Abstract
Objective:
To compare the efficacy and safety of 1-mg and 4-mg doses of preservative-free intravitreal triamcinolone with observation for eyes with vision loss associated with macular edema secondary to perfused central retinal vein occlusion (CRVO).
Methods:
Multicenter, randomized, clinical trial of 271 participants.
Main Outcome Measure:
Gain in visual acuity letter score of 15 or more from baseline to month 12.
Results:
Seven percent, 27%, and 26% of participants achieved the primary outcome in the observation, 1-mg, and 4-mg groups, respectively. The odds of achieving the primary outcome were 5.0 times greater in the 1-mg group than the observation group (odds ratio [OR],5.0; 95% confidence interval [CI], 1.8-14.1; P=.001) and 5.0 times greater in 4-mg group than the observation group (OR,5.0; 95% CI, 1.8-14.4; P=.001); there was no difference identified between the 1-mg and 4-mg groups (OR, 1.0; 95% CI, 0.5-2.1; P=.97). The rates of elevated intraocular pressure and cataract were similar for the observation and 1-mg groups, but higher in the 4-mg group.
Conclusions:
Intravitreal triamcinolone is superior to observation for treating vision loss associated with macular edema secondary to CRVO in patients who have characteristics similar to those in the SCORE-CRVO trial. The 1-mg dose has a safety profile superior to that of the 4-mg dose.
Application to Clinical Practice:
Intravitreal triamcinolone in a 1-mg dose, following the retreatment criteria applied in the SCORE Study, should be considered for up to 1 year, and possibly 2 years, for patients with characteristics similar to those in the SCORE-CRVO trial.
Trial Registration:
clinicaltrials.gov Identifier: NCT00105027
Central retinal vein Occlusion (CRVO) is an important cause of vision loss worldwide.1-4 The prevalence of CRVO was estimated to be 0.4% in the Blue Mountains Eye Study.2 The 15-year cumulative incidence of CRVO was 0.5% in the Beaver Dam Eye Study.3 In the Beaver Dam cohort, central and branch retinal vein occlusion accounted for 12% of eyes that developed severe vision loss over a 15-year period.4
Macular edema is a frequent cause of vision loss in eyes with CRVO.5-7 The natural history of macular edema secondary to CRVO was delineated in the Central Vein Occlusion Study (CVOS), which found no significant difference in visual outcome between the treatment (grid photocoagulation) and observation groups at any follow-up point.7 Although there was a definite decrease in macular edema on fluorescein angiography in the treatment group when compared with the observation group, this did not translate to a direct visual improvement. Therefore, at present there is no proven, effective therapy for vision loss associated with macular edema secondary to CRVO.
During the last decade, a number of additional treatments for macular edema secondary to CRVO have been investigated. Such treatments include vitrectomy surgery, radial optic neurotomy, intravitreal injection of tissue plasminogen activator, and intravitreal injection of aptamers or antibodies targeted at vascular endothelial growth factor (VEGF).8-15 Treatment of macular edema secondary to CRVO with intravitreal injection(s) of triamcinolone acetonide (hereafter referred to as intravitreal triamcinolone) has been evaluated recently. Beginning in 2002, a few case series suggested intravitreal triamcinolone as a potentially efficacious therapy for vision loss and retinal thickening in patients with CRVO, but suggested that some patients develop steroid-related complications such as elevated intraocular pressure (IOP) and cataract; injection-related complications such as retinal detachment and endophthalmitis were also reported.16-19 Most of these case series lacked long-term follow-up and adequate numbers of study participants. Despite the shortcomings of these case series, intravitreal triamcinolone is currently in use for treatment of CRVO.
The rationale for the use of corticosteroids to treat macular edema secondary to CRVO follows the observation that the increase in retinal capillary permeability that results in macular edema may be caused by a breakdown of the blood retina barrier mediated in part by VEGF, a 45-kDa glycoprotein.20-22 Therefore, attenuation of the effects of VEGF, noted to be upregulated in eyes with CRVO, may reduce macular edema associated with CRVO.23,24 Corticosteroids have been demonstrated to inhibit the expression of VEGF and therefore may be an effective therapy for macular edema.25,26 Inflammation may also contribute to the pathology of CRVO, and the anti-inflammatory properties of corticosteroids may play a role in the attenuation of the disease process.27 Additionally, corticosteroids may also have a neuroprotective effect that may be beneficial in eyes with CRVO.28
Intravitreal triamcinolone is used by ophthalmologists in the clinical setting as a readily available pharmacologic agent (Kenalog 40; Bristol-Myers Squibb, Princeton, New Jersey, or Triesence; Alcon Inc, Fort Worth, Texas), though use for the treatment of macular edema is off label. Other formulations such as compounded preservative-free triamcinolone acetonide are also used in the clinical setting.
The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study, sponsored by the National Eye Institute, is a clinical trial designed to compare 1-mg and 4-mg doses of intravitreal triamcinolone with standard care for treatment of vision loss associated with macular edema secondary to perfused CRVO and branch retinal vein occlusion (BRVO).29,30 This article describes findings from the SCORE-CRVO trial. A companion article that compares intravitreal triamcinolone with grid photocoagulation, when applicable, for the treatment of vision loss associated with macular edema secondary to BRVO is published concurrently (the SCORE-BRVO trial).31 The standard-care treatment for CRVO was observation at the time the SCORE Study was planned in 2003. The 2 primary study objectives of the SCORE-CRVO trial are (1) to determine whether intravitreal triamcinolone at doses of 1 mg or 4 mg produces greater visual benefit, with an acceptable safety profile, than observation for the treatment of vision loss associated with macular edema secondary to CRVO, and (2) to compare the efficacy and safety of the 1-mg and 4-mg triamcinolone doses.
METHODS
DESIGN
The SCORE-CRVO trial was designed as a multicenter, prospective, randomized clinical trial and adhered to the tenets of the Declaration of Helsinki. Approval for the protocol was obtained from either a central (Jaeb Center for Health Research) or local institutional review board. Health Insurance Portability and Accountability Act–compliant informed consent forms were obtained before screening any participants. Study oversight was provided by an independent data and safety monitoring committee. Eligibility for the SCORE-CRVO trial was determined at the clinical sites (Table 1). All participants were randomized within 21 days of screening or rescreening. One eye per participant was enrolled in the trial. Participants and physicians were masked to the intravitreal triamcinolone dose (1 mg vs 4 mg) but not to the treatment assignment of observation vs intravitreal triamcinolone. The prespecified primary efficacy evaluation was performed at month 12. The primary outcome measure was the proportion of participants who experienced a gain in visual acuity letter score of 15 or more from baseline to month 12, as assessed by the electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) method.32
Table 1.
Study Eye Major Inclusion and Exclusion Criteria
| Inclusion Criteria |
| Best-corrected ETDRS visual acuity letter score of ≤73 (approximate Snellen equivalent, 20/40 or worse) and ≥19 (20/400 or better)a |
| Center-involved macular edema secondary to CRVO present on clinical examination |
| Mean central subfield retinal thickness of 2 OCT fast macular scans, ≥250 μm |
| Media clarity, pupillary dilation, and participant cooperation sufficient for adequate fundus photographs |
|
|
| Exclusion Criteria |
| Macular edema due to a cause other than CRVO |
| An ocular condition such that visual acuity would not improve from resolution of the edema (eg, foveal atrophy) |
| Substantial cataract estimated to have reduced visual acuity by 3 lines or more |
| Prior treatment with intravitreal corticosteroids at any time or peribulbar steroid injection within 6 mo prior to randomization |
| History of focal or grid macular photocoagulation within 15 wk (3.5 mo), panretinal photocoagulation within 4 mo prior to randomization, or anticipated need for panretinal photocoagulation within the 4 mo following randomization |
| Prior pars plana vitrectomy |
| Major ocular surgery (including cataract extraction) within prior 6 mo or anticipated within the next 6 mo following randomization |
| Yttrium aluminum garnet capsulotomy performed within 2 mo prior to randomization |
| IOP ≥25 mm Hg, open-angle glaucoma (either primary open-angle glaucoma or other cause of open-angle glaucoma), steroid-induced IOP elevation that required IOP-lowering treatment, or pseudoexfoliation |
| Aphakia |
Abbreviations: CRVO, central retinal vein occlusion; ETDRS, Early Treatment Diabetic Retinopathy Study; IOP, intraocular pressure; OCT, optical coherence tomography.
The original lower limit of visual acuity was expanded from 34 or more to 24 or more 5 months after accrual began and then from 24 or more to 19 or more 12 months after accrual began.
RANDOMIZATION
Within baseline visual acuity strata of good (visual acuity letter score, 73-59; Snellen equivalent, 20/40 to 20/63); moderate (visual acuity letter score, 58-49; Snellen equivalent, 20/80 to 20/100); and poor (visual acuity letter score, 48-19; Snellen equivalent, 20/125 to 20/400), participants were randomly assigned centrally through a Web-based data entry system maintained at the data coordinating center (EMMES Corporation, Rockville, Maryland), with equal probability to receive standard care (observation group), 1 mg of intravitreal triamcinolone, or 4 mg of intravitreal triamcinolone using a permuted blocks design with random block sizes.
VISIT SCHEDULE
Study visits were planned for every 4 months through 36 months. At baseline and at each follow-up visit, the best-corrected visual acuity letter score was measured at 3 m by a masked certified tester using the E-ETDRS method.32 A standardized refraction was performed at baseline, month 4, and at the annual visits (months 12, 24, and 36). All participants who received intravitreal triamcinolone had additional safety visits at 4 days (±3 days) and at 4 weeks (±1 week) following each injection; visual acuity, IOP measurement, and an eye examination including a dilated fundus examination were recorded for the study eye at these visits. At all other visits, participants had an eye examination, E-ETDRS testing, IOP measurement, and an optical coherence tomography (OCT) scan (OCT2 or Stratus OCT; Carl Zeiss Meditech, Dublin, California). These tests were performed on both eyes at all visits except months 8, 16, 20, 28, and 32, where OCT was performed only on the study eye. Stereoscopic color fundus photographs (7 fields) were taken of the study eye at baseline and at the annual visits. Three-field photographs were taken of the study eye at the month 4, 8, 16, 20, 28, and 32 visits, and of the fellow eye at the baseline and annual visits. Lens opacities for both eyes were assessed at baseline and at the annual visits using the modified Age-Related Eye Disease Study grading method.33 Fluorescein angiograms were performed at baseline and at the month 4, 12, and 24 visits. All images were sent to the reading center (University of Wisconsin Fundus Photograph Reading Center, Madison, Wisconsin) foranalysis, where they were graded in a masked fashion. Blood pressure was measured at baseline and at the annual visits.
Participating study personnel such as physician investigators and study coordinators were certified by the data coordinating center. Photographers and technicians who performed the fundus photographs and OCT images for this study were certified by the reading center.
INTRAVITREAL INJECTIONS
The optimal dose of intraocular corticosteroid to maximize efficacy and simultaneously minimize adverse effects was not known when the SCORE Study was initiated. The most commonly used dose of triamcinolone for treating eyes with macular edema secondary to CRVO is 4 mg, delivered in a volume of 0.1 mL. However, the rationale for using this dose has been based on clinical feasibility (limiting volume of 0.1 mL of the first available triamcinolone formulation; Kenalog) rather than scientific principles. Clinical experience with other triamcinolone doses ranging from 1 to 20 mg34-36 is limited.
The SCORE-CRVO trial evaluated 1-mg and 4-mg doses of triamcinolone. The 1-mg dose was evaluated because this dose is likely to exceed the concentration necessary to saturate the glucocorticoid receptors in the cell cytoplasm.37,38 In addition, it was anticipated that, compared with the 4-mg dose, the 1-mg dose would have a lower risk of steroid-related adverse events. Insufficient data were available at the time of protocol development to warrant evaluating a dose higher than 4 mg.
A preservative-free, nondispersive formulation of triamcinolone was used in the current study in an effort to avoid the postinjection intraocular inflammation described with Kenalog and some of the compounded triamcinolone formulations, thought to be attributable to the excipients in Kenalog, endotoxins, or to a particle dispersion phenomenon.39,40 The study drug was manufactured as a sterile, preservative-free, single-use, intravitreal injectable (Allergan Inc, Irvine, California; 4-mg brand name, Trivaris) in 1-mg and 4-mg doses. Both doses were in a volume of 0.05 mL. All study eyes assigned to the intravitreal triamcinolone groups received a standardized ocular surface preparation procedure prior to injection consisting of an eyelid speculum, topical anesthetic, administration of topical antibiotics on the day of injection, and asepsis with povidone iodine.41
Following the preparation procedure, either 1 mg or 4 mg of triamcinolone acetonide was injected into the vitreous cavity via the pars plana 3 to 4 mm posterior to the limbus. The eyelid speculum was removed, and indirect ophthalmoscopy was performed to confirm the intravitreal location of the triamcinolone and to confirm a perfused central retinal artery. Participants were instructed to use topical antibiotics 4 times daily for 3 days following the injection.
RETREATMENT CRITERIA
Retreatment criteria were identical for both intravitreal triamcinolone groups. Participants were retreated at 4-month intervals (minimum, 105 days from the last treatment) according to the original treatment assigned at randomization, except when specific reasons not to retreat were encountered. However, even if these reasons for not retreating were present, investigators were not prohibited from retreating. The 3 reasons to consider deferral of retreatment were (1) treatment was successful (either the investigator believed that the macula was flat, with an OCT-measured central subfield thickness of ≤225 μm, the visual acuity letter score was 79 or more [Snellen equivalent, 20/25 or better], or there was substantial improvement in macular edema from the prior treatment and further improvement from the prior treatment might be expected); (2) treatment was contraindicated because, in the judgment of the investigator, the participant had a significant adverse effect due to prior treatment (eg, IOP rise that required treatment); or (3) additional treatment was considered “apparently futile.” Treatment was considered apparently futile if a period of 8 or more months transpired during which there were 2 intravitreal triamcinolone treatments but there was no evidence of at least borderline improvement. Borderline improvement was present if, when compared with findings at the beginning of the period, there was an increase in visual acuity letter score of 5 or more or there was a decrease in calculated retinal thickening (actual thickness minus mean normal thickness42) that was at least 50 μm and represented at least a 20% reduction in retinal thickening compared with the findings at the beginning of the period.
ALTERNATE TREATMENT CRITERIA
In the SCORE-CRVO trial, eyes assigned to observation could receive intravitreal triamcinolone when there was a loss from baseline in best-corrected visual acuity letter score of 15 or more that was present at 2 consecutive 4-month–interval visits. The decrease in visual acuity had to be a result of persistent or recurrent macular edema (ie, not because of cataract or other abnormality) that was documented on OCT. If the above criteria were met, eyes assigned to observation could receive (but were not required to receive) intravitreal triamcinolone (4-mg dose, study formulation); all such eyes were analyzed as originally assigned in the observation group, even if they received treatment with intravitreal triamcinolone.
