic due to nonunion, infection, implant failure and bone stress fracture. Allotransplantation of living musculoskeletal tissue remains experimental, due to unacceptable risks of long-term immune modulation currently required to maintain tissue viability 1.
A possible solution to this problem could be to develop a neoangiogenic circulation within a transplanted bone by implantation of recipient-derived vessels. Our method combines primary anastomosis of donor bone nutrient vessels with simultaneous intramedullary implantation of an arteriovenous pedicle from the recipient animal. Short-term (2 weeks) immunosuppression (IS) is used during the process of angiogenesis from these recipient-derived vessels. This approach allows the allografted bone to develop its blood supply largely or entirely with recipient blood vessels and permits the graft to survive after IS withdrawal and subsequent nutrient vessel thrombosis. Our results, reported previously, demonstrate the ability of this method to maintain bone blood flow and osteocyte viability 2. Investigation of the mechanisms underlying these observations requires study of transplant chimerism, defined as the movement of cells from the recipient into the transplanted bone. For this purpose, we determined the relative proportion of the Sry gene (recipient-derived) to an autosomal housekeeper gene (cyclophilin) in a sex-mismatched transplantation model. We hypothesize that: 1) investigation of transplant chimerism is possible in tissue allotransplantation with our methods, 2) this process is moderated by short term IS and promoted by patent AV bundle implantation and 3) transplant chimerism correlates with bone blood flow and bone viability.
Methods
Vascularized femoral allotransplantations were performed from female Dark Agouti (DA: genetic expression: RT1a) rats to male Piebald Virol Glaxo (PVG: genetic expression: RT1c) rats, representing a major immunohistocompatability mismatch. Sex-mismatched transplantation was performed to aid in later identification of cellular lineage within the transplanted tissue. An 18 week survival period was chosen, based upon our previous work, which demonstrated substantial repopulation of transplanted rat femora at this time period3. All experiments were performed under the direction of the Mayo Clinic Institutional Animal Care and Use Committee (IACUC).
Surgical Procedure
The complete surgical procedure has been described previously 2. In all recipient animals, the allogeneic femur was transplanted to a subcutaneous abdominal pocket, with microvascular restoration of the nutrient vessel circulation. The graft and recipient femoral vessels were anastomosed and the contralateral recipient saphenous artery and vein were implanted through the intramedullary canal as an arteriovenous pedicle. After microsurgical anastomosis the bone was covered with a flexible silicone wrap to prevent angiogenesis from surrounding tissue. This then allowed evaluation of the effect of AV bundle implantation on bone revascularization.
At surgery, each recipient rat was randomly allocated to one of 4 groups, which differed in the use of immunosuppression (none versus short-term Tacrolimus, a macrolide immunosuppressant drug) and patency of implanted recipient vessels for neoangiogenesis (patent versus ligated saphenous AV bundles) (Table 1).
Table 1. Demographics.
Group 1 (patent AV bundle, no immunosuppression (IS); group 2 (ligated AV bundle, no IS); group 3 (patent, Tacrolimus IS); group 4 (ligated AV bundle, Tacrolimus IS).
| Group | AV-Bundle | Immunosuppression | n | N left for relative expression ratio measurement* |
|---|---|---|---|---|
| 1 | Patent | None | 11 | 10 |
| 2 | Ligated | None | 7 | 4 |
| 3 | Patent | TACROLIMUS, 2 weeks | 11 | 10 |
| 4 | Ligated | TACROLIMUS, 2 weeks | 9 | 7 |
Specimens with enough tissue left for DNA extraction.
