Abstract
Behavioral momentum theory proposes that operant behavior is the product of two separable processes: its rate of occurrence and its resistance to change. Generally speaking, operant situations providing more densely spaced or greater magnitudes of reinforcement should be more resistant to disruption. Attempts to disrupt ongoing behavior by manipulating the availability of food or deprivation level typically have supported the predictions of behavioral momentum. Tests with pharmacological disruptors, however, have yielded mixed results. Most investigations of pharmacological disruption of operant behavior have evaluated momentum across situations that differ in rate of reinforcement. The present experiment was an attempt to systematically replicate prior work, but under conditions of differing reinforcement magnitudes. Pigeons were trained to key peck on a multiple fixed-ratio thirty schedule of food presentation, where different components programmed 2, 4, or 8-s access to grain. Resistance to rate-decreasing effects of drugs was evaluated with several compounds drawn from distinct pharmacological classes: chlordiazepoxide, cocaine, clonidine, haloperidol, morphine, and ethanol were tested. Additionally, disruption by pre-feeding and extinction was examined. Generally, resistance to change by drug administration was not modulated by reinforcement magnitude. Pre-feeding and extinction tests, however, replicated previous work, indicating that our procedure was sensitive to more common disruptors. The results give additional support to the notion that pharmacological disruptors may not behave in the manner predicted by behavioral momentum theory.
Keywords: behavioral momentum, reinforcement magnitude, fixed ratio, resistance to change, pigeon, key peck
Behavioral momentum is a development in learning theory that proposes behavior is the result of two separable processes: its rate of occurrence and its resistance to change (Nevin, 1992; Nevin & Grace, 2000; Nevin, Tota, Torquato, & Shull, 1990). The theory is an analogy of behavior to the momentum of a physical body. Response rate is analogous to velocity of a moving body, and resistance to change is analogous to the body’s mass. Behavioral momentum, then, is the product of these two orthogonal quantities, just as the momentum of the physical body is the product of its velocity and mass. The distinction between response rate and resistance to change, moreover, may also be said to be the distinction between performance and learning (Nevin, 1974). Parsing performance measures from learning measures gives the model intuitive appeal. Consider a pianist at different times playing two pieces of music, one rapid and upbeat and the other slow and melancholy. One would be hesitant to say the upbeat piece is better “learned” simply because notes are emitted at a higher rate.
Response rate and resistance to change are each thought to stem from different relationships imbedded in the three-term contingency. Response rate is a result of response-reinforcer relationships. Resistance to change, on the other hand, is thought due to stimulus-reinforcer relationships, and so generally speaking, environments arranging denser reinforcer deliveries should enhance the behavioral “mass” and promote greater resistance to perturbations over environments arranging leaner reinforcer densities (Nevin & Grace, 2000; Nevin et al., 1990). Attempts to perturb or disrupt ongoing behavior have been implemented in a variety of ways, some of the most common include the delivery of response-independent reinforcement (Nevin, 1974; Nevin, Mandell, Atak, 1983; Shahan & Burke, 2004), satiation to the reinforcer (Nevin et al., 1990), and extinction of operant behavior (Nevin, 1974; Nevin et al., 1990). Across a wide variety of procedures, the literature supports the prediction that responding maintained under circumstances of denser reinforcement, to the exclusion of differences in response rate, will be more resistant to disruption (see, Nevin, 1974; Nevin, 1992; Nevin & Grance, 2000).
The support for behavioral momentum theory, however, is less unanimous when resistance to pharmacological disruptors is considered. On the one hand, there are reports of consistency. Harper (1999a) examined haloperidol and clozapine on multiple variable-interval (VI) schedules. In one component of the schedule, extra, response-independent food deliveries were programmed by a concomitant variable-time (VT) schedule. The results showed that resistance to the rate-decreasing effects of both drugs was greatest for behavior maintained under the component with the added food deliveries. A similar experiment replicated those results with quinpirole and fluoxetine, but not d-amphetamine, which was attributed to a specific disruption in stimulus control of the latter (Harper, 1999b). Additionally, Egli, Schaal, Thompson, and Cleary (1992) examined the effects of methadone and buprenorphine and concluded that resistance to disruptions in responding was greater under conditions providing a denser schedule of reinforcement.
On the other hand, contrary findings have been reported. Cohen (1986) examined several compounds from a variety of drug classes (d-amphetamine, haloperidol, cholecystokinin-octapeptide, and pentobarbital) on three different procedural arrangements where operant responding produced food (chained random-interval (RI), multiple fixed-interval (FI), and multiple RI schedules). The results failed to find general support for behavioral momentum. More recently, Jimenez-Gomez and Shahan (2007) examined the resistance to change of ethanol-maintained responding of rats. Ethanol was available by a multiple variable-interval (VI) 15 s VI 45 s schedule. Consistent with predictions of momentum theory, behavior maintained by the richer schedule of ethanol was more resistant to extinction than behavior maintained by the leaner schedule. Although behavior maintained by the denser schedule of ethanol was shown to be more resistant to extinction, tests of the rate-decreasing effects of naltrexone did not reveal any greater resistance to change under the component arranging a denser schedule of reinforcement.
Recent work from our lab has provided some additional support to the notion that pharmacological disruptors do not operate as do the more traditional tests. Lamb and Ginsburg (2005) trained pigeons to respond on a three-component multiple schedule. In each component, food was made available following completion of a fixed-ratio (FR) 30 schedule of food delivery. Each component arranged a different duration of food access, either 2, 4, or 8 s. Once stable performance was attained, each pigeon was examined under a range of doses of the serotonin reuptake blocker fluvoxamine and the norepinephrine reuptake blocker desipramine. As resistance to change should be greater under a denser, compared to a leaner, schedule of reinforcement, so should resistance to change be greater under larger, compared to smaller, magnitudes of reinforcement, as both can be equated to duration of access per unit time (Nevin, 1974; Harper & McLean, 1992). Lamb and Ginsburg, however, did not find any greater resistance of behavior maintained by the longer duration of access to the rate-decreasing effects of either fluvoxamine or desiprimine.
Comparing the disparate results across the literature, one noticeable difference between different reports is the schedule of reinforcement maintaining operant behavior. Studies that obtained results consistent with behavioral momentum theory generally used multiple VI schedules (e.g., Egli et al, 1992; Harper, 1999a; Harper, 1999b; but see data from Cohen, 1986 with multiple random-interval (RI) schedules), whereas inconsistent results were obtained with a variety of fixed schedules and chained RI schedules (e.g., Cohen, 1986; Lamb & Ginsburg, 2005). Such differences raise the possibility that the lack of differential effect of fluvoxamine or desipramine found by Lamb and Ginsburg may have been due to the choice of a multiple FR schedule.