ASSESSMENT OF MACULAR EDEMA
The reading center graders, without knowledge of treatment assignment or participant clinical data, followed a standardized protocol to grade the area of macular edema and retinal hemorrhage using stereoscopic fundus photographs.43 The OCT scans were evaluated for both quantitative data (eg, central subfield thickness), using the macular fastmap scan consisting of 6 radially oriented scans, and qualitative data (eg, presence or absence of vitreomacular traction, subretinal fluid, and cystoid spaces), using the 2 scan crosshair images.44 Center point thickness was used for analysis instead of central subfield thickness because this permitted correction of errors in the measurement of the inner and outer retinal boundaries. The correlation between center point thickness and central subfield thickness is 0.98.45 Fluorescein angiograms were graded for area of nonperfusion and leakage in disc areas.
STATISTICAL ANALYSIS
The primary efficacy outcome measure of the SCORE-CRVO trial is the proportion of participants experiencing a gain in EETDRS visual acuity letter score of 15 or more from baseline to month 12. The primary outcome measure was examined for each of 3 pairwise comparisons: (1) the 1-mg triamcinolone group vs the observation group; (2) the 4-mg triamcinolone group vs the observation group; and (3) the 1-mg vs the 4-mg triamcinolone group.
The SCORE-CRVO trial was designed assuming efficacy of 15% in the observation arm, estimated from the CVOS,7 and 30% in both the 1-mg and 4-mg triamcinolone arms, estimated from studies of case series for treatment of macular edema with intravit-real triamcinolone.16-19,46 The original target sample size was 630 participants, to be divided equally among the 3 treatment arms. After 10% attrition, this would yield 90% power independently at α=.025, 2-tailed, for 2 of the 3 primary pairwise comparisons: (1) the 1-mg triamcinolone group vs the observation group; and (2) the 4-mg triamcinolone group vs the observation group. A priori, a treatment difference between the 1-mg and the 4-mg triamcinolone groups was not expected. Slow recruitment prompted a downward revision of the sample size from 630 to 486 participants, granting 80% power. Later, a series of conditional power analyses convinced the data and safety monitoring committee that the trial should continue, even though only about 50% of the revised sample size would be attained. A common closeout date of February 28, 2009, was subsequently established to allow at least 12 months of follow-up of all participants.
The primary analysis of the SCORE-CRVO trial is based on an observed case analysis that analyzed participants based on the arm to which they were randomized (consistent with the intention-to-treat principle) and treated missing 12-month observations as missing completely at random. To be included in the primary analysis, a study participant must have had 12-month visual acuity within a window ranging from 2 months before the target date to 3 months after the target date, with the target date defined as 12 months after the date of randomization. The statistical significance of the 3 pairwise comparisons was calculated using closed testing procedures47 modified for sequential testing, with family-wide error controlled at no more than α=.05. Logistic regression modeled the effects of the treatment assignment on the primary outcome while adjusting for the stratification factor of baseline visual acuity (good, moderate, or poor) used in the design of the SCORE-CRVO trial. P values for the 3 primary study group comparisons and for the simultaneous comparison of all 3 arms (1 mg of triamcinolone vs 4 mg of triamcinolone vs observation) were calculated. P values were then adjusted to account for simultaneous inference at a single time point as well as for interim monitoring. For interim monitoring, an O'Brien-Fleming–type boundary using a Lan and DeMets α-spending function was specified so that, for all but the final comparison, family-wide error would be no more than .005 and the amount of family-wide α spent at the final comparison would be between .045 and .05.
Analyses were also performed to assess the consistency of the primary efficacy results. This included a last observation carried forward analysis and a per protocol analysis that included only study eyes with 12-month visual acuity data and excluded participants who, before 12 months, received an alternative treatment (ie, treatment crossovers) or a nonprotocol treatment, who did not meet the eligibility criteria, or who did not receive the treatment assigned at randomization. Additional analyses assessing the consistency of results are presented elsewhere (eTable; http://www.archophthalmol.com).
Secondary statistical analyses were performed, with analysis of variance and Kruskal-Wallis tests for continuous data and χ2 tests for categorical data. To examine changes from baseline in visual acuity for various subgroups, 95% confidence intervals (CIs) for the mean changes were provided. Presentations of continuous data included median (interquartile range) instead of or in addition to mean (standard deviation) to allow a description of the distribution of the data. Only the primary analysis was adjusted for multiple testing. Thus, P values and CIs for secondary findings are intended primarily to give a sense of the variability inherent in the data. Adverse events reported by the clinical centers were coded per the Medical Dictionary for Regulatory Activities, version 11.0, by trained staff at the data coordinating center. SAS version 9.1.3 (SAS Inc, Carey, North Carolina) was used to conduct all statistical analyses. All analyses included data available as of April 1, 2009.
RESULTS
BASELINE CHARACTERISTICS
Between November 8, 2004, and February 29, 2008, 271 patients with CRVO were enrolled from 66 clinical sites (Table 2). The mean duration of macular edema (based on patient history or ophthalmologic diagnosis) prior to enrollment was 4 months; 39% of participants had macular edema for less than 3 months and 81% for 6 months or less. The mean baseline visual acuity letter score was 51 (Snellen equivalent, approximately 20/100), and eyes had a mean center point thickness of 659 μm based on OCT. A more detailed description of the SCORE-CRVO participant population can be found elsewhere.41
Table 2.
Baseline Characteristics by Treatment Group
| No. (%) |
||||
|---|---|---|---|---|
| Characteristic | Observation | 1 mga | 4 mga | Total |
| Participants | 88 | 92 | 91 | 271 |
| Demographic characteristics | ||||
| Mean (SD) age, y | 69.2 (12.8) | 67.4 (12.4) | 67.5 (12.0) | 68.0 (12.4) |
| Minimum/maximum | 35/93 | 32/88 | 27/91 | 27/93 |
| Women | 40 (45) | 43 (47) | 40 (44) | 123 (45) |
| White race | 81 (92) | 84 (91) | 82 (90) | 247 (91) |
| Study eye characteristics | ||||
| Mean (SD) E-ETDRS visual acuity letter score (Snellen equivalent) | 52.1 (13.1) | 50.6 (14.9) | 51.0 (14.4) | 51.2 (14.1) |
| 73-59 (20/40 to 20/63) | 33 (38) | 33 (36) | 34 (37) | 100 (37) |
| 58-49 (20/80 to 20/100) | 20 (23) | 19 (21) | 19 (21) | 58 (21) |
| 48-19 (20/125 to 20/400) | 35 (40) | 40 (43) | 38 (42) | 113 (42) |
| Duration of macular edema, mo | 4.2 (3.1) | 4.5 (4.2) | 4.2 (3.6) | 4.3 (3.7) |
| <3 | 29 (33) | 36 (39) | 40 (44) | 105 (39) |
| 3-6 | 43 (49) | 38 (41) | 34 (37) | 115 (42) |
| 7-12 | 14 (16) | 14 (15) | 15 (16) | 43 (16) |
| >12 | 2 (2) | 4 (4) | 2 (2) | 8 (3) |
| IOP, mm Hg | 15.4 (3.2) | 15.3 (3.2) | 15.8 (3.2) | 15.5 (3.2) |
| IOP-lowering medication | 9 (10.0) | 4 (4.3) | 7 (7.7) | 20 (7.4) |
| Phakic | 66 (75) | 77 (84) | 76 (84) | 219 (81) |
| Imaging data, mean (SD) | ||||
| OCT center point thickness, μm | 695 (208) | 643 (226) | 641 (248) | 659 (229) |
| Total macular volume, mean (SD), mm3 | 10.4 (1.7) | 10.6 (2.0) | 10.0 (2.1) | 10.3 (2.0) |
| Area of retinal thickening within the grid, mean (SD), DAb | 13.0 (4.6) | 12.2 (4.8) | 11.8 (5.1) | 12.3 (4.8) |
| Area of retinal hemorrhage within the grid, mean (SD), DAb | 3.6 (3.0) | 3.1 (3.2) | 3.4 (3.5) | 3.4 (3.3) |
| Area of fluorescein leakage within the grid, mean (SD), DAb | 11.6 (4.8) | 10.9 (5.0) | 10.4 (5.1) | 10.9 (5.0) |
| >10 DA of capillary nonperfusion in the eyec | 0 (0) | 2 (3) | 1 (2) | 3 (2) |
| Mean (SD) nonstudy eye E-ETDRS visual acuity letter score | 80.8 (15.0) | 81.2 (12.6) | 81.5 (10.3) | 81.2 (12.7) |
| Other clinical characteristics | ||||
| Diabetes mellitus | 22 (25) | 17 (18) | 23 (25) | 62 (23) |
| Hypertension | 70 (80) | 63 (68) | 64 (70) | 197 (73) |
| Coronary artery disease | 20 (23) | 17 (18) | 19 (21) | 56 (21) |
| History of cancer | 14 (16) | 19 (21) | 25 (27) | 58 (21) |
Abbreviations: DA, disc area; E-ETDRS, electronic Early Treatment Diabetic Retinopathy Study; IOP, intraocular pressure; OCT, optical coherence tomography.
Indicates dosage of intravitreal triamcinolone acetonide.
The grid is defined as the 9 ETDRS subfields centered in the macula and measures 16 DAs.
Capillary nonperfusion in the eye was assessed in 59, 72, and 65 eyes at baseline in the observation, 1-mg, and 4-mg triamcinolone acetonide dosage groups, respectively.
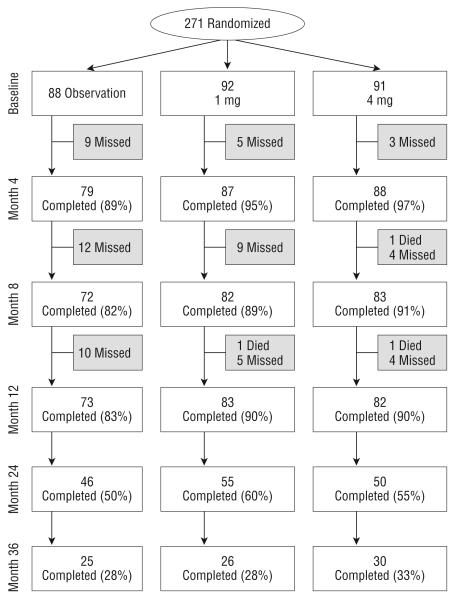
FOLLOW-UP
Figure 1 shows study follow-up of all participants at 4-month intervals through month 12, and then annually through month 36. The month 12 primary outcome visit was completed by 83%, 90%, and 90% in the observation, 1-mg, and 4-mg groups, respectively. At the time of study completion, 56% of participants had month 24 outcomes assessed and 30% had month 36 outcomes assessed.
Figure 1.
Flowchart of participant progress in the SCORE-CRVO Study. Missed visits include those who missed a visit but came back for other visits and those who prematurely withdrew from the study.
STUDY TREATMENTS
Prior to month 12, the average number of injections was similar in the triamcinolone groups, with 2.2 in the 1-mg triamcinolone group (95% CI, 2.1-2.4) and 2.0 in the 4-mg triamcinolone group (95% CI, 1.8-2.1) (Table 3). The success of the prior triamcinolone treatment was the primary reason for not giving additional injections prior to 12 months (66%). Less frequent reasons cited for not giving retreatment prior to 12 months included futility of the treatment (11%), treatment contraindication (7%), participant refusal (3%), and other reasons (11%).
Table 3.
Number of Protocol Treatments and Percentage of Participants Treated
| Participants, No. (%) |
|||
|---|---|---|---|
| Observation (n=88) |
1 mga (n=92) |
4 mga (n=91) |
|
| Injections | |||
| Baseline to prior to 12 mo, mean (95% CI) | 0.1 (0.0-0.2) | 2.2 (2.1-2.4) | 2.0 (1.8-2.1) |
| Prior to 12 mo | |||
| 3 | 0 (0) | 45 (49) | 29 (32) |
| 2 | 0 (0) | 24 (26) | 30 (33) |
| 1 | 8 (9)b | 23 (25) | 31 (34) |
| 0 | 80 (91) | 0 (0) | 1 (1)c |
| Visit | |||
| Baseline | 88 (0) | 92 (100) | 91 (99) |
| Month 4 | 81 (1) | 87 (77)d | 88 (57) |
| Month 8 | 74 (9) | 82 (57) | 83 (46) |
| Month 12 | 73 (11) | 83 (36) | 82 (35) |
| Month 16 | 66 (8) | 71 (21) | 69 (32) |
| Month 20 | 52 (10) | 63 (22) | 60 (22) |
| Month 24 | 46 (9) | 55 (16) | 50 (26) |
Abbreviation: CI, confidence interval.
Indicates dosage of intravitreal triamcinolone acetonide.
The 8 participants receiving an injection in the observation group represent crossovers from observation to 4 mg, per protocol guidelines.
One participant was randomized to the 4-mg arm, but withdrew before initial treatment and therefore never received an injection.
Percentage indicates participants who received injections of those who attended the follow-up visit.
Few treatment protocol deviations were noted prior to 12 months. These entailed intravitreal injections of (1) anti-VEGF drug in four 1-mg triamcinolone participants, two 4-mg triamcinolone participants, and 2 participants in the observation group; and (2) non–study formulation triamcinolone in two 1-mg triamcinolone participants and 1 participant in the observation group. These participants were included in the primary analyses, but not in the per protocol analysis.
OUTCOMES
Visual Acuity
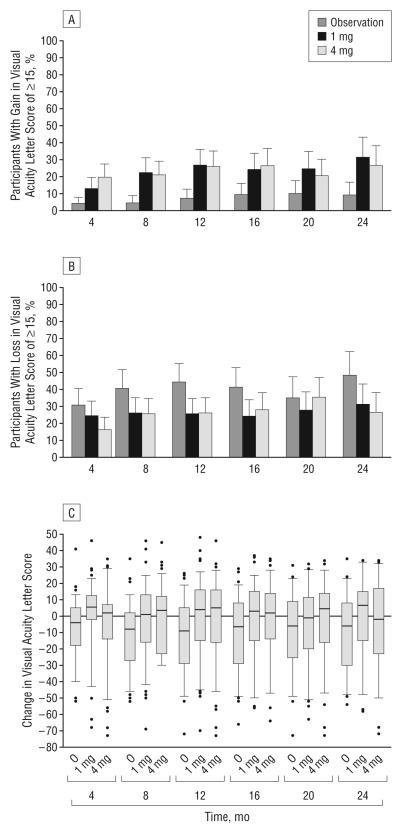
The primary outcome of the SCORE-CRVO trial, the percentage of participants with a gain in visual acuity letter score of 15 or more from baseline to month 12, was 6.8%, 26.5%, and 25.6% for the observation, 1-mg, and 4-mg groups, respectively (Table 4; Figure 2A). The odds ratio (OR) estimates for a gain in visual acuity letter score of 15 or more (after adjusting for baseline visual acuity) comparing the 1-mg and 4-mg triamcinolone groups, respectively, with the observation group, were 5.0 (95% CI, 1.8-14.1; P=.001) and 5.0 (95% CI, 1.8-14.4; P=.001) and, comparing the 1-mg with the 4-mg triamcinolone group, was 1.0 (95% CI, 0.5-2.1; P=.97). The last observation carried forward approach and the per protocol analysis gave results that were qualitatively similar to the primary analysis, with both triamcinolone groups similar to each other and significantly better than the observation group.
