Abstract
Objectives
To determine the feasibility of conducting a randomized clinical trial designed to compare two methods of manual therapy (myofascial physical therapy (MPT) and global therapeutic massage (GTM)) among patients with urologic chronic pelvic pain syndromes.
Materials and Methods
Our goal was to recruit 48 subjects with chronic prostatitis/chronic pelvic pain syndrome or interstitial cystitis/painful bladder syndrome at six clinical centers. Eligible patients were randomized to either MPT or GTM and were scheduled to receive up to 10 weekly treatments, each 1 hour in duration. Criteria to assess feasibility included adherence of therapists to prescribed therapeutic protocol as determined by records of treatment, adverse events which occurred during study treatment, and rate of response to therapy as assessed by the Patient Global Response Assessment (GRA). Primary outcome analysis compared response rates between treatment arms using Mantel-Haenszel methods.
Results
Twenty-three (49%) men and 24 (51%) women were randomized over a six month period. Twenty-four (51%) patients were randomized to GTM, 23 (49%) to MPT; 44 (94%) patients completed the study. Therapist adherence to the treatment protocols was excellent. The GRA response rate of 57% in the MPT group was significantly higher than the rate of 21% in the GTM treatment group (p=0.03).
Conclusions
The goals to judge feasibility of conducting a full-scale trial of physical therapy methods were met. The preliminary findings of a beneficial effect of MPT warrants further study.
Keywords: Chronic Prostatitis, Urologic Pelvic Pain Syndrome, Interstitial Cystitis, Painful Bladder Syndrome, Physical Therapy
INTRODUCTION
The Urologic Chronic Pelvic Pain Syndromes (UCPPS), which include Interstitial Cystitis/Painful Bladder Syndrome (IC/PBS) in men and women and Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) in men, are characterized by pelvic pain with concurrent urinary symptoms.
PBS, as defined by the International Continence Society, is “the complaint of suprapubic pain related to bladder filling, accompanied by other symptoms, such as increased daytime and night-time frequency, in the absence of proven urinary infection or other obvious pathology.” 1 These symptoms may be related to interstitial cystitis (IC),2 with additional characteristic findings of glomerulations and/or ulcers present at cystoscopy and hydrodistension. The underlying pathophysiology of these disorders has not been elucidated, and the relationship between PBS and IC is not clear.
Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS), or NIH type IIIA/IIIB prostatitis, is also characterized by pelvic pain and lower urinary tract symptoms, in the absence of proven urinary tract infection or other obvious pathology. CP/CPPS is also a clinical description based on symptoms, and does not depend on urodynamic or cystoscopic findings.
Estimates of national prevalence of these UCPPS syndromes vary widely according to the populations studied and the survey methodology. In 1990, interstitial cystitis (IC) was thought to affect as many as 500,000 U.S. citizens, with 25% of patients under age 25.3 Recent estimates vary between 0.2% and 3.4% of the population.4–9 In 2000, annual national expenditures in the USA for IC/PBS were estimated at $797 million.10 Estimates of the prevalence of symptoms characteristics of CP/CPPS vary similarly and may be higher: community based surveys demonstrate prevalence of 8–11.5% of men younger than age 50.11–12
On examination, tension and tenderness of the pelvic floor musculature and other somatic tissues are commonly present in UCPPS patients13–20 It is thought that these myofascial abnormalities contribute significantly to the pain of UCPPS, but it is not known whether these musculoskeletal abnormalities are a consequence of lower urinary tract symptoms, or are a primary disorder which gives rise to secondary urinary symptoms. Frequently found abnormalities include “myofascial trigger points”, defined as taut bands or tender nodules, which evoke twitch responses or reproduce the character and location of symptoms during careful palpation.21 Importantly, there have been several reports of UCPPS symptom relief by therapeutic efforts directed at those muscular abnormalities.16–20 In practice, those therapeutic interventions are typically carried out by a physical therapist skilled in manual therapy techniques. Although widely practiced, no randomized trials have established the effectiveness of specialized external and pelvic floor physical therapy for treatment of UCPPS.
Since there have been very few prospective randomized clinical trials involving physical therapies to guide us, we designed a study to determine whether a randomized study of physical therapy for treatment of UCPPS is feasible. The criteria to assess feasibility were whether UCPPS patients are willing to be randomized between two forms of manual physical therapy, whether physicians are capable of identifying relevant myofascial abnormalities during their evaluation of UCPPS patients, to determine whether we can assure that the manual therapy treatments are similar in nature and quality at several study sites, to assess the safety of manual therapies for treatment of UCPPS, and to determine the response rate to manual physical therapy.
Methods
The Urological Pelvic Pain Collaborative Research Network (UPPCRN) is a cooperative network of investigators from 20 clinical centers and a Data Coordinating Center (DCC), funded by the National Institutes of Diabetes, Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (http://www.cceb.med.upenn.edu/uppcrn).
The study received Institutional Review Board approval at all enrolling sites and the Data Coordinating Center (DCC), located at the University Of Pennsylvania School Of Medicine. The UPPCRNData and Safety Monitoring Board oversees aspects of clinical trial design, conduct, and analyses of trial data.
Study Design
This was a randomized single-blind clinical trial in which 8 participants each were to be recruited by a subset of six of the UPPCRN clinical centers, for a total sample size of 48. Subjects who met eligibility criteria and did not have any of the exclusion factors (see Table 1) were randomized in equal number to either Myofascial Physical Therapy (MPT) or Global Therapeutic Massage (GTM) and were not informed whether the treatment they were receiving was MPT or GTM. MPT treatment targeted internal (pelvic) and external trigger point work, focusing on the muscles and connective tissues of the pelvic floor, hip girdle, and abdomen; whereas, GTM was considered a non-specific somatic treatment with full-body Western massage and was included as a comparison treatment arm. Patients were scheduled for 10 weekly treatments, each one hour in duration. Participation ended when a subject completed treatment and outcome assessment, voluntarily withdrew, or was withdrawn by their physician for medical reasons.
Table 1.