Yet, previous research has shown that resistance to change of operant behavior maintained by multiple FR schedules can conform to the predictions of behavioral momentum. Cohen, Riley, and Weigle (1993), for example, showed that behavior during a component arranging a small FR schedule was more resistant to the effects of pre-feeding and extinction than behavior in a component correlated with a larger FR schedule. Related to this outcome, Hoffman, Branch, and Sizemore (1986) showed that behavior maintained in a component correlated with a small FR schedule was less sensitive to the rate-decreasing effects of cocaine than behavior maintained in components correlated with larger FR values, which is consistent with the predictions of behavioral momentum (see also Pinkston & Branch, 2004). So, at least when FR parameter has been manipulated in a multiple schedule context, resistance to change in the face of traditional (e.g., pre-feeding) and pharmacological (e.g., cocaine) disruptors have been shown to conform to the predictions of behavioral momentum theory.
With regard to Lamb and Ginsburg (2005), the data suggest that it was not the choice of FR schedules, per se, that may account for the lack of differential effects of fluvoxamine and desiprimine. Another distinct feature of Lamb and Ginsburg’s procedure was the manipulation of reinforcement magnitude, rather than reinforcement rate, across schedule components. Control by reinforcement magnitude has found mixed effects in the literature (see, Bonem & Crossman, 1988), so perhaps the procedure used by Lamb and Ginsburg simply did not permit differential sensitivity to any form of disruption. Thus, the present experiment had two goals. First, it was important to evaluate the procedures used by Lamb and Ginsburg (2005) under tests of more traditional disruptors of operant behavior (e.g. pre-feeding and extinction) to assess resistance to change of a single FR parameter under differing conditions of reinforcement magnitude. Second, as reinforcer magnitude has received less attention in studies of pharmacological disruption, we examined several additional compounds under this procedure to explore further the pharmacological specificity of our earlier effects. Compounds were selected across a wide array of target systems, and included some compounds from classes previously tested in resistance to change research (i.e., chlordiazepoxide, cocaine, haloperidol, and morphine) and some novel compounds (i.e., clonidine and ethanol).
METHOD
Subjects
Ten adult White Carneau pigeons were used in the present experiment. Some pigeons participated in a previously published experiment (Lamb & Ginsburg, 2005) examining the effects of desiprimine and fluvoxamine (see Table 1); others were drug naïve. Each pigeon was housed individually and maintained at 80% of its laboratory free-feeding weight. Supplemental food was given as needed after each session to maintain body weight. Pigeons were housed in a colony room maintained on a 12:12 light:dark cycle; pigeons were tested during the light portion of the cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Table 1.
Order of conditions for each pigeon.
| Pigeon | Conditions |
|---|---|
| R131 | Ethanol, Cocaine, Pre Feed, EXT2, Chlordiazepoxide, Morphine, Clonidine, Haloperidol |
| R141 | Ethanol |
| R151 | Ethanol, Cocaine, Pre Feed, EXT, Chlordiazepoxide, Morphine, Clonidine, Haloperidol |
| R161 | Ethanol, Cocaine, Pre Feed, EXT, Chlordiazepoxide, Morphine, Clonidine, Haloperidol |
| R171 | Ethanol |
| R26 | Cocaine, Pre Feed, EXT, Chlordiazepoxide, Morphine, Clonidine, Haloperidol |
| R28 | Clonidine, Pre Feed, EXT, Cocaine, Haloperidol |
| R30 | Pre Feed, EXT, Morphine, Chlordiazepoxide, Haloperidol |
| R31 | Pre Feed, EXT, Morphine, Chlordiazepoxide, Cocaine, Clonidine |
| R33 | EXT |
Previous experience with fluvoxamine and desiprimine
Extinction
Apparatus
Experimental sessions were conducted in Gerbrands G7410S test chambers (Alderston, MA, USA). Each chamber was 27.5 cm × 26.4 cm × 27.5 cm. One wall of the chamber was made of aluminum and served as the intelligence panel; the remaining walls and ceiling were made of Plexiglas™. The floor was made of a steel grid. On the intelligence panel, three response keys were located 18 cm above the chamber floor, equidistant from each other. Only the center key was used in the present experiment. The key could be illuminated from behind by 24-V bulbs. Bulbs were covered with white, green, or red caps. Below the center key a square aperture permitted access to a food hopper. When raised, the hopper provided access to a standard pigeon diet (Purina Checkers, Purina Mills, St Louis, MO, USA). Each chamber was enclosed in a light- and sound- attenuating shell (Gerbrands, G7211) equipped with an exhaust fan. Experimental events and data recording were accomplished by a computer running Med-PC IV (Med Associates, Georgia, VT).
Procedure
Each pigeon was trained to key peck under a three-component multiple FR 30 schedule of food presentation. Session onset was signaled by the illumination of the houselights. Components differed with respect to the duration of food access permitted following the completion of the ratio. When the key was lit green, completion of the ratio permitted access to food for 2 s. When the key was lit white, food access was 4 s following ratio completion; and when lit red, each completed ratio provided 8-s access to the food hopper. Each component had a 60-s limited hold (i.e., if the 30-response requirement was not met within 60 s, the component ended). When either the available food was collected or the limited hold period expired, a 60-s time out was imposed where all lights were extinguished and responses had no programmed consequences. At the end of the time out, one of the other schedule components was selected. Selection of components was determined by first compiling the list of the six possible sequences by which 2-s, 4-s, and 8-s components could be ordered. For each component block, one of these possibilities was selected from the list randomly without replacement. Experimental sessions were 60 min in duration and were conducted Monday-Friday.
Once responding stabilized on the multiple schedule, each pigeon was tested across a variety of conditions examining several drugs. One to three weeks separated successive conditions. In the pre-feeding condition, each pigeon was fed a controlled amount of food 30 min prior to the daily session. The amount of food was 0, 5, 10, 20, 40, or 80 g. The testing of different food amounts followed the scheme by which doses of drug were tested (see below); 0 g of food can be considered the “vehicle” in translating from the dosing scheme to the pre-feeding condition and served as a control measurement. The extinction (EXT) condition was conducted in a single week. Each pigeon was exposed to the multiple schedule as normal on the Monday of the condition. On the Tuesday, Wednesday, and Thursday of the week, all components of the multiple schedule was changed to extinction. During EXT sessions, components were selected as before and presented for 60 s. During these periods, pecks were recorded, but the feeder cycle was suspended. Following each component presentation, the 60-s time out period was programmed as before. Values from the preceding Monday served as control for EXT sessions. On Friday, the original multiple schedule was reinstated. The order of testing conditions for each pigeon can be seen in Table 1.