Bone Blood Flow
At 18 weeks the hydrogen washout method, as described and subsequently validated by our laboratory 4, was used to determine bone blood flow. Animals were anesthetized with a combination of ketamine (90 mg/kg IM), and xylazine (10 mg/kg IM). The rat was given a breathing mix of 30% oxygen and 70% hydrogen. A shallow 0.36 mm hole was drilled in the transplant cortex and a reference electrode was inserted into a subcutaneous pocket. Next, a hydrogen sensing electrode (Unisense Hydrogen Microsensor: Unisense, Aarhus, Denmark) was placed in the cortical hole with a micromanipulator. The hydrogen concentration was measured and allowed to reach a state of equilibrium (tissue saturation). At this point, hydrogen inhalation was stopped and the rate of tissue hydrogen signal decrease plotted from which the regression line was determined using LabVIEW software (National Instruments Corporation, Austin, TX). The rate of hydrogen washout thus measured is used to calculate cortical bone blood flow 4
Histology
After euthanization, the transplant was carefully removed. A transverse section was decalcified and stained with Hematoxylin/Eosin. Bone viability was measured using a grading system based upon osteocyte counts (the percentage of lacunae either vacant or occupied by an osteocyte) on the complete transverse histologic cross-sections (20 × magnification). Two samples were graded from each animal and the average taken. A four-point scale was used, ranging from a score of 0 (no osteocyte necrosis) to 3 (all lacunae empty) 2.
Laser Capture Microdissection
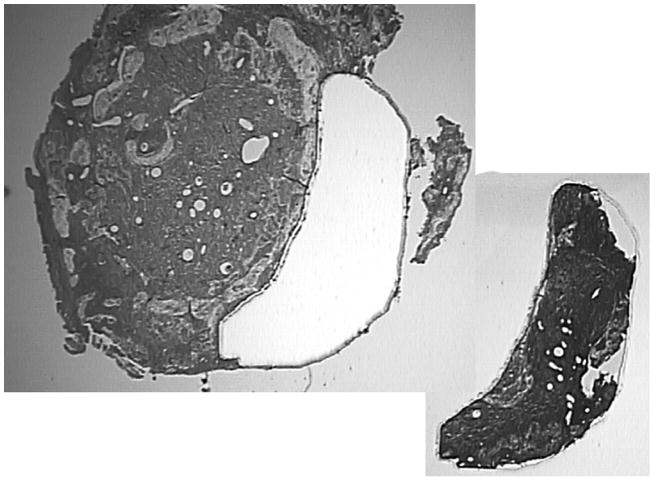
Decalcified, formalin-fixed and paraffin-embedded 8 μ sections were placed on metal-framed polyethylene naphthalate (PEN) membrane slides (Arcturus Bioscience, Inc., Mountain View, CA). After deparaffinization for 2 minutes in xylene and air drying, the membrane slide was placed in the Veritas Laser Capture Microdissection System (Model #704, Molecular Devices, Inc., Sunnyvale, CA). Using the UV laser cutting feature, a region of cortical bone averaging 1.21 μm2 was taken from the samples (Figure 1). The cortical bone samples were captured on a specialized cap (CapSure Macro LCM caps, Arcturus Bioscience, Inc., Mountain View, CA) and were then ready for DNA extraction. Using stable Proteinase K as an extraction agent, (PicoPure DNA Extraction Kit, Arcturus Bioscience, Inc., Mountain View, CA) and 16 hours of incubation at 65°C, DNA was recovered from the sample.
Figure 1.
Specimen after microdissection showing the captured section used for real-time qPCR.
Spin columns (Performa spin columns - Catalog # 13266, Edge Bio Systems, Gaithersburg, MD) were used to further purify the extracted product, which averaged 13.9 ng/μl DNA. This procedure involved preparing a Performa gel filtration cartridge by centrifuging at 750 × g for 2 minutes and then transferring the cartridge to a 1.5 ml microcentrifuge tube. Afterwards, the sample was added drop-wise to the center of the packed column and centrifuged again for 2 minutes at 750 × g. The eluate was retained and frozen in a -20° C freezer for further evaluation.