Inclusion, exclusion and deferral criteria
Inclusion Criteria:
|
Exclusion Criteria:
|
Exclusion criteria for males only:
|
Exclusion criteria for females only:
|
| Deferral criteria: |
| For men: within the past 24 weeks underwent aTURP, TUIP, TUIBN, TUMT, TUNA, balloon dilation of the prostate, open prostatectomy or any other prostate or treatment, such as cryotherapy or thermal therapy. |
| For women: within the past 24 weeks has had vaginal delivery or C-section, transvaginal surgery, hysterectomy, prolapse surgery. |
Study measures
Since this was a pilot study to establish feasibility of comparing two manual therapies, many of the trial outcomes are related to study conduct. These measures included:
Proportion of patients who consented to join the study from among all eligible patients approached.
Number of patients deemed eligible by physician, based on history and clinical examination, who were considered ineligible by physical therapist due to lack of relevant physical abnormalities.
Adherence of therapist to prescribed therapeutic protocol, as determined by records of treatment.
The primary measures related to patient outcomes, including safety and efficacy, are as follows:
Adverse Events, including Serious Adverse Events (AEs/SAEs)
Patient Global Response Assessment (GRA) (see Table 2), with patients classified as ‘responders’ if they state that, compared to how they were before treatment, their symptoms are now ‘moderately’ or ‘markedly’ improved
Change in several validated symptom scales, described subsequently.
Table 2.
Primary symptom outcome assessment tool
The Global Response Assessment (GRA) consists of the following question: “As compared to when you started the study, how would you rate your overall symptoms now?”
|
| Participants who indicated they were 6: “moderately” or 7: “markedly” improved are considered intervention responders. |
Eligibility Assessment
Recruitment was conducted from among patients who attended urology and urogynecology clinics at each of the designated clinical sites based on the inclusion and exclusion criteria outlined in Table 1. Briefly, potential participants had to be adult and have clinical diagnosis of either PBS/IC or CP/CPPS. Based on personal experience, the investigators hypothesized that the time to response to physical therapies is proportional to the duration of symptoms. Since treatment was limited to 10 sessions in this protocol, we restricted entry to subjects with symptoms present for less than 3 years. The patient must have previously undergone at least one course of another form of therapy for his/her symptoms. We also excluded patients who are intolerant to digital vaginal or rectal examination, i.e., would be unable to tolerate the MPT treatments. Participants who had previously undergone myofascial physical therapy for their symptoms also were ineligible.
Certification of treating therapists
Certification of treating therapists took place in several steps. Up to two physical therapists were involved at each clinical site. As a pre-requisite, the licensed physical therapists and their collaborating investigator physicians attested that they already routinely treat patients with UCPPS using MPT techniques similar to those utilized in the study. To standardize MPT and GTM treatments, therapists received study materials, including the study protocol, description of the MPT and GTM treatments, and DVDs demonstrating MPT and GTM therapies. They attended a certification weekend session during which the study protocol and treatments were reviewed and demonstrated on volunteers. A licensed Massage therapist (RH-P) instructed therapists in the proper performance of a traditional Western-style massage.22 For preliminary certification, candidate therapists demonstrated full understanding and competence in the execution of the steps involved in completion of MPT and GTM treatments. After subsequently completing and attesting to correct performance of 5 treatments of each type, therapists were then certified to participate in the protocol.
Pre-intervention assessments
Clinically-identified potential participants were counseled about study procedures and were administered informed consent. During the first study visit, patients completed symptom scales, including a rating of their average pelvic/bladder discomfort or pain, rating of severity of urinary urgency and urinary frequency, all rated on average over the past 4 weeks. Other symptom scales included the IC Symptom and Problem Index,23 NIH-Chronic Prostatitis Symptom Index (males),24 SF-12 Health Status Questionnaire,25 and a gender-specific sexual function index (Female Sexual Function Index26 or Sexual Health Inventory for Men27). Patients also underwent pelvic examination by their study physician, including transvaginal or transrectal examination of the soft tissues of the pelvic floor (levator ani, obturator internus and tissues of the urogenital diaphragm). Patients were eligible to continue with study participation if some pelvic floor tenderness was elicited in any of the designated areas during this baseline pelvic examination. Patients without such tenderness were excluded from further participation.
At the second study visit, patients underwent a more complete examination of the musculoskeletal system and soft tissues by their study physical therapist. Patients were eligible to continue participation in the study only if the therapist confirmed that there was tenderness present on pelvic examination. The location of the tenderness did not have to correspond to that found by the PI. Other pre-treatment assessments by the physical therapist included mapping of any scars and connective tissue restrictions, and evaluation of all soft tissues of the back, hip girdle, abdominal wall and pelvic floor.
Participants meeting all eligibility criteria were randomly assigned in equal proportions within each of the six strata defined by Clinical Site, via a pre-specified sequence distributed in a series of sealed envelopes, to receive either MPT or GTM. Each participant underwent 10 weekly one-hour treatments by the physical therapist. Patients were contacted by telephone by their study coordinator every week between treatments and asked about any adverse events. Study coordinators remained masked to study treatment assignment.
Study treatments
Patients randomized to the MPT group underwent connective tissue manipulation (CTM) to all body wall tissues of the abdominal wall, back, buttocks and thighs that clinically were found to contain connective tissue abnormalities and/or myofascial trigger point release to painful myofascial trigger points. CTM was applied bilaterally to the patient in the prone position, posteriorly from inferior thoracic level 10 to the popliteal crease. This was done until a texture change was noted in the treated tissue layer. Manual techniques, such as trigger point barrier release, with or without active contraction or reciprocal inhibition, manual stretching of the trigger point region and myofascial release were utilized on the identified trigger points.
Once adequate changes were noted in the posterior tissues, the patient was repositioned into the supine position for CTM to bilateral anterior tissues. This allowed the inclusion of the thighs; laterally, anteriorly, and medially from the knee up to, and including, the thigh crease. CTM was performed on the abdominal wall from the supra-pubic rim to the anterior costal cartilages, with a concentration of manual interventions to focus on the peri-umbilical tissues. Manual trigger point release techniques were utilized to treat any noted trigger points or scars in the anterior or posterior lower quadrants. It was presumed that external episiotomy scars found in the perineum or external pelvic floor were especially relevant.