Drugs
Cocaine hydrochloride and morphine sulfate (obtained from NIDA) and chlordiazepoxide hydrochloride and clonidine hydrochloride (Sigma, St. Louis, MO) were dissolved in physiological saline to provide an injection volume of 1.0 ml/kg. Haloperidol was dissolved into an acidified saline vehicle (1:9 ratio of 1 N acetic acid to saline) to an injection volume of 1.0 ml/kg. With the exception of ethanol, each compound was administered im 10 min prior to experimental sessions. Injections were made into the pectoral muscle. All doses are expressed in terms of the salt. Ethanol (Aaper Alcohol & Chemical Company, Shelbyville, KY) was dissolved into tap water to a volume of 2 ml and administered 10 min prior to the session by oral gavage. Drugs were administered on Tuesdays and Fridays, and vehicle was administered on Thursdays. Vehicle was also given on Tuesdays and Fridays in place of the scheduled dose; these determinations served as control points. Once the dose-response tests were completed, each dose was re-determined a second time.
Data Analysis
In the analysis of the drug effects, we first examined absolute response rates. We conducted a three-way repeated-measures ANOVA (Reinforcer magnitude X Dose X Determination) for each compound tested. For some compounds, not all pigeons were tested at each dose (see Results). In those cases, the ANOVA’s were conducted once excluding pigeons with missing data, and again including data from all pigeons, but omitting any dose that was not equally represented. Our main interest in these preliminary ANOVA’s was assessing any systematic change in the dose-response functions from the first to the second determination. None of the preliminary analyses revealed either a main effect of determination or a significant interaction of determination with other terms. As the order of determinations had no significant effect, multiple determinations were averaged for each dose. The average dose-response function was then converted to a percentage of values obtained after determination of the vehicle (those obtained on Tuesdays or Fridays). Scaling the dose-response functions by the rate obtained under vehicle tests provided a common footing for the examination of resistance to change across the dose-response function. Data from the non-drug conditions were also normalized to control response rates.
A second approach to data analysis was the computation of ED50’s (the dose at which responding is reduced by 50% of control values) from each dose response function. ED50’s were computed for each subject from the descending limb of the dose-response function that ranged between 80% and 20% of values obtained under vehicle administration. Points outside of this range were used when only those two points defined the line crossing a 50% decrease in responding. The only exception was the data from R31 under tests of chlordiazepoxide. The highest doses tested, 32 mg/kg, only decreased responding to 50% of control. In that single case, the function ranging from 100% to 50% was identified and the ED75 was calculated (see Table 2). The calculation of the ED75 for that single function was for descriptive purposes, and was not included in any further summaries or statistical comparisons. The computation of the ED50 was made by regressing the descending portion of the function against the log10 of the dose. The linear equation from the fit was solved for the x-value yielding a 50% reduction in rate and that value was exponentiated to obtain the dose. Normalized response rate and ED50 values were examined with repeated-measures ANOVA. Post-hoc analyses of linear trend or paired t-tests were used to evaluate specific comparisons when appropriate. In the case of multiple t-tests, alpha levels were adjusted by the Bonferroni method to keep the experiment-wise alpha level equal to .05.
Table 2.
Effects of different pre-session food amounts on behavior maintained by different durations of gain access expressed as a proportion of control values.
| Pigeon | 80% Wt. (g) | Access (s) | 20 g | 40 g | 80 g |
|---|---|---|---|---|---|
| R13 | 464 | 2 | 0.87 | 0.64 | 0.22 |
| 4 | 0.92 | 0.82 | 0.48 | ||
| 8 | 0.83 | 0.83 | 0.51 | ||
| R15 | 482 | 2 | 0.73 | 0.19 | 0.09 |
| 4 | 0.87 | 0.58 | 0.29 | ||
| 8 | 0.87 | 0.73 | 0.29 | ||
| R16 | 485 | 2 | 0.61 | 0.51 | 0.13 |
| 4 | 0.82 | 0.91 | 0.47 | ||
| 8 | 0.73 | 0.80 | 0.48 | ||
| R26 | 511 | 2 | 0.94 | 0.89 | 0.13 |
| 4 | 0.98 | 0.97 | 0.48 | ||
| 8 | 1.02 | 1.03 | 0.57 | ||
| R28 | 480 | 2 | 0.69 | 0.36 | 0.00 |
| 4 | 0.79 | 0.48 | 0.00 | ||
| 8 | 0.61 | 0.46 | 0.00 | ||
| R30 | 497 | 2 | 0.52 | 0.22 | 0.03 |
| 4 | 0.89 | 0.66 | 0.43 | ||
| 8 | 0.92 | 0.76 | 0.46 | ||
| R31 | 525 | 2 | 1.06 | 0.67 | 0.56 |
| 4 | 1.00 | 0.82 | 0.86 | ||
| 8 | 0.97 | 0.92 | 0.91 |
RESULTS
Analysis of Baseline Responding and Non-Pharmacological Disruptors
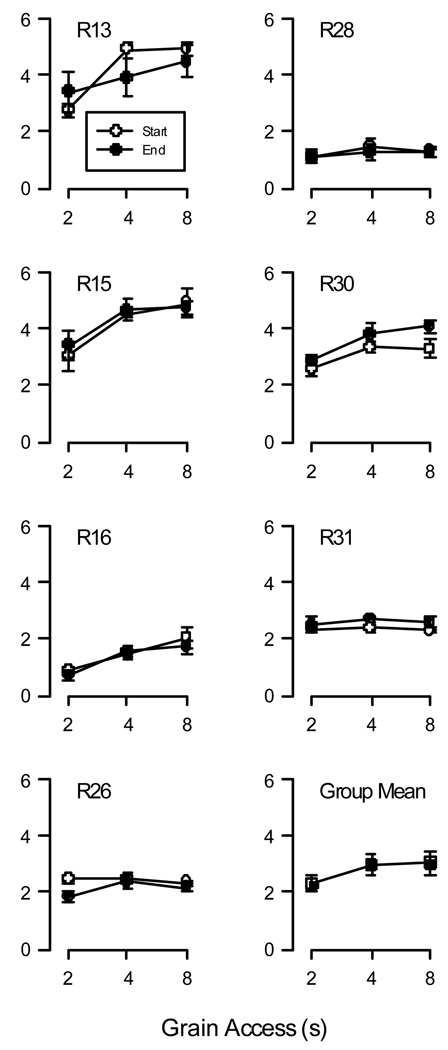
Before examining the drug effects, we will consider the baseline rates of responding. Although absolute response rate should not impact resistance to change, it is useful to establish that performance was stable throughout various phases of testing. Changes in the baseline performance could indicate shifts in the units of behavior, or irreversible drug effects that could confound interpretation of normalized data. Figure 1 shows data from the seven pigeons that participated in more than one condition (R14 and R17 died soon after the end of the last condition and not enough data were available to evaluate once conditions concluded; R33 was only exposed to the week-long Extinction procedure). Data were taken from the last five sessions the week prior to the first testing condition and the last five sessions from the week following the end of the final condition. In general, responding was stable throughout the experiment as suggested by Figure 1. Also, response rate appeared generally to be an increasing function of reinforcer magnitude. A two-way repeated-measures ANOVA revealed that there was no significant difference between baseline responding at the start and end of the experiment (F(1,6) = .005, p < .94), but there was a significant effect of food access duration (F(2,12) = 9.83, p < .003). The interaction was not significant. To further compare differences in rate due to access times, the individual functions in Figure 1 were averaged. Data from the three remaining pigeons over the week prior to testing were averaged and included in the group-wise analysis. The combined data are shown in the lower right graph in Figure 1. Repeated-measures ANOVA on the mean response rate revealed a significant effect of food access (F(2,18) = 12.30, p < .001). Paired t-test comparisons between each of the three presentation times indicated that responding maintained by 2-s grain access was significantly different from that maintained by 4-s and 8-s access (p < .02 and p < .005, respectively). The latter were not significantly different from each other.