Quantitative real time Polymerase Chain Reaction
Quantitative real-time polymerase chair reaction (real-time qPCR) was performed using a Bio-Rad MyiQ Real-Time Instrument and Bio-Rad Sybr Green Super mix (Bio-Rad Laboratories catalog # 170-8880, Hercules, CA.) Genomic DNA was extracted from paraffin slide sections using the laser capture methods described earlier. Real-time qPCR was carried out using primer sets for Sry (gene of interest) and Cyclophilin, a commonly used housekeeper gene. The Sry gene is located on the Y-chromosome, and therefore it is used in sex-mismatched transplantation models to detect recipient or donor specific cells with molecular techniques5. Sequences used were Rattus norvegicus Sry (NM 012772.1) and Cyclophilin (M19533.1). Primer sets were designed using Beacon Designer software (Premier Biosoft International, Palo Alto CA.). All sequences were confirmed using the Basic Local Alignment and Search Tool (BLAST) from the National Center for Biotechnology Information (NCBI, Bethesda, MD). Sry primers used were: 5′Sry: 5′- GGG ACA ACA ACC TAC ACA CTA TC -3′ and 3′Sry: 5′-CTG GTG CTG CTG TTT CTG C - 3′. Cyclophilin primers used were 5′cyclophilin: 5′- ATC AAA CCA TTC CTT CTG TAG CTC - 3′ and 3′cyclophilin: 5′ - GGA ACC CAA AGA ACT TCA GTG AG - 3′.
Temperature, primer concentration and DNA concentration were optimized using a Bio-Rad I cycler with a gradient block. Real-time qPCR amplicons were run on a 3% agarose gel to confirm proper size. They were then extracted and sequenced on an Applied Biosystems Incorporated 3730XL DNA analyzer (Foster City, CA) to confirm product. Real-time qPCR reactions were then run using the Bio-Rad MyiQ system with sybr green and melt curve analysis using the following conditions: (i) 3 minutes denaturation at 95 degrees for 1 cycle, (ii) 15 seconds of denaturation at 95 degrees, 1 minute of annealing and extension at 66 degrees for 51 cycles followed by (iii) generation of a melting curve. Melt curves were performed as follows: (i) 1 minute at 95°C, (ii) 1 minute at 55°C, (iii) 81 repeats at 55°C with reading of fluorescence every 10 seconds. Samples that did not contain enough DNA for real-time qPCR after laser capture microdissection were excluded.
Standard Curve
A standard curve was run for both Sry and Cyclophilin using the synthetic amplicon. A standard curve was calculated using linear regression analysis. The amplicon was diluted using 10 fold serial dilutions from 101-108 molecules/μl. The dynamic range of the curve spanned at least six orders of magnitude. The amount of product in a particular sample was determined by interpolation from a standard curve of Ct values generated from the synthetic amplicon dilution series. Efficiencies were all 90-100%, coefficients 0.990-1.000 and standard curve slopes -3.2 to -3.5.
Data analysis
The raw data from experimental samples produced by the MyiQ real-time instrument and program were transferred to Linereg Software 5 to calculate the efficiency for each well6.
Relative quantification was determined by calculating the relative Expression Rate (rER) with the Gene Expression Ct Difference (GED) formula according to Schefe 6. This calculation takes the individual efficiencies of amplification for each well into account, and allows for normalization to a reference sample (male control).
Three threshold cycle values (Ct1, Ct2, and Ct3) were obtained from separate amplification products of each gene, thus producing three rER values for each specimen to verify a normal distribution. On each real-time qPCR run female and male control samples were also included in triplicate. A total of 8 male and 8 female controls were tested. In each calculation, the male-only control sample served as the reference sample (ref). We averaged these three relative expression ratios (rER), with individual PCR efficiencies (E) included, according to the formula:
where Rnorm is the relative quantity of the Gene of interest (GOI: Sry) to the Housekeeper gene (HKG: Cyclophilin) by clustering the corresponding values as indicated in figure 2. We assumed that the calculated rERs for one sample-of-interest (SOI) are part of a normal distribution (as the Ct and E values are), thus allowing calculation of the mean value and the standard deviation of these rERs (Figure 2) 6. A rER close to 1.0 reflects a predominately recipient (male) derived cell population, while lower rERs represent a majority of surviving donor (female) bone cells. A higher rER thus reflects a higher rate of transplant chimerism.