Transvaginal/transrectal treatment of the soft tissues of the pelvic floor with CTM of periurethral tissues, white line, muscle origins and insertions was also performed. Myofascial manipulation to each muscle group was performed with the focus on restrictive bands and trigger points. Neuromuscular re-education, focusing on lengthening the pelvic floor musculature, was performed in conjunction with myofascial manipulation, including post-isometric relaxation.
During the time between visits, when deemed appropriate by the therapist, patients were asked to double-void two to three times after each void (i.e. after voiding, to remain seated on the toilet and to relax or drop the pelvic floor again as if to initiate voiding). This was meant to facilitate a proprioceptive awareness of the movement of the pelvic floor during voiding, hoping to utilize recent recall to make dropping their pelvic floor easier. As their ability to drop their pelvic floor improves the patients could add five pelvic ‘drops’ to the end of the exercise. Squatting was also taught as a position to use to facilitate and practice pelvic floor drops.
The treating physical therapist was permitted to vary the exact content of the hour-long MPT treatment based on the physical abnormalities present, and on the response of tissues to manipulation. Initial treatments devoted at least half of the treatment time to external myofascial therapy. As connective tissue changes became evident with repetitive treatments, less time is typically needed for treatment of external tissues, and more time devoted to internal (transvaginal, transrectal) work. When severity of symptoms prohibited transvaginal/transrectal myofascial trigger point release or CTM (even though initial examination and inclusion of the patient was possible), this variance was allowed.
In order to maximize the potential for a treatment effect, each therapist typically offered appropriate home exercises to each patient randomized to the MPT arm. Each therapist was provided with a catalog of stretches and/or exercises specifically chosen for this study and the appropriate exercise/stretch was given to the patient when desired by the therapist. Importantly, these were not “Kegel” exercises which can increase the irritability of myofascial trigger points and exacerbate symptoms if practiced during an early phase of therapy. Later, after muscle control is achieved, a focus on improving muscle strength may be more appropriate and was permitted by the protocol.
Patients randomized to the GTM group received weekly massages consisting of full body Western massage for one hour. Unlike the MPT arm, in which the therapists tailored the focus of therapy to target individual patient needs, GTM was employed according to a common study protocol. This differs from clinically practiced “therapeutic massage”, as the participating therapists were neither permitted to deviate from the GTM regimen, nor to tailor the massage techniques to individual patients. Techniques employed include: effleurage, petrissage, friction, tapotement, vibration and kneading. These techniques were applied in upper and lower limbs, trunk, buttocks, abdomen, head and neck, each for prescribed time periods (e.g. 10 minutes massage to head and neck). Patients randomized to GTM were not provided with a home exercise program.
Statistical analysis
To ensure balance across treatment arms, a stratified randomization was used. Within each of the six (6) strata defined by clinical site, subjects were further stratified by gender and randomly allocated in equal proportions to the two treatment arms.
Although this was a pilot study, for which comparison of treatment efficacy was a secondary outcome, the small number of participants (24 per arm) was adequate to provide approximately 80% power to detect a 40% absolute difference (e.g, 70% vs. 30%) in response rates between MPT and GTM, using a two-sided test at the 5% level. With a total of 24 subjects per treatment arm, 95% confidence intervals for rates (such as response and adverse events) have a maximum width of ±20%, ignoring slight adjustments for variability due to clinical site.
An intent-to-treat analysis, including all randomized participants, was implemented. Participants who discontinued treatment during the trial, particularly in the case of an adverse event, were not considered withdrawals from study, unless they withdrew consent for further follow-up. However, randomized participants who withdrew prior to the final assessment at twelve (12) weeks were considered to be treatment failures, and were included in the denominator for evaluation of response rates based on the GRA.
The primary efficacy endpoint was response rate determined based on the GRA(Table 2). An analysis comparing response rates between treatment arms, utilizing the exact conditional test (ECT) version of Mantel-Haenszel methods to adjust for within-center clustering, was implemented within the Proc-StatXact software system.28 Outcome measures between treatment arms were analyzed with Wilcoxon rank sum tests, and outcome scores within treatment groups and disease states were determined with paired t-tests. Statistical significance was at p < 0.05.
RESULTS
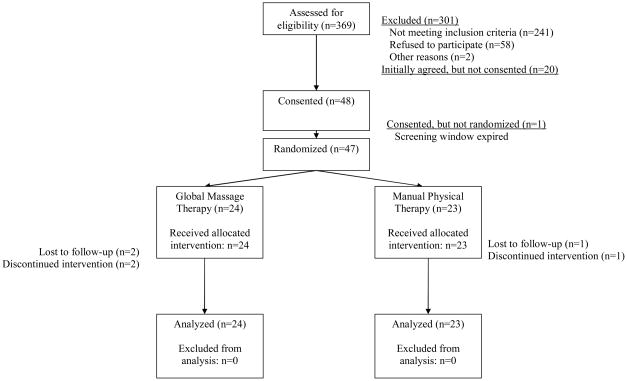
As detailed in Figure 1, 369 patients with UCPPS were reviewed for study participation, of whom 68 (18%) agreed to participate and 47 were randomized, including 23 (49%) men and 24 (51%) women. Recruitment took place over 6 months. All patients identified as eligible by their study physician were also considered eligible by the study physical therapist, on the basis of the presence of tenderness of the pelvic floor. Twenty-four (51%) patients were randomized to GTM, 23 (49%) to MPT. IC/PBS patients included 24 women and 2 men; 21 men had a CP/CPPS diagnosis. Forty-four (94%) patients completed the study, with 2 patients withdrawing from the GTM treatment arm and one withdrawal from MPT.
Figure 1.
CONSORT diagram
The baseline demographic characteristics of study participants overall, and by treatment assignment, are presented in Table 3. The mean age was 43 years (range 22–76); 41 (87%) were Caucasian. Patient self-reports of medical history differed between the two disorders, with a higher incidence of migraine headaches, GI disorders, drug allergies and sinusitis among IC/PBS patients than among CP/CPPS patients (p<0.05).
Table 3.