Figure 1.
Responses per second are plotted as a function of duration of food access. Data are shown for each pigeon that participated in more than two conditions; as well as the group mean for all 10 pigeons (bottom right). For individual data plots, open symbols plot the baseline rates of responding in the week prior to the onset of the first conditions; closed symbols plot rates after the end of the final condition. Error bars denote standard deviation for individual data and the standard error for the group data.
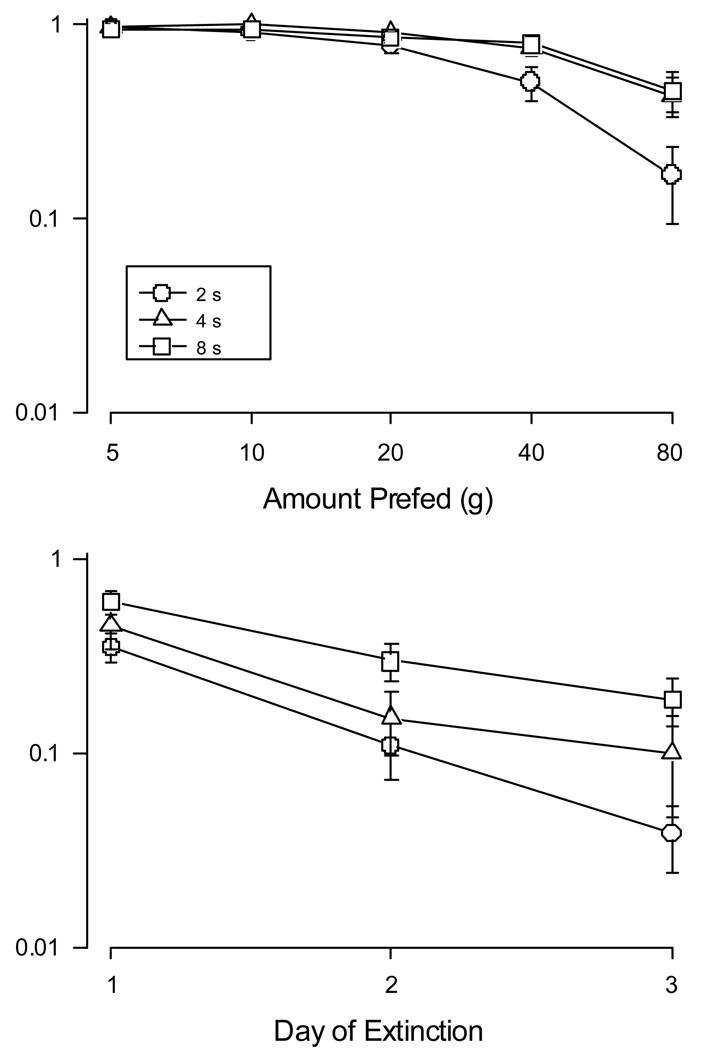
Having established the reliability of behavior over the experiment, we turn our attention to the non-pharmacological disruptors evaluated on our procedure. The effect of pre-feeding various amounts of food can be seen in top graph of Figure 2. For comparison, experimental weights for each pigeon are shown in Table 2. Responding was decreased more rapidly in the component that provided 2-s access of food relative to the other components. A repeated-measures ANOVA comparing the effects of the pre-fed amount and the duration of food access revealed a significant effect of pre-feeding (F(4,24) = 35.93, p < .001) and a significant effect of reinforcer magnitude (F(2,12) = 14.50 p < .001); additionally, the interaction was significant (F(8, 48) = 9.36, p < .001), consistent with the steeper decline of responding maintained by the shortest access time. An analysis of the individual subject data shows that the apparent trends reflect order at the level of individual pigeons. Table 2 shows values obtained for selected pre-fed amounts; the data were chosen to represent the part of the function encompassing the largest changes. For all pigeons, pre-feedings of 40 g or 80 g suppressed behavior more when it was maintained by 2-s access to grain compared to that seen under with either 4-s or 8-s durations; the only exception being the complete elimination of R28’s responding by 80g. Effects on behavior maintained by 4-s access to grain generally fell in the middle (10/14 cases).
Figure 2.
(Upper) Key pecks per minute, expressed a proportion of control rates, are plotted against amounts of food fed to pigeons 30 min prior to experimental sessions. Note the double logarithmic axes. Circles represent data obtained from the multiple schedule component arranging 2-s access to food, triangles represent similar data from the component arranging 4 s of food, and squares the component arranging 8-s access to food. Error bars denote the standard error. (Lower). Relative response rates are plotted for each schedule component over successive sessions of extinction. Note the axes are log-linear. Details are the same as in the upper graph.
Under extinction, responding decreased across successive days, as shown in the bottom graph of Figure 2. Decreases, furthermore, appeared greater when shorter, rather than longer, durations of food access were provided for schedule completion. A two-way repeated-measures ANOVA revealed a significant decrease across days (F(2, 14) = 43.90 p < .0001) and significant difference among durations of grain access (F(2, 14) = 4.50 p < .031). The interaction was not significant, indicating the functions declined at the same rate. Post-hoc tests showed a significant linear trend for the effect of day (F(1,7) = 46.8, p < .0001) and for the effect of grain duration (F(1,7) = 12.4, p < .01). Together these effects show that although the functions declined at the same rate, they were consistently ordered across days. The response proportions collected for each day may be seen in Table 3. Here again, the effects seen at the level of grouped data reflect order at the individual level. On the first day of extinction, there was no clear effect of reinforcer magnitude. On the second session and third sessions, responding maintained by the 2-s grain duration was suppressed consistently below that maintained by the 8-s grain presentation (7/8 cases on the second day; 8/8 on the third day). Responding during the component programming 4-s access to grain was less consistently ordered, but generally approximated levels seen under the component providing 2-s access to grain.
Table 3.