Figure 2.
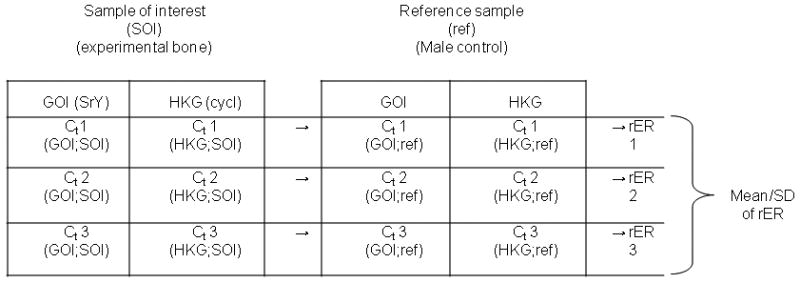
Algorithm for calculation of the relative expression ratio (rER) from the cycle threshold (Ct) values of each sample. Since the measurements were done in triplicates we used 3 values (Ct1, Ct2, Ct3) for each probe.
Statistics
A Kruskal-Wallis test was used to detect differences across the four treatment groups. Pairwise comparisons using the Wilcoxon rank sum test were performed to further clarify the variables driving significant variances. Male and female control data were included.
Results
We have previously demonstrated the ability of an implanted recipient derived AV-bundle to replace a rat femoral nutrient circulation after microsurgical transplantation when combined with short term immunosuppression 2.
In this study the bone blood flow was significantly higher in those animals in which both short-term immunosuppression (IS) and a patent AV bundle were used, when compared to groups who received no immunosuppression or had their AV bundles ligated. The median blood flow (range) measured in animals with patent AV bundles with no immunosuppression (Group 1) 0.00 (0.00 - 0.21) and 0.12 (0.00 - 0.27) with short-term immunosuppression (Group 3). In those animals with ligated AV bundles, median cortical blood flow measured 0.00 (0.00 - 0.07)(Group 2) and 0.00(0.00 - 0.09) in Group 4 (p<0.01, Figure 3).
Figure. 3.
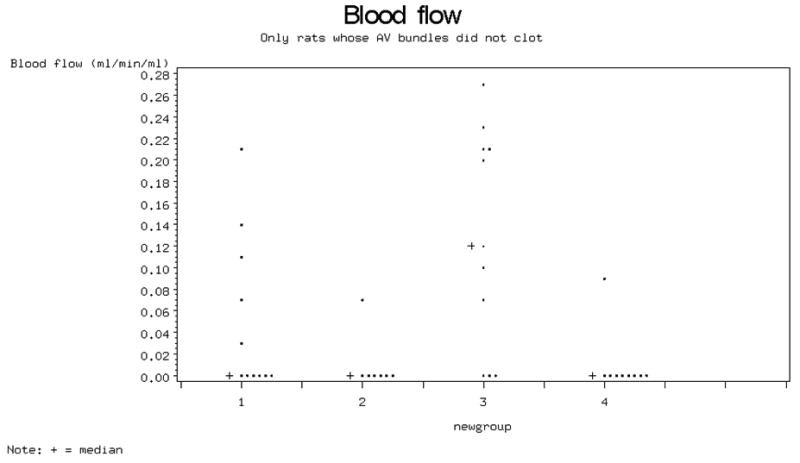
Bone blood flow (mL/min/100 g).
Bone viability scores on whole histologic specimens showed that the lowest (best) median value was obtained in the short-term IS and patent AV-bundle group (Figure 4).
Figure 4.