Table 4.
Change in Visual Acuity Letter Score at Month 12
| Change in Visual Acuity Letter Score | Observation (n=73) |
1 mga (n=83) |
4 mga (n=82) |
|---|---|---|---|
| Distribution of change at month 12b | |||
| Gain | |||
| ≥15c | 6.8 | 26.5 | 25.6 |
| 10-14 | 8.2 | 14.5 | 9.8 |
| 5-9 | 11.0 | 9.6 | 13.4 |
| Same ±4 | 19.2 | 14.5 | 18.3 |
| Loss | |||
| 5-9 | 6.8 | 4.8 | 3.7 |
| 10-14 | 4.1 | 4.8 | 3.7 |
| ≥15 | 43.8 | 25.3 | 25.6 |
| Mean (95% Cl)d | −12.1 (−17.1 to −7.1) | −1.2 (−6.4 to 4.1) | −1.2 (−6.3 to 4.0) |
| Median (IQR) | −9.0 (−29.0 to 5.0) | 5.0 (−16.0 to 16.0) | 4.0 (−15.0 to 16.0) |
Abbreviations: CI, confidence interval; IQR, interquartile range.
Indicates dosage of intravitreal triamcinolone acetonide.
Indicates percentage of observed cases (ie, observation group, 73; 1-mg group, 83; 4-mg group, 82).
Odds ratios (ORs) and closed-testing P values for pairwise comparisons with a gain in visual acuity letter score of 15 or more are 1 mg vs observation: OR, 5.0; 95% CI, 1.8-14.1; P=.001; 4 mg vs observation: OR, 5.0; 95% CI, 1.8-14.4; P=.001; 4 mg vs 1 mg: OR, 1.0; 95% CI, 0.5-2.1; P=.97. Odds ratios are adjusted for baseline visual acuity. Confidence intervals are not adjusted for simultaneous testing or interim monitoring.
Analysis of variance comparing the means among the 3 groups at month 12; P=.004.
Figure 2.
Change from baseline in electronic Early Treatment Diabetic Retinopathy Study visual acuity letter score at each 4-month follow-up visit. The histograms show the percentages of participants with a gain (A) or loss (B) in visual acuity letter score of 15 or more from baseline. The dashed line from each bar represents the upper 95% confidence limit. C, Box plot with whiskers represents the 5th and 95th percentiles; the line in the box represents the median; dots, values outside the whiskers; O, observation; 1 mg and 4 mg, doses of intravitreal triamcinolone acetonide.
Both triamcinolone groups had a similar change from baseline to month 12 in mean visual acuity letter score (an approximately 1-2–letter loss) compared with a mean loss of 12 in the observation group (Table 4). Across visits (Table 5; Figure 2), the percentage with a gain in visual acuity letter score of 15 or more was consistently lower in the observation group than the triamcinolone groups and similar for the 1-mg and 4-mg triamcino-lone groups (Figure 2A). At month 24, a loss in visual acuity letter score of 15 or more (Figure 2B) was noted in approximately 48% of participants in the observation group compared with approximately 30% of participants in the triamcinolone groups (P=.06, χ2 test).
Table 5.
Change From Baseline in Visual Acuity Letter Score by Follow-up Visit and Treatment Group
| Visit | Participants Who Attended, No. From Observation/ 1-mg/4-mg Group |
Change From Baseline in Visual Acuity Letter Score |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) |
Gain of ≥15, % (95% CI) |
Loss of ≥15, % |
||||||||
| Observation | 1 mga | 4 mga | Observation | 1 mga | 4 mga | Observation | 1 mga | 4 mga | ||
| Month 4 | 79/87/88 | −7.8 (−11.6 to −3.9) | −4.8 (−9.6 to 0.0) | 1.5 (−2.6 to 5.7) | 4 (0-8) | 13 (6-20) | 19 (11-28) | 30 | 24 | 16 |
| Month 8 | 72/82/83 | −11.7 (−16.2 to −7.2) | −3.0 (−8.2 to 2.3) | −1.9 (−6.7 to 2.9) | 4 (0-9) | 22 (13-31) | 20 (12-29) | 40 | 26 | 25 |
| Month 12 | 73/83/82 | −12.1 (−17.1 to −7.1) | −1.2 (−6.4 to 4.1) | −1.2 (−6.3 to 4.0) | 7 (1-13) | 27 (17-36) | 26 (16-35) | 44 | 25 | 26 |
| Month 16 | 66/71/69 | −11.4 (−16.9 to −6.0) | −1.5 (−6.7 to 3.7) | −3.6 (−9.3 to 2.1) | 9 (2-16) | 24 (14-34) | 26 (16-36) | 41 | 24 | 28 |
| Month 20 | 52/62/60 | −9.8 (−16.0 to −3.7) | −2.5 (−8.7 to 3.8) | −5.5 (−11.6 to 0.7) | 10 (2-18) | 24 (14-35) | 20 (10-30) | 35 | 27 | 35 |
| Month 24 | 46/55/50 | −10.7 (−17.4 to −4.1) | −4.4 (−11.5 to 2.8) | −2.4 (−9.3 to 4.4) | 9 (1-17) | 31 (19-43) | 26 (14-38) | 48 | 31 | 26 |
Abbreviation: CI, confidence interval.
Indicates dosage of intravitreal triamcinolone acetonide.
In an analysis limited to eyes that were pseudophakic at baseline, the mean gain in visual acuity was 2 letters in the 1-mg triamcinolone group, while there was a mean loss in visual acuity letter score of 1 in the 4-mg triamcinolone group and 14 in the observation group (P=.09, analysis of variance). Other analyses that examined 12-month visual acuity outcomes for prespecified baseline subgroups categorizing duration of macular edema, visual acuity letter score, and OCT-measured center point thickness also demonstrated results consistent with those of the overall 12-month analysis (Table 6).
Table 6.
Twelve-Month Change From Baseline in Visual Acuity Letter Score Among Subgroups
| Subgroup | Participants Who Attended, No. From Observation/ 1-mg/4-mg Group |
Change From Baseline in Visual Acuity Letter Score |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) |
Gain of ≥15, % |
Loss of ≥15, % |
||||||||
| Observation | 1 mga | 4 mga | Observation | 1 mga | 4 mga | Observation | 1 mga | 4 mga | ||
| Baseline visual acuity letter score (Snellen equivalent) | ||||||||||
| 73-59 (20/40-20/63) | 28/28/33 | −10.6 (−18.6 to −2.7) | −5.3 (−16.5 to 6.0) | −5.1 (−12.7 to 2.5) | 4 | 25 | 9 | 39 | 29 | 24 |
| 58-49 (20/80-20/100) | 16/19/16 | −13.3 (−26.6 to 0.0) | 7.8 (−1.9 to 17.6) | 2.8 (−10.2 to 15.9) | 6 | 47 | 38 | 44 | 11 | 25 |
| 48-19 (20/125-20/400) | 29/36/33 | −12.8 (−20.9 to −4.7) | −2.8 (−9.8 to 4.3) | 0.9 (−7.9 to 9.6) | 10 | 16 | 36 | 48 | 31 | 27 |
| Baseline center point thickness, μm | ||||||||||
| <500 | 6/21/24 | −9.3 (−31.1 to 12.5) | 4.1 (−6.0 to 14.2) | 7.4 (−1.0 to 15.7) | 17 | 33 | 33 | 50 | 19 | 4 |
| ≥500 | 66/61/56 | −12.5 (−17.9 to −7.1) | −2.1 (−8.2 to 4.0) | −4.1 (−10.5 to 2.2) | 6 | 25 | 23 | 44 | 26 | 34 |
| Duration of macular edema at baseline, mo | ||||||||||
| ≤3 | 41/43/43 | −9.3 (−16.2 to −2.4) | −3.4 (−11.9 to 5.0) | −0.8 (−8.5 to 6.9) | 7 | 33 | 28 | 39 | 33 | 23 |
| >3 | 32/40/39 | −15.6 (−23.2 to −8.1) | 1.3 (−5.1 to 7.6) | −1.5 (−8.6 to 5.5) | 6 | 20 | 23 | 50 | 18 | 28 |
| Pseudophakic at baseline | 18/10/10 | −13.6 (−23.9 to −3.3) | 1.9 (−11.9 to 15.7) | −1.2 (−12.8 to 10.4) | 6 | 20 | 20 | 56 | 10 | 30 |
Abbreviation: CI, confidence interval.
Indicates dosage of intravitreal triamcinolone acetonide.
Imaging
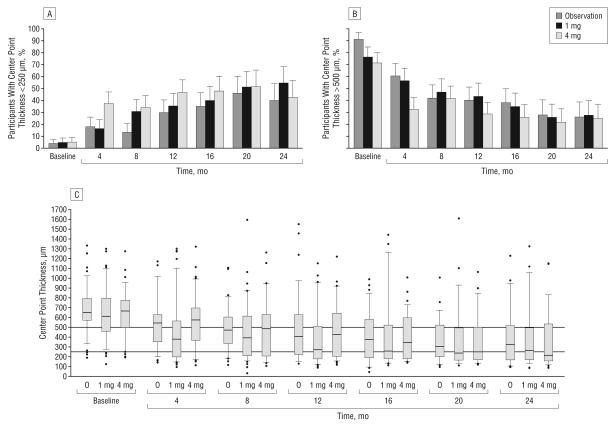
All 3 study groups showed OCT-measured center point thickness decreases from baseline through follow-up (Table 7; Figure 3). At the month 4 visit, the median decrease was greater in the 4-mg triamcinolone group (196 μm decrease) than the 1-mg (77 μm decrease) and the observation groups (125 μm decrease; P<.001, Kruskal-Wallis test). By the scheduled follow-up visit, the percentage of participants with a center point thickness of less than 250 μm was similar for the 3 study groups, with the exception of the month 4 visit, at which a greater percentage of participants in the 4-mg triamcinolone group had such a decrease (P=.002, χ2 test).
Table 7.
OCT-Measured Center Point Thickness
| Visit | Participants Who Attended, No. From Observation/ 1-mg/4-mg Group |
Center Point Thickness, μm |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) |
Median Change From Baseline (IQR) |
<250 μm, % |
||||||||
| Observation | 1 mga | 4 mga | Observation | 1 mga | 4 mga | Observation | 1 mga | 4 mga | ||
| Baseline | 87/91/89 | 651 (572-794) | 666 (501-777) | 612 (459-798) | NA | NA | NA | 3 | 4 | 4 |
| Month 4b | 75/82/84 | 543 (352-627) | 575 (366-697) | 379 (199-565) | −125 (−290 to −29) | −77 (−218 to 84) | −196 (−359 to −47) | 16 | 16 | 36 |
| Month 8 | 70/73/80 | 472 (334-604) | 485 (206-630) | 393 (211-615) | −198 (−326 to −102) | −173 (−306 to −17) | −219 (−351 to −63) | 12 | 28 | 33 |
| Month 12 | 68/72/78 | 408 (224-629) | 427 (204-643) | 272 (178-509) | −277 (−418 to −40) | −196 (−390 to −62) | −261 (−407 to −79) | 28 | 32 | 45 |
| Month 16 | 61/61/59 | 377 (188-581) | 343 (181-597) | 258 (178-523) | −315 (−427 to −145) | −242 (−426 to −108) | −259 (−396 to −91) | 33 | 36 | 44 |
| Month 20 | 44/55/47 | 304 (200-522) | 249 (169-501) | 238 (165-493) | −343 (−458 to −102) | −296 (−450 to −60) | −249 (−362 to −102) | 41 | 48 | 47 |
| Month 24 | 43/48/45 | 325 (168-515) | 215 (157-534) | 265 (169-495) | −304 (−465 to −108) | −286 (−458 to −119) | −236 (−421 to −63) | 38 | 50 | 39 |
Abbreviations: IQR, interquartile range; NA, not applicable; OCT, optical coherence tomography.
Indicates dosage of intravitreal triamcinolone acetonide.
Kruskal-Wallis test comparing the distribution of change from baseline among the 3 groups at month 4; P<.001. The χ2 test compared the percentages of participants with a thickness of less than 250 μm at month 4, P=.002.
Figure 3.
Optical coherence tomography–measured center point thickness at each 4-month follow-up visit. The histograms show the percentages of participants with center point thicknesses of less than 250 μm (A) and greater than 500 μm (B). The line from each bar represents the upper 95% confidence limit. C, Box plots with whiskers represent the 5th and 95th percentiles; the line in the box represents the median; dots, values outside the whiskers; O,observation; 1 mg and 4 mg, doses of intravitreal triamcinolone acetonide. Horizontal reference lines at 250 and 500 μm are presented.
Changes in OCT-measured center point thickness from baseline in all 3 study groups showed moderate negative correlation with changes from baseline in visual acuity letter score over time. The Pearson correlation coefficients were −0.29, −0.19, and −0.45, at month 4 and −0.39, −0.32, and −0.32 at month 12 for the observation, 1-mg, and 4-mg groups, respectively.
For all 3 study groups, disc areas of fluorescein leakage within the grid were smaller at the 12-month visit than at baseline, although at month 4 there was more leakage in the observation group than in the 1-mg and 4-mg triamcinolone groups (P=.002, Kruskall-Wallis test) (Table 8). There were few eyes with more than 10 disc areas of capillary nonperfusion in the eye at months 4, 12, and 24, with little difference between groups.
Table 8.
Area of Fluorescein Leakage and Capillary Nonperfusion by Fluorescein Angiogram
| Fluorescein Leakage Within the Grid, DAa |
Capillary Nonperfusion Within the Eye, DAb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit | Patients Who Attended, No. From Observation/ 1-mg/4-mg Group |
Median (IQR) |
Visit | Patients Who Attended, No. From Observation/ 1-mg/4-mg Group |
>10 DA, % |
||||
| Observation | 1 mgc | 4 mgc | Observation | 1 mgc | 4 mgc | ||||
| Baseline | 87/91/90 | 13 (7-16) | 12 (6-16) | 12 (6-16) | Baseline | 59/72/65 | 0 | 3 | 2 |
| Month 4d | 78/84/84 | 12 (6-16) | 9 (4-15) | 5 (2-14) | Month 4 | 60/70/65 | 0 | 7 | 6 |
| Month 12 | 63/77/76 | 7 (4-14) | 6 (2-14) | 7 (2-13) | Month 12 | 50/62/57 | 10 | 15 | 7 |
| Month 24 | 39/50/45 | 9 (4-13) | 5 (2-12) | 9 (2-15) | Month 24 | 34/40/33 | 21 | 15 | 3 |
Abbreviations: DA, disc areas; ETDRS, Early Treatment Diabetic Retinopathy Study; IQR, interquartile range.