Effects of extinction on key pecking previously maintained by different durations of grain access expressed as a proportion of control values.
| Pigeon | Access (s) | Day 1 | Day 2 | Day 3 |
|---|---|---|---|---|
| R13 | 2 | 0.31 | 0.05 | 0.01 |
| 4 | 0.30 | 0.06 | 0.04 | |
| 8 | 0.31 | 0.12 | 0.04 | |
| R15 | 2 | 0.52 | 0.18 | 0.02 |
| 4 | 0.41 | 0.17 | 0.05 | |
| 8 | 0.55 | 0.49 | 0.13 | |
| R16 | 2 | 0.17 | 0.07 | 0.01 |
| 4 | 0.29 | 0.04 | 0.06 | |
| 8 | 0.97 | 0.45 | 0.26 | |
| R26 | 2 | 0.64 | 0.18 | 0.01 |
| 4 | 0.44 | 0.14 | 0.01 | |
| 8 | 0.65 | 0.20 | 0.07 | |
| R28 | 2 | 0.39 | 0.01 | 0.09 |
| 4 | 1.22 | 0.51 | 0.46 | |
| 8 | 0.66 | 0.56 | 0.40 | |
| R30 | 2 | 0.21 | 0.01 | 0.07 |
| 4 | 0.38 | 0.04 | 0.04 | |
| 8 | 0.57 | 0.21 | 0.12 | |
| R31 | 2 | 0.23 | 0.08 | 0.09 |
| 4 | 0.38 | 0.20 | 0.13 | |
| 8 | 0.20 | 0.14 | 0.42 | |
| R33 | 2 | 0.31 | 0.29 | 0.00 |
| 4 | 0.21 | 0.06 | 0.00 | |
| 8 | 0.85 | 0.20 | 0.08 |
Analysis of Pharmacological Disruptors
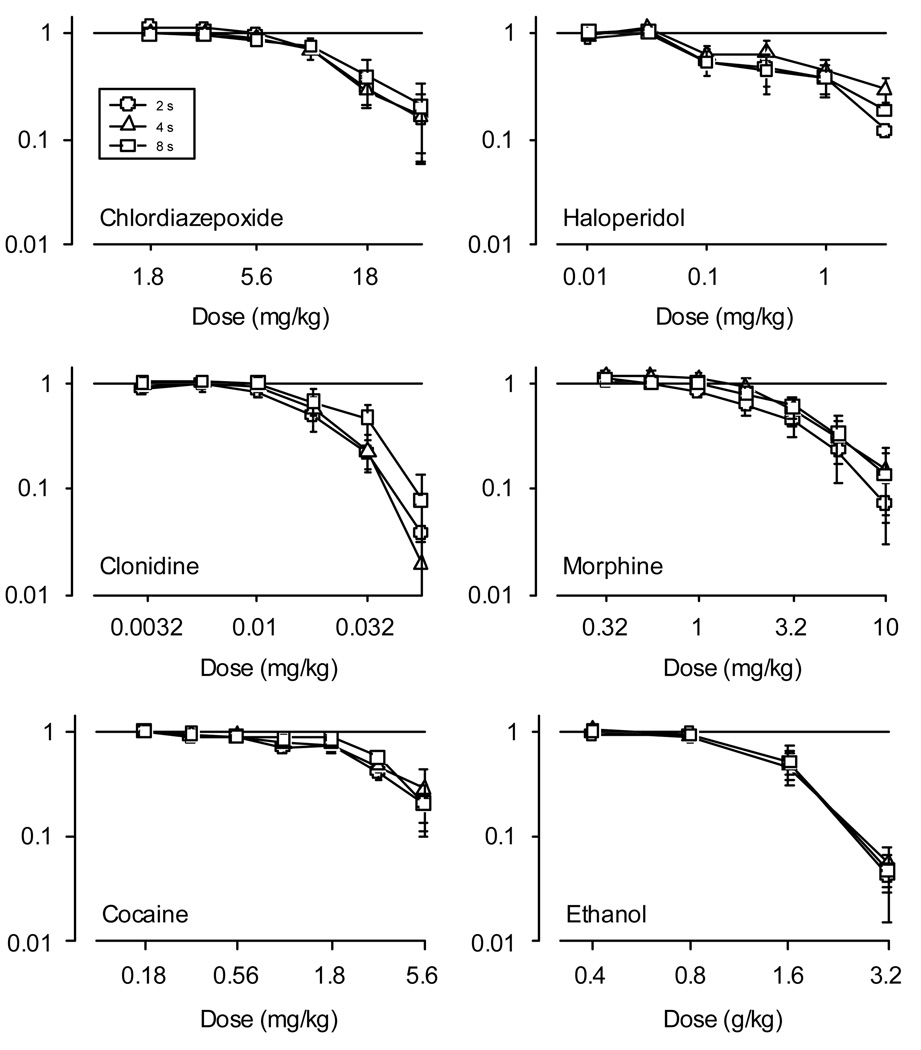
Attempts to disrupt schedule-controlled behavior with various pharmacological compounds are shown in Figure 3. Recall that we tested several compounds across a wide range of classes: sedatives (chlordiazepoxide and ethanol), stimulants (cocaine), antipsychotics (haloperidol), opioids (morphine), and alpha-2 agonists (clonidine). All drugs dose-dependently decreased operant behavior. The specific effects of each drug are discussed in turn below. Corresponding ED50 estimates from the dose response functions are shown in Table 4.
Figure 3.
Relative response rates are plotted against dose in mg/kg or g/kg (ethanol) for each of six drugs tested (see Table 1 for details). Note the double logarithmic axes. Each point is the mean of the average dose-response function obtained from each pigeon. Circles, triangles, and squares denote data obtained from multiple schedule components arranging 2 s, 4 s, or 8 s of food access, respectively. Horizontal lines at 1 indicate no change from control values. Error bars denote the standard error.
Table 4.