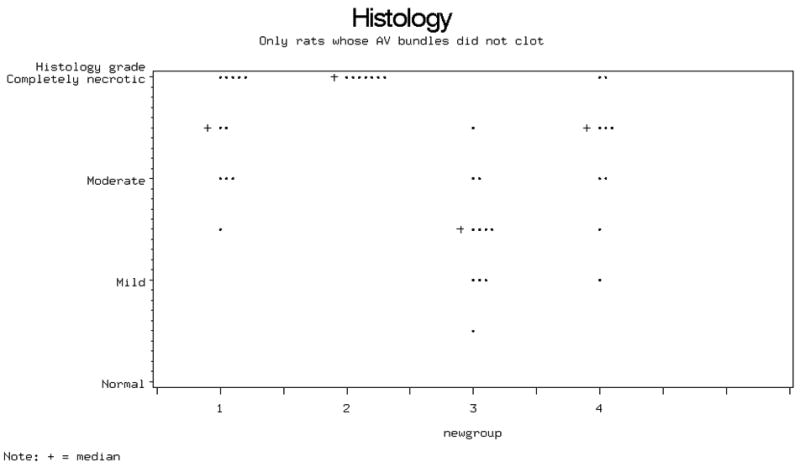
Histological grading of bone viability. Bone necrosis was least severe in group 3 specimens (with patent AV bundle and Tacrolimus IS).
In determination of transplant chimerism, we excluded samples from which no DNA could be extracted (Table 1). We measured a relative expression ratio (rER) of 1.04 ± 0.06 for male and 0.03 ± 0.04 for female controls (Mean ± SD). These values demonstrate male controls to have essentially equal expression of cyclophilin and Sry genes (100%), while female controls had no Sry expression. The mean rER was lowest in group 4 (0.77 ± 0.06) and highest in group 2 (0.92 ± 0.12), while group 3 had a rER of 0.81 ± 0.05 and group 1 had a rER of 0.88±0.1. (Figure 5)
Figure 5.
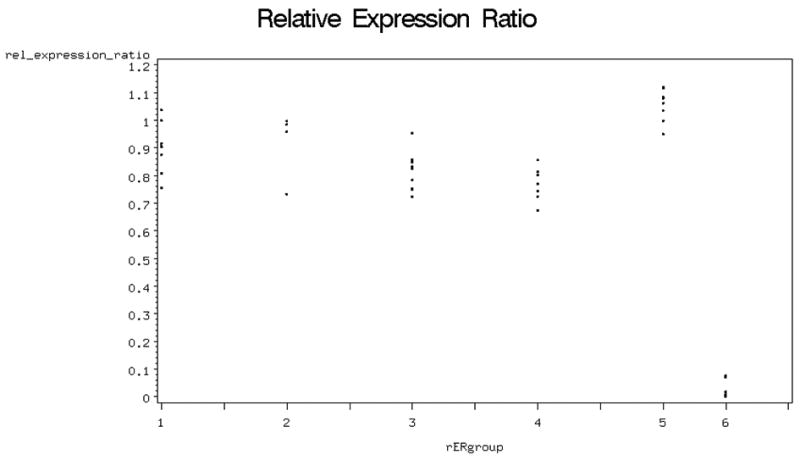
Relative expression ratio in the different groups (5=male; 6= female)
Only four of seven animals treated with no immunosuppression and a ligated AV-Bundle (Group 2) had DNA left for analysis, due to bone necrosis (Table 1). When the four groups were compared, a significant difference was found between groups 1 (88%; no IS; patent AV bundle) and 4 (77%; IS, ligated AV bundle) (p=0.02; Fig.5). There was no significant correlation between relative Expression Rates and bone blood flow or histology.
