Abstract
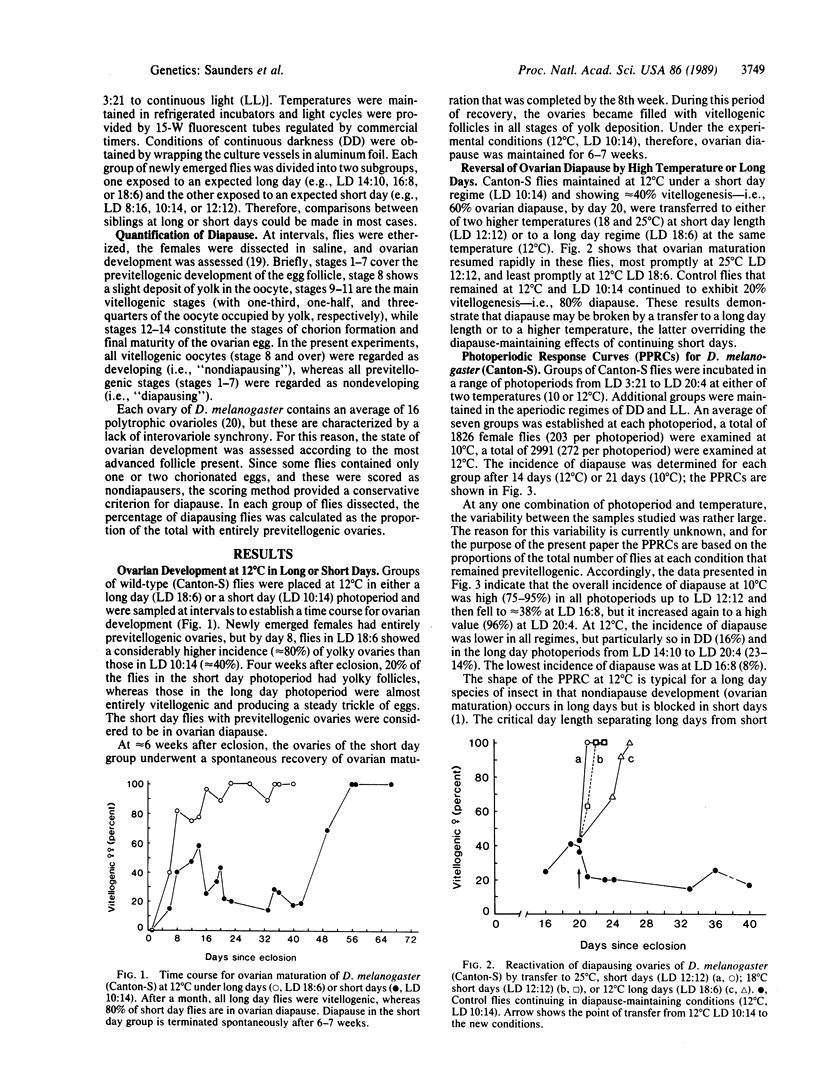
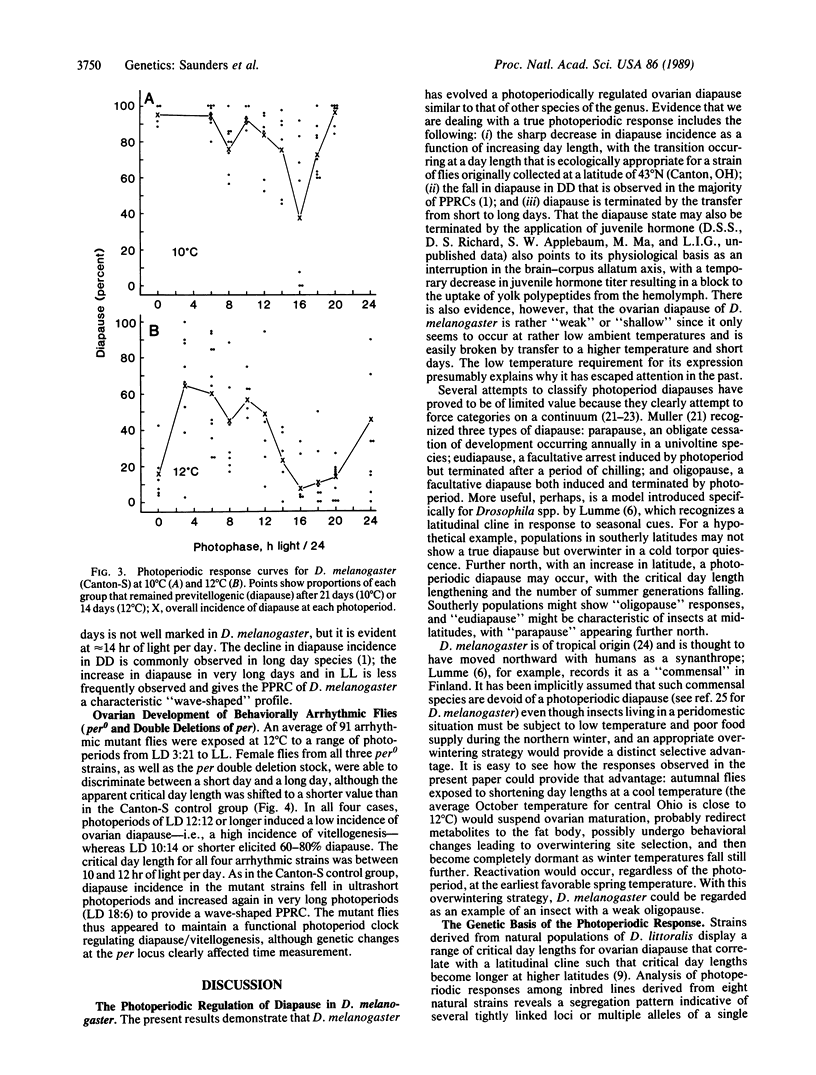
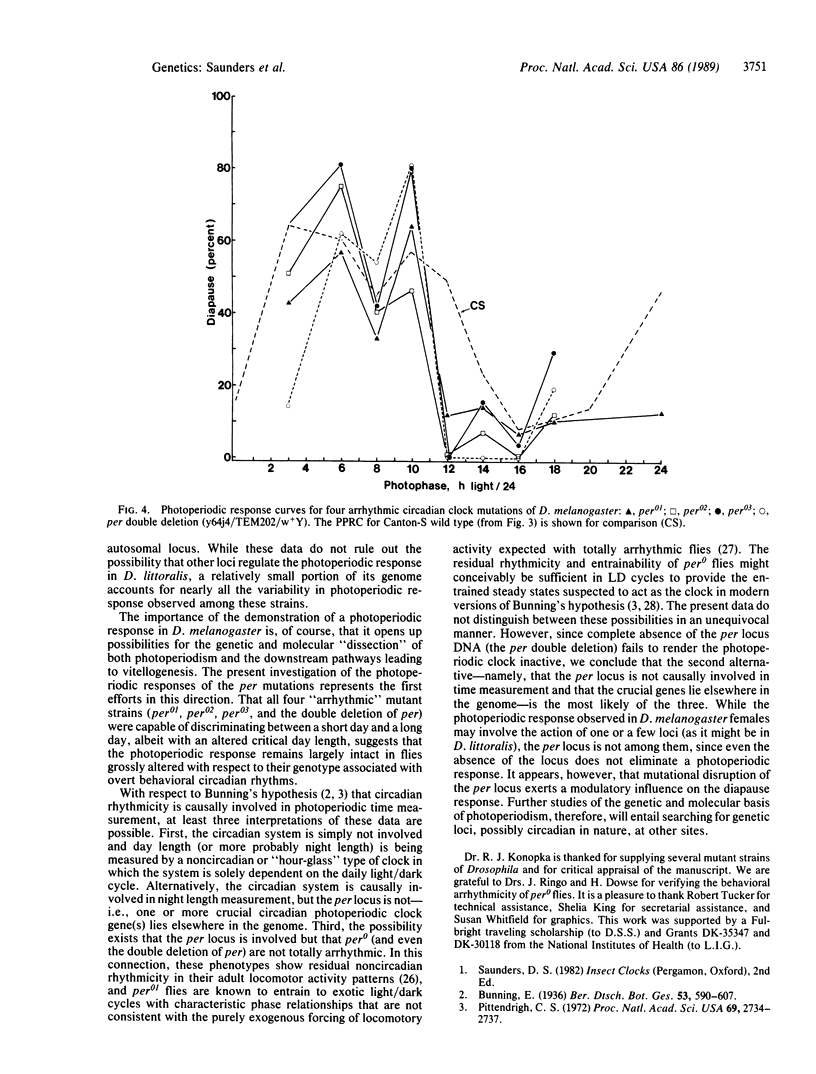
The fruit fly Drosophila melanogaster displays an ovarian diapause that is regulated by photoperiod. Newly eclosed female flies (Canton-S wild type) exposed to short days (less than 14 hr of light per day) at 12 degrees C (or 10 degrees C) enter a fairly shallow reproductive diapause. Females exposed to long days (16 hr of light per day) at the same low temperature undergo ovarian maturation. The short day induced diapause continues for 6-7 weeks under a 10:14 light/dark cycle at 12 degrees C but is terminated rapidly after a transfer to higher temperature (18 or 25 degrees C) or to long days (18:6 light/dark cycle). Females from three strains homozygous for alleles of the period (per) locus, reportedly arrhythmic for behavioral circadian rhythms, and females that possessed two overlapping deletions of per were also capable of discriminating between long and short days, although, when compared with the wild-type flies, the critical day length was shifted to shorter values by approximately 2 hr. It is concluded that the period locus is not causally involved in photoperiod time measurement.