ED50 values (mg/kg or g/kg [ethanol]).
| Pigeon | Component | ||
|---|---|---|---|
| 2-s | 4-s | 8-s | |
| Chlordiazepoxide | |||
| R13 | 17.2 | 16.3 | 13.8 |
| R15 | 12.6 | 13.7 | 14.6 |
| R16 | 10.7 | 9.7 | 9.2 |
| R26 | 14.4 | 12.6 | 10.3 |
| R30 | 9.40 | 7.5 | 10.1 |
| R31* | 7.0 | 7.0 | 7.1 |
| Average | 12.9 | 12.0 | 11.6 |
| Clonidine | |||
| R13 | 0.014 | 0.025 | 0.035 |
| R15 | 0.027 | 0.032 | 0.049 |
| R16 | 0.028 | 0.026 | 0.030 |
| R26 | 0.015 | 0.017 | 0.018 |
| R28 | 0.008 | 0.007 | 0.005 |
| R31 | 0.018 | 0.023 | 0.042 |
| Average | 0.018 | 0.022 | 0.030 |
| Cocaine | |||
| R13 | 3.51 | 2.57 | 4.70 |
| R15 | 1.73 | 2.43 | 7.62 |
| R16 | 1.28 | 8.03 | 2.35 |
| R26 | 3.00 | 3.13 | 3.29 |
| R28 | 2.11 | 1.69 | 5.26 |
| R31 | 5.92 | 6.38 | 3.41 |
| Average | 2.93 | 4.04 | 4.44 |
| Haloperidol | |||
| R13 | 0.28 | 0.59 | 0.24 |
| R15 | 1.00 | 1.17 | 2.20 |
| R16 | 1.62 | 0.89 | 0.31 |
| R26 | 0.20 | 0.89 | 0.63 |
| R28 | 0.08 | 0.09 | 0.08 |
| R30 | 0.67 | 0.67 | 0.57 |
| Average | 0.64 | 0.72 | 0.67 |
| Morphine | |||
| R13 | 1.03 | 1.62 | 2.45 |
| R15 | 5.20 | 5.58 | 7.20 |
| R16 | 1.34 | 2.00 | 1.95 |
| R26 | 5.90 | 6.18 | 6.75 |
| R30 | 1.87 | 3.29 | 1.63 |
| R31 | 2.39 | 9.50 | 6.70 |
| Average | 2.96 | 4.70 | 4.45 |
| Ethanol | |||
| R13 | 2.57 | 2.32 | 2.27 |
| R14 | 2.12 | 1.92 | 2.07 |
| R15 | 1.39 | 1.65 | 1.87 |
| R16 | 1.30 | 2.02 | 2.06 |
| R17 | 1.27 | 1.20 | 1.18 |
| Average | 1.73 | 1.53 | 1.89 |
Indicates ED75; value not included in group mean.
Chlordiazepoxide
The plot of the normalized data indicates that the rate of suppression was similar across components. A two-way repeated-measures ANOVA (Dose X Reinforcer Magnitude) revealed a significant main effect of Dose (F(5, 25) = 44.25 p < .001), but no significant effect of either the reinforcer magnitude or the interaction term. Similarly, repeated-measures ANOVA found no significant difference among ED50 values.
Clonidine
Generally, responding maintained by shorter access durations appeared to be suppressed at lower doses than responding maintained by longest food duration. Two-way repeated measures ANOVA identified a main effect of dose (F(5, 25) = 32.93, p < .0001) and a main effect of the reinforcer magnitude (F(2,10) = 6.33, p < .017). The interaction was not significant. Despite the differences in means, post hoc tests failed to distinguish differences among components at conventional levels of significance, although a post-hoc test for linear trend on the magnitude term approached significance (F(1, 5) = 6.56, p < .052). Similarly, repeated-measures ANOVA on the ED50 values identified an effect of reinforcer magnitude (F(2, 10) = 5.19, p < .028), but post hoc pairwise comparisons did not distinguish the effects of reinforcer magnitude further. On the individual level, ED50’s were ordered with respect to grain duration for 4 out of the 6 pigeons; and the largest ED50’s were obtained for behavior maintained by the 8-s duration of grain access in 5 out of 6 pigeons.
Cocaine
In tests of cocaine, it is important to note that pigeon R31 was not tested with the 0.18 or 3.2 mg/kg doses, so some adjustment was made in the statistical analyses. Initially, the ANOVA was conducted for all pigeons, but excluded the incompletely determined doses. The result indicated a significant effect of dose (F(4, 20) = 22.23, p < .001), but no significant effect of reinforcer magnitude or of the interactions term. The ANOVA was performed a second time including all doses, but excluding the data from pigeon R31; the same pattern of results were obtained. Correspondingly, analysis of the ED50’s revealed no significant effect of food access duration.
Haloperidol
The rate-decreasing effects of haloperidol were not changed systematically by reinforcer magnitude. Pigeons R15 and R16 were not tested with the 0.032, 0.32 or 3.2 mg/kg doses of haloperidol. Pigeon R31 was not tested with 0.01 mg/kg haloperidol. Thus, only two doses (0.1 and 1.0 mg/kg) were determined in all subjects. Two-way repeated measures ANOVA performed on the data represented by all subjects revealed a significant effect of dose (F(1, 5) = 7.1, p < .04). There was no significant effect of reinforcer magnitude or the interaction term. Other ANOVA’s were conducted using other doses, but excluding the data from pigeons with incomplete dose-response functions. All indicated no effect of the grain access duration as described above. The ED50’s from the dose-response functions parallel the plotted data and ANOVA’s; that is, ED50’s did not differ significantly across the different food durations.
Morphine
Visual inspection of the graph first suggests that responding across the middle part of the dose range was decreased more when behavior was maintained by 2-s access to food relative to longer access times. Two-way repeated-measures ANOVA, however, indicated only a significant effect of the dose (F(6, 30) = 27.19. p < .001); there was no significant effect of reinforcer magnitude or of the interaction term. Similarly, ANOVA conducted on the ED50 values also found no significant effect of reinforce magnitude. As can be seen in Table 4, however, data from 5 out of 6 pigeons showed the lowest obtained ED50 values were correlated with the 2-s grain access component, supporting the apparent trend in Figure 3.
Ethanol
In tests of ethanol, R14 and R17 were tested only once with 3.2 g/kg ethanol, and so contributed only a single determination at that dose; all points were determined twice for the rest of the pigeons. Inspection of the data shown in Figure 3 indicates, on average, nearly identical effects of ethanol on performance in each of the schedule components. Two-way repeated-measures ANOVA confirm the impression of the figure. Dose-dependent decreases were significant (F(3, 12) = 22.86, p < .001), but there was no main effect of either the reinforcer magnitude or the interaction. Likewise, repeated-measures ANOVA on the ED50 values found no significant effect of reinforcer magnitude.
DISCUSSION
The present experiment extends our previous work in two important ways. First, the inclusion of the pre-feeding and extinction conditions showed that responding maintained by the larger magnitudes of reinforcement were more resistant to change than responding maintained by the smallest magnitude. The results, moreover, were reliable and evident at the level of individual subjects. So, the negative findings of Lamb and Ginsburg (2005) were not likely due to failure of their procedure to establish a “mass” differential across schedule components. Additionally, the data obtained with traditional disruptors are important in extending the generality of the predictions of behavioral momentum theory to FR performance, which has not been examined as extensively as that of other schedules. Our data, in combination with others (e.g., Cohen, Riley, & Weigle, 1993), indicate that the use of FR schedules per se does not imply an inability to obtain results consistent with the predictions of behavioral momentum.