The grid is defined as the 9 ETDRS subfields centered in the macula and measures 16 DA.
Within the eye is 210 DA.
Indicates dosage of intravitreal triamcinolone acetonide.
Kruskal-Wallis test comparing the distribution of change from baseline in area of fluorescein leakage within the grid among the 3 groups at month 4, P=.002.
Safety
More eyes in the 4-mg triamcinolone group (35%) initiated IOP-lowering medication through 12 months compared with the 1-mg triamcinolone (20%) and observation groups (8%) (P=.02 for the observation vs 1 mg comparison; P<.001, observation vs 4 mg; and P=.02, 1 mg vs 4 mg, χ2 test) (Table 9). During the first 12 months of the study, 2 participants in the 1-mg triamcinolone group received tube shunt surgery and, between 12 and 24 months, 2 participants in the 4-mg triamcinolone group received tube shunt surgery; the surgery in all participants was deemed by the investigator to be necessary because of the underlying disease (neovascular glaucoma) rather than to steroid-related IOP elevation.
Table 9.
Ocular Adverse Events
| No. (%) |
|||
|---|---|---|---|
| Characteristic | Observation (n=88) |
1 mga (n=92) |
4 mga (n=91) |
| Events Through 12 Months | |||
| Elevated IOP or glaucoma | |||
| Initiation of IOP-lowering medicationb | 7 (8) | 18 (20) | 32 (35) |
| IOP >35 mm Hg | 1 | 5 | 8 |
| IOP >10 mm Hg above baseline | 2 | 15 | 24 |
| Laser peripheral iridotomyc | 0 | 0 | 1 |
| Trabeculectomy | 0 | 0 | 0 |
| Tube shuntd | 0 | 2 | 0 |
| Cataract | |||
| Baseline phakic eyes | 66 | 77 | 76 |
| Lens opacity onset or progressione | 12 (18) | 20 (26) | 25 (33) |
| Cataract surgery | 0 | 0 | 4 |
| Other ocular adverse events | |||
| At least 1 of the following adverse events: | 9 | 11 | 6 |
| Infectious endophthalmitis | 0 | 0 | 0 |
| Noninfectious endophthalmitis | 0 | 0 | 0 |
| Retinal detachment | 0 | 0 | 0 |
| Iris neovascularization or neovascular glaucoma | 2 | 9 | 4 |
| Retinal neovascularization | 4 | 2 | 2 |
| Vitreous hemorrhage | 4 | 4 | 0 |
| Other ocular surgical procedures | |||
| YAG capsulotomy | 1 | 0 | 0 |
| Sector or panretinal scatter photocoagulation | 5 | 9 | 3 |
| Pars plana vitrectomy | 1 | 2 | 0 |
|
| |||
| Selected Events at 12-24 Months | |||
| Glaucoma procedures | |||
| Laser peripheral iridotomy | 0 | 0 | 0 |
| Trabeculectomy | 0 | 0 | 0 |
| Tube shuntd | 0 | 0 | 2 |
| Cataract | |||
| Cataract surgery | 0 | 3 | 21 |
Abbreviation: IOP, intraocular pressure.
Indicates dosage of intravitreal triamcinolone acetonide.
Percentages are of the total sample size. P<.001 based on an overall χ2 test. For the 3 pairwise comparisons, adjusting for multiple testing, P=.02 for the observation vs 1-mg comparison; P<.001, observation vs 4 mg P=.02, 1 mg vs 4 mg.
Laser peripheral iridotomy was performed for angle-closure glaucoma.
A tube shunt was performed for these participants for treatment of neovascular glaucoma.
P=.14, χ2 test.
Among eyes that were phakic at baseline, the estimate through month 12 of new-onset lens opacity or progression of an existing opacity in the observation group, based on assessment at the clinical center, was 18% compared with 26% and 33% for the 1-mg and 4-mg triamcinolone groups, respectively (P=.14) (Table 9). While no eyes in the observation or 1-mg triamcinolone groups had cataract surgery through month 12, 4 eyes in the 4-mg group received cataract surgery. Similarly, cataract surgery was more frequent between months 12 and 24 in the 4-mg group, with 21 eyes receiving cataract surgery compared with 3 in the 1-mg group and 0 in the observation group (for the data between 1 and 2 years, P=.12 for the observation vs 1 mg comparison; P<.001, observation vs 4 mg; and P<.001, 1 mg vs 4 mg, log-rank test).
Through month 12, there were no reports of infectious or noninfectious endophthalmitis or retinal detachment in any of the 3 study groups (Table 9). Iris neovascularization/neovascular glaucoma, retinal neovascularization, and vitreous hemorrhage occurred at low frequencies in each of the groups. Other surgical procedures through 12 months including sector/ panretinal scatter photocoagulation, pars plana vitrectomy, and yttrium aluminium garnet capsulotomy, were also uncommon.
Minor ocular adverse events related to the injection procedure were evaluated (data not shown), with vitreous floaters and conjunctival hemorrhage reported in a similar proportion of participants in both triamcinolone groups through 12 months (vitreous floaters, 24% for the 1-mg group and 33% for the 4-mg group; conjunctival hemorrhage, 29% for the 1-mg group and 28% for the 4-mg group). Silicone oil droplets in the vitreous were also reported through 12 months in 20% of the 1-mg group and 13% of the 4-mg group. A separate article provides more detailed information on the incidence of intravitreal silicone oil in the SCORE Study, which decreased precipitously following the introduction of a luer cone needle design in place of a staked-on needle design.48
Reports of systemic adverse events (not shown) were similar among the SCORE-CRVO trial groups. The Medical Dictionary for Regulatory Activities system/ organ class of infection and infestations had the highest percentage of incidence through 12 months, with 10%, 15%, and 19% of participants reporting at least 1 event for the observation, 1-mg, and 4-mg study groups, respectively. Three deaths were reported before 12 months of follow-up (1 in the 1-mg group and 2 in 4-mg group), and 5 more deaths after 12 months of follow-up (1 in the observation group, 1 in the 1-mg group, and 3 in the 4-mg group). The causes of the 8 deaths, as reported by the clinical centers, include for the observation group, complications from a broken hip; the 1-mg group, respiratory failure and brain hemorrhage; and the 4-mg group, myocardial infarction, lung cancer (n=2), liver cancer, and unknown cause.
COMMENT
The results of the SCORE-CRVO trial demonstrate that the likelihood of a gain in visual acuity letter score of 15 or more at 12 months is 5 times greater with intravitreal triamcinolone than observation for eyes with vision loss associated with macular edema secondary to perfused CRVO. At all time points through 12 months, mean visual acuity was better in the triamcinolone groups than in the observation group. Compared with the natural history of macular edema secondary to CRVO, the effect of intravitreal triamcinolone on visual acuity was consistent across all prespecified subgroups.
In contrast to the visual acuity results, there was no difference between groups in retinal thickness at 12 months. At month 4, there was a greater reduction in OCT-measured center point thickness in the 4-mg triamcinolone group than the other 2 groups (P<.001). These results suggest that triamcinolone has an effect on macular edema but reinforces the observation that there is only a moderate correlation between OCT-measured thickness and visual acuity in patients with retinal vein occlusion.45 A possible explanation for the discordance between visual acuity and retinal thickness such that all 3 groups showed a constant decline in median thickness until 12 months but the triamcinolone-treated groups had visual acuity results superior to that of the observation group could be a neuroprotective, anti-inflammatory, or other effect of corticosteroids in eyes with CRVO.27,28,49,50
A recent meta-analysis by Mohamed et al51 concluded that there is no level I evidence to support any intervention to improve visual acuity over the natural history of untreated macular edema in eyes with CRVO. However, the results from the SCORE-CRVO trial showed that intravitreal triamcinolone can alter the natural history of CRVO beneficially with respect to visual acuity. The results from the SCORE-CRVO trial showed that the natural history of untreated CRVO is poor, with only 7% of participants showing a gain in visual acuity letter score of 15 or more at 12 months. In the CVOS, the visual acuity inclusion criteria were broader than in the SCORE Study, but the result was similar; 6% of patients in the untreated arm of CVOS group M had a gain in visual acuity letter score of 15 or more at 12 months.
The adverse effects of the intravitreal injections were manageable, particularly with the 1-mg dose. There were no cases of infectious or noninfectious endophthalmitis with the intravitreal injection procedure and triamcinolone formulation used in the SCORE-CRVO trial. The lack of cases of noninfectious endophthalmitis in 586 injections performed in this trial, all of which were evaluated within 1 week for postinjection complications, may be because of the preservative-free, micronized, nondispersive triamcinolone formulation (the triamcinolone crystals were suspended in a hyaluronate matrix gel) used in this trial. Silicone oil droplets in the vitreous following intravitreal injections were noted (13%-20%, depending on study group) but no adverse effects were attributable to the droplets. Intravitreal silicone oil due to the use of siliconized syringes is a recognized occur-rence.52,53 There was a dose-dependent higher frequency of initiating IOP-lowering medications in the triamcinolone groups compared with the observation group (Table 9). However, no participants in any of the 3 groups received filtration surgery during the 12 months. Two participants in the 1-mg triamcinolone group received tube shunt surgery but these procedures were performed because of complications from neovascular glaucoma, not steroid-related IOP elevation. Four cataract surgeries were performed in the 4-mg triamcinolone group, with none in either the 1-mg triamcinolone or the observation groups up to month 12. These results indicate that the 1-mg triamcinolone group has a superior adverse event profile than the 4-mg triamcinolone group. Additionally, these results indicate that the most serious consequences of steroid-related adverse effects, cataract surgery and glaucoma surgery, were seen with similar frequency when comparing the 1-mg triamcinolone group with the observation group at month 12 and at 2 years (Table 9), suggesting that the safety profile of 1-mg triamcinolone is comparable with observation with respect to these surgical complications.
It is important to note that the treatment effect in this trial was achieved by a regimen that encouraged frequent retreatment. Participants were retreated with triamcinolone unless they improved significantly with respect to vision or OCT-measured retinal thickness or had an adverse event that precluded further retreatment. Thus, undertreatment with triamcinolone in the SCORE-CRVO trial was minimized as much as possible.
The results of the current study indicate that, as judged at 12 months, intravitreal triamcinolone is an effective therapy compared with observation for patients with vision loss associated with macular edema secondary to CRVO who are similar to those enrolled in the SCORE-CRVO trial. Despite the observed benefit to visual acuity with either the 1-mg or 4-mg dose of triamcinolone relative to observation, three-fourths of eyes that received intravitreal triamcinolone did not have a gain in visual acuity letter score of 15 or more from baseline to 12 months, one-fourth of treated eyes had vision loss of a similar magnitude, and more than half of patients had an OCT-measured center point thickness greater than 250μm at 12 months. The search for other treatments would be beneficial and should be explored in the future to improve on the outcomes demonstrated by the current study.
Twelve-month data are important in a disease with an acute nature, and may be sufficient follow-up, but the longer-term role of triamcinolone is also important. A shortcoming of the current study is the lack of definitive data at 2 years. Difficulty with the recruitment of participants over an extended period shortened the duration of follow-up for many participants. From the data available between month 12 and 2 years, the beneficial effect of both doses of intravitreal triamcinolone on visual acuity, as determined by mean change in visual acuity, attenuated between 12 months and 2 years. It is unknown how much progressive cataract formation reduced visual acuity during this time period and whether a lower threshold for performing cataract surgery would have improved visual acuity at 2 years in eyes treated with intravitreal triamcinolone. However, to minimize the effect of cataract on the results, the protocol encouraged cataract surgery at all follow-up visits as soon as clinically indicated in the judgment of the investigator. Regardless of the effect of cataract between month 12 and 2 years, the visual acuity result at 2 years was the same as at 12 months, significantly favoring the triamcinolone groups; this finding supports continuation of therapy to 2 years, although the smaller number of patients available for the 2-year visit precludes a definitive recommendation for 2 years of therapy with intravitreal triamcinolone. With respect to safety concerns at 2 years, the rates of cataract and glaucoma complications parallel the results at month 12. Furthermore, the adverse event profile at 2 years for the triamcinolone groups in the concurrent SCORE-BRVO trial is similar to that in the SCORE-CRVO trial, and the consistency of the adverse event results in the 2 trials strengthens the data regarding the 2-year adverse event rates noted in the SCORE-CRVO trial.
In conclusion, intravitreal triamcinolone in both a 1-mg and 4-mg dose had better visual acuity outcomes over 12 months than the untreated natural history of macular edema secondary to perfused CRVO. Beyond 12 months, the greater likelihood of visual acuity gain with triamcinolone persists, although there is a mild attenuation of the effect of triamcinolone with respect to mean change in visual acuity, possibly because of cataract. The superior safety profile of the 1-mg dose compared with the 4-mg dose, particularly with respect to glaucoma and cataract, renders it the preferred dose. Based on the results of the SCORE-CRVO trial, intravitreal triamcinolone in a 1-mg dose and following the retreatment criteria used in this study should be considered for up to 1 year, and possibly 2 years, in patients with vision loss associated with macular edema secondary to CRVO who have characteristics similar to the participants studied in this trial.
Supplementary Material
Acknowledgments
Funding/support: This study was supported by National Eye Institute (National Institutes of Health, Department of Health and Human Services) grants 5U10EY014351, 5U10EY014352, and 5U10EY014404; and in part by Allergan, Inc.
Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Investigators
Resource Centers
Office of the Study Chair at the University of Wisconsin: Michael S. Ip, MD (study chair); Marian Fisher, PhD (statistical consultant); Barbara Nardi (assistant to the study chair); past participating personnel, Nancy T. Cahill. Office of the Study Co-Chair at Penn State College of Medicine: Ingrid U. Scott, MD, MPH (study co-chair). Data Coordinating Center at The EMMES Corporation: Paul C. VanVeldhuisen, PhD (principal investigator); Neal L. Oden, PhD (statistician); Maria J. Figueroa, MBA (project director); Anne Charlton (data manager); Eileen Guan, MS (programmer); Oscar Moreno (clinical systems analyst); Janet Van Dyke (regulatory/administrative coordinator); past participating personnel, Tracy Bailey, Chanele Robinson, Michael Frasketi. Fundus Photograph Reading Center at the University of Wisconsin: Barbara A. Blodi, MD (principal investigator); Debra J. Christianson; Julee Elledge; Vonnie Gama; Kathleen E. Glander; Anne Goulding; Dawn J. Myers; Susan B. Reed; Pam Vargo; Kelly J. Warren, MS.
Funding Agency
National Eye Institute, National Institutes of Health, Department of Health and Human Services, Maryann Redford, DDS, MPH (Program Officer).