Summary of demographic characteristics by treatment arm. Treatment groups were similar with respect to all demographic characteristics at baseline. All but two patients in the IC/PBS group were female.
| Treatment | |||
|---|---|---|---|
| GTM | MPT | Total | |
| Number of Subjects | 24 | 23 | 47 |
| Gender (p=0.66) | |||
| Male | 11 (46%) | 12 (52%) | 23 (49%) |
| Female | 13 (54%) | 11 (48%) | 24 (51%) |
| Age(yrs) (p=0.28) | |||
| Mean ± s.d. | 44.9 ± 14.0 | 41.1 ± 11.4 | 43.0 ± 12.8 |
| Median | 45.1 | 40.9 | 43.3 |
| Range | 22 to 76 | 23 to 66 | 22 to 76 |
| Ethnicity/Race (p1=0.42) | |||
| North American Indian/North Native | 0 | 0 | 0 |
| Asian/Asian-American | 0 | 1 (4%) | 1 ( 2%) |
| Black/African-American | 0 | 2 (8%) | 2 ( 4%) |
| White/Caucasian | 22 ( 92%) | 19 (83%) | 41 ( 87%) |
| Native Hawaiian/Other Pacific Islander | 0 | 0 | 0 |
| Other | 2 (8%) | 1 (4%) | 3 (6%) |
| Diagnosis (p=0.67) | |||
| IC/PBS | 14 (58%) | 12 (52%) | 26 (55%) |
| CP/CPPS | 10 (42%) | 11 (48%) | 21 (45%) |
| Educational Level (p=0.13) | |||
| Less than High school | 0 | 0 | 0 |
| High School/GED | 5 (21%) | 1 (5%) | 6 (13%) |
| Some college | 7 (29%) | 7 (30%) | 14 (30%) |
| Graduated college or above | 12 (50%) | 15 (65%) | 27 (57%) |
| Employment (p=0.15) | |||
| Employed | 14 (58%) | 17 (74%) | 31 (66%) |
| Unemployed/Retired | 7 (29%) | 2 (9%) | 9 (19%) |
| Fulltime Homemaker | 3 (13%) | 2 (9%) | 5 (11%) |
| Disabled | 0 (0%) | 2 (9%) | 2 (4%) |
| Annual Family Income (p=0.89) | |||
| <$10,000 | 1 (7%) | 1 (7%) | 2 (7%) |
| $10,001 – $25,000 | 1 (7%) | 0 (0%) | 1 (3%) |
| $25,001 – $50,000 | 3 (20%) | 4 (29%) | 7 (24%) |
| $50,001 – $100,000 | 4 (27%) | 3 (21%) | 7 (24%) |
| >$100,000 | 6 (40%) | 6 (43%) | 12 (41%) |
| Missing | 9 | 9 | 18 |
p-value for white versus non-white.
Patients’ symptoms (Table 4) prior to treatment were rated moderate to severe (range 4–10 on a scale of 10) for pain in 96%, urgency in 91% and frequency in 89% of participants.
Table 4.
Baseline symptom characteristics by treatment group at baseline. Pain, urgency and frequency values represent means of scores at baseline visits 1 and 2. Treatment groups were similar at baseline with respect to symptom severity. p-value corresponds to test comparing scores by treatment group.
| GTM | MPT | Total | |
|---|---|---|---|
| Number of Subjects | 24 | 23 | 47 |
| Average Pain Severity Score (p=0.17) | |||
| None (0) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mild (1–3) | 2 (8%) | 0 (0%) | 2 (4%) |
| Moderate (4–6) | 11 (46%) | 9 (39%) | 20 (43%) |
| Severe (7–10) | 11 (46%) | 14 (61%) | 25 (53%) |
| Average Urgency Severity Score (p=0.15) | |||
| None (0) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mild (1–3) | 4 (17%) | 0 (0%) | 4 (9%) |
| Moderate (4–6) | 8 (33%) | 9 (39%) | 17 (36%) |
| Severe (7–10) | 12 (50%) | 14 (61%) | 26 (55%) |
| Average Frequency Severity Score (p=0.16) | |||
| None (0) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mild (1–3) | 4 (17%) | 1 (4%) | 5 (11%) |
| Moderate (4–6) | 6 (25%) | 5 (22%) | 11 (23%) |
| Severe (7–10) | 14 (58%) | 17 (74%) | 31 (66%) |
The median (range) number of treatment visits administered in the MPT group was 10 (4–10) and in the GTM group was 10 (1–10); 87% of patients in each arm received at least 7 of their assigned treatments. Therapist adherence to the treatment protocols was excellent: during GTM treatments, therapists recorded having performed massage to all body parts during all treatments as prescribed by the protocol, except for omission of head and neck and/or abdominal massage during most treatments in 1 patient (protocol violation). Therapists reported they applied all of the allowed therapeutic interventions at least some of the time, and varied treatments as allowed by the protocol. The most commonly utilized interventions were connective tissue manipulation of external tissues of lower limbs, buttocks, abdominal wall, pelvic floor, and trigger point treatments to abdominal wall and pelvic floor. Individualized home stretch/exercise programs were prescribed for 21 (48%) patients, of which one patient performed none, 5 performed some, and 15 performed all. After treatment, 42 of 44 (95%) patients had correctly identified the study group to which they were assigned.
Adverse events (AEs; see Table 5) were reported by 5 (21%) of patients in the GTM group and 12 (52%) of patients in the MPT group. Pain was the most common class of AEs, reported by 14 (30%) participants, of which 3 pain AEs were rated as severe (1 in GTM group and 2 in MPT group).
Table 5.