Second, disruption of responding by acute drug administration generally was not related to reinforcement magnitude; and this was true for five of the six drugs tested, although the data from morphine tests revealed a trend consistent with differential effectiveness of reinforcement magnitude. Lamb and Ginsburg (2005) reported that the rate-decreasing effects of fluvoxamine and desiprimine also did not depend on reinforcement magnitude maintaining FR 30 performance in their experiment. The results of the present work are consistent with those effects and suggest that pharmacological disruption may not meet the predictions of behavioral momentum when behavior is maintained by FR schedules programming different magnitudes of reinforcement.
Out of all the drugs tested, only clonidine provided effects consistent with the predictions of behavioral momentum. Previous research has shown that clonidine may be effective at aiding in sustained attention in tests of working memory (Ramos & Arnsten, 2007; Southwick, et al., 1999) and has been effective in treatment of attention-deficit hyperactivity disorder (Arnsten, Scahill, & Findling, 2007). Presumably, such effects are due to clonidine’s action at post-synaptic alpha-2 adrenoreceptors (Ji, Ji, Zhang, & Li, 2008; Robbins, 1997), but such improvement could also reflect pre-synaptic mechanisms. In any case, the effects obtained in the present experiment might be related to enhanced attentional or stimulus control effects garnered by clonidine. It must be stressed, however, that even those effects were modest, so much so that post-hoc tests strained to identify specific differences for either response rate decrements or ED50 values.
In a review of the literature, we were unable to find additional data showing differential effects of clonidine on resistance to change. Halevy, Feldon, and Weiner (1986), for example, examined clonidine’s effects on rats’ running speeds in a straight alleyway. Different groups of rats received food after either every trip down the alley (continuous reinforcement, CRF) or for half of the trips, on average (partial reinforcement, PRF). Later extinction was implemented for both groups. Clonidine was administered across groups in different phases of training, either prior to extinction, during extinction, or both. Clonidine given during extinction increased resistance to extinction, but did so for both CRF and PRF groups. In a different study, Smith (1990) examined clonidine’s effects on fixed-ratio responding, either FR 10 or FR 40, in separate groups of rats. Weekly tests across a range of doses found similar rate-decreasing effects of clonidine on local response rates of each schedule (effects of clonidine on overall rate were not reported). The prior work, however, must be interpreted cautiously with respect to our data, because much of the prior research on clonidine used between-subjects designs. Cohen (1998) has shown that the predictions of momentum are better met using within-subject methods where different conditions alternate rapidly within a single session (e.g., a multiple schedule). So, the lack of corroborating data may reflect more the use of between-subject manipulations, rather than differential effects of clonidine on resistance to change.
While the results obtained with clonidine may provide modest support for the predictions of behavioral momentum theory applied to the disruptive effects of drugs, recall that the majority of the present findings are in contradiction. Here, resistance to change due to haloperidol administration did not covary with reinforcement magnitude (see also Cohen, 1986), which stands in opposition to the findings of Harper (1999a). Neither were the disruptive effects of morphine strongly correlated with reinforcer magnitude, which is in some opposition to the findings of Egli et al. (1992) in their examination of methadone. Overall, the literature provides no clear indications of the boundary conditions under which behavioral momentum will hold for pharmacological disruptors. Harper (1999b) has suggested that behavioral momentum may be decreased under conditions where the drug reduces stimulus control over behavior. While such reductions may occur, as shown with Harper’s results for d-amphetamine, it does not seem that single account could be true unilaterally when one considers the variety of drugs tested on our procedure: fluvoxamine, desiprimine, cocaine, haloperidol, morphine, ethanol, and chlordiazepoxide are drugs with very different pharmacological profiles. It seems unlikely that they all operate by analogous reduction of stimulus control (see also Robbins, 2002).
While the present experiment does not resolve the discrepancies in the literature, we can suggest two avenues for future research. First, it seems necessary to develop a deeper appreciation for how different schedules control behavior, and subsequently how different drugs affect those controlling mechanisms (see, Branch, 1984) in relation to resistance to change. Consider the differing effects of haloperidol observed between our work and that of Harper (1999a). While we found little support for behavioral momentum when examining pharmacological disruption using FR schedules, Harper did obtain outcomes consistent with behavioral momentum using VI schedules. Interestingly, it is now apparent that both types of procedures yield results consistent with momentum theory under traditional disruptors. Such differences seem to imply something special about the relationship between haloperidol and each schedule type, beyond any simple disruptive qualities. Ratio schedules differ in many respects from interval schedules. The feedback function relating behavior and reinforcement, for example, has been considered fundamental in determining the outcome of scheduled reinforcement (e.g., Baum, 1981; Nevin & Baum, 1980). It may be that haloperidol’s effects are related to the shape of the feedback function, which is linear for ratio schedules and negatively accelerated for interval schedules. Or other response characteristics, unknown at present, may play a role. In general, performance under ratio schedules may be more sensitive to disruption than that of interval schedules (Thompson et al., 1984), meaning that it would be more difficult to separate the effects of behavioral “mass” on resistance to change. Knowledge of such drug-schedule interactions appear necessary to understand resistance to change in the face of pharmacological disruption.
A second possibility extends from side effects inherent in the use of pharmacological disruptors themselves. Ostensibly, rate-decreasing effects of drugs have been the focus of pharmacological disruptions because they formally parallel the rate-decreasing effects of more traditional disruptors (e.g., extinction or response-independent food). Yet, doses along the descending limb of the dose-response function may induce several additional changes, such as sedative effects, direct motor effects, degradation in stimulus control (Harper, 1999b), etc. Such extraneous processes complicate the analysis of the drug’s action on resistance to change, and it is not clear that behavioral momentum can easily distinguish among them (nor was it designed to).
On the other hand, pharmacological manipulations are positioned uniquely to examine resistance to change in terms of rate-increasing effects. This parallel side of momentum theory has received little study, as suggested above disruptors used predominantly decrease operant behavior. Behavioral pharmacology, however, has identified several circumstances where drug administration increases behavior (Dews & Wenger, 1977; Sanger & Blackman, 1976). Recent work from our lab, moreover, has suggested some rate-increasing effects conform to the predictions of behavioral momentum theory. Lamb and Ginsburg (2008) examined the rate-dependent effects of fluvoxamine and desipramine when behavior was maintained by multiple fixed-interval schedules where each component signaled different durations of food access, similar to the present experiment. Rate-dependency analyses suggested that larger magnitude of reinforcement attenuated the rate-dependent effects of both drugs. Specifically, lower response rates were increased more when behavior was maintained by smaller, compared to larger, reinforcer magnitudes. Additionally, Harper (1999b) found that low doses of fluoxetine increased behavior under multiple VI schedules, and behavior under the component providing lower amounts of reinforcement was less resistant to the increasing effects. In sum, rate-decreasing effects may not provide a “behaviorally clean” preparation for the investigation of resistance to change by pharmacological disruptors because they introduce other unwanted effects. On the other hand, resistance to rate-increasing effects of certain drugs may provide a relatively unused testbed of the predictions of behavioral momentum, as well a unique application of behavioral pharmacology to the development of operant learning theory.