Discussion
Identification of the lineage of cells (recipient or transplant origin) is important in understanding the mechanisms by which viability is maintained in tissue transplantation. In previous work, we employed a competitive polymerase chain reaction, simultaneously amplifying a Y-chromosome-specific gene (Sry) and an autosomal gene to quantify changes in cell populations, after isolating DNA from homogenized whole bone samples. These studies have been conducted in both isograft 3, 7 and allograft 8 living bone transplants. The gender mismatch applied in these models permits the study of cell lineage. Such procedures are common in clinical transplantation, and may contribute to immunogenicity. Examples of poorer outcome exist for both male-to-female and female-to-male transplants9, although only the former has a proven H-Y antigen minor histocompatibility mismatch.
We have demonstrated that a substantial repopulation of the transplanted rat femora occurs by 18 weeks after transplantation, with cells of recipient lineage (transplant chimerism) 3. This gradual replacement of donor cells by recipient-derived cells occurs such that by 24 weeks only 10% of the remaining cells are of donor origin as shown in a previous study 7. These studies confirmed that vascularized bone allotransplants are largely repopulated over time by cells of recipient origin, but were unable to clarify what type of cells the chimeric male cells were, nor their specific location within the bone 7. Efforts to determine lineage of single cells with molecular methods in bone can be difficult. For example, in-situ hybridization should allow analysis of individual cells on microscopic inspection. In practice, however, the method is technically challenging in calcified tissue. In this paper, we report a new analysis of bone allotransplant chimerism, using laser capture microdissection to select cells of interest, followed by their analysis using real-time qPCR. Selective sampling of areas of cortical bone should provide better specificity of cell type than use of whole-bone samples.
The identity of viable osteocytes is of particular interest, as this would provide evidence for the underlying mechanism permitting cellular survival. Should the osteocytes themselves prove to be chimeric, as our data seem to suggest, it would be likely that the transplanted bone was able to successfully maintain its structure and function by first replacing the allogenic endosteal blood supply with a recipient-derived neoangiogenic circulation, followed by a process of remodelling and new bone formation from circulation-derived osteoprogenitor cells. A finding of viable allogeneic osteocytes would imply a different mechanism of transplant survival. For example, in graft adaptation 10, 11, the transplanted bone may acquire properties that allow it to survive in a non-tolerant but immunologically competent recipient 12. In osseous tissue, allogeneic matrix and cells may be sequestered by new bone formation, providing protection similar to diminished blood flow 13. Short-term immunosuppression at times permits longer-term tissue survival after its withdrawal 14, 15. Kuroki et al. demonstrated prolonged limb allograft survival in rats after short-term 14 day Tacrolimus therapy 16. Muramatsu et al. also reported an average 31 day delay in hind limb allograft rejection after short-term Tacrolimus therapy (1mg/kg/day for 30 days) in rats 15. The mechanism that permits tissue survival has been presumed to be sequestration of immunogenic matrix by new bone formation, but this assumption has not been demonstrated experimentally 13, 17. A similar finding would be expected with induction of donor-specific tolerance or with successful drug-mediated immunosuppression.
Some evidence from solid organ transplantation suggests that tolerance is associated with the development of mixed chimerism (survival of transplant lymphocytes in recipient lymphoid tissue) 18, 19, although a causal relationship has not been proven. Future studies of bone and composite tissue allotransplantation should include analysis of recipient animal lymphoid tissue for mixed chimerism, as well as analysis of osteocytes for quantification of transplant chimerism. We believe our methods have considerable potential in answering these important questions.
Our results demonstrate that, while varying proportions of remaining donor DNA were identified in all groups, most of the DNA proved to be male (recipient derived) in origin. Our samples, obtained by laser capture microdissection typically included a relatively large number of cells. While efforts to sample osteocytes alone was the goal, it is possible that the samples included other types of recipient-derived (male) cells, DNA remnants from both male and female nonviable cells as well as surviving transplanted female cells. We believe that the lineage results demonstrate an ongoing remodelling process, however, mediated primarily by recipient animal cells via the recipient-derived neoangiogenic blood supply.