Cumulative adverse events (AEs) by body system and by treatment arm. Body systems are not included if no related AEs were reported. Table includes all adverse events, regardless of their relationship to study intervention. Patients are counted once in each category and included in the column relating to their worst toxicity grade. Patients may have had events in more than one body system category. An adverse event was not counted if the same event is observed at baseline with an equal or greater toxicity grade.
| Toxicity Grade, GTM (n=24) | Toxicity Grade, MPT (n=23) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body System | None (Gr0) | Mild (Gr1) | Mod (Gr2) | Severe (Gr3) | **Total (%) | None (Gr0) | Mild (Gr1) | Mod (Gr2) | Severe (Gr3) | **Total (%) |
| Highest grade of adverse event | 19 | 3 | 1 | 1 | 5 (21%) | 11 | 8 | 2 | 2 | 12 (52%) |
| Cardiovascular (Arrhythmia) | 24 | 0 | 0 | 0 | 0 | 22 | 0 | 1 | 0 | 1 (4%) |
| Constitutional symptoms | 22 | 2 | 0 | 0 | 2 (8%) | 22 | 1 | 0 | 0 | 1 (4%) |
| Hemorrhage | 24 | 0 | 0 | 0 | 0 | 22 | 1 | 0 | 0 | 1 (4%) |
| Infection | 23 | 0 | 1 | 0 | 1 (4%) | 23 | 0 | 0 | 0 | 0 |
| Musculoskeletal | 24 | 0 | 0 | 0 | 0 | 22 | 1 | 0 | 0 | 1 (4%) |
| Pain (unspecified) | 21 | 1 | 1 | 1 | 3 (13%) | 12 | 8 | 1 | 2 | 11 (48%) |
| Pain – Abdomen/Intestine | 1 | 1 | 0 | |||||||
| Pain – Back | 0 | 1 | 0 | 1 | 0 | 0 | ||||
| Pain – Bladder/Rectum/Pelvis/Genital | 1 | 2 | 1 | 5 | 1 | 2 | ||||
| Genitourinary | 23 | 1 | 0 | 0 | 1 (4%) | 19 | 4 | 0 | 0 | 4 (17%) |
The overall GRA response rates and rates for subgroups of subjects with IC/PBS and CP/CPPS are shown in Table 6. The overall GRA response rate of 57% in the MPT group was significantly higher than the rate of 21% in the GTM treatment group (p=0.03). As detailed in Table 6, a significant difference between treatment arms was present in the PBS/IC group, but not in the CP/CPPS group since many patients with CP/CPPS responded to both GTM and MPT.
Table 6.
Global Response Assessment (GRA) by Treatment Group. Frequencies represent number of patients (n) or n (%) as appropriate. For the GRA portion of the response assessment, responders are defined as those reporting “Markedly Improved” or “Moderately Improved” on the GRA. Patients for whom the GRA value is missing are considered non-responders, and are included in the denominator for the assessment of response rates. Subjects who withdrew are also included in the denominator.
| Therapy | |||
|---|---|---|---|
| GTM | MPT | Total | |
| Number of Subjects Randomized | 24 | 23 | 47 |
| Response Rate Based on GRA (p=0.03) | |||
| Responders | 5 (21%) | 13 (57%) | 18 (38%) |
| Non-responders | 19 (79%) | 10 (43%) | 29 (62%) |
| Response Rate for IC/PBS Subgroup (n=26; p= 0.03) | |||
| Responders | 1 (7%) | 6 (50%) | 7 (27%) |
| Non-responders | 13 (93%) | 6 (50%) | 19 (73%) |
| Response Rate for CP/CPPS Subgroup (n=21; p= 0.39) | |||
| Responders | 4 (40%) | 7 (64%) | 11 (52%) |
| Non-responders | 6 (60%) | 4 (36%) | 10 (48%) |
| Global Assessment of Response: | |||
| Markedly Improved | 3 | 8 | 11 |
| Moderately Improved | 2 | 5 | 7 |
| Slightly Improved | 10 | 6 | 16 |
| No Change | 5 | 2 | 7 |
| Slightly Worsened | 1 | 0 | 1 |
| Moderately Worsened | 1 | 1 | 2 |
| Markedly Worsened | 0 | 0 | 0 |
| Missing or Withdrawn | 2 | 1 | 3 |
Tables 7 and 8 show baseline and 12-week symptom scores according to clinical diagnosis of IC/PBS and CP/CPPS, respectively, stratified by treatment assignment. MPT resulted in improved (decreased) symptom scores for both IC/PBS and CP/CPPS patients (Table 7; p < 0.05). GTM did not provide any significant relief of symptom scores for the IC/PBS group, but was associated with improvements in the CP/CPPS group in domains of pain, quality of life and ICSI (Table 8; p < 0.05). The SF-12 physical and mental component scores in both disorder groups were unaffected by GTM. Sexual function improved with MPT for the IC/PBS group, but not CP/CPPS.
Table 7.
IC/PBS patients: baseline and endpoint symptom scores. Comparison within and between treatment groups
| MPT | GTM | MPT vs GTM p value† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base line (N=12) | 12 weeks (N=11) | Mean change (%) | p Value* | Baseline (N=14) | 12 weeks (N=12) | Mean change (%) | p value* | ||
| Pain (0–10) | 6.8±2.0 | 4.2±2.9 | −2.5 (−36.8) | 0.005 | 6.7±1.6 | 5.9±2.0 | −0.9 (−13.4) | 0.09 | 0.07 |
| Urinary Urgency (0–10) | 6.8±1.4 | 4.0±2.7 | −2.7 (−39.7) | 0.01 | 6.7±2.0 | 6.3±2.5 | −0.8 (−11.9) | 0.26 | 0.14 |
| Urinary Frequency (0–10) | 7.2±1.4 | 3.5±2.5 | −3.6 (−50.0) | .003 | 7.6±1.7 | 6.8±1.8 | −1.2(−15.8) | 0.09 | 0.06 |
| ICSI | 13.0 ± 4.8 | 8.1 ± 4.9 | −4.6 (−35.4) | 0.02 | 12.8 ± 4.0 | 12.9 ± 4.7 | 0 (0) | 0.94 | 0.01 |
| ICPI | 12.1 ± 3.3 | 7.3 ± 4.7 | −4.7 (−38.8) | .006 | 11.5 ± 3.0 | 10.8 ± 4.0 | −1.3 (−11.3) | 0.09 | 0.04 |
| FSFI Total§ | 21.3 ±7.1 | 25.3 ± 6.8 | 5.0 (23.5) | .002 | 18.4 ± 10.6 | 21.7 ± 8.8 | 1.4 (7.6) | 0.75 | 0.54 |
| SF-12 physical§ | 40.8 ± 11.3 | 42.9 ± 13.2 | 1.3 (3.2) | 0.51 | 39.6 ± 10.0 | 38.2 ± 11.6 | −4.4 (−11.1) | 0.33 | 0.23 |
| SF-12 mental§ | 33.5 ± 12.8 | 40.6 ± 9.1 | 6.2 (18.5) | 0.11 | 38.2 ± 12.1 | 39.2 ± 11.8 | 1.8 (4.7) | 0.50 | 0.44 |
Paired t-test, comparison of scores between baseline and week 12
Wilcoxon rank sum test, comparison of change scores from baseline to week 12 for MPT and GTM groups.