SCORE Study Committees
Executive Committee: Barbara A. Blodi, MD; Maria J. Figueroa, MBA; Michael S. Ip, MD; Neal L. Oden, PhD; Maryann Redford, DDS, MPH; Ingrid U. Scott, MD, MPH; Paul C. VanVeldhuisen, PhD; J. Michael Jumper, MD (clinic investigator representative); Neal Oestreich (clinic coordinator representative). Data and Safety Monitoring Committee: John C. Connett, PhD (chair); Deborah R. Barnbaum, PhD; Harry W. Flynn Jr, MD; Robert Frank, MD; Saul Genuth, MD; Lee Jampol, MD; Steven Wisniewski, PhD; Maryann Redford, DDS, MPH (nonvoting); past participating member, Jeanette Resnick. SCORE Advisory Group: Barbara A. Blodi, MD; Mark S. Blumenkranz, MD; Neil M. Bressler, MD; Karl Csaky, MD, PhD; Matthew D. Davis, MD; Michael Gorin, MD, PhD; Julia A. Haller, MD; Michael S. Ip, MD (study chair); Mark W. Johnson, MD; Anne Lindblad, PhD; Bernard H. Doft, MD; Sharon Fekrat, MD; Maria J. Figueroa, MBA; Daniel Finkelstein, MD; Neal L. Oden, PhD; Maryann Redford, DDS, MPH; Ingrid U. Scott, MD, MPH; Paul C. VanVeldhuisen, PhD.
Clinical Centers
Personnel are listed as PI for principal investigator; Sub-I, subinvestigator; C, coordinator; P, photographer; O, optical coherence tomography technician; VA, visual acuity tester; and R, refractionist. Atlantic Retina Center: Joseph Schwartz, MD (PI), James Rial, MD (Sub-I), Jessica Adams (C, O, VA, R), Brandi Farlow (P), Paige Lincoln (VA, R), Holly Farrell (R), Jessica Phoebus (VA, R); Barnes Retina Institute: Gaurav Shah, MD (PI), Kevin Blinder, MD (Sub-I), Nicholas Engelbrecht, MD (Sub-I), Matthew Thomas, MD (Sub-I), Daniel Joseph, MD (Sub-I), Nancy Holekamp, MD (Sub-I), Rhonda Weeks (C), Pamela Light (C), Jarrod Wehmeier (P, O), Matt Raeber (P, O), Tim Wright (P, O), Virginia Nobel (VA, R), Lynda Boyd (VA, R), Carol Walters, COT (VA, R), Tammy Ressel (VA, R); Bay Area Retina Associates: Subhransu Ray, MD (PI), Allen Verne, MD (Sub-I), Craig Leong, MD (Sub-I), Stewart Daniels, MD (Sub-I), T. Daniel Ting, MD (Sub-I), Kathleen Dowell (C), Maria Aguilos (C), Cindy Moreci (C), Fred Hanamoto (P, O), Nicole Hom (VA, R), Megan Williams (VA, R), Sumi Takahara (VA, R), Rouella Tejada (VA, R), William Combs (VA, R), Sean Teshima-McCormick (VA, R); California Retina Consultants: Dante Pieramici, MD (PI), Ma'an Nasir, MD (Sub-I), Robert Avery, MD (Sub-I), Alessandro Castellarin, MD (Sub-I), Robert See, MD (Sub-I), Stephen Couvillion, MD (Sub-I), Elizabeth Robbins (C), Tamara Norton (C), Sarah Davies (C), Joan Turner (C), Stephanie Cook (C), Jessica Basefsky (C, O, VA, R), Robin Smith (C), Liz Tramel (P, O, VA, R), Matthew Guist (P, O), Melissa Kruzel, COA (P, O, VA, R), Karen Boyer (O), Amy Sterling (O), Kelly Avery (VA, R), Liza Magana (VA, R); Carolina Eye Associates: Gregory Mincey, MD (PI), Mara-Leigh Schafer, CCRP (C), Judy Fleming (C), Lisa Fulghum (C), William McVerry (P, O), S. Jill Pierce (VA, R), A. Kristy Taylor (VA, R), Tracey Coley (VA, R), Melissa West (VA, R), Megan Cole (VA, R), Jaime Dick (VA, R); Carolina Retina Center: Jeffrey Gross, MD (PI), Barron Fishburne, MD (Sub-I), Michael Magee, MD (Sub-I), Jennifer Enlow (C), Peggy Cummings (C), Amy Flowers (C, VA, R), Carlton Bequette (C), Kayla Henry (C), Randall Price (P, O), Heather Purvis (P, O), Richard Christoff (O), Christophe Mallet (O), Stephanie Hoerner (VA), Heather Hawkins (VA, R), Heidi Lovit (VA, R), Cori Fore (VA, R), Regina Gabriel (VA, R), Kristin Bland (VA, R); Casey Eye Institute: Andreas Lauer, MD (PI), Thomas Hwang, MD (Sub-I), David Wilson, MD (Sub-I), J. Timothy Stout, MD (Sub-I), Michael Klein, MD (Sub-I), Christina Flaxel, MD (Sub-I), Susan Pope (C, VA, R), Deby Martin, OT (C), Ann Lundquist (C, VA, R), Kelly West (P, O), Joseph Rossi (P, O), Scott Pickell (P, O), Jessica Gaultney (P, O), Patrick Wallace (P, O), Ellen Redenbo (P, O), Christopher Howell (P, O), Peter Steinkamp (P, O), Patrick Rice (P, O), Maureen Toomey (VA, R), Susan Nolte (VA, R), Shelley Hanel (VA), Deborah Vahrenwald, COT (VA, R), Shirley Ira (VA, R), Chad Indermill (VA, R); Center for Retina & Macular Disease: Michael Tolentino, MD (PI), David Misch, MD (Sub-I), Adam Berger, MD (Sub-I), Suk Moon, MD (Sub-I), Richard Hamilton, MD (Sub-I), Janelle Anderson, CCRC (C), Jason Strickland (P, O), Vera Dilts (P, O, VA, R), Donald True-man, COT (C, P, O, VA, R), Gina Cassa (O, VA, R), Cindy Williams (VA, R), Laura Holm, LPN (VA, R), Shelly Cooper (VA, R); Central Florida Retina: Suzanne Demming, MD (PI), Preston Richmond, MD (Sub-I), C. Durham Barnes, MD (Sub-I), Douglas Lieb, MD (Sub-I), Saad Shaikh, MD (Sub-I), John Olson, MD (Sub-I), Neil Okun, MD (Sub-I), L. Denise Davila (C, O, VA, R), Ginny Bell (C, O, P), Trudy Thornton (P, O), Joyce Treutel (VA, R), Cathy Huertas (VA, R), Clarivel Diaz (VA, R); Central Florida Retina Institute: Scott Friedman, MD (PI), Oren Plous, MD (Sub-I), Steve Carlton (C, P, O, VA), Kelly Blackmer (C, O, VA), Jolleen Key (C, O, VA, R), Jessica Maldonado (O), Allen McKinney (O, VA, R), Sheila Walters-Treon (P, O), Damanda Fagan (VA, R), Virginia Gregory, COA (VA, R), Karen Sjoblom (VA, R), Yvette Fraser-Neumann (VA, R); Charlotte Eye Ear Nose and Throat: Andrew Antoszyk, MD (PI), David Browning, MD (Sub-I), Jason Sanders, MD (Sub-I), Justin Brown, MD (Sub-I), Angella Karow (C, VA, R), Jennifer Helms (C, VA, R), Angela Price (C, VA, R), Stephanie Niemi (C), Alison Stallings (C), Melissa Cowen, CRC (C, VA, R), Roderick Walker, CPO, CRC (C, VA, R), Michael McOwen, CRA (P, O), Brian Lutman (P, O), Linda Davis, COA, CRA (P, O), Donna McClain, COA (P, O), Lorraine Clark (O), Debora Jordan (O), Amanda Vittitow (O), Uma Balasubramaniam (O), Richard George (O), Heather Murphy, OA (VA, R), Jeanie Goad, COA (VA), Lisa Chatari (VA, R), Danielle Brooks, CRC (VA), Rachel Pierce, COA (VA, R); Cincinnati Eye Institute: Robert Hutchins, MD (PI), Stewart Krug, MD (Sub-I), Frances Bowman (C), Carrie Johnson (P, O, VA, R), Teresa Mistler (P, O, VA, R), Nicole Conley (O), Elizabeth Nelson-Parker (VA), Amy Quesnelle (VA); Cleveland Clinic Foundation: Peter Kaiser, MD (PI), Hilel Lewis, MD (Sub-I), Jonathan Sears, MD (Sub-I), Laura Holody (C), Tami Fecko (P, O), Nicole Brugnoni (P, O), Deborah Ross (O), Sharon Allen (O), Anthony Fattori (VA, R), Judy Niskanen (VA, R); Connecticut Retina Consultants: Wayne Larrison, MD (PI), Philip Falcone, MD (Sub-I), James Weisz, MD (Sub-I), Nicole Griffith (C), Alan Karnolt (P, O, R), Jennifer Kurtz (VA); Delaware Valley Retina Associates: Darmakusuma Ie, MD (PI), Jeffrey Lipkowitz, MD (Sub-I), Kekul Shah, MD (Sub-I), Susan Geraghty (C, P, O), Beverly Sannazzaro (C), Linda McCall (P, O), Morgan Harper (VA, R), Lydia Polanco-Weaver (VA, R), Diana Napoli (VA, R); Denver Health Medical Center: Antonio Ciardella, MD (PI), Jon Braverman, MD (PI), Dorothy Thomas (C), Graciela Gallardo (C), Brenner Dixon (C), Rebecca Doherty (C), Melissa Stillberger (C), Leif Ryman (C), Debra Brown (P, O), Colleen Smith (VA, R), Janelle Zapata, COMT (VA, R), Rosemary Rhodes, COT (VA, R); Duke Eye Center: Sharon Fekrat, MD (PI), Michael Cooney, MD (Sub-I), Eric Postel, MD (Sub-I), Sunil Srivastava, MD (Sub-I), Tamer Mahmoud, MD (Sub-I), Srilaxmi Bearelly, MD (Sub-I), Brooks McCuen, MD (Sub-I), Tamara Fackler, MD (Sub-I), Prithvi Mruthyunjaya, MD (Sub-I), Cynthia Toth, MD (Sub-I), Glen Jaffe, MD (Sub-I), Ivan Suner, MD (Sub-I), John Gross, MD (Sub-I), Mikki O'Neal (C), Noreen McClain, COT (C), Gregg Hoffmeyer (P, O), Terry Hawks (P, O), Russell Burns (P, O), Michael Kelly (P, O), Jeanne Lee (P, O), Allan Laurens (P, O), Merritt Gammage (P, O), Marriner Skelly (P, O), Jeffrey Napoli (O), Megan Wood (VA, R), Joyce Bryant (VA, R), Sheila Poland (VA), Neeru Sarin, COA (VA, R); Eldorado Retina Associates, PC: Mary Lansing, MD (PI), Hong Zhuan Chen (C, P, O, VA, R), Lauren Fox (C, P, O, VA, R), Ryan Odil (P, O, VA, R), Kimberly Alexander (P, O), Ramona Smith, COT (P), Denis Roussel (P, O, VA, R), Jeanne Ross, RN (VA, R); Elman Retina Group, PA: Michael Elman, MD (PI), Robert Raden, MD (Sub-I), Michelle Sloan (C), JoAnn Starr (C, VA, R), Tammy Butcher (C), Terri Cain (P, O), Peter Sotirakos (P, O), Giorya Andreani (P, O), Dena Salfer-Firestone (VA, R), Teresa Coffey (VA, R), Pamela Singletary (VA, R), Nancy Gore (VA, R); Eye Foundation of Kansas City: Nelson Sabates, MD (PI), Felix Sabates, MD (Sub-I), Michael Cassell, MD (Sub-I), Abraham Poulose, MD (Sub-I), Komal Desai, MD (Sub-I), Mickie Keeling, RN (C), Damon Fletcher (C, VA, R), Gary Gallimore, COMT (P, O), Yolanda Konior, COT (P, O), Candice Whitehead (P, O, VA, R), Keith Kunkelman, CCRP (C, VA, R), Aisha Sayers (VA, R), Erin Grisafe, COA (VA, R), LaGalia Afola, COA (VA, R); George Washington University: Jeevan Mathura Jr, MD (PI), B. Eric Jones, MD (PI), Ronald Olszowy, MD (C, VA), Yasmeen Mahmood (C, VA), Jean Zalamea, MD (C, VA), Smitha Shekar (C, VA), Bertrand Miskell (P, O), Marc Taylor (P, O), Mirsad Ibisevic, MD (O), Nancy Brockman (VA, R), Nikka Aberman (VA), Abdullah Habib, MD (VA, R), Eva Karlsson, COT (VA, R), Ram K. C. (VA, R), Alexandre Gauthier (VA), Jerry Tsong (VA); Georgia Retina, PC: Mark Rivellese, MD (PI), Atul Sharma, MD (Sub-I), Jay Stallman, MD (Sub-I), Sean Koh, MD (Sub-I), Scott Lampert, MD (Sub-I), Michael Jacobson, MD (Sub-I), Adam Martidis, MD (Sub-I), Robert Stoltz, MD, PhD (Sub-I), John Miller, MD (Sub-I), Starr Hendricks, COA (C, VA, R), Emily Butler-Grant (C), Leslie Marcus, CRC (C), Cotina Pauley (P), Rebecca McManus (O), Christy Starnes (O), Alana Simpson (VA, R), Shelly Fulbright (VA, R), Jaimee Kenes (VA, R), Grethel Betanzos (VA, R), Meg Redmond (VA, R), Leslie Ellorin, COA (VA, R); Henry Ford Health Care: Paul Edwards, MD (PI), Michael Ober, MD (Sub-I), J. David Carey, MD (Sub-I), Janet Murphy, COT (C, VA, R), Sheila Rock, COT (C, VA, R), Mary Monk (C, VA, R), Damon Fletcher (C, VA, R), Julianne Hall (C, VA, R), Mark Croswell, CRA (P, O), Tracey Troszak, CRA (P, O), Lisa Schillace, CRA (P, O), Steven Ogilvy (P, O), Bradley Stern (P, O), Brian Rusinek (O), Alexis Smith (O), Elizabeth Carnegie (VA), Dorena Wilson (R), Gayle Roberts (VA, R); Horizon Eye Care: Miriam Ridley, MD (PI), Frederick Weidman, III, MD (Sub-I), April Glessner (C), Mara-Leigh Schafer, RN (C, VA), Susan Doran, COA, CRC (C), David Peterson, COT (P, O), Jennifer Lummis, COA (P, O), Jeanine Cisco (P, O), Amy Brogdon, COA (VA, R); Illinois Retina Associates: John Pollack, MD (PI), Joseph Civantos, MD (Sub-I), Barbara Ciscato (C, VA), Dan Muir (P, O), Robin Mikota (VA, R), Patti Midlock (VA, R), Belinda Kosinski (VA, R), Harvey Kourous Rezaei, MD (PI), David Orth, MD (Sub-I), Joseph Civantos, MD (Sub-I), Sohail Hasan, MD, PhD (Sub-I), Susan Brown (C, VA, R), Shannya Townsend-Patrick (P, O), Kiersten Nelson (P, O), Linda Arredondo, RN (VA), Toni Lofton, RN (VA), Gina Shelton (VA), Celeste Figliulo (VA, R); Kresge Eye Institute: Dean Eliott, MD (PI), Tamer Mahmoud, MD, PhD (PI), Gary Abrams, MD (Sub-I), Raymond Iezzi, MD (Sub-I), James Puklin, MD (Sub-I), Asheesh Tewari, MD (Sub-I), Laura Schulz, CCRC (C), Sandeep Randhawa, MD (C), David Griffith (P, O), Kyohei Abe (P, O), Zlatan Sadikovic (P, O), Kenneth Christopherson (P, O), Elizabeth Silvis, CRA (P, O), Kit Morehead (P, O), Karen Voltz (P, O), Dena McDonald (P, O), Lisa Schillace (P, O), Melanie Bailey (O), Vicki Krzeminski, COA (VA, R), Cheryl Milanovic, COA (VA, R); Lahey Clinic: Jeffrey Marx, MD (PI), Fleming Wertz III, MD (PI), Gregory Blaha, MD, PhD (Sub-I), Patricia Pahk, MD (Sub-I), Fina Barouch, MD (Sub-I), Elisha Tilton, MD (Sub-I), Avon Stewart (C), Ellen Casazza (P, O), Richard Selter (P, O), Jonathan Rosen (P, O), Tracy Scrivano (VA, R), Patti-Ann Morse (VA), Stephen Kelly, Jr (VA), Sean Mahoney (VA, R), Molly Concannon (VA, R), Michael Johnson (R); Loma Linda University: Michael Rauser, MD (PI), Joseph Fan, MD (Sub-I), Mukesh Suthar, MD (Sub-I), Anu Diekmann, MPH (C), Arun Chakrabarty, MD (C), Carrousel Corliss (C, O, VA, R), Alice Ortega (C, VA), Sarina Osuna (C, O, VA, R), Gisela Santiago (C), Kara Rollins (C, P, O, VA, R), Christy Quesada (C, VA, R), Cara Davidson (C, VA, R), Gene Saldana (P, O), William Milam (P), Jonathan Cloud (P), William Kiernan, OD (VA, R); Manhattan Eye, Ear, and Throat: Richard Spaide, MD (PI), K. Bailey Freund, MD (Sub-I), James Klancnik, MD (Sub-I), Jason Slakter, MD (Sub-I), John Sorenson, MD (Sub-I), Leandro Maranan (C, VA, R), Cindy Novalis (P, O), Eugene Agresta (P, O), Namrata Saroj (VA, R), Maria Scolaro (VA, R); Medical College of Wisconsin: Judy Kim, MD (PI), Dennis Han, MD (Sub-I), Thomas Connor, Jr, MD (Sub-I), William Wirostko, MD (Sub-I), David Weinberg, MD (Sub-I), Kimberly Stepien, MD (Sub-I), Christine Lange (C, VA), Troy Drescher, CCRC (C, VA, R), Kelly Reiter (C, VA), Dawn Alvarez, CRC (C, VA), Sharon Rekow, CCRC (C, VA), Jeanette Graf, CRC (C, VA), Krissa Packard, CRC (C), Joseph Beringer (P, O), Kathy Selchert (P, O), Dennis Backes (P, O), Kristy Keller (O), Vicki Barwick (VA,R), Judy Flanders (VA, R); Mid-America Retina Consultants, PA: William Rosenthal, MD (PI), Ivan Batlle, MD (Sub-I), Jeffry Gerson, OD (C, VA, R), Lois Swafford (C), Barbara Johnson, RN (C, VA), Karla Batlle (C), R. Scott Varner (P, O), Gwen Williams, COA (O, VA, R), Felicia Ziolo (VA, R), Ericka Breit, RN (VA, R), Debbie Lee, RN (VA), Michelle Parks (VA, R); Midwest Eye Institute: Raj Maturi, MD (PI), Donald Wilson, MD (Sub-I), Nicholas Hrisomalos, MD (Sub-I), John Minturn, MD (Sub-I), Thomas Ciulla, MD (Sub-I), Laura Bleau, LPN (C, O, VA, R), Tom Steele (P), Jama Poston, COA (O, VA, R), Kelly Love (VA, R), Denise Samaniego (VA, R), Michelle Storie, COA (VA, R), Carolee Novak, COA (VA, R); National Ophthalmic Research Institute: A. Thomas Ghuman, MD (PI), Glenn Wing, MD (Sub-I), Joseph Walker, MD (Sub-I), Paul Raskauskas, MD (Sub-I), Cheryl Kiesel, CCRC (C, P, O), Eileen Knips, RN (C, P, O), Cheryl Ryan, CCRC (C), Crystal Peters (C), Danielle Dyshanowitz (VA, R), Jennifer Banks (VA, R); New England Eye Center: Caroline Baumal, MD (PI), Jackie Nguyen, MD (Sub-I), Jay Duker, MD (Sub-I), Elias Reichel, MD (Sub-I), Catherine Milch, MD (Sub-I), Adam Rogers, MD (Sub-I), Dal Chun, MD (Sub-I), Paul Yates, MD (Sub-I), David Eichenbaum, MD (Sub-I), Tina Scheufele, MD (Sub-I), Torsten Wiegand, MD (Sub-I), Michelle Serfass (C), Theresa Peterson (C), Linda Merry (C), Beverly Snell (C), Jennifer Layzer (P, O), Jonathan Rosen (P), Christine Kiernan (P), Maureen Thibault (O), David Schultz (O), David McMahon (O), Julie Wilson (O), Robyn McDonough (VA, R), Leah Mullen (VA, R); New York Eye and Ear Infirmary: Richard Rosen, MD (PI), Belinda Shirkey, MD (Sub-I), Ronald Gentile, MD (Sub-I), Eric Fitz, MD (Sub-I), Juan Romero, MD (Sub-I), Katy Tai (C, VA, R), Kenneth Boyd (P), Nancy Gonzalez (P), Noelle Vallet (O), Robert Masini (O), Sapna Kapoor (VA, R), Roma Ovase (VA, R), Peggy Guerrero (VA, R), Dipika Joshi (VA, R), Jenny Gallardo, BA (VA, R); Northern Illinois Retina: Susan Fowell, MD (PI), James Watson (C, P, O, VA, R), Maureen Cain, RN (C), Nancy Mercurio (C), Christine Gomez (VA, R), Jacquie Button (VA, R), Elizabeth Watson (VA, R); Northwestern University Ophthalmology: Alice Lyon, MD (PI), Manjot Gill, MD (Sub-I), Jeevan Mathura, MD (Sub-I), Rukhsana Mirza, MD (Sub-I), Laima O'Donnell (C, VA, R), Annie Munana (C), Zuzanna Rozenbajgier (C), Jonathan Shankle (P, O), Dawn Ryan (P, O), Evica Simjanoski (O), Lori Kaminski (VA, R); Ophthalmic Consultants of Boston: Trexler Topping, MD (PI), Janet Chieh, MD (Sub-I), Michael Morley, MD (Sub-I), Rubin Kim, MD (Sub-I), Lisa Schocket, MD (Sub-I), Dal Chun, MD (Sub-I), Tina Cleary, MD (Sub-I), Paul Yates, MD (Sub-I), David Eichenbaum, MD (Sub-I), Jackie Nguyen, MD (Sub-I), Torsten Wiegand, MD (Sub-I), Lori Tyler (C), Joy Bankert (C), Victoria Hurley (C), Paula Zand (C), Lindsey Williams (C), Cullen Michael Jones (P, O), Margaret Graham (P, O), Mary Doherty (VA, R), Katie Marino (VA, R), Taneika Howard (VA, R), Heather Davis, COA (VA, R), Sandy Chong (VA, R), Emily Corey (VA, R), Katie Moses (VA, R), Reena Zachariah (R); Orange County Retina Medical Group: Sanford Chen, MD (PI), Mohit Nanda, MD (Sub-I), John Maggiano, MD (Sub-I), Timothy You, MD (Sub-I), Marinel Casiano (C, O), Aileen Buddemeier (P, O), Adrian Vazquez (P), Aileen Buddemeier (VA, R), Nicole Zimmer (VA, R), Erika Agustin (VA, R), Renielle Agustin (VA, R); Paducah Retinal Center: Carl Baker, MD (PI), Tracey Caldwell, CRC (C, P), Denise Smith (P, O), Alecia Travis (O), Lynnette Lambert (VA, R), Mary Palmer (VA, R), Tracey Martin (O, VA, R); Palmetto Retina Center: John Wells, MD (PI), W. Lloyd Clark, MD (Sub-I), Robbin Spivey (C, O, VA, R), Mark Evans (P, O), Mallie Taylor (P, O), Amy Hickman (P, O), Marcia Gridine, BS (VA, R), Peggy McDougal (VA, R); Penn State Hershey Medical Center: Kimberly Neely, MD (PI), Thomas Gardner, MD (Sub-I), David Quillen, MD (Sub-I), Ingrid Scott, MD, MPH (Sub-I), Susan Chobanoff (C, VA, R), Mary Wilmarth, COMT (C, VA, R), Timothy Bennett (P, O), James Strong (P, O), Laura Walter (VA, R), Ernesto Rodriguez (VA, R), Mary Frawley (VA, R); Retina & Vitreous Consultants of WI Jonathan Hershey, MD (PI), Sharath Raja, MD (Sub-I), Susan Larson, RN (C, O), Jacqueline Dugdale (C, O, VA, R), Kevin Lonergan (P, O), Ruth Picchiottino (P), Lori Willman (VA, R); Retina and Vitreous of Texas: Joseph Khawly, MD (PI), H. Michael Lambert, MD (Sub-I), Arthur Willis, MD (Sub-I), Roberto Diaz-Rohena, MD (Sub-I), Pam Miller (C, VA, R), Cindy Mendenhall (C), Paula Uribe (C), Susan Busch (C, O, VA, R), Joseph Morales (P, O), Donald Lowd (P, O), Alllison Schmidt (P, O), Kristopher Chase (P, O), Pam Miller (VA, R), Valerie Lazarte (VA, R), Debbie Fredrickson (VA, R), Mikki O'Neal (VA, R); Retina Associates of Cleveland, Inc: Lawrence Singerman, MD (PI), Michael Novak, MD (Sub-I), Hernando Zegarra, MD (Sub-I), Z. Nicholas Zakov, MD (Sub-I), Scott Pendergast, MD (Sub-I), David Miller, MD (Sub-I), Mithlesh Sharma, MD (Sub-I), Joseph Coney, MD (Sub-I), Stephanie Schura (C), Susan Rath (C), Josel Collins (C, O), Lori Revella (C, P, O), Larraine Stone, RN, BA (C), Gregg Greanoff (P, O), John Dubois (P, O), Shelia Smith-Brewer (P, O), Tamara Cunningham (P, O), Vivian Tanner (VA, R), Kimberly DuBois (VA, R), Mary Ilc (VA, R), Jacqueline Hursky (VA, R), Maureen Cunningham (VA, R), Tammy Brink (VA, R), Connie Keller, COA (VA, R), Elizabeth McNamara (VA, R), Trina Nitzsche (VA, R); Retina Associates of New Jersey: Steven Madreperla, MD, PhD (PI), Richard Klein, MD (Sub-I), Michael Harris, MD (Sub-I), Stuart Noorily, MD (Sub-I), Leonard Feiner, MD, PhD (Sub-I), Kathleen Alworth, BA (C), Diane Deininger (C), Helen Hetherington (C, VA, R), Edwin Turton, CRA (P, O), Howard Radzyner (P, O), Sally Pogosky (O), Kara McLeod (VA), Jeanine Parke (VA), Veronica Irizarry (VA, R); Retina Consultants: Paul Greenberg, MD (PI), Harold Woodcome, MD (PI), Robert Janigian, MD (Sub-I), Magdalena Krzystolik, MD (Sub-I), Caldwell Smith, MD (Sub-I), Emma German (C), Sylvia Varadian (C), Collin DuCoty (C), Mark Hamel (P, O), Alex Nagle (P, O), Erika Banalewicz (VA, R), Claudia Salinas (VA, R), Sandra Henriques (VA); Retina Consultants of Charleston: Eric Jablon, MD (PI), D. Virgil Alfaro, MD (Sub-I), Ben Geer (C), Elizabeth Rodriguez, MD (C), Gretchen Graterole (C), Monica Rodriguez, MD (C), Nirav Parikh (C, O), Gerardo Zapada, MD (C), Rian McMillan (P), Michelle Rothen (O), Molly Hughes, COT (VA, R); Retina Group of Florida: William Thompson, MD (PI), Ronald Glatzer, MD (Sub-I), Jaclyn Lopez (C), Alicia Tardif (C), Cindy Fernandez (C), Grettel Cousins (C), Michele Earl (P, O), Brian Fernandez (P, O), Karen McHugh (P, O), Patricia Aramayo (P, O), Melissa Ritchie (O), Jamie Mariano (VA, R), Janet Benton-Murray (VA, R), Giddel Rouvier (VA, R), Antonio Bolet (VA, R), Clifford Sherley (VA, R), Evelyn Quinchia (VA, R); Retina Research Center: Brian Berger, MD (PI), Isaac Loose, MD (Sub-I), Margaret Rodriguez (C), Steven Jeffers (C), Elisabeth Durham (C), Erin Scrivner (C, VA, R), Linda Nguyen (C, VA, R), Renee Morris (C, VA, R), Ginger Manhart (C, VA, R), Quinn Krzykowski (C, VA, R), Ben Ostrander (P, O), Kimberly Solalinde (VA, R), Bobbi Gallia (VA, R), Melissa Talbert (VA, R), Yong Ren (VA, R), Jamie Sun (VA, R), Nicole Callen (VA, R), Michael Gartner (VA, R); Retina Vitreous Center: Daniel Roth, MD (PI), Stuart Green, MD (Sub-I), Steven Leff, MD (Sub-I), Bruce Keyser, MD (Sub-I), Jonathan Prenner, MD (Sub-I), David Yarian, MD (Sub-I), Eric Friedman, MD (Sub-I), H. Matthew Wheatley, MD (Sub-I), Cheryl Hambrock, RN (C), Thea Tantum, COT (C, V, R), Jane Deinzer, RN (C, V, R), Tina LaPrise, RN (C, O, V, R), Finn Anderson (P, O), Tina LaPrise, RN (OCT Operator), Michele Manochio, COA (VA, R), Lynn Campanaro (VA, R), Sandra Parker (VA, R); Retina Vitreous Consultants: Robert Bergren, MD (PI), Bernard Doft, MD (Sub-I), Lars Freisberg, MD (Sub-I), Pamela Rath, MD (Sub-I), Louis Lobes, MD (Sub-I), Karl Olsen, MD (Sub-I), Shannon Reilly, BS (C), Frances Casillo (C), Candace Depp, CRC (C), Nicole Lucko, CRC (C), Justine Kulasa (C), Barbara Mack (C), Jamie Jones, CRC (C), Alan Campbell, CRA (P, O), David Steinberg, CRA (P, O), Sharon Murajda-Jumba (P, O), Shawnique Latham (VA, R), Grace Rigoni, COA (VA, R), Linda Wilcox, COA (VA, R), Dawn Matthews (VA, R), Keri Harkleroad, CRC (VA, R), Julie Pakulski (VA, R), Ann Marie Borthwick (VA, R); Retina Vitreous Surgeons: G. Robert Hampton, MD (PI), Paul Torrisi, MD (Sub-I), Sam Spalding III, MD (Sub-I), Bryan Rutledge, MD (Sub-I), Cynthia Grinnell, RN (C), Fayth DiSano (C, VA, R), Peter Hay (P, O), Lynn Capone (P, O), Jeanne Burke (O), Kenneth Fyles (O), Robert Corey (O), Kelly Harrison (O), Lynn Kwasniewski (VA, R), Tanya Czajak (VA, R); Retinal Consultants of Arizona: Derek Kunimoto, MD (PI), Edward Quinlan, MD (Sub-I), Jack Sipperley, MD (Sub-I), Pravin Dugel, MD (Sub-I), Donald Park, MD (Sub-I), Kean Oh, MD (Sub-I), Judy Liu, MD (Sub-I), Jennifer Cornelius (C, VA, R), Jennifer Cavanagh (C, VA, R), Aimee Scalzo (C, VA, R), Sandra Arenas (C, VA, R), Pat Dawson (C, VA, R), Siru Adhikari (C, VA, R), John Bucci (P, O), Gabe Balea (P, O), John Martin (P, O), Norma Jimenez (P, O), Danielle Smith (P, O), Don Doherty (P, O), Cheryl Tuttle (P, O), Brenda Laizure (P, O), Roger Weckter (VA, R), Mia Chavez (VA, R), Heather Dunlap (VA, R), Joy Wilson (VA, R), Elena Bay (VA, R), Jessica Miner (VA, R), Sarah Mobley (VA, R), Georgina Lopez (VA, R); Rocky Mountain Retina Consultants: Roy Goodart, MD (PI), David Faber, MD (PI), Douglas Mehr, MD (Sub-I), Peter Hurlbut-Miller, MD (Sub-I), Saad Ahmad, MD (Sub-I), Hollie Murphy (C), Jeremy Gleed, RN (C), Donna Knight, COT (P, O, VA, R), Richard Osguthorpe, CRA (P, O, VA, R), Trisha Perkins (P, O, VA, R), Charles Carofanello (VA, R), Barbara