Short-term Tacrolimus immunosuppression seems to play a key role in maintaining osteocyte viability during development of a neoangiogenic circulation using our bone transplantation method. This is reflected in the histologic scores. For example, group 4 (IS; ligated AV bundle) transplants have no blood supply after withdrawal of the immunosuppression, and so have fewer viable cells than group 3. Furthermore, while only group 4 had a significantly lower rER than group 1, groups 3 and 4 both reveal a trend towards a lower rER than groups 1 and 2, presumably due to the use of immunosuppression. The difference between groups 4 and 2 emphasizes this trend. The lack of statistical significance is due at least in part to the reduced sample size in group 2.
These results indicate that short term immunosuppression may cause relatively less donor cell death resulting in decreased repopulation of the transplant by male recipient cells. However, this protection of donor cell viability by immunosuppression seems to be undermined by the presence of a patent recipient arteriovenous bundle, reflected by the higher rER in group 3 compared to group 4. Long term maintenance of blood supply may result in increased exposure of donor cell surface alloantigens to the recipient immune system, resulting in increased rejection of donor cells. This could allow for more repopulation with recipient cells whose migration is facilitated by the neoangiogenic recipient blood supply.
Further study will be required, with identification and sampling of osteocytes in regions of new cortical bone formed following transplantation. It may be that bone formation follows a bi-modal distribution, beginning initially by surviving allogeneic osteoblasts, but eventually predominated by osteogenesis from recipient-derived cells invading the transplant 20. With our unique transplantation method, one might expect the microsurgical anastomoses in animals receiving no immunosuppression to thrombose quickly, and the female osteocytes to die. Thus, most DNA isolated from the bone would be from viable male cells engaged in creeping substitution of necrotic bone. Our histologic and lineage data would support this hypothesis. All other groups would likely have at least some female (transplanted) cells surviving, via nutrition provided from either a patent AV bundle (if not ligated), a patent nutrient vessel (if IS used), or both. This is indeed what our data suggest. A more detailed study performed with multiple fluorochrome labeling at early and late time points would help to demonstrate if such a bimodal distribution of osteocyte lineage can be found in femora treated with both IS and AV-bundle implantation.
Acknowledgments
Funding: This project was funded by a National Institute of Health Grant - AR49718. Tacrolimus (FK-506) was kindly donated by Fugisawa Pharmaceutical Co, Ltd., Osaka, Japan.
Footnotes
No authors report any conflicts of interest.
Contributor Information
Michael Pelzer, The Department of Orthopedic Surgery, Microvascular Research Laboratory, Mayo Clinic, Rochester, Minnesota.
Mikko Larsen, The Department of Orthopedic Surgery, Microvascular Research Laboratory, Mayo Clinic, Rochester, Minnesota.
Patricia F. Friedrich, The Department of Orthopedic Surgery, Microvascular Research Laboratory, Mayo Clinic, Rochester, Minnesota
Ross A. Aleff, The Department of Orthopedic Surgery, Microvascular Research Laboratory, and Molecular Core Facility, Mayo Clinic, Rochester, Minnesota
Allen T. Bishop, The Department of Orthopedic Surgery, Microvascular Research Laboratory, Mayo Clinic, Rochester, Minnesota
References