The sample sizes shown are slightly smaller for some secondary outcomes due to missing values. FSFI Total baseline sample sizes are 10 and 12 for MPT and GTM respectively; 12-week sample sizes are 9 for both MPT and GTM. Baseline sample sizes for SF-12 physical and SF-12 mental are 13.
Table 8.
CP/CPPS patients: baseline and endpoint symptom scores. Comparison within and between treatment groups
| MPT | GTM | MPT vs GTM p value† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (N=11) | 12 weeks (N=11) | Mean change (%) | p Value* | Baseline (N=10) | 12 weeks (N=9) | Mean change (%) | p value* | ||
| NIH-CPSI Total (0–43) | 33.5± 4.3 | 19.2 ± 11.1 | −14.4 (−43.0) | 0.0003 | 25.8 ± 5.7 | 18.2± 9.6 | −6.8 (−26.4) | 0.03 | 0.07 |
| Pain (0–21) | 14.2 ± 2.5 | 8.0 ± 5.7 | −6.2 (−43.7) | 0.0007 | 12.7± 2.8 | 8.2 ± 5.6 | −4.4 (−34.6) | 0.03 | 0.39 |
| Urinary (0–10) | 8.9 ± 1.4 | 5.0 ± 2.8 | −3.9 (−43.8) | 0.0002 | 4.6 ± 2.7 | 3.9 ± 2.4 | −0.3 (−6.5) | .65 | 0.007 |
| QOL (0–12) | 10.4 ± 2.0 | 6.2 ± 3.8 | −4.2 (−40.4) | 0.002 | 8.5 ± 2.0 | 6.1 ± 3.2 | −2.1 (−24.7) | 0.05 | 0.10 |
| ICSI§ | 11.3 ± 4.3 | 6.7 ± 4.8 | −5.3 (−46.9) | 0.004 | 5.6 ± 3.0 | 4.3 ± 2.7 | −2.3 (−41.1) | 0.02 | 0.09 |
| ICPI§ | 10.4 ± 4.2 | 5.4 ± 5.0 | −5.9 (−56.7) | 0.008 | 3.8 ± 2.9 | 3.5 ± 3.0 | −1.3 (−34.2) | 0.48 | 0.11 |
| SHIM | 18.8 ± 7.0 | 16.4 ± 9.7 | −2.5 (−13.3) | 0.26 | 17.1 ± 5.7 | 18.6 ± 6.6 | 2.2 (12.9) | 0.37 | 0.28 |
| SF-12 physical§ | 40.4 ± 8.2 | 48.9 ± 10.0 | 6.1 (15.1) | 0.03 | 46.0 ± 9.0 | 50.2 ± 8.3 | 4.2 (9.1) | 0.08 | 0.73 |
| SF-12 mental§ | 38.8 ±10.7 | 43.7 ± 12.5 | 5.6 (14.4) | 0.10 | 37.2 ± 10.0 | 42.9 ± 9.8 | 5.8 (15.6) | 0.06 | 1.00 |
Paired t-test, comparison of scores between baseline and week 12.
Wilcoxon rank sum test, comparison of change scores from baseline to week 12 for MPT and GTM group
The sample sizes shown are slightly smaller for some secondary outcomes due to missing values. ICSI and ICPI baseline sample sizes are 9 for GTM; 12-week sample sizes are 7 for MPT and 6 for GTM. SF-12 physical and SF-12 mental baseline sample sizes are 10 for MPT and 9 for GTM; the 12-week sample sizes are 10 for MPT.
The physicians’ baseline physical exam of internal muscle tenderness/pain to palpation across left and right side within each muscle group (total score for each muscle group range 0–6) resulted in an average total score of 10.96 across the 4 muscle groups, significantly less than the average PT assessment of 17.78 at baseline (p<0.001). Table 9 presents the level of tenderness/pain to palpation by disorder and treatment arm at baseline and at the final 12-week physician evaluation for each of the four muscle groups. MPT resulted in significant relief of tenderness/pain of the internal muscle groups in the IC/PBS patients compared with GTM (p<0.05). The painful tender points in CP/CPPS patients were relieved by MPT; however, some muscle groups (anterior and posterior levator) were similarly relieved after GTM. Minimal urogenital diaphragm tenderness did not change with either therapeutic approach.
Table 9.
Average level of tenderness/pain to palpation across left and right side within muscle groups (sum of both sides range 0–6) in pelvic floor exam by physician at baseline versus primary endpoint
| IC/PBS | CP/CPPS | |||||
|---|---|---|---|---|---|---|
| Baseline | Endpoint | p-value | Baseline | Endpoint | p-value | |
| MPT | (N=12) | (N=11) | ||||
| Anterior levator | 3.67 | 2.00 | 0.02 | 3.72 | 1.64 | 0.003 |
| Posterior levator | 3.83 | 2.00 | 0.01 | 2.45 | 1.27 | 0.090 |
| Obturator internus | 3.17 | 1.73 | 0.01 | 2.36 | 0.55 | <0.001 |
| Urogenital diaphragm | 1.64 | 1.30 | 0.13 | 1.70 | 0.55 | 0.13 |
| GTM | (N=14) | (N=10) | ||||
| Anterior levator | 3.21 | 2.75 | 0.64 | 3.1 | 2.0 | 0.02 |
| Posterior levator | 3.29 | 2.42 | 0.12 | 2.9 | 0.78 | 0.001 |
| Obturator internus | 3.07 | 2.33 | 0.10 | 2.6 | 1.11 | 0.13 |
| Urogenital diaphragm | 2.07 | 1.42 | 0.33 | 1.4 | .22 | 0.08 |
p-values are paired t-test
DISCUSSION
This is the first published randomized trial comparing MPT and GTM for UCPPS treatment. We achieved our objective of demonstrating that such a clinical trial was feasible. Patients were willing to be randomized between two forms of manual treatment, with 38% (48/128) of those screened and found to be eligible for study inclusion agreeing to be randomized. We were also able to standardize both treatment approaches, with reports of almost complete adherence to prescribed patterns for GTM and reports of appropriate, tailored myofascial physical therapy applied to patients assigned to the MPT group. All AEs were more common in the MPT group, as expected, but our low study withdrawal rate and low rate of severe adverse events suggests that patients found study treatments highly acceptable. Finally, the overall response rate of 57% in the MPT group suggests that MPT represents a clinically meaningful treatment option, supporting the findings of others.16–20
A feasibility study was necessary, as there was some skepticism regarding patient willingness to be randomized between two forms of manual therapy for UCPPS. First, myofascial physical therapy can be painful and/or seem unduly invasive. Second, UPPCRN sites are regional referral centers and patients often expect immediate resolution of their symptoms and may be unwilling to risk randomization to treatment with an unproven therapy. Study participation can be further challenged by the logistical obstacles of time and distance in such referral patients, as this was a demanding treatment protocol that required multiple treatment visits.