AKNOWLEDGEMENTS
This work was supported by Grant AA012337. The authors thank Gerardo Martinez for his assistance.
Footnotes
The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha.
REFERENCES
- Arnsten AF, Scahill L, Findling RL. Alpha2-adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. Journal of Child and Adolescent Psychopharmacology. 2007;17:393–406. doi: 10.1089/cap.2006.0098. [DOI] [PubMed] [Google Scholar]
- Baum WH. Optimization and the matching law as accounts of instrumental behavior. Journal of the Experimental Analysis of Behavior. 1981;36:387–403. doi: 10.1901/jeab.1981.36-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonem M, Crossman EK. Elucidating the effects of reinforcement magnitude. Psychological Bulletin. 1988;3:348–362. doi: 10.1037/0033-2909.104.3.348. [DOI] [PubMed] [Google Scholar]
- Branch MN. Rate dependency, behavioral mechanisms, and behavioral pharmacology. Journal of the Experimental Analysis of Behavior. 1984;42:511–522. doi: 10.1901/jeab.1984.42-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SL. A pharmacological examination of the resistance-to-change hypothesis of response strength. Journal of the Experimental Analysis of Behavior. 1986;46:363–379. doi: 10.1901/jeab.1986.46-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SL. Behavioral momentum: the effects of the temporal separation of rates of reinforcement. Journal of the Experimental Analysis of Behavior. 1998;69:29–47. doi: 10.1901/jeab.1998.69-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SL, Riley DS, Weigle PA. Test of behavioral momentum in simple and multiple schedules with rats and pigeons. Journal of the Experimental Analysis of Behavior. 1993;60:255–291. doi: 10.1901/jeab.1993.60-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews PD, Wenger GR. Rate-dependency of the behavioral effects of amphetamine. In: Thompson T, Dews’ PB, editors. Advances in Behavioral Pharmacology. Vol 1. New York: Academic Press; 1977. pp. 167–227. [Google Scholar]
- Egli M, Schaal DW, Thompson T, Cleary J. Opiod-induced response-rate decrements in pigeons responding under variable-interval schedules: Reinforcement mechanisms. Behavioural Pharmacology. 1992;3 581-191. [PubMed] [Google Scholar]
- Halevy G, Feldon J, Weiner I. The effects of clonidine on the partial reinforcement extinction effect (PREE) Psychopharmacology. 1986;90:95–100. doi: 10.1007/BF00172878. [DOI] [PubMed] [Google Scholar]
- Harper DN. Behavioral resistance to haloperidol and clozapine. Behavioural Processes. 1999a;46:1–13. doi: 10.1016/S0376-6357(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Harper DN. Drug-induced changes in responding are dependent on baseline stimulus-reinforcer contingencies. Psychobiology. 1999b;27:95–104. [Google Scholar]
- Harper DN, McLean AP. Resistance to change and the Law of Effect. Journal of the Experimental Analysis of Behavior. 1992;57:317–337. doi: 10.1901/jeab.1992.57-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman SH, Branch MN, Sizemore GM. Cocaine tolerance: acute versus chronic effects as dependent upon fixed-ratio size. Journal of the Experimental Analysis of Behavior. 1987;47:363–376. doi: 10.1901/jeab.1987.47-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XH, Ji JZ, Zhang H, Li BM. Stimulation of α2–adrenoreceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology. 2008;33:2263–2271. doi: 10.1038/sj.npp.1301603. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gomez C, Shahan TA. Resistance to change of alcohol self-administration: Effects of alcohol-delivery rate on disruption by extinction and naltrexone. Behavioural Pharmacology. 2007;18:161–169. doi: 10.1097/FBP.0b013e3280f2756f. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Ginsburg BC. Fluvoxamine and desipramine on fixed-ratio responding: Effects of reinforcement magnitude. Behavioural Pharmacology. 2005;16 doi: 10.1097/01.fbp.0000181594.01244.a2. 573-378. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Ginsburg BC. Reinforcement magnitude modulates the rate-dependent effects of fluvoxamine and desipramine on fixed-interval responding in the pigeon. Behavioural Pharmacology. 2008;19:51–60. doi: 10.1097/FBP.0b013e3282f3d093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. Response strength in multiple schedules. Journal of the Experimental Analysis of Behavior. 1974;21:389–408. doi: 10.1901/jeab.1974.21-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. An integrative model for the study of behavioral momentum. Journal of the Experimental Analysis of Behavior. 1992;57:301–316. doi: 10.1901/jeab.1992.57-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Grace RC. Behavioral momentum and the Law of Effect. Behavioral and Brain Sciences. 2000;23:73–90. doi: 10.1017/s0140525x00002405. [DOI] [PubMed] [Google Scholar]
- Nevin JA, Baum WH. Feedback functions for variable interval reinforcement. Journal of the Experimental Analysis of Behavior. 1980;34:207–217. doi: 10.1901/jeab.1980.34-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Mandell C, Atak JR. The analysis of behavioral momentum. Journal of the Experimental Analysis of Behavior. 1983;39:49–59. doi: 10.1901/jeab.1983.39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Tota ME, Torquato RD, Shull RL. Alternative reinforcement increases resistance to change: Pavlovian or operant contingencies? Journal of the Experimental Analysis of Behavior. 1990;53:359–379. doi: 10.1901/jeab.1990.53-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston JW, Branch MN. Repeated post- or pre-session cocaine administration: role of dose and fixed-ratio schedule. Journal of the Experimental Analysis of Behavior. 2004;81:169–188. doi: 10.1901/jeab.2004.81-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AFT. Andrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacology & Therapeutics. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biological Psychology. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioral pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:262–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Blackman DE. Rate-dependent effects of drugs: a review of the literature. Pharmacology, Biochemistry, & Behavior. 1976;4:73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Burke KA. Ethanol-maintained responding of rats is more resistant to change in a context with added non-drug reinforcement. Behavioural Pharmacology. 2004;15:279–285. doi: 10.1097/01.fbp.0000135706.93950.1a. [DOI] [PubMed] [Google Scholar]
- Smith JB. Effects of fixed-ratio requirement on observed tolerance to decreased responding by clonidine. Pharmacology, Biochemistry, & Behavior. 1990;36:993–995. doi: 10.1016/0091-3057(90)90112-u. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremmer JD, Rasmusson A, Morgan CA, III, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Thompson T, Honor J, Verchota S, Cleary J. Interval and ratio reinforcement contingencies as determinants of methadone’s effects. Pharmacology, Biochemistry, & Behavior. 1984;21:743–747. doi: 10.1016/s0091-3057(84)80013-1. [DOI] [PubMed] [Google Scholar]