- 1.Bishop AT, Pelzer M. Vascularized bone allotransplantation: current state and implications for future reconstructive surgery. Orthop Clin North Am. 2007;38:109–122. vii. doi: 10.1016/j.ocl.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Pelzer M, Larsen M, Chung YG, et al. Short-term immunosuppression and surgical neoangiogenesis with host vessels maintains long-term viability of vascularized bone allografts. J Orthop Res. 2007;25:370–377. doi: 10.1002/jor.20313. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu K, Bishop AT, Sunagawa T, Valenzuela RG. Fate of donor cells in vascularized bone grafts: identification of systemic chimerism by the polymerase chain reaction. Plast Reconstr Surg. 2003;111:763–772. doi: 10.1097/01.PRS.0000041532.11604.B5. [DOI] [PubMed] [Google Scholar]
- 4.Pelzer M, Larsen M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout Technique-Part I: quantitative evaluation of tissue perfusion in the laboratory rat. J Orthop Res. 2008;26:741–745. doi: 10.1002/jor.20562. [DOI] [PubMed] [Google Scholar]
- 5.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 6.Schefe JH, Lehmann KE, Buschmann IR, et al. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression's C (T) difference” formula. J Mol Med. 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 7.Muramatsu K, Valenzuela RG, Bishop AT. Detection of chimerism following vascularized bone allotransplantation by polymerase chain reaction using a Y-chromosome specific primer. J Orthop Res. 2003;21:1056–1062. doi: 10.1016/S0736-0266(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 8.Muramatsu K, Bishop AT. Microchimerism following vascularized bone allotransplantation. Transplant Proc. 2002;34:2722–2724. doi: 10.1016/s0041-1345(02)03387-0. [DOI] [PubMed] [Google Scholar]
- 9.Csete M. Gender issues in transplantation. Anesth Analg. 2008;107:232–238. doi: 10.1213/ane.0b013e318163feaf. [DOI] [PubMed] [Google Scholar]
- 10.Medawar P. Transplantation of tissues and organs: introduction. Br Med Bull. 1965;21:97–99. [Google Scholar]
- 11.Woodruff MFA, Woodruff HG. The transplantation of normal tissue: with special reference to auto- and hemotransplants of thyroid and spleen in the anterior chamber of the eye, and subcutaneously, in guinea-pig. Philosoph Trans R Soc Lond (Biol) 1950;234:559–581. [PubMed] [Google Scholar]
- 12.Pober JS. Is host endothelium a silver lining for allografts. Lancet. 2001;357(9249):2–3. doi: 10.1016/S0140-6736(00)03558-3. [DOI] [PubMed] [Google Scholar]
- 13.Burchardt H, Glowczewskie FP, Enneking WF. Allogeneic segmental fibular transplants in azathioprine-immunosuppressed dogs. J Bone Joint Surg Am. 1977;59:881–894. [PubMed] [Google Scholar]
- 14.Lee WP, Pan YC, Kesmarky S, et al. Experimental orthotopic transplantation of vascularized skeletal allografts: functional assessment and long-term survival. Plast Reconstr Surg. 1995;95:336–349. [PubMed] [Google Scholar]
- 15.Muramatsu K, Doi K, Akino T, et al. Longer survival of rat limb allograft. Combined immunosuppression of FK-506 and 15-deoxyspergualin. Acta Orthop Scand. 1997;68:581–585. doi: 10.3109/17453679708999031. [DOI] [PubMed] [Google Scholar]
- 16.Kuroki H, Ishida O, Daisaku H, et al. Morphological and immunological analysis of rats with long-term-surviving limb allografts induced by a short course of FK 506 or cyclosporine. Transplant Proc. 1991;23:516–520. [PubMed] [Google Scholar]
- 17.Muramatsu K, Doi K, Kawai S. Limb allotransplantation in rats: combined immunosuppression by FK-506 and 15-deoxyspergualin. J Hand Surg [Am] 1999;24:586–593. doi: 10.1053/jhsu.1999.0586. [DOI] [PubMed] [Google Scholar]
- 18.Foster RD, Ascher NLM, McCalmont TH, et al. Mixed allogeneic chimerism as a reliable model for composite tissue allograft tolerance induction across major and minor histocompatibility barriers. Transplantation. 2001;72:791–797. doi: 10.1097/00007890-200109150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 20.de Potzolli O. Evaluation des Schicksals integrierter Knochentransplantate durch kombinierten Einsatz klinischer, radiologischer und nuklearmedizinischer Verfahren. Medizinische Fakultät der Universität Ulm; Stuttgart: 2005. [Google Scholar]