We made several interesting observations. There was a striking difference in the response rate on the GTM arm between success rates in CP/CPPS (all men) and IC/PBS (all but 2 of whom were women), at 40% in CP/CPPS and 7% in PBS/IC. This suggests either that CP/CPPS responds differently to GTM, or that men respond differently to GTM, or that other, unmeasured factors are important. Since all the study therapists were women, male patients might have responded to receiving non-sexual therapeutic touch administered by a woman. This hypothesis could be tested, for example, by studying similar therapies administered to men by male therapists. At a minimum, these results suggest that therapeutic massage may merit further study as a therapeutic alternative for UCPPS.
There were a number of strengths of our study. They include the standardization of treatment arms across a number of centers, the recruitment of men and women from sites located throughout the US, the high rate of adherence to the regimens in each treatment group, and the high rate of assessment of the primary endpoint (low rate of loss to follow-up) and the assessment of a broad range of domains as outcomes.
There were several potential limitations of our study. First, it was not possible to blind study participants to their treatment assignment, as over 90% were aware of their treatment group when queried at the end of the study. To compensate for this shortcoming, we attempted to keep study coordinators unaware of the participants’ treatment assignment. In order to make the GTM arm more similar to the MPT arm and minimize unblinding among participants, we originally considered including weekly internal pelvic assessments in patients undergoing GTM; we ultimately decided that internal manipulation, which had no therapeutic intent, could not be justified. Secondly, GTM was administered by physical therapists who may have consciously or unconsciously biased the outcome of therapy. In order to avoid such bias, we considered having massage therapists administer GTM, while physical therapists administered MPT; we abandoned this idea since the confounding of therapist with treatment would be an added disadvantage.
Importantly, this randomized controlled trial was not designed to assess whether myofascial physical therapy (MPT) is superior to massage therapy for treatment of UCPPS. Such a study would have to allow for optimization of MPT according to the physical abnormalities that are present, and according to response to treatments and performance of massage therapy treatments by licensed massage therapists. Although physical therapists receive some training in massage therapy, and are all licensed to practice massage, this is not a therapeutic modality that is routinely employed by most physical therapists. We elected to have our physical therapists perform both MPT and GTM treatments, in order to avoid the confounding of ‘therapist’ and ‘treatment’ that would have been present if we had decided to have physical therapists perform the MPT and massage therapists perform GTM. Importantly, the GTM treatment utilized in this trial does not represent the standard of care for massage treatment, and the results of this study should not be taken to mean that MPT is superior to massage therapy as would be practiced by an expert for UCPPS.
We also considered alternative comparison treatments, including treatment with oral medication or with procedures such as sacral neuromodulation or acupuncture. We abandoned those study designs, because alternative treatments that do not involve bodywork cannot provide us with an estimate of the treatment effect that is present simply through meeting weekly with a caring therapist who administers therapeutic touch.
In conclusion, our initial encouraging results suggest that a full-scale clinical trial of myofascial physical therapy methods is possible, and that MPT may offer meaningful clinical benefit to patients with UCPPS. In order to determine if our findings can be replicated, we are now conducting a second small study comparing MPT and GTM (with sample size of approximately 90) at 11 sites.
Abbreviations and Acronyms
- CP
Chronic prostatitis
- CPPS
Chronic pelvic pain syndrome
- CPSI
Chronic Prostatitis Symptom Index
- CTM
Connective Tissue Manipulation
- GRA
Global Response Assessment
- GTM
Global Therapeutic Massage
- IC
Interstitial Cystitis
- ICSI
O’Leary-Sant IC Symptom Index
- ICPI
O’Leary-Sant IC Problem Index
- PBS
Painful Bladder Syndrome
- MPT
Myofascial Physical Therapy
- NIH
National Institutes of Health
- QOL
Quality of Life
- UCPPS
Urologic Chronic Pelvic Pain Syndromes
Urological Pelvic Pain Collaborative Research Network (UPPCRN)Study Group
Northwestern University/Rehab. Institute of Chicago
Colleen Fitzgerald, MD (Co-Investigator)
Suzanne Badillo, PT
Cynthia E. Neville, PT
Darlene Marko, RN
Cleveland Clinic
Jeannette Potts, MD (PI)
Elizabeth O’Dougherty PT
Donel Murphy, RN
William Beaumont Hospital
Kenneth Peters, MD (PI)
Lisa K. Odabachian, MPT, RN, BSN
Andrea Sanfield, PT
Eleanor Anton, RN
Loyola University
Mary Pat Fitzgerald, MD (PI)
Rhonda K. Kotarinos, MS, PT
Carole Fortman PT
Rick Halle-Podell LMT
Judith Senka, RN
Janet Rindels, RN
Lucia Raducanu, RN
Stanford University
Rodney U. Anderson, MD (PI)
Annemarie Cosby, PT
Angie Morey, MS
Christopher K. Payne, MD (PI)
Laura C. Fraser, MPT
Debra Clay, RN
Anna Ramakrishnan, MS
University of Michigan Medical Center
J. Quentin Clemens, MD (PI)*
University of Pennsylvania School of Medicine
J. Richard Landis, PhD (PI)
Keith Mickelberg, RN, BSN
Marie Durborow
Ted Barrell, BS
Shannon Chuai, MS
Liyi Cen, MS
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
John W. Kusek, PhD (Project Officer)