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargiello T. A., Young M. W. Molecular genetics of a biological clock in Drosophila. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2142–2146. doi: 10.1073/pnas.81.7.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowse H. B., Hall J. C., Ringo J. M. Circadian and ultradian rhythms in period mutants of Drosophila melanogaster. Behav Genet. 1987 Jan;17(1):19–35. doi: 10.1007/BF01066008. [DOI] [PubMed] [Google Scholar]
- Hall J. C., Rosbash M. Genetic and molecular analysis of biological rhythms. J Biol Rhythms. 1987 Fall;2(3):153–178. doi: 10.1177/074873048700200301. [DOI] [PubMed] [Google Scholar]
- Helfrich C., Engelmann W. Evidences for circadian rhythmicity in the per0 mutant of Drosophila melanogaster. Z Naturforsch C. 1987 Nov-Dec;42(11-12):1335–1338. doi: 10.1515/znc-1987-11-1231. [DOI] [PubMed] [Google Scholar]
- KING R. C., RUBINSON A. C., SMITH R. F. Oogenesis in adult Drosophila melanogaster. Growth. 1956 Jun;20(2):121–157. [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumme J., Oikarinen A., Lakovaara S., Alatalo R. The environmental regulation of adult diapause in Drosophila littoralis. J Insect Physiol. 1974 Oct;20(10):2023–2033. doi: 10.1016/0022-1910(74)90109-7. [DOI] [PubMed] [Google Scholar]
- Pittendrigh C. S. Circadian surfaces and the diversity of possible roles of circadian organization in photoperiodic induction. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2734–2737. doi: 10.1073/pnas.69.9.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh C. S., Takamura T. Temperature dependence and evolutionary adjustment of critical night length in insect photoperiodism. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7169–7173. doi: 10.1073/pnas.84.20.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. F., Konopka R. J. Circadian clock phenotypes of chromosome aberrations with a breakpoint at the per locus. Mol Gen Genet. 1981;183(2):243–251. doi: 10.1007/BF00270625. [DOI] [PubMed] [Google Scholar]
- Tyshchenko V. P., Goryshin N. I., Azarian A. G. Rol' tsirkadnykh protsessov v fotoperiodizme nasekomykh. Zh Obshch Biol. 1972 Jan-Feb;33(1):21–31. [PubMed] [Google Scholar]
- Zehring W. A., Wheeler D. A., Reddy P., Konopka R. J., Kyriacou C. P., Rosbash M., Hall J. C. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984 Dec;39(2 Pt 1):369–376. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]