Breehl (VA, R), Serena Cleverly (VA, R), Stephanie Colborn (VA, R), Katie Jo Farnsworth (VA, R); Sarasota Retina Institute: Keye Wong, MD (PI), Melvin Chen, MD (PI), John Niffenegger, MD (Sub-I), Marc Levy, MD (Sub-I), Christine Holland (C), Peggy Jelemensky (C), Rosa Miller (P, O), Mark Sneath (P, O, VA, R), Marianne Cottrill (O), Karen Hagin (VA, R), Hasseema Shelton (VA, R); Scheie Eye Institute: Alexander Brucker, MD (PI), Joan DuPont (C), William Nyberg (P, O), James Berger (P, O), Laurel Weeney (O), Sheri Drossner (VA, R), Tanya Metelitsina, MD (VA, R), Monique McRay (VA, R); Southeast Retina Center: Dennis Marcus, MD (PI), Harinderjit Singh, MD (Sub-I), June Benson, COA (C, VA, R), Graciela Zapata (C), Kimbi Overton (C, VA, R), Carrie McAteer (C), Belinda Freeman (P, O), Linda Cortez (P, O), Mark Evans, COT (P, O), Victoria Oldag, COT (O, VA, R), Elizabeth Price, COA (VA, R), Ken Ivey (P, O, VA, R), Judith Hendrickson (VA, R), Carrie Hill (VA, R), Julie Coxville (VA, R), Kasie Leverett (VA, R); Southern California Desert Retina Consultants: Clement Chan, MD (PI), Steven Lin, MD (Sub-I), Asha Nuthi, DO (Sub-I), David Salib, MD (Sub-I), William Koch, COA (C), Celeste Campbell (C), Kelly Sage (C), Eric Dickerson (C), Trina Keith (C), Teri Andresen (C), Tina Wiskirchen (C), Sherri Judd (C), Kimberly Walther (C), Isela Aldana (C), Richard Evans, CRA (P, O), Kenneth Huff, COA (P, O), Sabrina Bretz (P, O), Donna Chesbrough (P, O, VA, R), Dariusz Tarasewicz, MD (VA, R), Sarah Warren (VA, R), Lisa Lehrack, COT (VA, R), Kara Rollins (VA, R), Sandra Castillo (O, VA, R), Lenise Myers (VA, R); St Johns Clinic Eye Specialists: X. Kathryn Sun, MD, PhD (PI), Thomas Essman, MD (Sub-I), Pearlena Hamlet, BSN, RN (C), Susan McLaughlin (C), Brenda Kendall (P, O), Ester Dorweiler (VA, R), Crystal Trythall (VA, R); Texas Retina Associates, Lubbock: Michel Shami, MD (PI), Stephen Smith, MD (Sub-I), Phyllis Pusser, COA (C), Carrie Tarter (C, VA, R), Thom Wentlandt (P, O), Lydia Barragan, COA (O, VA), Erinn Anderson (O), Lynda Squires (VA, R), Travis Bryant (VA), Natalie Garcia (VA, R); Texas Retina Associates, Arlington: David Callanan, MD (PI), Wayne Solley, MD (Sub-I), Gary Fish, MD, JD (Sub-I), Jodi Creighton, COA (C, VA, R), Janay Elmore (C), Cheryl Grimes (C), Michael Henson (C), Patricia Bradley, COT (C), Bob Boleman (P, O), Keith Gray (O), Tina Bell (O), Wendi Sams (O), Yolanda Garcia (VA, R); University of North Carolina Department of Ophthalmology: M. Elizabeth Hartnett, MD (PI), Travis Meredith, MD (Sub-I), Maurice Landers, MD (Sub-I), Seema Garg, MD, PhD (Sub-I), Cassandra Barnhart (C, VA, R), Fatima N'Dure (C, VA, R), Kelly Shields (P, O), Rona Esquejo-Leon (P, O), Debra Cantrell, COA (P, O); University of California, Irvine: Baruch Kuppermann, MD (PI), Heikki Kostamaa, MD (Sub-I), Faisal Jehan, MD (Sub-I), Luiz Hagemann, MD (Sub-I), Bogdan Alexandrescu, MD (Sub-I), Luis de Aguiar Marques, MD (Sub-I), Babak Fardin, MD (Sub-I), Leandro Zacharias, MD (Sub-I), Tony Huynh, MD (Sub-I), Stephanie Lu, MD (Sub-I), Jeff Grijalva, COT, CCRA (C, VA, R), Bret Trump (P, O), Rosie Magallon (VA, R); University of Chicago: Kourous Rezaei, MD (PI), William Mieler, MD (PI), Seenu Hariprasad, MD (PI), James Green, MD (Sub-I), Sumit Bhatia, MD (Sub-I), Eric Chiu, MD (Sub-I), Joseph Benevento, MD (Sub-I), Rama Jager, MD (Sub-I), Richard Lin, MD (Sub-I), Michael Grassi, MD (Sub-I), Theodore Lin, MD (Sub-I), Veeral Sheth, MD (Sub-I), James Strom, RN, BSN (C), Fran Lietz (C), Sophie Gen, CCRP (C), James Marks (C, VA, R), Ian Cadena (C), Kimberly Vernon (C), Stephanie Mallinga (C), Irina Pateva (C), Kashka Pierce (C), Rosita Lopez (C, VA, R), Ashley Baker (C), James Rago (P, O), Louise Sclafani, OD (VA, R), Melissa Pimentel (VA, R); University of Kentucky: P. Andrew Pearson, MD (PI), Peter Blackburn, MD (Sub-I), Michele Reg (C), Phyllis Gillespie (P, O), Mike Hanson (P, O), Philip Moss, COT (VA, R), Susie Craig, COA (VA, R), Felipe Drucker (VA, R), Renee James (VA, R), Leeann Roberts (VA, R), Leah Lewis (VA, R); University of Medicine and Dentistry of New Jersey: Neelakshi Bhagat, MD (PI), Marco Zarbin, MD, PhD (Sub-I), Monique Roy, MD (Sub-I), Rakesh Ahuja (C, VA, R), Nilang Patel (C), Leslie Garay (C), Chetna Shah, MD (C), Analin Alvir (C, VA, R), George Elkomos (C, VA, R), Meiling Chin (C, VA, R), Catherine Fay (C, VA, R), Tatiana Forofonova (P,O), Beth Malpica (P,O), Michael Lazar (O), Amanda Oliveira (VA), Paul Edema-Sillo (VA, R), Catherine Horan (VA, R); University of Michigan: Mark Johnson, MD (PI), Susan Elner, MD (Sub-I), Stephen Saxe, MD (Sub-I), David Zacks, MD (Sub-I), Linda Fournier (C, VA, R), Linda Goings (P, O), Richard Hackel (P, O), Robert Prusak (P, O), Julie Gothrup (VA, R); University of Minnesota: Timothy Olsen, MD (PI), Todd Klesert, MD, PhD (PI), Pamela Rath, MD (Sub-I), Joseph Terry, MD (Sub-I), Sally Cook (C), Ann Holleschau (C), Jamie Walski (C), Pat Harvey (P, O), Mark Cohen (P, O), David Philiph (VA, R), Pamela Patterson (VA, R), Sabrina Rolfer (VA, R); University of Nebraska Medical Center: Eyal Margalit, MD (PI), Thomas Hejkal, MD, PhD (Sub-I), Marguerite Kohlhepp, MD (Sub-I), Monica Milleson (C), Susan Galata (C), Allen Katz, BFA (P, O), Donna Neely (VA, R); University of Pittsburgh Eye Center: Andrew Eller, MD (PI), Evan Waxman, MD (Sub-I), Thomas Friberg, MD (Sub-I), Michael Gorin, MD, PhD (Sub-I), Wael Abdelghani, MD (Sub-I), Barbara Mack (C, VA, R), Barbara Fink (C, VA, R), Faith Bivins (C, VA, R), Phyllis Ostroska (P, O), Janice Campbell (P, O), William Lucas (P, O), Diane Curtin (P, O), Sharon MurajdaJumba (O), Kristy Truman (VA, R), Mina Owlia (VA, R), Linda Calgaro (VA, R); University of Rochester Eye Institute: David DiLoreto Jr, MD, PhD (PI), Mina Chung, MD (Sub-I), Diane O'Brien (C), Nancy Fedick, MS, CCRC (C, VA), Ann Stoutenburg (C, VA), Christine Arcara (C, VA), Gina Crowley (C, O, VA, R), Dawn Lafferty (C, VA), Kari Steinmetz (C, VA, R), Dorothea Castillo (P, O), William Fischer (P, O), Julie Tutko (P, O), Rachel Grunhaus (P, O), Laura Smoral (VA), Malinda Goole (VA, R), Terrance Schaefer (VA, R), Lynne Addams (VA, R), Margaret Embrey (VA, R); University of Wisconsin: Michael Altaweel, MD (PI), T. Michael Nork, MD (Sub-I), Justin Gottlieb, MD (Sub-I), Barbara Blodi, MD (Sub-I), Suresh Chandra, MD (Sub-I), Michael Ip, MD (Sub-I), Thomas Stevens, MD (Sub-I), Jennie Perry-Raymond (C), Barbara Soderling (C, VA, R), Kristine Dietzman (C, VA, R), Denise Krolnik (P, O), John Peterson (P, O), Gene Knutson (P, O), Erika Christianson (VA, R), Alyson Pohlman (Skoldberg) (VA, R), Guy Somers (VA, R), Kathryn Burke (VA, R), Michelle Olson (VA, R), Angela Wealti (VA, R); Valley Retina Institute: Vincent Vann, MD, PhD (PI), Victor Gonzalez, MD (PI), Yu-Tang Su, MD (Sub-I), Nehal Patel, MD (Sub-I), Imtiaz Chaudhry, MD (Sub-I), Jessica Herrera, CCRC (C, O), Dina Garcia (C), Sally Gallegos (C), D. Aaron Guel (C), Sandra Ozuna (C), ReAnna McNames (C), Jackie Saldivar (C), Rosie Corona (P), Daniel Cuellar (O), Cassandra Garza (O), Francisco Gonzalez (O), Alma Herrera (VA, R), Gloria Garcia-Garza, COA (VA, R), Maria Martinez (VA, R), Marlene Lopez (VA, R), Maria Trevino (VA, R), Monica Cantu (VA, R); Vanderbilt University Medical Center: Paul Sternberg, MD (PI), Franco Recchia, MD (Sub-I), Anita Agarwal, MD (Sub-I), Genise Mofield (C), Sandy Owings (C, VA, R), Mary Taylor-Ward (C, VA, R), Kamila Kinder (C, VA, R), Gail Lanier (C), Christine Franklin (C, VA, R), Tony Adkins (P, O), G. E. Rocky Munn Jr (P, O); Virginia Eye Institute: Byron Ladd, MD (PI), James Combs, MD (Sub-I), Eleanore Ebert, MD (Sub-I), George Sanborn, MD (Sub-I), Melissa Vaughan (C, VA, R), Karen Sullivan (C), Elona Miller-Long (P, O), Michael Palcynski (P, O), Mark Zalewski (P, O), Megan Walsh (P, O), Christopher Wong (P, O), Chad Hoyle-Harris (P, O), Kristin Spawn (VA, R), Robin Driver (VA, R); Vision Research Foundation: Alan Ruby, MD (PI), George Williams, MD (Sub-I), Antonio Capone, MD (Sub-I), Tarek Hassan, MD (Sub-I), Michael Trese, MD (Sub-I), Bruce Garretson, MD (Sub-I), Sunita Yedavally, MD (Sub-I), Virginia Regan (C, VA, R), Candice DuLong (C, VA, R), Craig Bridges (P, O), Tony Medina, CRA (P, O), Thomas Treuter (P, O), Marissa Guenther (P, O), Fran McIver (P, O), Peter Roberts (P, O), Christina Consolo (P, O), Mary Zajechowski (VA, R), Lisa Staples (VA, R); Vitreoretinal Consultants: David Brown, MD (PI), Tien Wong, MD (Sub-I), Richard Fish, MD (Sub-I), Rosa Kim, MD (Sub-I), Matthew Benz, MD (Sub-I), Eric Chen, MD (Sub-I), Jennifer Hallett (C), Xiaozhou Sher Tang (C), Celia Hutchison (C), Rebecca De La Garza (C), Leslie Jasso (C), Andrew Strickler (C), Jennifer Norris (C), Margaret Rodriguez (C), Leslie Garcia (C), Laura Shawver (C), Eric Kegley (P, O), Amanda Williamson (P, O), Lela Johnstone, CRA (P, O), Marriner Skelly (P, O), Mark Evans (P, O), Hang Bui (O), Beau Richter (O), Karin Mutz (VA, R), Amanda Kimbrough (VA, R), Shayla Hay (VA, R), Dallas Kubecka (VA, R), Debra Goates (VA, R), Stacy McGilvra (VA, R); VitreoRetinal Surgery: Sundeep Dev, MD (PI), Robert Ramsay, MD (Sub-I), Edwin Ryan, MD (Sub-I), David Williams, MD (Sub-I), Robert Mittra, MD (Sub-I), Herbert Cantrill, MD (Sub-I), Jill Johnson, MD (Sub-I), Steven Bennett, MD (Sub-I), Polly Quiram, MD (Sub-I), Neal Oestreich, COT (C, O, VA), Julianne Enloe, CCRP (C, VA), Holly Cheshier, COA (P), Julene Gamblain, COT (P), Debbie Erickson, COT (O), Tori Jones, COT (O), Gennaro Follano, COT (O), Martha Moos, COMT (VA, R), Ryan Neist, COMT (VA, R), Kristen Hodgden, COMT (VA, R), Lisa Mayleben, COMT (VA, R), Stephanie Schindledecker, OA (VA, R); Wake Forest University Eye Center: Craig Greven, MD (PI), M. Madison Slusher, MD (Sub-I), Joan Fish, RN, CCRC (C, VA, R), Marshall Tyler, CRA (P, O), David Miller, CRA (P, O), Mark Clark, CRA (P, O), Frances Ledbetter, LPN, COT (VA, R), Cara Everhart, COA (VA, R), Lori Cooke, RN (VA, R); West Coast Retina Medical Group, Inc: J. Michael Jumper, MD (PI), H. Richard McDonald, MD (Sub-I), Everett Ai, MD (Sub-I), Robert Johnson, MD (Sub-I), Sam Yang, MD (Sub-I), Arthur Fu, MD (Sub-I), Brandi Teske, COA (C, O, VA, R), Sarah Huggans (P, O, VA, R), Jeremy Miller (P, O), Sean Grout (P), Rona Lynn Esquejo (P, O), Amanda Bye (P, O), Dawn Ryan (O), Margaret Stolarczuk, OD (VA, R), Silvia Linares (VA, R); West Texas Retina Consultants: Sunil Patel, MD (PI), S. Young Lee, MD (Sub-I), Brandi Dunn, RN (C, P, O, VA, R), Kristen Garcia, COA (C, O), Larry Varnadore (P), Brenda Arrington, COA (P, O, VA, R), Tammy Jones, COA (P, O, VA, R), Leah Adams (O, VA, R), Angela Jaimes (O, VA, R), Misty McArthur (O, VA, R), Gwyn Nafe (VA, R), Tamara Bartlett (VA, R); Wilmer Eye Institute: Quan Nguyen, MD, MSc (PI), Sharon Solomon, MD (Sub-I), Julia Haller, MD (Sub-I), Andrew Schachat, MD (Sub-I), Ingrid Zimmer-Galler, MD (Sub-I), James Handa, MD (Sub-I), Susan Bressler, MD (Sub-I), Peter Gehlbach, MD (Sub-I), Daniel Finkelstein, MD (Sub-I), Jennifer Sung, MD (Sub-I), Morton Goldberg, MD (Sub-I), Peter Campochiaro, MD (Sub-I), Ovais Shaikh, MD (C), Amy LeBow (C), Karen Klima (C, VA, R), Harold Henson (C), Anita Baird (C, VA, R), Judith Belt (P), Jackie McDonald (P), Mark Herring (P), Dave Emmert (P), Rachel Falk (P), Dennis Cain (P), Syed Mah-mood Shah (O), Janis Grawl (O), Jennifer Simmons-Denton (O, VA, R), Pamela Singletary (VA, R), Lisa Greer (VA, R).