Leroy M. Nyberg, PhD, MD
Christopher Mullins, PhD
*Dr. Clemens conducted this research while at Northwestern University
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. Standardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourology and Urodynamics. 2002;21:167. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Hanno PM, editor. Interstitial Cystitis. London: Springer-Verlag; [Google Scholar]
- 3.Held PJ, Hanno PM, Wein AJ, Paul MV, Cahn MA. Epidemiology of interstitial cystitis. In: Hanno PM, editor. Interstitial Cystitis. London: Springer-Verlag; 1990. pp. 29–48. [Google Scholar]
- 4.Temml C, Wehrberger C, Riedl C, Ponholzer A, Marszalek M, Madersbacher S. Prevalence and correlates for interstitial cystitis symptoms in women participating in a health screening project. Eur Urol. 2003;51:803–9. doi: 10.1016/j.eururo.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Nickel JC, Teichman JM, Gregoire M, Clark J, Downey J. Prevalence, diagnosis, characterization and treatment of prostatitis, interstitial cystitis, and epididymitis in outpatient urological practice: the Canadian PIE study. Urology. 2005;66:935–40. doi: 10.1016/j.urology.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Clemens JQ, Link Cl, Eggers PW, Kusek JW, Nyberg LM, McKinlaqy JB for the BACH Survey Investigators. Prevalence of painful bladder symptoms and effect on quality of life in Black, Hispanic and White men and women. J Urol. 2007;177:1390–4. doi: 10.1016/j.juro.2006.11.084. [DOI] [PubMed] [Google Scholar]
- 7.Leppilahti M, Tammela TLJ, Huhtala H, Auvinen A. Prevalence of symptoms related to interstitial cystitis in women: A population based study in Finland. J Urol. 2002;168:139–43. [PubMed] [Google Scholar]
- 8.Clemens JQ, Meenan RT, O’Keeffe MC, Gao SY, Calhoun EA. Prevalence and incidence of interstitial cystitis in a managed care population. J Urol. 2005;173:98–102. doi: 10.1097/01.ju.0000146114.53828.82. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim IA, Diokno AC, Killinger KA, Carrico DJ, Peters KM. Prevalence of self-reported interstitial cystitis (IC) and interstitial-cystitis-like symptoms among adult women in the community. Int Urol Nephrol. 2007;39:489–95. doi: 10.1007/s11255-007-9181-2. [DOI] [PubMed] [Google Scholar]
- 10.Payne CK, Joyce GF, Wise M, Clemens JQ the Urologic Diseases in America Project. Interstitial cystitis and painful bladder syndrome. J Urol. 2007;177:2042–9. doi: 10.1016/j.juro.2007.01.124. [DOI] [PubMed] [Google Scholar]
- 11.Collins MM, Stafford RS, O’Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. Journal of Urology. 1998;159:1224. [PubMed] [Google Scholar]
- 12.Nickel JC, Downey J, Hunter D, Clark J. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. Journal of Urology. 2001;165:842. [PubMed] [Google Scholar]
- 13.Peters KM, Carrico DJ, Kalinowski SE, Ibrahim IA, Diokno AC. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70(1):16–8. doi: 10.1016/j.urology.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 14.Shoskes DA, Berger R, Elmi A, Landis JR, Propert KJ, Zeitlin S the Chronic Prostatitis Collaborative Research Network Study Group. J Urol. 2008;179:556–60. doi: 10.1016/j.juro.2007.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lilius HG, Oravisto KJ, Valtonen EJ. Origin of pain in interstitial cystitis. Effect of ultrasound treatment on the concomitant levator ani spasm syndrome. Scandinavian Journal of Urology & Nephrology. 1973;7:150. doi: 10.3109/00365597309133690. [DOI] [PubMed] [Google Scholar]
- 16.Weiss JM. Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. Journal of Urology. 2001;166:2226. doi: 10.1016/s0022-5347(05)65539-5. [DOI] [PubMed] [Google Scholar]
- 17.Oyama IA, Rejba A, Lukban JC, Fletcher E, Kellogg-Spadt S, Holzberg AS, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862. doi: 10.1016/j.urology.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 18.Clemens JQ, Nadler RB, Schaeffer AJ, Belani J, Albaugh J, Bushman W. Biofeedback, pelvic floor re-education, and bladder training for male chronic pelvic pain syndrome. Urology. 2000;56:951. doi: 10.1016/s0090-4295(00)00796-2. [DOI] [PubMed] [Google Scholar]
- 19.Cornel EB, van Haarst EP, Schaarsberg RW, Geels J. The effect of biofeedback physical therapy in men with Chronic Pelvic Pain Syndrome Type III. European Urology. 2005;47:607. doi: 10.1016/j.eururo.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RU, Wise D, Sawyer T, Chan C. Integration of myofascial trigger point release and paradoxical relaxation training treatment of chronic pelvic pain in men. Journal of Urology. 2005;174:155. doi: 10.1097/01.ju.0000161609.31185.d5. [DOI] [PubMed] [Google Scholar]
- 21.Travell J, Simons D. Myofascial Pain and Dysfunction: The Trigger Point Manual. 2. 1 and 2. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 22.Benjamin PJ, Tappan FM. Tappan’s Handbook of Healing Massage Techniques: Classic, Holistic, and merging Methods. 4. Prentice Hall; 2005. [Google Scholar]
- 23.O’Leary MP, Sant GR, Fowler FJ, Jr, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 24.Litwin MS, McNaughton-Collins M, Fowler FJJ, Nickel JC, Calhoun EA, Pontari MA, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Network. J Urol. 1999;162:369. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 25.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 27.Schover LR, Fouladi RT, Warneke CL, Neese L, Klein EA, Zippe C, et al. Defining sexual outcomes after treatment for localized prostate carcinoma. Cancer. 2002;95:1773. doi: 10.1002/cncr.10848. [DOI] [PubMed] [Google Scholar]
- 28.Cox DR, Oakes DO. Analysis of Survival Data. Boca Raton, FL: Chapman and Hall/CRC [Imprint]; CRC Press LLC; 1984. [Google Scholar]