Footnotes
Group Information: A complete list of the SCORE Study Research Group members is provided on pages 1112 and 1113.
Financial Disclosure: None reported.
Additional Information: The eTable is available at http://www.archophthalmol.com.
REFERENCES
- 1.David R, Zangwill L, Badarna M, Yassur Y. Epidemiology of retinal vein occlusion and its association with glaucoma and increased intraocular pressure. Ophthalmologica. 1988;197(2):69–74. doi: 10.1159/000309923. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. Arch Ophthalmol. 1996;114(10):1243–1247. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Moss SE, Meuer SM, Klein BEK. The 15-year cumulative incidence of retinal vein occlusion. Arch Ophthalmol. 2008;126(4):513–518. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15 year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142(4):539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Central Vein Occlusion Study Group Baseline and early natural history report. Arch Ophthalmol. 1993;111(8):1087–1095. doi: 10.1001/archopht.1993.01090080083022. [DOI] [PubMed] [Google Scholar]
- 6.The Central Vein Occlusion Study Group Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115(4):486–491. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- 7.The Central Vein Occlusion Study Group Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion: the Central Vein Occlusion Study Group M report. Ophthalmology. 1995;102(10):1425–1433. doi: 10.1016/s0161-6420(95)30849-4. [DOI] [PubMed] [Google Scholar]
- 8.Opremcak EM, Bruce RA, Lomeo MD, Ridenour CD, Letson AD, Rehmar AJ. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina. 2001;21(5):408–415. doi: 10.1097/00006982-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Zambarakji HJ, Ghazi-Nouri S, Schadt M, Bunce C, Hykin PG, Charteris DG. Vitrectomy and radial optic neurotomy for central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2005;243(5):397–405. doi: 10.1007/s00417-004-1046-0. [DOI] [PubMed] [Google Scholar]
- 10.Glacet-Bernard A, Kuhn D, Vine AK, Oubraham H, Coscas G, Soubrane G. Treatment of recent onset central retinal vein occlusion with intravitreal tissue plasminogen activator: a pilot study. Br J Ophthalmol. 2000;84(6):609–613. doi: 10.1136/bjo.84.6.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elman MJ, Raden RZ, Carrigan A. Intravitreal injection of tissue plasminogen activator for central retinal vein occlusion. Trans Am Ophthalmol Soc. 2001;99:219–223. doi: 10.1097/00006982-200306000-00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa M, Kumagai K, Ogino N, Uemura A, Larson E. Long-term visual outcomes of vitrectomy for cystoid macular edema due to nonischemic central retinal vein occlusion. Eur J Ophthalmol. 2006;16(6):841–846. doi: 10.1177/112067210601600609. [DOI] [PubMed] [Google Scholar]
- 13.Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26(3):279–284. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Glacet-Bernard A, Coscas G, Chabanel A, Zourdani A, Lelong F, Samama MM. A randomized, double-masked study on the treatment of retinal vein occlusion with troxerutin. Am J Ophthalmol. 1994;118(4):421–429. doi: 10.1016/s0002-9394(14)75791-5. [DOI] [PubMed] [Google Scholar]
- 15.Wroblewski JJ, Wells JA, III, Adamis AP, et al. Pegaptanib in Central Retinal Vein Occlusion Study Group. Pegaptanib sodium for macular edema secondary to central retinal vein occlusion. Arch Ophthalmol. 2009;127(4):374–380. doi: 10.1001/archophthalmol.2009.14. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg PB, Martidis A, Rogers AH, Duker JS, Reichel E. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2002;86(2):247–248. doi: 10.1136/bjo.86.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusion. Arch Ophthalmol. 2002;120(9):1217–1219. [PubMed] [Google Scholar]
- 18.Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240(9):782–783. doi: 10.1007/s00417-002-0529-0. [DOI] [PubMed] [Google Scholar]
- 19.Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol. 2003;136(3):419–425. doi: 10.1016/s0002-9394(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 20.Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46(9):1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 21.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. J Biol Chem. 1999;274(33):23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 22.Senger DR, Galli SJ, Dvorak AM, Peruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor (VPF) that promotes accumulation of as-cites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 23.Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997;12(1):99–109. [PubMed] [Google Scholar]
- 24.Pe'er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998;105(3):412–416. doi: 10.1016/S0161-6420(98)93020-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Bao S, Lai D, Rapkins RW, Gillies MC. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. 2008;57(4):1026–1033. doi: 10.2337/db07-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Wang Y, Gao L, Li X, Li M, Guo J. Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expression. Biol Pharm Bull. 2008;31(8):1541–1546. doi: 10.1248/bpb.31.1541. [DOI] [PubMed] [Google Scholar]
- 27.Lee HB, Pulido JS, McCannel CA, Buettner H. Role of inflammation in retinal vein occlusion. Can J Ophthalmol. 2007;42(1):131–133. doi: 10.1139/i06-101. [DOI] [PubMed] [Google Scholar]
- 28.Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc Natl Acad Sci USA. 2008;105(12):4862–4867. doi: 10.1073/pnas.0709102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn HW, Jr, Scott IU. Intravitreal triamcinolone acetonide for macular edema associated with diabetic retinopathy and venous occlusive disease: it's time for clinical trials. Arch Ophthalmol. 2005;123(2):258–259. doi: 10.1001/archopht.123.2.258. [DOI] [PubMed] [Google Scholar]
- 30.Blumenkranz MS. New therapy for central retinal vein occlusion: are intravitreal steroids a possible answer? Arch Ophthalmol. 2005;123(2):259–261. doi: 10.1001/archopht.123.2.259. [DOI] [PubMed] [Google Scholar]
- 31.The SCORE Study Research Group A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 6. Arch Ophthalmol. 2009;127(9):1115–1128. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 33.The Age-Related Eye Disease Research Group The age-related eye disease study (AREDS) system for classifying cataracts from photographs. Am J Ophthalmol. 2001;131(2):167–175. doi: 10.1016/s0002-9394(00)00732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chieh JJ, Roth DB, Liu M, et al. Intravitreal triamcinolone acetonide for diabetic macular edema. Retina. 2005;25(7):828–834. doi: 10.1097/00006982-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Jonas JB, Kreissig I, Spandau UH, Harder B. Infectious and noninfectious endophthalmitis after intravitreal high-dosage triamcinolone acetonide. Am J Ophthalmol. 2006;141(3):579–580. doi: 10.1016/j.ajo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Kim JE, Pollack JS, Miller DG, Mittra RA, Spaide RF, Isis Study Group ISIS-DME: a prospective, randomized, dose-escalation intravitreal steroid injection study for refractory diabetic macular edema. Retina. 2008;28(5):735–740. doi: 10.1097/IAE.0b013e318163194c. [DOI] [PubMed] [Google Scholar]
- 37.Then Bergh F, Grasser A, Trenkwalder C, Backmund H, Holsboer F, Rupprecht R. Binding characteristics of the glucocorticoid receptor in peripheral blood lymphocytes in multiple sclerosis. J Neurol. 1999;246(4):292–298. doi: 10.1007/s004150050349. [DOI] [PubMed] [Google Scholar]
- 38.Schottelius A, Wedel S, Weltrich R, et al. Higher expression of glucocorticoid receptor in peripheral mononuclear cells in inflammatory bowel disease. Am J Gastroenterol. 2000;95(8):1994–1999. doi: 10.1111/j.1572-0241.2000.02188.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen SD, Lochhead J, Patel CK. Diffuse intraocular dispersion of triamcinolone particles as a cause of sterile endophthalmitis [letter] Arch Ophthalmol. 2004;122(11):1733. doi: 10.1001/archopht.122.11.1733-a. [DOI] [PubMed] [Google Scholar]
- 40.Moshfeghi DM, Kaiser PK, Bakri SJ, et al. Presumed sterile endophthalmitis following intravitreal triamcinolone acetonide injection. Ophthalmic Surg Lasers Imaging. 2005;36(1):24–29. [PubMed] [Google Scholar]
- 41.Ip MS, Oden NL, Scott IU, et al. SCORE Study Report 3: study design and baseline characteristics. Ophthalmology. doi: 10.1016/j.ophtha.2009.03.022. published online July 21, 2009. doi:10.1016/j.ophtha.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massin P, Erginay A, Haouchine B, Mehidi AB, Paques M, Gaudric A. Retinal thickness in healthy and diabetic subjects measures using optical coherence tomography mapping software. Eur J Ophthalmol. 2002;12(2):102–108. doi: 10.1177/112067210201200205. [DOI] [PubMed] [Google Scholar]
- 43.Fundus Photograph Reading Center Imaging Procedures for the Standard Care vs COrticosteroid for REtinal Vein Occlusion (SCORE) Study . National Eye Institute; Bethesda, MD: 2008. (NTIS order number PB2008-113740). [Google Scholar]
- 44.Domalpally A, Blodi BA, Scott IU, et al. SCORE Study Report 4: grading methodology of optical coherence tomography images. Arch Ophthalmol. doi: 10.1001/archophthalmol.2009.277. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott IU, VanVeldhuisen PC, Oden NL, et al. SCORE Study Investigator Group. SCORE Study Report 1: baseline association between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology. 2009;116(3):504–512. doi: 10.1016/j.ophtha.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ip MS, Gottlieb JL, Kahana A, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol. 2004;122(8):1131–1136. doi: 10.1001/archopht.122.8.1131. [DOI] [PubMed] [Google Scholar]
- 47.Marcus R, Peritz E, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63(3):655–660. [Google Scholar]
- 48.Scott IU, Oden NL, VanVeldhuisen PC, et al. SCORE Study Report 7: incidence of intravitreal silicone oil droplets associated with staked-on versus luer cone syringe design. Am J Ophthalmol. doi: 10.1016/j.ajo.2009.06.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vane J, Botting R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987;1(2):89–96. [PubMed] [Google Scholar]
- 50.Barkana Y, Belkin M. Neuroprotection in ophthalmology: a review. Brain Res Bull. 2004;62(6):447–453. doi: 10.1016/S0361-9230(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 51.Mohamed Q, McIntosh RL, Saw SM, Wong TY. Interventions for central retinal vein occlusion. Ophthalmology. 2007;114(3):507–519, 524. doi: 10.1016/j.ophtha.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Freund KB, Laud K, Eandi CM, Spaide RF. Silicone oil droplets following intravitreal injection. Retina. 2006;26(6):701–703. doi: 10.1097/01.iae.0000223177.08438.2b. [DOI] [PubMed] [Google Scholar]
- 53.Bakri SJ, Ekdawi NS. Intravitreal silicone oil droplets after intravitreal drug injections. Retina. 2008;28(7):996–1001. doi: 10.1097/IAE.0b013e31816c6868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.